ABSTRACT
Hepatitis C virus (HCV)-induced chronic liver disease is a leading cause of hepatocellular carcinoma (HCC). However, the molecular mechanisms underlying HCC development following chronic HCV infection remain poorly understood. MicroRNAs (miRNAs) play an important role in homeostasis within the liver, and deregulation of miRNAs has been associated with liver disease, including HCC. While host miRNAs are essential for HCV replication, viral infection in turn appears to induce alterations of intrahepatic miRNA networks. Although the cross talk between HCV and liver cell miRNAs most likely contributes to liver disease pathogenesis, the functional involvement of miRNAs in HCV-driven hepatocyte injury and HCC remains elusive. Here we combined a hepatocyte-like cell-based model system, high-throughput small RNA sequencing, computational analysis, and functional studies to investigate HCV-miRNA interactions that may contribute to liver disease and HCC. Profiling analyses indicated that HCV infection differentially regulated the expression of 72 miRNAs by at least 2-fold, including miRNAs that were previously described to target genes associated with inflammation, fibrosis, and cancer development. Further investigation demonstrated that the miR-146a-5p level was consistently increased in HCV-infected hepatocyte-like cells and primary human hepatocytes, as well as in liver tissue from HCV-infected patients. Genome-wide microarray and computational analyses indicated that miR-146a-5p overexpression modulates pathways that are related to liver disease and HCC development. Furthermore, we showed that miR-146a-5p has a positive impact on late steps of the viral replication cycle, thereby increasing HCV infection. Collectively, our data indicate that the HCV-induced increase in miR-146a-5p expression both promotes viral infection and is relevant for pathogenesis of liver disease.
IMPORTANCE HCV is a leading cause of chronic liver disease and cancer. However, how HCV induces liver cancer remains poorly understood. There is accumulating evidence that a viral cure does not eliminate the risk for HCC development. Thus, there is an unmet medical need to develop novel approaches to predict and prevent virus-induced HCC. miRNA expression is known to be deregulated in liver disease and cancer. Furthermore, miRNAs are essential for HCV replication, and HCV infection alters miRNA expression. However, how miRNAs contribute to HCV-driven pathogenesis remains elusive. Here we show that HCV induces miRNAs that may contribute to liver injury and carcinogenesis. The miR-146a-5p level was consistently increased in different cell-based models of HCV infection and in HCV patient-derived liver tissue. Furthermore, miR-146a-5p increased HCV infection. Collectively, our data are relevant to understanding viral pathogenesis and may open perspectives for novel biomarkers and prevention of virus-induced liver disease and HCC.
INTRODUCTION
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease and hepatocellular carcinoma (HCC) worldwide. While there is no vaccine to prevent HCV infection, tremendous progress has been made in the management of chronic hepatitis C (1). However, recent evidence indicates that individuals who have achieved viral cure remain at risk for development of HCC (2). This suggests that the virus triggers changes in host cell networks that drive liver disease and carcinogenesis and that persist even after viral elimination. However, the molecular mechanisms underlying HCV-induced liver disease and HCC development remain poorly understood.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression at a posttranscriptional level. They play an important role in cellular homeostasis within the liver, and hence alterations in intrahepatic miRNA networks have been associated with liver disease, including fibrosis, cirrhosis, and HCC (3, 4). Notably, HCV infection is intricately linked to miRNAs, as the most abundant miRNA of the liver, miR-122, is essential for HCV replication (4–7). In addition to using host miRNAs for viral replication, HCV may also modulate host cell miRNA profiles to favor its persistence and thereby induce liver disease (8). Accumulating evidence highlights a complex cross talk between HCV and miRNAs in liver fibrosis, steatosis, and HCC (8). However, the functional involvement of miRNAs in HCV-mediated hepatocyte injury and liver disease pathogenesis remains to be elucidated.
Several pieces of evidence have shown HCV-mediated deregulation of miRNAs in hepatoma cell lines (9). Although a few ex vivo studies have investigated miRNA patterns in HCV-associated HCC tissues, no clear picture has yet emerged regarding the modulation of miRNAs upon HCV-induced liver disease. Indeed, these studies differ largely in their methodological approaches, sampling sizes and features, and the ethnicity of patients (9, 10). Most importantly, only limited information is available about the differential expression of miRNAs in preneoplastic liver nodules compared to HCC (11, 12), underscoring the current lack of a proper model that closely recapitulates the progression of HCV-associated HCC. To date, the majority of cell culture models to study the molecular virology and cell biology of HCV infection have relied on human hepatoma cells (reviewed in reference 13). However, given their transformed phenotype, these model systems may preclude systematic identification of the changes in host cell circuits that are relevant for virus-induced liver disease. Human hepatocytes are the natural target cells of HCV, but primary cells are not well suited for large-scale and long-term analysis of infection. It was reported previously that dimethyl sulfoxide (DMSO)-induced differentiation of Huh7-derived human hepatoma cell lines induces a hepatocyte-like phenotype and that these cells are amenable to long-term HCV infection (14–16). In this study, we combined this hepatocyte-like cell-based model system, high-throughput small RNA sequencing (RNA-Seq), computational analysis, and functional studies to investigate HCV-miRNA interactions that may contribute to liver disease and HCC.
MATERIALS AND METHODS
Reagents.
DMSO was obtained from Sigma-Aldrich. DAPI (4′,6-diamidino-2-phenylindole) was obtained from Life Technologies. The HCV E2-specific AP33 antibody (mouse) has been described previously (17). The inhibitor 2-amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-(4-piperidinyl)-3-pyridinecarbonitrile (ACHP) was obtained from Tocris. The direct-acting antivirals (DAAs) daclatasvir (DCV; NS5A inhibitor), sofosbuvir (SOF; NS5B inhibitor), and simeprevir (SMV; NS3/4A inhibitor) were synthesized by Acme Bioscience. A miR-146a-5p mimic was obtained from Sigma-Aldrich. Anti-miR-146a-5p was synthesized by Integrated DNA Technology (IDT). A nontargeting control small interfering RNA (siRNA), a miR-122-5p mimic, anti-miR-122, and siRNAs targeting CD81 or apolipoprotein E (ApoE) were from Dharmacon (GE Healthcare). siRNA targeting signal transducer and activator of transcription 3 (STAT3) was obtained from Qiagen.
Cell culture.
The sources and culture conditions for Huh7.5.1, Huh7, and HEK293T cells were described previously (18). For proliferation arrest and differentiation in long-term infection experiments, 2.5 × 104 Huh7.5.1 cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 1% DMSO as described for Huh7 and Huh7.5 cells (14–16). Primary human hepatocytes (PHH) were isolated and cultured as described previously (19).
Human subjects.
Human material from patients undergoing liver biopsy or surgical resection was obtained with informed consent from all patients. The respective protocols were approved by the Ethics Committee of the University Hospital Basel, Switzerland (EKBB 13, December 2004; liver biopsy specimens), and the University of Strasbourg Hospitals, France (CPP 10-17; primary human hepatocytes).
Liver biopsy specimens.
Histopathological grading and staging of the liver biopsy specimens according to the Metavir classification system were performed at the Pathology Institute of the University Hospital Basel as described previously (20) and are summarized in Table 1. All patients who donated liver tissue were males between 30 and 70 years old and females between 31 and 76 years old (Table 1).
TABLE 1.
Characteristics of patients and patient-derived liver biopsies included in this studya
| Biopsy ID | HCV genotype | Viral load (106 IU/ml) | Diagnosis | Metavir score | Age at biopsy (yr) | Gender |
|---|---|---|---|---|---|---|
| C270 | 1b | 1.8 | Chronic HCV infection | A3/F3 | 76 | F |
| C238 | 1b | ND | Chronic HCV infection | A1/F1 | 54 | F |
| C257 | 1a | 0.1 | Chronic HCV infection | A1/F1 | 30 | M |
| B823 | 4 | 3.2 | Chronic HCV infection | A1/F1 | 50 | M |
| C669 | 3a | 19.9 | Chronic HCV infection | A2/F2 | 48 | M |
| C672 | 4 | 11.9 | Chronic HCV infection | A1/F1 | 45 | M |
| C561 | 1b | 1.1 | Chronic HCV infection | A3/F3 | 70 | M |
| C579 | 1a | 1.10 | Chronic HCV infection | A2/F3 | 50 | M |
| C703 | 1b | ND | Chronic HCV infection | A2/F2 | 48 | F |
| C716 | 3a | 3.5 | Chronic HCV infection | A3/F4 | 49 | F |
| C582 | 1a | 2.9 | Chronic HCV infection | A2/F2 | 60 | F |
| C748 | 1a | 9.4 | Chronic HCV infection | A1/F2 | 56 | M |
| C567 | 1b | 1.3 | Chronic HCV infection | A2/F3 | 64 | F |
| C757 | 1 | 2 | Chronic HCV infection | A1/F2 | 71 | F |
| C685 | 3a | 4.7 | Chronic HCV infection | A1/F2 | 55 | F |
| C651 | 4 | 1.2 | Chronic HCV infection | A1/F1 | 55 | M |
| C663 | 4 | ND | Chronic HCV infection | A2/F3 | 55 | M |
| C766 | 1a | 0.4 | Chronic HCV infection | A1/F1 | 40 | F |
| C262 | NA | NA | ASH, cirrhosis | NA | 59 | F |
| C344 | NA | NA | Steatosis (5%), siderosis grade I | NA | 43 | M |
| C332 | NA | NA | NASH | NA | 49 | F |
| C330 | NA | NA | Minimal steatosis | NA | 47 | M |
| C305 | NA | NA | Minimal unspecific hepatitis | NA | 44 | M |
| C298 | NA | NA | Steatohepatitis (NASH) | NA | 50 | M |
| C237 | NA | NA | Steatosis, ASH, siderosis | NA | 51 | M |
| C187 | NA | NA | Normal liver parenchyma | NA | 31 | F |
| C145 | NA | NA | Normal liver parenchyma | NA | 43 | F |
ASH, alcoholic steatohepatitis; F, female; M, male; NA, not applicable; NASH, nonalcoholic steatohepatitis; ND, not determined.
