ABSTRACT
Human cytomegalovirus (HCMV) may cause disseminated/end-organ disease in congenitally infected newborns and immunosuppressed transplant recipients. Two glycoprotein complexes, gH/gL/gO and gH/gL/pUL128/pUL130/pUL131 (gH/gL/pUL128L; referred to as the pentamer), are required for HCMV entry into fibroblasts and endothelial/epithelial cells, respectively, in the presence of the viral fusion protein gB. In addition, gH/gL/gO was recently reported to also be required for infection of endothelial/epithelial cells. Virus entry into human fibroblasts involves fusion of the virus envelope with the plasma membrane, whereas entry into endothelial/epithelial cells involves macropinocytosis or endocytosis and low-pH-dependent fusion with endosomes. A large set of neutralizing monoclonal antibodies (MAbs), directed to gH, gB, and multiple components of the pentamer, was developed. In addition, novel anti-gO human monoclonal antibodies were recently isolated. It is known that epithelial cell infection with a wild HCMV strain at a high multiplicity of infection produces a large number of syncytia. Incubation of heavily HCMV VR1814-infected ARPE-19 epithelial cells with neutralizing MAbs to one, two, or three components of the pUL128L portion of the pentamer blocked syncytium formation at an antibody concentration of 10 μg/ml, whereas only a partial inhibitory effect was displayed for MAbs to gO, gH, or gB at the same concentration. A blocking effect was also exhibited by convalescent-phase sera from primary HCMV infections. These findings indicate that the pentamer is required for syncytium formation in epithelial cells.
IMPORTANCE Human cytomegalovirus (HCMV) mostly infects epithelial and endothelial cells in vivo. Recently, the pentamer protein complex (gH/gL/pUL128L) was identified as being required for infection of these cells, in association with the other protein complex, gH/gL/gO. In primary infections, HCMV migrates to endothelial cells and then to leukocytes, which disseminate the infection throughout the body. The virus then spreads to organs and tissues, mostly infecting either single cells or multinucleated epithelial giant cells (syncytia), depending on the viral load. Potent neutralizing human MAbs directed to distinct binding sites of the pUL128L portion of the pentamer were shown in the past to block virus dissemination. In the present study, MAbs to pUL128L were shown to block syncytium formation with a higher potency than that of MAbs to gO, gH, or gB, thus suggesting their role in limiting virus dissemination. This finding provides additional information useful for the development of anti-HCMV therapeutic antibodies and subunit vaccines.
INTRODUCTION
Human cytomegalovirus (HCMV) is still a major cause of morbidity and mortality in immunocompromised transplant recipients and congenitally infected fetuses. Development of an HCMV vaccine has been given the highest priority by the Institute of Medicine of the United States (1). Following its initial isolation from human embryonic lung fibroblasts, HCMV was found to infect several different cell types in vivo, such as epithelial and endothelial cells and fibroblasts (2–4).
HCMV entry into epithelial/endothelial cells requires a pentamer complex consisting of gH/gL and the UL128/130/131 locus (UL128L) gene products. While gH and gL were previously known, the UL128L locus was more recently identified as being indispensable for virus entry and growth in endothelial cells and for virus transfer to leukocytes (5). Subsequently, Wang and Shenk showed that the UL131 open reading frame (ORF) is required for epithelial cell tropism (6) and that the virion protein complex gH/gL/pUL128/pUL130 is required for infection of epithelial/endothelial cells but does not affect the efficiency of fibroblast infection (7). Finally, Ryckman et al. (8) identified the gH/gL/pUL128L pentamer as the entry complex for epithelial/endothelial cells, thus suggesting the presence of a cell-type-specific receptor. On the other hand, HCMV entry into fibroblasts is mediated by the gH/gL/gO complex. Mass spectrometry and mutagenesis analyses revealed that gL-Cys144 forms disulfide bonds with gO-Cys351 in gH/gL/gO and with UL128-Cys162 in the pentamer. Introduction of a double mutation at the disulfide bond level of the pentamer (UL128-Cys162Ser/gL-Cys144Ser) impaired syncytium formation (SF) and reduced interference with HCMV entry into epithelial cells (9). As a result, gH/gL/gO and the pentamer were considered to represent two mutually exclusive cell entry complexes (9). However, gH/gL/gO was also found to be required for viral entry into epithelial cells (10, 11), as the pentamer would broaden virus tropism, possibly by binding to cell-type-specific receptors, while other postentry mechanisms, possibly involving the UL135 and UL136 gene products (12), may contribute to the determination of cell tropism. In addition, it was recently shown that the relative amounts of the two complexes in the virion envelope are regulated by the UL148 gene product and correlate with differences in HCMV strain cell tropism (13).