HCV production and infection assays.
HCV pseudoparticles (HCVpp) expressing envelope glycoproteins from strain J6 (genotype 2a) and harboring a luciferase reporter gene were generated as described previously (21, 22). Huh7.5.1 cells were incubated with HCVpp, and HCV entry was assessed by measuring luciferase activity 3 days later, as described previously (19). Vesicular stomatitis virus pseudoparticles (VSVpp) were assessed in parallel as an unrelated virus control (21, 22). Cell culture-derived (HCVcc) Jc1, Jc1E2FLAG, and JcR2a (all derived from the J6/JFH1 chimera Jc1 [genotype 2a/2a]) were generated in Huh7.5.1 cells as described previously (23–25). The infectivity of HCVcc was determined by calculating the 50% tissue culture infective dose (TCID50) as described previously (26). Titers of HCVcc Jc1 and JcR2a used across experiments were ∼106/ml, and the titer of Jc1E2FLAG for the RNA-Seq experiment was 1.6 × 107/ml. Fifty percent confluent Huh7.5.1 cultures were infected with HCVcc Jc1 or Jc1E2FLAG for 7 days. Eighty percent confluent PHH monolayers were exposed to HCVcc Jc1 infection for 3 days. The FLAG peptide (100 μg/ml) or cell culture supernatants from mock-electroporated cells were used for control experiments. HCV infection was assessed by reverse transcription-quantitative PCR (RT-qPCR) analysis of intracellular HCV RNA, immunostaining using the HCV E2-specific AP33 antibody, or determination of luciferase activity as described previously (25, 27, 28).
RNA purification.
For total RNA purification, Huh7.5.1 cells, PHH, and human liver tissues were lysed using Tri reagent (MRC) and RNA purified either according to the manufacturer's instructions or in combination with a Direct-zol RNA miniprep kit (Zymo Research). RNA quantity and quality were assessed using a NanoDrop spectrophotometer (Thermo Scientific) and a Bioanalyzer 2100 instrument (Illumina), with a quality cutoff RNA integrity number of >8. HCV RNA was purified from cell supernatants by use of a QIAamp viral RNA minikit (Qiagen) according to the manufacturer's instructions.
Small RNA library preparation and deep sequencing.
The preparation and sequencing of small RNA libraries were performed by the IGBMC Microarray and Sequencing Platform (Strasbourg, France) as described previously (29). Libraries were prepared using 1.2 μg of total RNA, which was processed using a TruSeq RNA sample prep kit according to the Illumina protocol. Large-scale small RNA-Seq was performed using an Illumina HiSeq 2500 instrument, with a read length of 50 nucleotides (nt).
RT-qPCR.
Quantitative real-time PCRs were established as described previously (30), using a Corbett Rotor-gene 6000 real-time PCR system (Qiagen). For mature miRNA analysis, total RNA (100 ng) was first polyadenylated and reverse transcribed by using a miScript II RT system (Qiagen) according to the manufacturer's instructions. The obtained cDNA (2 μl of a 10−1 dilution) was subjected to RT-qPCR using a miScript SYBR green PCR kit (Qiagen). Primers were the mature miRNA sequence for the forward primers (as indicated at the Sanger miRBase database, v19.0 [31]) and the universal miScript primer (Qiagen) for the reverse primer. To check for RT-qPCR efficiency, standard curves were performed using 10-fold dilution series of total RNA from naive Huh7.5.1 cells. Data were analyzed using the ΔΔCT method, using small nucleolar RNA, C/D box 61 (SNORD61) as an endogenous reference and the noninfected samples as a calibrator (32).
miRNA transfection.
Reverse transfection was performed using RNAiMax reagent according to the manufacturer's instructions (Life Technologies). Unless otherwise indicated, all siRNAs and miRNA mimics were used at a concentration of 20 nM, while anti-miRNAs were used at a concentration of 100 nM.
Small-molecule inhibitor and DAA treatments.
To inhibit nuclear factor kappa B (NF-κB) signaling, control or miR-146a-5p-transfected differentiated Huh7 or Huh7.5.1 cells were incubated in the presence or absence of 6.25 μM ACHP for 24 h. Subsequently, cells were infected using HCVcc (Jc1) for 5 days and then lysed, and RNA was purified for miRNA expression analysis. Noninfected cells were analyzed in parallel as controls. The concentration of ACHP used here was not cytotoxic to Huh7.5.1 cells, as assessed using Presto Blue reagent according to the manufacturer's recommendations (Life Technologies). To counteract HCV infection in vitro, differentiated Huh7.5.1 cells were infected with HCVcc (Jc1E2FLAG) for 7 days and subsequently treated for 4 weeks with a cocktail of 5 nM DCV, 1 μM SOF, and 0.5 μM SMV. After two additional weeks of washout, HCV RNA loads and miR-146a-5p expression levels in cell supernatants and cell lysates, respectively, were assessed by RT-qPCR as described above. HCV-infected untreated cells were analyzed in parallel as controls. Cell culture media containing or not containing DAAs were changed three times per week.
Analysis of cell proliferation and cell cycle.
Differentiated Huh7.5.1 cells were reverse transfected with a nontargeting control siRNA, a miR-146a-5p mimic, or siSTAT3 as described above. At 24, 48, 72, and 96 h posttransfection, cells were detached and cell proliferation was assessed by cell counting using a Bio-Rad TC20 automated cell counter (Bio-Rad). To analyze the cell cycle, cells were fixed at 24, 48, 72, and 96 h posttransfection in cold 70% ethanol for 30 min, and DNA staining was performed for 30 min at 37°C, using 50 μg ml−1 propidium iodide (Sigma-Aldrich) and 0.1 mg ml−1 RNase A (Sigma-Aldrich) in phosphate-buffered saline (PBS). Samples were acquired on a FACScan flow cytometer with a doublet discrimination module on the FL3 channel (Becton Dickinson).
Microarray assay.
Genome-wide mRNA profiling in differentiated hepatocyte-like Huh7.5.1 cells overexpressing miR-146a-5p was performed using a GeneChip Human Gene 1.0 ST array (Affymetrix TSA) at the IGBMC Microarray and Sequencing Platform (Illkirch, France). Briefly, biotinylated single-stranded cDNA targets were prepared as described previously (33). Following fragmentation and end labeling, hybridization of 2.07 μg of cDNAs was performed using a GeneChip fluidics station 450 instrument (Affymetrix) for 16 h at 45°C. The array strips were imaged using a GeneChip Scanner 3000 7G instrument (Affymetrix) at a resolution of 0.7 μm. Three biological replicates were run for each condition.
Effect of miR-146a on the HCV life cycle.
To assess the impact of miR-146a on the viral life cycle, Huh7.5.1 cells were transfected with a miR-146a-5p mimic, anti-miR-146a-5p, or a nontargeting control siRNA as described above. siCD81, anti-miR-122, and siApoE were used as controls to inhibit HCV entry, translation/replication, and assembly, respectively, and a miR-122 mimic was used to promote HCV translation/replication (5, 22, 34, 35). To test the involvement of miR-146a-5p in HCV entry, cells were incubated with HCVpp at 48 h posttransfection. HCV entry was assessed by measuring intracellular luciferase activity 72 h later, as described previously (19, 22). To study the effect of miR-146a-5p on HCV translation, cells were transfected with the indicated siRNAs, miRNAs, and anti-miRNAs and then, 48 h later, with HCV RNA (JcR2a) bearing a Renilla luciferase reporter gene sequence. HCV RNA translation was assessed by measuring intracellular luciferase activity after 4 h (25, 28, 36). To assess the relevance of miR-146a-5p for viral replication, cells were electroporated with JFH1/ΔE1E2 RNA carrying a firefly luciferase reporter gene (23). Cells allowing HCV replication were transfected at 48 h postelectroporation, and HCV replication was assessed by measuring intracellular firefly luciferase activity 48 h later. Luciferase activity data were normalized to protein amounts, which were determined for the same lysates by using a DC protein assay (Bio-Rad) according to the manufacturer's instructions. To assess the effect of miR-146a-5p on HCV assembly/egress and infectivity, cells were transfected 48 h prior to infection with HCVcc (Jc1) for 4 h. HCVcc were then removed, and cells were incubated with complete medium for 48 h. Cells were lysed to determine intracellular HCV RNA levels and infectivity titers as described previously (26, 37). Extracellular HCV RNA levels and infectivity titers in cell culture supernatants were analyzed as described previously (26, 37). Specific infectivity was estimated as the ratio of TCID50/HCV RNA as described previously (28).
Bioinformatic and statistical analyses.
Processing and annotation of small RNA sequences have been described previously (20). Briefly, sequencing reads were preprocessed using FASTX_Toolkit (http://hannonlab.cshl.edu/fastx_toolkit). Reads of 15 to 40 nt were then mapped to the human genome (assembly version hg19; University of California Santa Cruz [UCSC] repository) by using Bowtie v0.12.8 (38). Small RNAs were annotated and quantified using ncPRO-seq software, version 1.5.1 (39), which uses miRBase release 20 and Rfam v.11. miRNA measures were normalized using DESeq2 software (40). Only miRNAs present in at least one of the libraries, with a minimum of 10 reads, were kept for further analysis of differential miRNA expression, which was performed using DESeq2 software (40).
Raw data (CEL intensity files) from genome-wide mRNA profiling were extracted from the scanned images by using Affymetrix GeneChip Command Console (AGCC), version 4.0. CEL files were further processed with Affymetrix Expression Console software, version 1.3.1, to calculate probe set signal intensities by use of robust multiarray average (RMA) algorithms with default settings. Differentially expressed genes were selected using the FCROS method (41), with the selection error set at 0.03. Functional annotation and gene network analyses of differentially regulated genes were performed by using Ingenuity Pathway Analysis (IPA; Qiagen) software. In parallel, modulation of molecular pathways under conditions of miR-146a-5p overexpression was determined in Molecular Signature Database (MSigDB; ver.4.0) (42) by using gene set enrichment analysis (GSEA) (43).
miRNA targeting analyses were performed using MiRSystem (44), which allows retrieval of both experimentally validated and predicted miRNA targets.
Statistical analyses were performed using the GraphPad Prism v.6 package. The normality of data set distributions was assessed using the D'Agostino and Pearson omnibus and the Shapiro-Wilk normality tests. Data sets with normal distributions are presented as means ± standard deviations (SD) and were analyzed by the unpaired t test with Welch's correction or one-way analysis of variance (ANOVA), as indicated in the figure legends. All other data sets are presented as medians with upper and lower quartiles and were analyzed using the nonparametric two-tailed Mann-Whitney test or Kruskal-Wallis test, as indicated in the figure legends.
Accession number.
Genome-wide mRNA and small RNA-Seq data reported in this paper were submitted to the Gene Expression Omnibus database under accession number GSE79341.
RESULTS
Persistent HCV infection induces the upregulation of miR-146a-5p in hepatocytes.
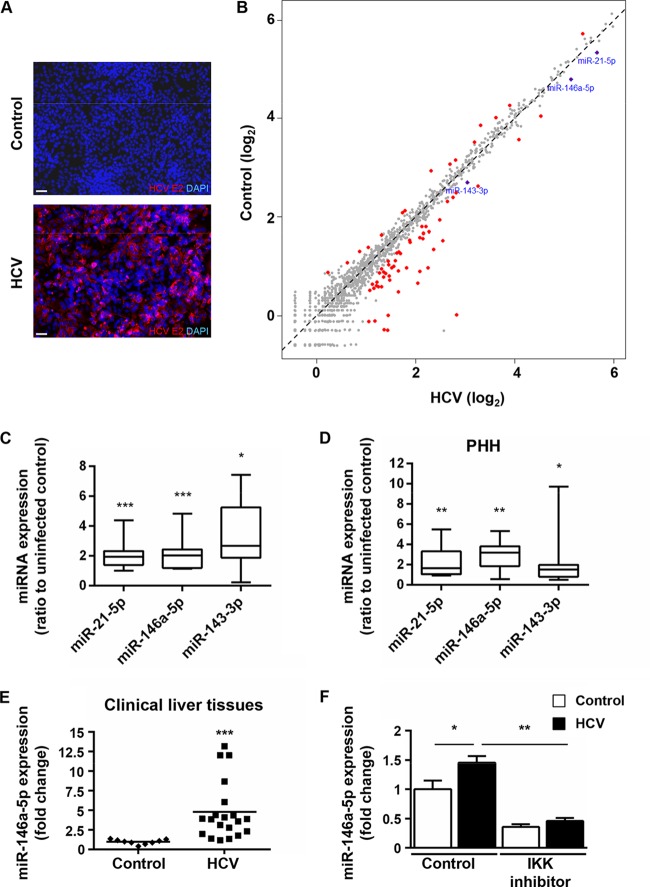
To systematically identify HCV-induced alterations of miRNA profiles of hepatocytes, we induced a poor proliferative state as well as expression of hepatocyte-specific genes in Huh7.5.1 cells, a state-of-the-art permissive cell line for HCV infection, by using 1% DMSO (14–16). These hepatocyte-like cells were then subjected to HCVcc (strain Jc1) infection for 7 days (20, 36), using highly purified virus particles in order to avoid any interference due to serum components or cytokines present in the cell culture medium. HCV infection of hepatocyte-like cells resulted in the establishment of pervasive infection as assessed by immunofluorescence staining of the viral antigen E2 (Fig. 1A). We then performed high-throughput small RNA cloning and deep sequencing of these persistently HCV-infected hepatocyte-like cells (20, 45). In total, 109,543,844 reads were generated for both cells at 7 days postinfection (dpi) and noninfected control cells. About 85% of total reads could be mapped to the human genome and categorized into RNA classes based on sequence annotation (data not shown). The large majority of reads from both HCV-infected and noninfected cells were annotated as mature miRNAs of about 22 nt. Interestingly, infected cells presented a slight increase in the number of reads annotated as rRNA compared to controls (data not shown). This is consistent with previous evidence of deregulated rRNA transcription in HCV-infected cultured liver cells (46). Profiling analyses indicated that HCV infection differentially regulated the expression of 72 miRNAs, among which 56 were upregulated at least 2-fold and 16 were downregulated 2-fold or more (Fig. 1B). Among the upregulated miRNAs, miR-21-5p was previously reported to be enhanced by HCV in both Huh7-derived cells and liver tissues (47–49). Interestingly, miR-21-5p appears to be highly expressed in HCC tumors and cell lines, in which it was shown to enhance cell proliferation, migration, and invasion (50, 51). Aiming to identify additional virus-mediated alterations of miRNA patterns that may be linked to liver disease and hepatocarcinogenesis, we chose to focus our investigation on miRNAs that were previously described to target genes associated with inflammation, fibrosis, and cancer development but have not yet been linked strongly to HCV-mediated hepatocyte injury (Table 2). Using RT-qPCR, we independently validated the expression of miR-146a-5p and miR-143-3p in chronically infected hepatocyte-like cells, along with that of miR-21-5p, which was assessed as a positive control. Similar to the RNA-Seq results, significant increases of these miRNAs were observed upon viral infection (Fig. 1C) (P = 0.00094, 0.00094, and 0.014, respectively; two-tailed Mann-Whitney test). To better understand the link between HCV and miRNA deregulation in the liver, we further studied the modulation of these three miRNAs by HCV in primary human hepatocytes (PHH), a model that most closely reflects the in vivo situation. In PHH cultures that were subjected to HCVcc (Jc1) infection, miR-21-5p, miR-146a-5p, and miR-143-3p were significantly upregulated (Fig. 1D) (P = 0.002, 0.005, and 0.03, respectively; two-tailed Mann-Whitney test), confirming that the HCV-mediated enhancement of miR-21-5p, miR-146a-5p, and miR-143-3p occurs in human hepatocytes. Interestingly, miR-146a-5p, which is a well-known immunoregulatory miRNA (52), was described to be upregulated in the sera and peripheral blood mononuclear cells (PBMC) of HCV-infected patients (53, 54). Moreover, a recent piece of evidence points to an involvement of miR-146a in liver fibrosis in mice and humans, independently of HCV (55). To gain insights into the clinical relevance of our findings, we studied miR-146a-5p expression in liver biopsy specimens from chronically HCV-infected patients and HCV-negative individuals (Table 1). Consistent with our in vitro data, upregulated miR-146a-5p was observed in HCV-positive liver tissues (Fig. 1E) (P < 0.0001; two-tailed Mann-Whitney test).
FIG 1.
Persistent HCV infection modulates host cell miRNA expression in hepatocyte-like Huh7.5.1 cells. (A and B) Huh7.5.1 cells were differentiated into hepatocyte-like cells by use of 1% DMSO (14–16), persistently infected using HCVcc, and subjected to molecular analyses at 7 dpi. (A) Immunodetection of HCV E2 protein in HCV Jc1-infected hepatocyte-like cells (HCV) and noninfected controls. Nuclei were counterstained with DAPI. Representative overlay images are shown. Bars, 50 μm. (B) Modulation of miRNA expression by HCV infection. Small RNAs (19 to 24 nt) from HCV Jc1E2FLAG-infected cells (HCV) or noninfected controls were subjected to RNA-Seq (see Materials and Methods). The scatterplot shows the 72 miRNAs with significant modulation upon HCV infection compared to control cells (red dots). miR-21-5p, miR-146a-5p, and miR-143-3p, which were enriched in HCV-infected cells, are highlighted in blue. (C) HCV increases the expression of miR-21-5p, miR-146a-5p, and miR-143-3p in hepatocyte-like cells. miRNA expression was analyzed by RT-qPCR assays of total RNA extracts from HCV-infected cells compared to noninfected controls. Results are for three independent experiments. (D) HCV increases the expression of miR-21-5p, miR-146a-5p, and miR-143-3p in PHH. miRNA expression was analyzed by RT-qPCR assay of total RNA extracts from HCV Jc1-infected PHH at 3 dpi compared to noninfected controls. Results are for five independent experiments. In panels C and D, the plots show sample lower quartiles (25th percentile; bottom of the box), medians (50th percentile; horizontal line in box), and upper quartiles (75th percentile; top of the box). The bottom and top whiskers indicate the 2.5 and 97.5 percentiles, respectively. (E) miR-146a-5p expression is enhanced in liver tissues from HCV-infected patients. Liver tissues from 20 HCV-infected patients (HCV) and 9 noninfected subjects (control) were analyzed for miR-146a-5p expression by RT-qPCR. Results for different biopsy specimens are shown as individual points. Median miRNA expression is shown as a black horizontal line. (F) HCV-induced upregulation of miR-146a-5p is mediated by NF-κB in hepatocyte-like cells. Cells were pretreated with the IKK inhibitor ACHP (6.25 μM) for 24 h and subjected or not subjected to HCV infection for 5 days. Nontreated cells (control) were analyzed in parallel. miR-146a-5p expression was assessed by RT-qPCR. The plots show mean fold changes ± standard errors of the means (SEM) for 4 independent experiments. In panels C, D, and E, statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.0001 (two-tailed Mann-Whitney test). In panel F, statistical significance is indicated as follows: *, P < 0.01; and **, P < 0.001 (one-way ANOVA).
TABLE 2.