It is known that infection of epithelial cells with HCMV at a low multiplicity of infection (MOI) causes plaque formation. In recent years, we had the opportunity to verify that all monoclonal antibodies (MAbs) directed to UL128L products of the pentamer were able to inhibit plaque formation, unlike MAbs to gH or gB. Similar results were obtained when the same MAbs were used to inhibit virus transfer to leukocytes from infected endothelial cells (14–16). In both cases, these results suggested that the pentamer was substantially involved in virus cell-to-cell spreading when cells of either the same or different types were tested. In addition, similar results were obtained when convalescent-phase sera from subjects with primary infection were tested. Since the predominant biological activity of MAbs raised in both humans and immunized animals is directed to the UL128L portion of the pentamer and is neutralizing in vitro (16–21), it can be hypothesized that the most prominent part of the pentamer (pUL128L), and possibly the receptor binding site, is blocked by neutralizing antibodies (Nt antibodies), thus preventing the activation of gB required for cell-to-cell fusion (9, 22). However, at present, binding of the pentamer to a receptor is just a hypothetical model.
It is widely agreed that there are two major regions for neutralizing antibody binding within the pentamer: one, relevant to gH/gL, is targeted by antibodies neutralizing both fibroblast and epithelial/endothelial cell infections, and the other, relevant to pUL128L, is targeted by potent neutralizing antibodies preventing infection of epithelial/endothelial cells (9, 16–21).
In this report, we describe the blocking activity of human and murine MAbs as well as human sera in preventing SF in epithelial cells.
MATERIALS AND METHODS
Human subjects.
Seven pregnant women with primary HCMV infection were selected among women referred to Fondazione IRCCS Policlinico San Matteo for suspected primary HCMV infection during pregnancy. Primary HCMV infection was diagnosed based on the presence of at least two of the following four criteria: HCMV-specific IgG seroconversion, presence of virus-specific IgM antibody, a low IgG avidity index, and DNAemia (15). Timing of infection onset was determined mostly based on HCMV seroconversion and/or serologic and virologic findings in association with the presence of clinical signs/symptoms. HCMV vertical transmission was diagnosed either antenatally, by detection of viral DNA in and virus isolation from amniotic fluid, or by virus isolation from urine collected within the first 2 weeks of life. Of the seven pregnant women selected, three transmitted the infection to the fetus and four did not. The study was approved by the Institutional Review Board of the Fondazione IRCCS Policlinico San Matteo, and written informed consent was obtained from the women enrolled.
Cells.
Two types of cell cultures were used in this study: human umbilical vein endothelial cells (HUVECs) and the retinal pigmented epithelial ARPE-19 cell line. HUVECs were obtained by trypsin treatment of umbilical cord veins and were used at passages 2 to 5. HUVECs were used for preparation of VR1814 virus stocks to be employed for ARPE-19 epithelial cell inoculation. The ARPE-19 (ATCC CRL-2302) epithelial cell line was used to perform all SF and SF inhibition (SFI) experiments conducted in this study.
Human and murine MAbs and their specificity.
Human MAbs were obtained from memory B cells isolated from peripheral blood mononuclear cells (PBMC) of a few immunocompetent subjects and one transplant patient following positive selection with CD22 magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), as reported previously (17). After immortalization of IgG+ memory B cells with Epstein-Barr virus and plating at 10 cells/well in 384-well plates, supernatants were tested for neutralizing activity on inoculated target cells by fluorescence microscopy, using an anti-p72 murine MAb as the non-neutralized-virus detector (15, 16). Finally, cells from antibody-positive cultures were cloned by limiting dilution. Antibodies were then purified and tested for neutralizing activity and epitope mapping in cross-competition experiments (17). Subsequently, neutralizing MAbs from mice immunized with soluble pentamer and isolated according to standard methods showed the same potency as human MAbs and targeted the same as well as additional antigenic sites on the pentamer (16). In addition, very recently, we isolated two anti-gO MAbs (CVB234 and CVB301) according to the same methodology.