HCV-regulated miRNAs with known involvement in inflammation, fibrosis, and cancera
| miRNA ID | miRNA target (gene symbol) | Molecular function in: |
||
|---|---|---|---|---|
| Inflammation | Fibrosis | Cancer | ||
| miR-122-5p | IGF1R | × | × | × |
| SRF | × | × | × | |
| CCNG1 | × | |||
| SLC7A1 | × | |||
| BCL2L2 | × | |||
| ADAM10 | × | × | × | |
| PRKRA | ||||
| WNT1 | × | × | × | |
| miR-210-3p | FGFRL1 | × | ||
| RAD52 | × | × | ||
| EFNA3 | × | |||
| BDNF | × | × | × | |
| PTPN1 | × | |||
| ISCU | × | |||
| E2F3 | × | |||
| MNT | × | × | × | |
| miR-338-5p | LRP1 | × | × | |
| miR-483-3p | SMAD4 | × | × | × |
| BBC3 | × | |||
| SRF | × | × | × | |
| MAPK3 | × | |||
| miR-574-5p | FOXN3 | × | ||
| miR-885-5p | CDK2 | × | × | |
| MCM5 | × | |||
| miR-1 | HCN4 | × | ||
| FOXP1 | × | |||
| HCN2 | × | × | ||
| PTMA | × | |||
| MET | × | × | × | |
| XPO6 | × | |||
| TAGLN2 | × | × | × | |
| LASP1 | × | × | ||
| KCNE1 | × | |||
| PNP | × | |||
| PIM1 | × | × | ||
| PAX3 | × | |||
| TWF1 | × | × | ||
| TWF2 | ||||
| FN1 | × | × | ||
| NOTCH3 | × | × | ||
| FABP3 | × | × | ||
| miR-10a-5p | HOXA1 | × | × | |
| USF2 | × | × | ||
| MAP3K7 | × | × | ||
| BTRC | ||||
| EPHA4 | × | × | ||
| miR-127-3p | PRDM1 | × | × | |
| BCL6 | × | × | ||
| miR-132-3p | CDKN1A | × | × | × |
| ARHGAP32 | ||||
| RB1 | × | × | ||
| HBEGF | × | × | × | |
| miR-133b | KCNH2 | × | ||
| FSCN1 | × | |||
| FAIM | × | |||
| EGFR | × | × | × | |
| FGFR1 | × | × | × | |
| HCN4 | × | |||
| miR-143-3p | KRAS | × | × | × |
| MYO6 | × | |||
| DNMT3A | × | × | × | |
| FNDC3B | × | |||
| MAPK7 | × | |||
| FSCN1 | × | |||
| SERPINE1 | × | × | × | |
| MACC1 | × | × | ||
| JAG1 | × | × | × | |
| AKT1 | × | × | × | |
| miR-146a-5p | CXCR4 | × | × | × |
| TLR2 | × | × | × | |
| TRAF6 | × | × | ||
| IRAK1 | × | × | ||
| BRCA1 | × | × | ||
| NFKB1 | × | × | ||
| EGFR | × | × | × | |
| CD40LG | × | × | × | |
| SMAD4 | × | × | × | |
| HNF1 | × | × | × | |
| SHP1 | × | × | × | |
| TLR4 | × | × | × | |
| miR-150-5p | MYB | × | × | × |
| EGR2 | × | × | ||
| MUC4 | × | × | × | |
| ZEB1 | × | × | × | |
| miR-206 | MET | × | × | × |
| NOTCH3 | × | × | ||
| ESR1 | × | × | ||
| PAX3 | × | |||
| miR-212-3p | MECP2 | × | × | × |
| PEA15 | × | |||
| PTCH1 | × | × | ||
| RB1 | × | × | ||
| miR-21-5p | RASGRP1 | × | ||
| BCL2 | × | × | × | |
| TIMP3 | × | × | × | |
| SOX5 | × | |||
| MTAP | × | × | × | |
| RECK | × | × | ||
| TGFBR2 | × | × | × | |
| PTEN | × | × | × | |
| E2F1 | × | × | × | |
| LRRFIP1 | × | × | ||
| TPM1 | × | |||
| NFIB | × | |||
| APAF1 | × | × | ||
| BTG2 | × | |||
| PDCD4 | × | × | × | |
| RHOB | × | × | ||
| SERPINB5 | × | × | ||
| BMPR2 | × | × | × | |
| DAXX | × | × | ||
| TP63 | × | |||
| MSH2 | × | × | ||
| MSH6 | × | |||
| ISCU | × | |||
| EIF4A2 | × | |||
| ANKRD46 | × | |||
| CDK2AP1 | × | |||
| DUSP10 | × | |||
| PPARA | × | × | × | |
| ANP32A | × | |||
| SMARCA4 | × | |||
| FASLG | × | × | × | |
| miR-494-3p | PTEN | × | × | × |
| CDK6 | × | × | ||
| BCL2L11 | × | |||
| miR-584-5p | ROCK1 | × | × | × |
| miR-663a | JUNB | × | × | |
| JUND | × | |||
The targets of miRNAs differentially regulated by HCV infection in hepatocyte-like Huh7.5.1 cells at 7 days postinfection were retrieved using the miRsystem tool. The involvement of these target genes in pathways related to inflammation, fibrosis, and cancer was assessed through miRsystem and a systematic screen of the available literature by use of PubMed. ×, involvement of miRNA target in the indicated process.
HCV-induced upregulation of miR-146a-5p is dependent on NF-κB signaling.
Next, we investigated the molecular mechanisms by which HCV induces the expression of miR-146a-5p in hepatocytes. Previous evidence revealed that inflammation and virus infection can trigger the induction of miR-146a through activation of NF-κB in monocytes, lymphocytes, and epithelial cells (56, 57). Since HCV infection was shown to have an impact on NF-κB signaling (58), we investigated whether the HCV-mediated induction of miR-146a-5p could be regulated by the NF-κB pathway. We used a specific inhibitor of the IκB kinase (IKK), ACHP, which prevents NF-κB DNA binding (59), to perturb NF-κB signaling in hepatocyte-like cells subjected or not subjected to HCV infection. The efficacy of NF-κB inhibition was confirmed by the markedly reduced expression of miR-146a-5p in ACHP-treated noninfected cells (Fig. 1F). Cells infected in the presence of the NF-κB inhibitor at a concentration that did not affect cell viability displayed a significant decrease in miR-146a-5p expression compared to control infected cells (Fig. 1F) (P < 0.0001), indicating that HCV-induced miR-146a-5p expression is NF-κB dependent. To test whether retinoic acid-inducible gene I (RIG-I), a known regulator of NF-κB activity (60), could be involved in this process, we analyzed HCV-induced miR-146a-5p expression in Huh7 cells—which exhibit functional RIG-I signaling, in contrast to Huh7.5.1 cells—in the presence and absence of the NF-κB inhibitor. As we observed comparable levels of inhibition of HCV-induced miR-146a-5p expression between hepatocyte-like Huh7.5.1 and hepatocyte-like Huh7 cells with the NF-κB inhibitor, RIG-I signaling does not appear to play a major role in this process (data not shown). Collectively, these data indicate that NF-κB signaling regulates miR-146a-5p expression in HCV-infected hepatocytes.
miR-146a-5p regulates cell metabolism and pathways that contribute to the pathogenesis of liver disease.
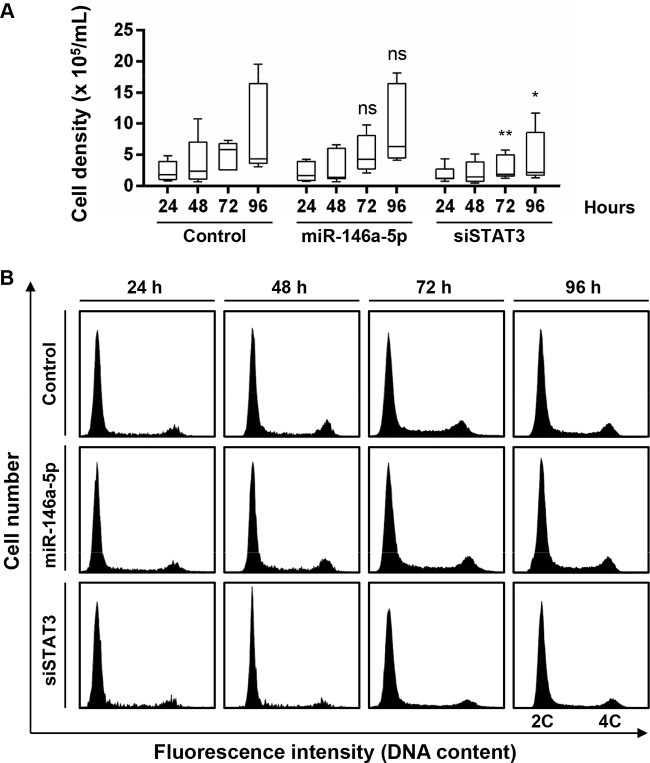
We then sought to explore the functional relevance of miR-146a-5p upregulation in hepatocytes. As miR-146a is well known to enhance cell proliferation in PBMC, we evaluated whether this miRNA promotes cell proliferation and cell cycle progression in hepatocyte-like cells. siRNA targeting STAT3 was used as a control to inhibit proliferation of hepatocyte-like cells (61). In contrast to cells silenced for STAT3, which displayed decreased cell proliferation over time, cells overexpressing miR-146a-5p did not show any significant alteration of cell proliferation within 96 h of transfection compared to control-transfected cells (Fig. 2A). To confirm this finding, we analyzed the cell cycle distribution of hepatocyte-like cells upon miR-146a-5p transfection by flow cytometry. While silencing of STAT3 resulted in markedly lower percentages of cells in the S and G2/M phases, overexpressing miR-146a-5p did not affect the overall cell cycle distribution (Fig. 2B). Taken together, these data suggest that miR-146a-5p does not promote cell proliferation of hepatocyte-like cells.
FIG 2.
Overexpression of miR-146a-5p does not alter proliferation and cell cycle distribution in hepatocyte-like cells. Differentiated Huh7.5.1 cells were transfected with control siRNA, a miR-146a-5p mimic, or siSTAT3. (A) Cell proliferation was assessed 24, 48, 72, and 96 h after transfection by direct cell counting. The plots show sample lower quartiles (25th percentile; bottom of the box), medians (50th percentile; horizontal line in box), and upper quartiles (75th percentile; top of the box). The bottom and top whiskers indicate the 2.5 and 97.5 percentiles, respectively. Results are for three independent experiments. *, P < 0.05; **, P < 0.01; ns, not significant (Kruskal-Wallis H test). (B) Cell cycle distribution was assessed at the different time points by flow cytometry following propidium iodide staining. Representative cell cycle profiles from one of three independent experiments are shown. 2C, cells in G0/G1 phase, with diploid DNA content; 4C, cells in G2/M phase, with tetraploid DNA content. The population of miR-146a-5p-overexpressing cells displayed a profile comparable to that of control cells. In contrast, the numbers of cells in the S and G2/M phases were markedly decreased upon knockdown of STAT3 compared with controls.
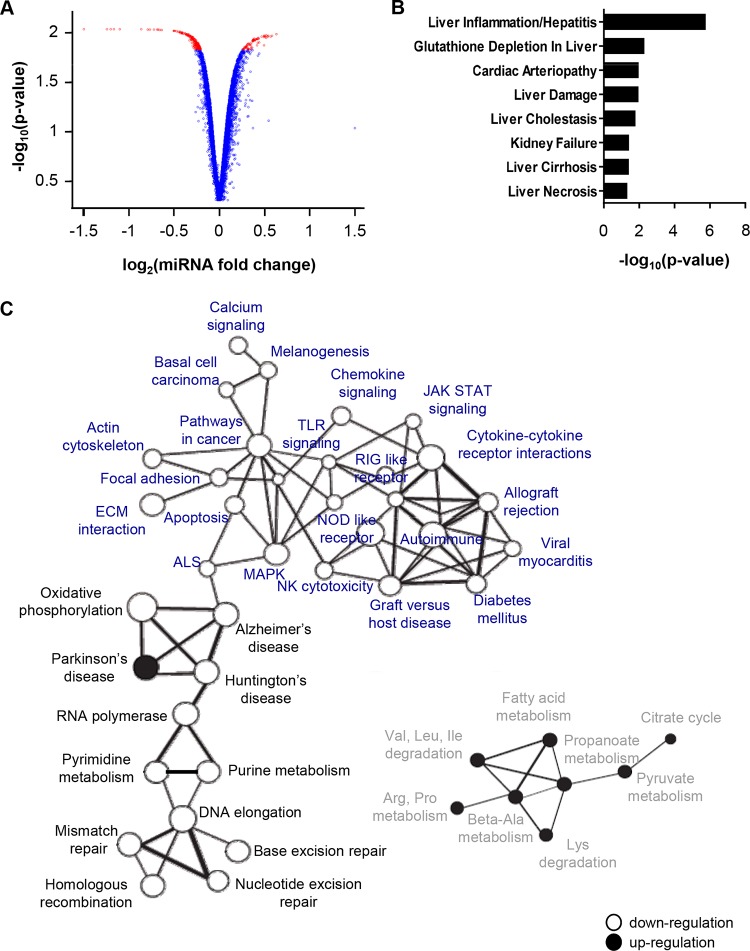
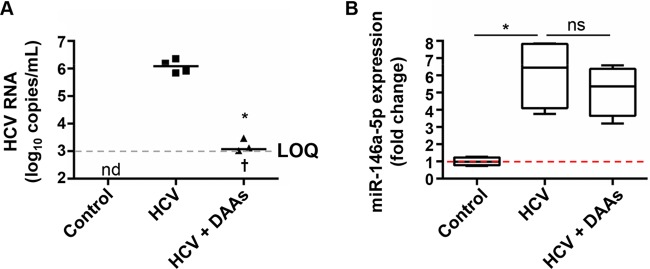
To investigate the pathways regulated by miR-146a-5p in our model in an unbiased manner, we performed a genome-wide transcriptomic analysis of hepatocyte-like cells upon miR-146a-5p overexpression (Fig. 3). Using RT-qPCR, we assessed a consistent increase of miR-146a-5p upon miRNA transfection prior to genome-wide microarray analysis (data not shown). Compared to the results for control cells, enhanced miR-146a-5p expression in hepatocyte-like cells resulted in a significant deregulation of 274 genes, including 63 upregulated and 211 downregulated genes (Fig. 3A) (P < 0.01; FCROS method). Interestingly, the interleukin-1 receptor-associated kinase 1 (IRAK1) and chemokine C-X-C motif chemokine ligand 8 (CXCL8) genes, two known targets of miR-146a-5p, were among the downregulated genes. In line with a previously shown implication of miR-146a-5p and IRAK1 in inflammatory responses in immune cells (52), our data suggest a role for miR-146a-5p in inflammation signaling in hepatocyte-like cells. Functional annotation using the IPA systems biology tool further showed an involvement of miR-146a-5p in gene networks related to inflammatory responses, cell death and survival, cell-to-cell signaling and interaction, and cancer-associated processes (data not shown). Moreover, on running all the significantly deregulated genes through Ingenuity's Tox Function prediction tool, the top toxicological functions that were most strongly associated with miR-146a-5p overexpression were related to liver disease, including liver inflammation and hepatitis, glutathione depletion (a process associated with liver disease progression), liver damage, cholestasis, cirrhosis, and necrosis (Fig. 3B). Together with previous evidence linking miR-146a deregulation to liver fibrosis (55), this piece of data suggests that the modulation of miR-146a-5p expression may contribute to liver disease pathogenesis. To gain further insights into the role of miR-146a-5p in hepatocyte injury and progression of liver disease, we chose to extend our computational analysis of miR-146a-5p-overexpressing cells by retrieving gene sets that shared common regulation by miR-146a-5p by using GSEA (43). In contrast to control-transfected hepatocyte-like cells, miR-146a-5p-overexpressing cells presented a gene profile underlying strong repression of inflammatory and immune response pathways along with an enrichment of defined metabolic regulatory networks, such as regulation of fatty acid metabolism and energetic metabolism (Fig. 3C). In particular, miR-146a-5p overexpression resulted in repression of oxidative phosphorylation, while it enhanced pyruvate metabolism and the citrate cycle (Fig. 3C), suggesting an induction of metabolic changes similar to those observed in tumor cells (62). Overall, our transcriptomic profiling indicates an involvement of miR-146a-5p in gene networks that are associated with liver disease and HCC development. Given that antiviral therapy leading to HCV cure does not eliminate the risk of hepatitis C patients developing HCC (2), we sought to determine the expression of miR-146a-5p in chronically HCV-infected hepatocyte-like cells treated with a state-of-the-art combination of DAAs. The DAA treatment, which decreased the viral load in cell supernatants to the limit of quantification of the RT-qPCR assay, did not restore the HCV-mediated overexpression of miR-146a-5p to its level in noninfected control cells (Fig. 4). These results suggest that overexpression of miR-146a, which is induced by HCV infection and is not reversed by antiviral therapy, may contribute to maintaining the increased risk of HCC development in patients cured of hepatitis C.
FIG 3.
miR-146a-5p modulates the expression of genes related to liver disease. (A) Modulation of mRNA expression by miR-146a-5p in hepatocyte-like cells. Hepatocyte-like cells were reverse transfected with a miR-146a-5p mimic or a nontargeting control siRNA (5 nM) 3 days prior to total RNA extraction and analysis of mRNA expression by microarray assay. Gene expression profiles are represented by a volcano plot, where the x axis represents the log2 fold change between miR-146a-5p-transfected cells and control cells and the y axis represents the −log10 P value obtained from the F-cross test for comparing miR-146a-5p-transfected cells to control cells. Red dots represent mRNAs whose values are considered to be statistically significant (P < 0.015; FCROS method). (B) Functional network analysis of genes with modified expression after miR-146a-5p overexpression in hepatocyte-like cells. The IPA toxicity algorithm was used to assess the enrichment of deregulated genes in specific toxicity pathways with altered expression in human disease. P values indicate the significance of enrichment of the input genes for each Tox Function pathway. (C) Coregulated gene networks in miR-146a-5p-overexpressing cells. Modulation of molecular pathways by miR-146a-5p was determined by using the KEGG database and GSEA (43). Significantly enriched gene networks involved in immune responses (blue), the cell cycle and DNA repair (black), and cell metabolism (gray) are shown. Gene sets downregulated by miR-146a-5p are depicted as open circles, while gene sets upregulated by the miRNA are represented by black circles. The thickness of each connecting line is proportional to the degree of overlapping genes between two different gene sets.
FIG 4.
Eradication of HCV infection does not restore the HCV-mediated overexpression of miR-146a-5p. Persistently HCV (Jc1E2FLAG)-infected hepatocyte-like cells were treated or not with a combination of DAAs (5 nM DCV, 1 μM SOF, and 0.5 μM SMV). Noninfected cells were analyzed as controls. (A) After 4 weeks of DAA treatment followed by 2 weeks of washout, HCV RNA loads were assessed by RT-qPCR assay of cell supernatants from HCV-infected nontreated cells (HCV) or HCV-infected DAA-treated cells (HCV + DAAs). The limit of quantification (LOQ), indicated by a dashed line, was 103 copies/ml. Results for 4 biological replicates are shown. Median HCV loads are shown as black lines. The cross indicates one sample that was HCV RNA negative. (B) miR-146a-5p expression was analyzed by RT-qPCR assay of total RNA extracts from the same samples as well as noninfected control cells. The plots show sample lower quartiles (25th percentile; bottom of the box), medians (50th percentile; horizontal line in box), and upper quartiles (75th percentile; top of the box). The bottom and top whiskers indicate the 2.5 and 97.5 percentiles, respectively. The red dashed line indicates a fold change of 1. *, P < 0.05 (two-tailed Mann-Whitney test); nd, not determined; ns, not significant.
Since the upregulation of miR-146a-5p results in the enhancement of fatty acid metabolism, which is instrumental to HCV replication, this piece of data suggests that in addition to modulating pathways associated with liver disease pathogenesis, miR-146a-5p may fine-tune the metabolic activity of hepatocytes upon viral infection and eventually act on the viral replication cycle itself.
miR-146a-5p promotes the production of infectious HCV particles without affecting viral entry or replication.