Western blotting.
The recombinant gH/gL/gO complex was subjected to SDS-PAGE under reducing conditions, followed by transfer to a polyvinylidene difluoride (PVDF) membrane, which was then incubated with MAbs against gH or gO. Antibody binding was detected with a horseradish peroxidase (HRP)-conjugated anti-human antibody (Sigma-Aldrich, St. Louis, MO) in combination with an enhanced chemiluminescence Western blotting detection reagent (GE Healthcare, Chicago, IL).
SPR experiments.
Surface plasmon resonance (SPR) experiments were carried out at 25°C on a ProteON XPR-36 instrument (Bio-Rad Laboratories, Hercules, CA) in phosphate-buffered saline (PBS; Gibco, Invitrogen, Thermo Fisher Scientific, Waltham, MA) with 0.05% Tween 20 (PBS-T). In summary, MAbs 13H11, CVB234, CVB301, and 15D8 were immobilized at 100 nM on a GLM ProteOn sensor chip (Bio-Rad Laboratories) through amine coupling, and a surface with no protein was created under identical coupling conditions for use as a reference. Recombinant gH/gL/gO was diluted in PBS-T and injected at a flow rate of 100 μl/min at different concentrations (100 nM, 75 nM, 50 nM, 25 nM, and 12.5 nM). The injection time was 180 s, and the dissociation time was 600 s. The data were processed with ProteOn Manager software and double referenced by subtraction of the blank surface and buffer injection-only values before local fitting of the data. Equilibrium dissociation constant (KD) values were calculated by applying the Langmuir fit model.
SFI.
The procedure for investigating SFI included preparation of confluent ARPE-19 epithelial cell monolayers grown on the bottom of shell vials and inoculated with HCMV VR1814 from passage 119 on HUVECs at an MOI of 10 PFU/cell (virus was titrated on either HUVECs or ARPE-19 cells, yielding overlapping results). Infected cell cultures were then centrifuged at 700 × g for 30 min (14–16) and incubated for 2 h at 37°C, and the medium was replaced with fresh medium containing serial log10 MAb or human serum dilutions (17). After 96 h, cells were fixed and stained with a p72 murine MAb (15, 16). In some experiments, cells were stained with PKH-26 membrane labeling dye, fixed with formaldehyde, and subsequently permeabilized with NP-40 and stained with p72 MAb or DAPI (4′,6-diamidino-2-phenylindole). Uninoculated ARPE-19 control cell cultures did not show the presence of any spontaneous SF. Syncytia consisting of more than 5 nuclei were counted in virus control and antibody-treated cultures, and the percent inhibition was calculated. This assay could not be performed on human fibroblasts, since the high MOI caused an extended cytopathic effect (CPE) within 24 h. The same antibodies had been tested previously for plaque formation inhibition (PFI) as well as leukocyte transfer inhibition (LTI) (15).
Neutralization assay.
Serial MAb or heat-inactivated human serum dilutions were incubated in duplicate for 60 min at 37°C with an equal virus volume containing 100 PFU of VR1814 (15). Virus-antibody mixtures were then added in duplicate to monolayers of ARPE-19 cells and centrifuged at 700 × g for 30 min. After 48 h of incubation, cells were fixed and stained for HCMV p72 by using a pool of murine MAbs. The antibody or serum concentration/dilution inhibiting virus infectivity by 50% or more with respect to the virus control was considered the 50% neutralizing antibody titer.
Measurements by ELISA of IgG antibodies to the pentamer, gH/gL/gO, and gB.
Levels of IgG antibodies to the pentamer, gH/gL/gO, and gB were measured in human serum samples by using a sandwich enzyme-linked immunosorbent assay (ELISA) as previously reported (15). Recombinant gH/gL complexes were produced by nucleofection of CHO cells as described previously (16), using cDNA encoding gH-2AgL in pEE6.4 and pUL128L or gO in pEE12.4 (Lonza Group, Basel, Switzerland). Briefly, half-area 96-well polystyrene plates were coated with a murine anti-gH MAb or an anti-gB MAb. The plates were then coated with cell culture supernatants containing different antigen preparations from transfected cells and incubated with serial 2-fold dilutions of human sera. Following addition of the enzyme-labeled conjugate and a substrate solution, the net optical density (OD) was calculated and specific titers determined.