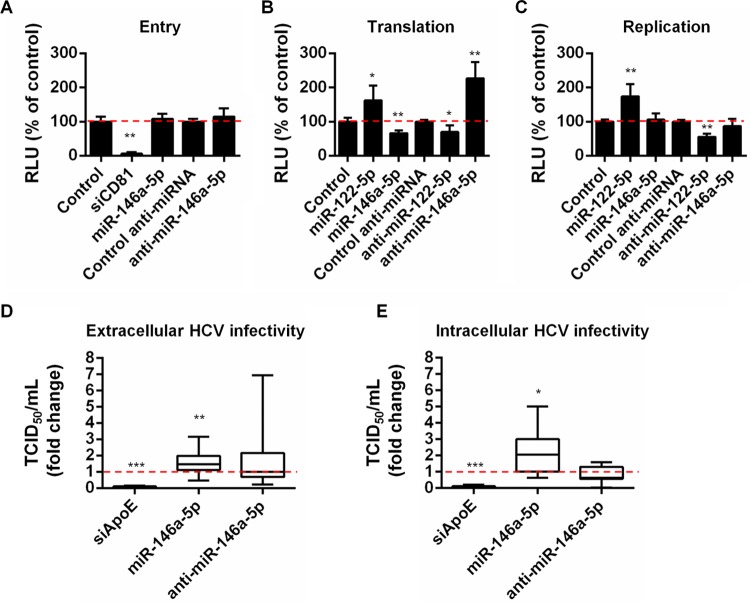
To assess the potential impact of miR-146a-5p on the HCV life cycle, we performed a series of gain-of-function and loss-of-function experiments using HCVpp, HCV/ΔE1E2 RNA, and HCVcc to investigate the role of miR-146a-5p in HCV entry, translation, replication, assembly, and infectivity. Loss-of-function perturbation of essential HCV host factors required for either viral entry, viral translation/replication, or viral assembly by use of siCD81, anti-miR-122, or siApoE, respectively, were used as controls. A miR-122 mimic was used as a gain-of-function control in translation/replication studies. To study the impact of miR-146a-5p on viral entry, Huh7.5.1 cells were first transfected with siRNAs, a miR-146a-5p mimic, or anti-miR-146a-5p and then incubated with luciferase reporter HCVpp or VSVpp, which were used as an unrelated control. In contrast to the silencing of CD81, which resulted in a dramatic decrease in HCVpp entry, modulating the expression of miR-146a-5p did not significantly affect this process (Fig. 5A). VSVpp entry was also not affected by the modulation of miR-146a-5p expression (data not shown). To assess the impact of miR-146a-5p on translation and replication, we used a Renilla luciferase reporter HCV genome (JcR2a) and a firefly luciferase reporter assembly-deficient HCV RNA (JFH1/ΔE1E2). The former was transfected into Huh7.5.1 cells which had previously been transfected with siRNA, miRNA, or anti-miRNA, while the latter was electroporated into cells prior to siRNA/miRNA transfection. In contrast to miR-122 and anti-miR-122, which significantly increased and decreased, respectively, both HCV translation and replication, miR-146a-5p and anti-miR-146a-5p did not significantly affect HCV replication, although they were able to modulate HCV translation when this step was studied independently of viral replication (Fig. 5B and C). Finally, to test whether miR-146a-5p may play a role in HCV assembly, we measured HCV RNA and viral infectivity in both lysates and supernatants of Huh7.5.1 cells that were infected with HCVcc (Jc1) following transfection of siRNA, miRNA, or anti-miRNA. In contrast to cells with silenced apoE, in which viral assembly was significantly impaired, overexpression of miR-146a-5p increased both extracellular and intracellular viral infectivities (Fig. 5D and E) (P = 0.005 and 0.036, respectively). In contrast, anti-miR-146a-5p had no significant effect on viral infectivity (Fig. 5D and E) (P = 0.7 and 0.08, respectively). Since the specific infectivity (as assessed by the TCID50/HCV RNA ratio) of the viral particles was not significantly affected by the modulation of the expression of miR-146a-5p (data not shown), these results suggest that miR-146a-5p promotes HCV assembly and particle release without increasing the specific infectivity of the produced virions. Taken together, these results revealed that ectopic miR-146a-5p expression promoted late steps of the HCV replication cycle, likely by increasing HCV assembly/egress (Fig. 5A to E). However, miR-146a-5p did not appear to be essential for HCV morphogenesis, since anti-miR-146a-5p did not inhibit the production of infectious viral particles (Fig. 5D and E). These results uncover miR-146a-5p as a new proviral host factor for HCV infection that increases the production of infectious viral particles.
FIG 5.
miR-146a-5p promotes HCV infection by enhancing production of infectious viral particles. (A) miR-146a-5p does not affect HCV entry. Huh7.5.1 cells were transfected with the indicated compounds and incubated with HCVpp for 72 h. HCVpp entry was assessed by determining the intracellular luciferase activity, which was expressed in relative light units (RLU). Results represent mean percentages ± SD for three independent experiments. (B) miR-146a-5p decreases HCV RNA translation. Huh7.5.1 cells were transfected with the indicated compounds and then, 48 h later, transfected with HCV RNA (JcR2a). HCV translation was assessed by determination of the luciferase activity 4 h later. Results are shown as mean percentages ± SD for three independent experiments. (C) miR-146a-5p does not modulate HCV replication. Huh7.5.1 cells were electroporated with assembly-deficient JFH1/ΔE1E2 RNA 48 h prior to transfection with the indicated compounds. HCV replication was assessed by determination of the luciferase activity 48 h later. Results are shown as mean percentages ± SD for four independent experiments. In panels A, B, and C, the dashed lines indicate an RLU level of 100% of the control level. (D and E) miR-146a promotes HCV assembly and egress. Huh7.5.1 cells were transfected with the indicated compounds 48 h prior to infection with HCVcc (Jc1). After 4 h, the viral inoculum was removed and cells were incubated with fresh medium for 48 h. Cells were lysed to determine the intracellular HCV RNA load and infectivity (TCID50/ml). Likewise, supernatants were used to determine the extracellular HCV RNA load and infectivity. The results represent extracellular (D) and intracellular (E) HCV infectivities as fold changes compared to control-transfected cells and are for nine independent experiments. The plots show sample lower quartiles (25th percentile; bottom of the box), medians (50th percentile; horizontal line in box), and upper quartiles (75th percentile; top of the box). The bottom and top whiskers indicate the 2.5 and 97.5 percentiles, respectively. The dashed lines indicate a fold change of 1. In panels A, B, and C, statistical significance is indicated as follows: *, P < 0.001; and **, P < 0.0001 (unpaired t test with Welch's correction). In panels D and E, statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.0001 (two-tailed Mann-Whitney test).
Collectively, our data indicate that the HCV-induced increase in miR-146a-5p expression promotes both virus infection and metabolic pathways associated with liver disease pathogenesis.
DISCUSSION
Progression of chronic hepatitis C to poor-prognosis liver disease and HCC is a multifactorial process that involves persistent hepatic inflammation, progressive liver fibrogenesis, development of neoplastic clones, and finally establishment of a carcinogenic tissue microenvironment (63). Since they are central players under both physiological and pathological conditions, miRNAs most likely contribute to HCV-driven liver disease (3, 8). Moreover, increasing evidence points to a role for miRNAs in fine-tuning HCV-hepatocyte interactions (4, 8). However, the lack of efficient and convenient model systems to investigate the biological circuits of virus-host interactions represents a major obstacle for the understanding of the mechanisms linking HCV infection, inflammation, and carcinogenesis. By performing high-throughput small RNA-Seq and computational analyses in a hepatocyte-like cell-based model system which enables persistent HCV infection (14–16), we identified 72 miRNAs significantly modulated by HCV that may have putative involvements in virus-driven liver disease and HCC (Fig. 1; Table 2). Indeed, 20 of these 72 miRNAs were already known to be implicated in inflammation, fibrosis, and cancer development. This subset of miRNAs included miR-122, which is a key player in HCV infection and liver disease (reviewed in reference 4); miR-21-5p, which has consistently been shown to be implicated in HCV infection and liver disease (47–49); and miR-146a-5p, a well-known immunoregulatory miRNA further described to be associated with liver fibrosis and HCC (54, 55, 64–66). Moreover, members of the miR-27 family of miRNAs, which contribute to the regulation of lipid metabolism, were recently reported to be increased by HCV and to contribute to liver steatosis (67, 68).
By pursuing our investigation in PHH and clinical liver tissues from HCV-infected patients (Fig. 1), we provided the first evidence that HCV induces upregulation of miR-146a-5p in hepatocytes. Interestingly, miR-146a has previously been associated with HCV infection: an increased expression of this miRNA was observed in the sera and PBMC of HCV-infected patients, suggesting a role for circulating miR-146-5p as a biomarker for hepatic injury (53, 54). In line with this, hepatitis B virus (HBV) has also been shown to increase miR-146a in human hepatoma cell lines and chronically HBV-infected patient-derived liver tissues (69). However, the cross talk between miR-146a-5p and HCV and its relevance to hepatocyte injury have not yet been evidenced. Taking advantage of a series of well-established functional assays to investigate distinct steps of virus-host interactions, we discovered that miR-146a-5p exerts a proviral effect in liver cells, likely by modulating HCV assembly/egress (Fig. 5D and E). In contrast to data reported for HepG2 cells, in which high ectopic expression of miR-146a was shown to target the epidermal growth factor receptor gene (EGFR) (70), encoding an HCV entry cofactor in liver cells (22), ectopic expression of miR-146a-5p under our experimental conditions did not affect EGFR expression (data not shown), in line with the absent modulation of the early steps of HCV infection upon miR-146a-5p overexpression (Fig. 5A). Collectively, our data uncover miR-146a-5p as a new proviral host factor for HCV infection. By overexpressing miR-146a-5p in liver cells, we observed its involvement in key metabolic pathways, including the proteasome pathway and fatty acid metabolism (Fig. 3C), which in turn were associated with HCV assembly (71–73). Moreover, we observed that miR-146a-5p likely modulates the anaerobic energetic metabolism in hepatocyte-like cells (Fig. 3). Since HCV has been shown to enhance cellular glucose consumption (74), miR-146a-5p may be implicated in the HCV-induced aerobic glycolysis shift that is also a hallmark of tumor cells. This piece of data suggests that by regulating liver cell metabolism, miR-146a-5p may be beneficial for HCV infection and potentially play a role in HCV-driven liver disease.
Beyond cell metabolism, our pathway enrichment analysis further suggests a pivotal role of miR-146a in downregulating inflammation signaling and immune responses in hepatocytes (Fig. 3). This is consistent with recent evidence that miR-146a contributes to the escape of HCC cells from antitumor immune responses in vivo (65). In the context of HCV infection, upregulation of miR-146a-5p may enable HCV-infected cells to escape from immune surveillance mechanisms, facilitating the maintenance of infection. It is noteworthy that our pathway analysis also highlighted a role for miR-146a-5p in promoting liver inflammation and disease progression (Fig. 3). Indeed, miR-146a was recently described as being involved in an inflammatory feedback circuit regulating the cross talk between hepatocytes and human stellate cells (HSC) (55). In rat models of liver fibrosis, cytokines were shown to increase the expression of both miR-146a and miR-21, which in turn sustain repression of hepatocyte nuclear factor 1a (HNF1a) in hepatocytes, thus enhancing hepatocellular inflammation, cytokine release, activation of HSC, and disease progression (55). This is also in line with previous studies showing an upregulation of miR-146a in the plasmas of mice subjected to liver inflammation induced by the cytidine-phosphate-guanosine (CpG) motif and lipopolysaccharide (LPS) (53). Interestingly, the same study highlighted enhanced miR-146a expression in mice with acetaminophen-induced liver injury characterized by little inflammation (53). Given its central role in liver cell homeostasis and signaling (Fig. 3), we propose that miR-146a-5p might serve as a molecular switch that fine-tunes stress signaling in hepatocytes, enabling modulation of metabolic activity in response to microenvironmental changes. Thus, impairment of miR-146a regulation and signaling may participate in hepatocellular injury. This may also provide clues about the putative modulation of miR-146a expression during the progression of liver disease. While enhanced expression of this miRNA was observed during chronic HCV infection (Fig. 1E) and fibrosis (55), its repression was associated with invasiveness of HCC tumors (54, 65), thus suggesting a tumor suppressor function for miR-146a. Further investigation will be necessary to better understand the molecular mechanisms of miR-146a-mediated liver disease pathogenesis and HCC development.
Taken together, the results of our study indicate a novel involvement of miR-146a-5p in promoting HCV infection and metabolic pathways associated with liver disease progression and contribute to a better understanding of HCV-induced liver disease pathogenesis, ultimately providing novel potential therapeutic targets for prevention of HCC in HCV-infected patients.
ACKNOWLEDGMENTS
We thank R. Bartenschlager (University of Heidelberg, Germany) for providing the plasmids for production of HCVcc, F. Chisari (The Scripps Research Institute, La Jolla, CA) for the gift of Huh7.5.1 cells, and A. Patel (MRC Virology Unit, Glasgow, United Kingdom) for the E2-specific MAb AP33. We acknowledge N. Van Renne, S. Coassolo, and A. Mawa (Inserm U1110, Strasbourg, France), as well as A. Weiss, L. Brino, and D. Dembele (IGBMC, Department of Functional Genomics and Cancer, Illkirch, France), for excellent technical assistance and helpful discussions regarding transfection protocols and computational analyses. We thank B. Campana (Department of Biomedicine, University Hospital Basel, Basel, Switzerland) for providing clinical information about the patients enrolled in the study. We thank S. Pfeffer (IBMC, Strasbourg, France) for helpful discussions regarding miRNA expression analyses. The small RNA-Seq and microarray analyses as well as bioinformatics were performed at the IGBMC Microarray and Sequencing Platform (Illkirch, France), a member of the France Genomique program.
T.F.B. and M.B.Z. initiated the project. M.B.Z. supervised research. S.B., S.P., and M.B.Z. designed experiments. S.B., S.P., H.E.S., S.C.D., E.C., M.A.O., J.B., and C.T. performed experiments. S.B., S.P., H.E.S., E.C., T.Y., J.B., T.F.B., and M.B.Z. analyzed data. I.F., C.S., M.H.H., and P.P. provided essential reagents. S.B., S.P., H.E.S., and I.F. designed figures and tables. S.B., S.P., and M.B.Z. wrote the paper. T.F.B. edited the manuscript. All authors read and approved the manuscript. The authors declare no conflicts of interest.
Funding Statement
This work was supported by the European Union (INTERREG-IV-Rhin Supérieur-FEDER-Hepato-Regio-Net grant to M.B.Z. and T.F.B., as well as ERC-2014-AdG-671231-HEPCIR, EU2010 HEPCAR, and a Seventh Framework Programme [FP7] HepaMab grant to T.F.B.), the Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) (grant 2012/239 to M.B.Z. and T.F.B. and grant 2013/108 to T.F.B.), the Fondation ARC pour la Recherche sur le Cancer (ARC), Paris, France, the Institut Hospitalo-Universitaire, Strasbourg, France (grant TheraHCC IHUARC IHU201301187 to T.F.B.), Inserm, and the University of Strasbourg. The IGBMC Microarray and Sequencing Platform was supported by the FG National Infrastructure, funded as part of the Investissements d’Avenir program managed by the Agence Nationale pour la Recherche (program ANR-10-INBS-0009). This work is published under the framework of the LABEX ANR-10-LABX-0028_HEPSYS program and benefits from funding from the state, managed by the French National Research Agency as part of the Investments for the Future program. S.P. and E.C. were supported by fellowships from the French Ministry of Research.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Chung RT, Baumert TF. 2014. Curing chronic hepatitis C—the arc of a medical triumph. N Engl J Med 370:1576–1578. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 2.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. 2012. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 3.Szabo G, Bala S. 2013. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol 10:542–552. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. 2015. miR-122—a key factor and therapeutic target in liver disease. J Hepatol 62:448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. 2005. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 6.Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Junemann C, Niepmann M. 2008. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J 27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Masaki T, Yamane D, McGivern DR, Lemon SM. 2013. Competing and noncompeting activities of miR-122 and the 5′ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc Natl Acad Sci U S A 110:1881–1886. doi: 10.1073/pnas.1213515110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrivastava S, Mukherjee A, Ray RB. 2013. Hepatitis C virus infection, microRNA and liver disease progression. World J Hepatol 5:479–486. doi: 10.4254/wjh.v5.i9.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gragnani L, Piluso A, Fognani E, Zignego AL. 2015. MicroRNA expression in hepatitis C virus-related malignancies: a brief review. World J Gastroenterol 21:8562–8568. doi: 10.3748/wjg.v21.i28.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan HX, Tang H. 2014. Complex interactions between microRNAs and hepatitis B/C viruses. World J Gastroenterol 20:13477–13492. doi: 10.3748/wjg.v20.i37.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varnholt H, Drebber U, Schulze F, Wedemeyer I, Schirmacher P, Dienes HP, Odenthal M. 2008. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology 47:1223–1232. doi: 10.1002/hep.22158. [DOI] [PubMed] [Google Scholar]
- 12.Ura S, Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Sunakozaka H, Sakai Y, Horimoto K, Kaneko S. 2009. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology 49:1098–1112. doi: 10.1002/hep.22749. [DOI] [PubMed] [Google Scholar]
- 13.Steinmann E, Pietschmann T. 2013. Cell culture systems for hepatitis C virus. Curr Top Microbiol Immunol 369:17–48. doi: 10.1007/978-3-642-27340-7_2. [DOI] [PubMed] [Google Scholar]
- 14.Sainz B Jr, Chisari FV. 2006. Production of infectious hepatitis C virus by well-differentiated, growth-arrested human hepatoma-derived cells. J Virol 80:10253–10257. doi: 10.1128/JVI.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauhofer O, Ruggieri A, Schmid B, Schirmacher P, Bartenschlager R. 2012. Persistence of HCV in quiescent hepatic cells under conditions of an interferon-induced antiviral response. Gastroenterology 143:429.e8–438.e8. doi: 10.1053/j.gastro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Xiao F, Fofana I, Thumann C, Mailly L, Alles R, Robinet E, Meyer N, Schaeffer M, Habersetzer F, Doffoel M, Leyssen P, Neyts J, Zeisel MB, Baumert TF. 2015. Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C. Gut 64:483–494. doi: 10.1136/gutjnl-2013-306155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, Patel AH. 2005. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol 79:11095–11104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. 2005. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A 102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger SE, Zeisel MB, Davis C, Thumann C, Harris HJ, Schnober EK, Mee C, Soulier E, Royer C, Lambotin M, Grunert F, Dao Thi VL, Dreux M, Cosset FL, McKeating JA, Schuster C, Baumert TF. 2010. Inhibition of hepatitis C virus infection by anti-claudin-1 antibodies is mediated by neutralization of E2-CD81-claudin-1 associations. Hepatology 51:1144–1157. doi: 10.1002/hep.23445. [DOI] [PubMed] [Google Scholar]
- 20.Mailly L, Xiao F, Lupberger J, Wilson GK, Aubert P, Duong FH, Calabrese D, Leboeuf C, Fofana I, Thumann C, Bandiera S, Lutgehetmann M, Volz T, Davis C, Harris HJ, Mee CJ, Girardi E, Chane-Woon-Ming B, Ericsson M, Fletcher N, Bartenschlager R, Pessaux P, Vercauteren K, Meuleman P, Villa P, Kaderali L, Pfeffer S, Heim MH, Neunlist M, Zeisel MB, Dandri M, McKeating JA, Robinet E, Baumert TF. 2015. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol 33:549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartosch B, Dubuisson J, Cosset FL. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med 197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoel M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF. 2011. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med 17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merz A, Long G, Hiet MS, Brugger B, Chlanda P, Andre P, Wieland F, Krijnse-Locker J, Bartenschlager R. 2011. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem 286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet MS, Longerich T, Diehl S, Ramirez F, Balla T, Rohr K, Kaul A, Buhler S, Pepperkok R, Lengauer T, Albrecht M, Eils R, Schirmacher P, Lohmann V, Bartenschlager R. 2011. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe 9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 27.Fofana I, Krieger SE, Grunert F, Glauben S, Xiao F, Fafi-Kremer S, Soulier E, Royer C, Thumann C, Mee CJ, McKeating JA, Dragic T, Pessaux P, Stoll-Keller F, Schuster C, Thompson J, Baumert TF. 2010. Monoclonal anti-claudin 1 antibodies prevent hepatitis C virus infection of primary human hepatocytes. Gastroenterology 139:953–964, 964.e1–964.e4. doi: 10.1053/j.gastro.2010.05.073. [DOI] [PubMed] [Google Scholar]
- 28.Da Costa D, Turek M, Felmlee DJ, Girardi E, Pfeffer S, Long G, Bartenschlager R, Zeisel MB, Baumert TF. 2012. Reconstitution of the entire hepatitis C virus life cycle in nonhepatic cells. J Virol 86:11919–11925. doi: 10.1128/JVI.01066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobuchon L, Marthey S, Le Guillou S, Laloe D, Le Provost F, Leroux C. 2015. Food deprivation affects the miRNome in the lactating goat mammary gland. PLoS One 10:e0140111. doi: 10.1371/journal.pone.0140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buck AH, Perot J, Chisholm MA, Kumar DS, Tuddenham L, Cognat V, Marcinowski L, Dolken L, Pfeffer S. 2010. Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection. RNA 16:307–315. doi: 10.1261/rna.1819210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths-Jones S. 2004. The microRNA Registry. Nucleic Acids Res 32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 33.Adamo A, Atashpaz S, Germain PL, Zanella M, D'Agostino G, Albertin V, Chenoweth J, Micale L, Fusco C, Unger C, Augello B, Palumbo O, Hamilton B, Carella M, Donti E, Pruneri G, Selicorni A, Biamino E, Prontera P, McKay R, Merla G, Testa G. 2015. 7q11.23 dosage-dependent dysregulation in human pluripotent stem cells affects transcriptional programs in disease-relevant lineages. Nat Genet 47:132–141. doi: 10.1038/ng.3169. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ, Elledge SJ. 2009. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci U S A 106:16410–16415. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benga WJ, Krieger SE, Dimitrova M, Zeisel MB, Parnot M, Lupberger J, Hildt E, Luo G, McLauchlan J, Baumert TF, Schuster C. 2010. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology 51:43–53. doi: 10.1002/hep.23278. [DOI] [PubMed] [Google Scholar]
- 36.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A 103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gastaminza P, Kapadia SB, Chisari FV. 2006. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol 80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CJ, Servant N, Toedling J, Sarazin A, Marchais A, Duvernois-Berthet E, Cognat V, Colot V, Voinnet O, Heard E, Ciaudo C, Barillot E. 2012. ncPRO-seq: a tool for annotation and profiling of ncRNAs in sRNA-seq data. Bioinformatics 28:3147–3149. doi: 10.1093/bioinformatics/bts587. [DOI] [PubMed] [Google Scholar]
- 40.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dembele D, Kastner P. 2014. Fold change rank ordering statistics: a new method for detecting differentially expressed genes. BMC Bioinformatics 15:14. doi: 10.1186/1471-2105-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. 2011. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu TP, Lee CY, Tsai MH, Chiu YC, Hsiao CK, Lai LC, Chuang EY. 2012. miRSystem: an integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS One 7:e42390. doi: 10.1371/journal.pone.0042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuddenham L, Jung JS, Chane-Woon-Ming B, Dolken L, Pfeffer S. 2012. Small RNA deep sequencing identifies microRNAs and other small noncoding RNAs from human herpesvirus 6B. J Virol 86:1638–1649. doi: 10.1128/JVI.05911-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raychaudhuri S, Fontanes V, Barat B, Dasgupta A. 2009. Activation of ribosomal RNA transcription by hepatitis C virus involves upstream binding factor phosphorylation via induction of cyclin D1. Cancer Res 69:2057–2064. doi: 10.1158/0008-5472.CAN-08-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Chen J, Wang H, Shi J, Wu K, Liu S, Liu Y, Wu J. 2013. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog 9:e1003248. doi: 10.1371/journal.ppat.1003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. 2008. Association of microRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res 14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marquez RT, Bandyopadhyay S, Wendlandt EB, Keck K, Hoffer BA, Icardi MS, Christensen RN, Schmidt WN, McCaffrey AP. 2010. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab Invest 90:1727–1736. doi: 10.1038/labinvest.2010.126. [DOI] [PubMed] [Google Scholar]
- 50.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. 2007. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L, Wu M, Su C, Chen J. 2013. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett 337:226–236. doi: 10.1016/j.canlet.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Taganov KD, Boldin MP, Chang KJ, Baltimore D. 2006. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bala S, Tilahun Y, Taha O, Alao H, Kodys K, Catalano D, Szabo G. 2012. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J Transl Med 10:151. doi: 10.1186/1479-5876-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Zhang Y, Sun XX, Ma X, Chen ZN. 2015. microRNA-146a inhibits cancer metastasis by downregulating VEGF through dual pathways in hepatocellular carcinoma. Mol Cancer 14:5. doi: 10.1186/1476-4598-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian H, Deng X, Huang ZW, Wei J, Ding CH, Feng RX, Zeng X, Chen YX, Ding J, Qiu L, Hu ZL, Zhang X, Wang HY, Zhang JP, Xie WF. 2015. An HNF1alpha-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells. Cell Res 25:930–945. doi: 10.1038/cr.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, Lin Z, Schaefer BC, Flemington EK. 2008. Epstein-Barr virus latent membrane protein 1 induces cellular microRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol 82:1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma X, Becker Buscaglia LE, Barker JR, Li Y. 2011. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong G, Waris G, Tanveer R, Siddiqui A. 2001. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A 98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanda T, Iida S, Ogura H, Asamitsu K, Murata T, Bacon KB, Ueda R, Okamoto T. 2005. Growth inhibition of multiple myeloma cells by a novel IkappaB kinase inhibitor. Clin Cancer Res 11:1974–1982. doi: 10.1158/1078-0432.CCR-04-1936. [DOI] [PubMed] [Google Scholar]
- 60.Zhang HX, Liu ZX, Sun YP, Zhu J, Lu SY, Liu XS, Huang QH, Xie YY, Zhu HB, Dang SY, Chen HF, Zheng GY, Li YX, Kuang Y, Fei J, Chen SJ, Chen Z, Wang ZG. 2013. Rig-I regulates NF-kappaB activity through binding to Nf-kappab1 3′-UTR mRNA. Proc Natl Acad Sci U S A 110:6459–6464. doi: 10.1073/pnas.1304432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, Karras JG, Zhang H. 2006. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res 12:7140–7148. doi: 10.1158/1078-0432.CCR-06-0484. [DOI] [PubMed] [Google Scholar]
- 62.Cantor JR, Sabatini DM. 2012. Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. 2014. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol 61:S79–S90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu K, Pan Q, Zhang X, Kong LQ, Fan J, Dai Z, Wang L, Yang XR, Hu J, Wan JL, Zhao YM, Tao ZH, Chai ZT, Zeng HY, Tang ZY, Sun HC, Zhou J. 2013. miR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis 34:2071–2079. doi: 10.1093/carcin/bgt160. [DOI] [PubMed] [Google Scholar]
- 65.Sun X, Zhang J, Hou Z, Han Q, Zhang C, Tian Z. 2015. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. Cell Cycle 14:243–252. doi: 10.4161/15384101.2014.977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng Q, Li S, Lao X, Chen Z, Li R, Deng Y, Qin X. 2014. The association of common functional polymorphisms in mir-146a and mir-196a2 and hepatocellular carcinoma risk: evidence from a meta-analysis. Medicine (Baltimore, MD) 93:e252. doi: 10.1097/MD.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shirasaki T, Honda M, Shimakami T, Horii R, Yamashita T, Sakai Y, Sakai A, Okada H, Watanabe R, Murakami S, Yi M, Lemon SM, Kaneko S. 2013. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol 87:5270–5286. doi: 10.1128/JVI.03022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singaravelu R, Chen R, Lyn RK, Jones DM, O'Hara S, Rouleau Y, Cheng J, Srinivasan P, Nasheri N, Russell RS, Tyrrell DL, Pezacki JP. 2014. Hepatitis C virus induced up-regulation of microRNA-27: a novel mechanism for hepatic steatosis. Hepatology 59:98–108. doi: 10.1002/hep.26634. [DOI] [PubMed] [Google Scholar]
- 69.Li JF, Dai XP, Zhang W, Sun SH, Zeng Y, Zhao GY, Kou ZH, Guo Y, Yu H, Du LY, Jiang SB, Zhou YS. 2015. Upregulation of microRNA-146a by hepatitis B virus X protein contributes to hepatitis development by downregulating complement factor H. mBio 6:e02459-14. doi: 10.1128/mBio.02459-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang S, He R, Rong M, Dang Y, Chen G. 2014. Synergistic effect of miR-146a mimic and cetuximab on hepatocellular carcinoma cells. Biomed Res Int 2014:384121. doi: 10.1155/2014/384121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki R, Matsuda M, Watashi K, Aizaki H, Matsuura Y, Wakita T, Suzuki T. 2013. Signal peptidase complex subunit 1 participates in the assembly of hepatitis C virus through an interaction with E2 and NS2. PLoS Pathog 9:e1003589. doi: 10.1371/journal.ppat.1003589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi A, Tazuma S, Nishioka T, Ohishi W, Hyogo H, Nomura S, Chayama K. 2005. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig Dis Sci 50:1361–1371. doi: 10.1007/s10620-005-2788-1. [DOI] [PubMed] [Google Scholar]
- 73.Syed GH, Amako Y, Siddiqui A. 2010. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab 21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramiere C, Rodriguez J, Enache LS, Lotteau V, Andre P, Diaz O. 2014. Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J Virol 88:3246–3254. doi: 10.1128/JVI.02862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]