RESULTS
Human MAbs and sequential serum samples from primary HCMV infections.
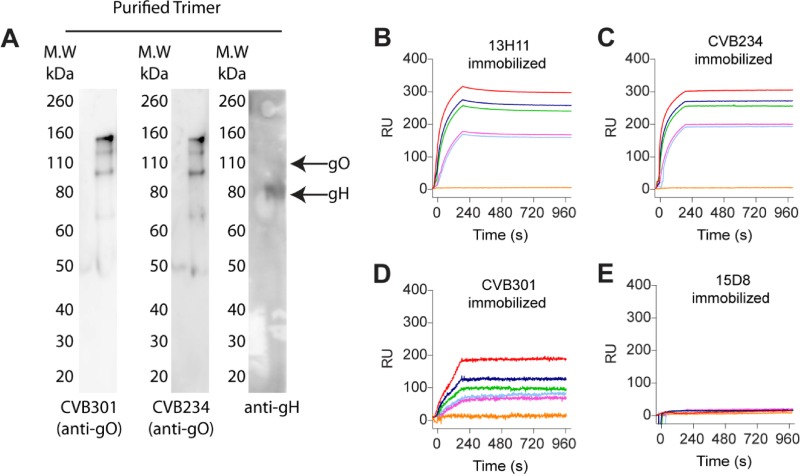
The human MAbs tested for SFI in this study were developed by our group some years ago (17) and were shown to display potent neutralizing activity by targeting multiple epitopes on the pentamer complex. By use of a neutralization assay, these MAbs were found to fall into two distinct groups: group 1, neutralizing HCMV infection of all target cells tested (fibroblasts and epithelial, endothelial, and myeloid cells), in the nanomolar range; and group 2, neutralizing HCMV infection of epithelial, endothelial, and myeloid cells, but not fibroblasts, in the picomolar range. By cross-competition experiments, three group 1 antibodies (3G16, 11B12, and 13H11) were found to bind to two distinct antigenic sites of gH, and six group 1 antibodies (2B11, 4H9, 5F1, 6B4, 7H3, and 10C6) recognized three distinct antigenic sites on gB. On the other hand, seven group 2 MAbs (1F11, 2F4, 4I22, 10F7, 4N10, 5A2, and 10P3) recognized three distinct antigenic sites on the pUL130/131 dimer (sites 2 to 4), while six group 2 MAbs (2C12, 6G4, 8C15, 8J16, 9I6, and 7I13) bound to two distinct antigenic sites on pUL128/130/131 (sites 5 and 6). Finally, a single group 2 MAb (15D8) bound a single protein of pUL128L (site 1), and a single MAb (8I21) recognized the gH/gL/pUL128/pUL130 tetramer (site 7). Of the two recently isolated anti-gO MAbs, one (CVB234) was able to neutralize HCMV infection of both fibroblasts and epithelial cells at concentrations of 1 to 3 μg/ml, i.e., in the nanomolar range (thus belonging to MAb group 1), and the other (CVB301) was nonneutralizing even at a concentration of 100 μg/ml. However, the two MAbs showed different binding affinities for gH/gL/gO, i.e., CVB234 showed a KD of 1 nM (similar to that of 0.5 nM for 13H11), whereas CVB301 showed a 100-fold lower affinity (KD = 100 nM). The characterization of the newly isolated anti-gO antibodies is shown in Fig. 1.
FIG 1.
Binding of anti-gO MAbs to gH/gL/gO as measured by Western blotting and SPR. (A) Western blot analysis of gH/gL/gO run under reducing conditions. (B) Binding of gH/gL/gO to immobilized anti-gH MAb 13H11, with a KD of 0.5 nM. (C) Binding of gH/gL/gO to immobilized anti-gO MAb CVB234, with a KD of 1 nM. (D) Binding of gH/gL/gO to immobilized anti-gO MAb CVB301, with a KD of 100 nM. (E) Absence of binding of gH/gL/gO to immobilized anti-pUL128 MAb 15D8. RU, relative units.
In addition, sequential serum samples from pregnant women with primary HCMV infection were collected and tested at different time intervals after infection onset.
SF following infection of ARPE-19 cells with VR1814 propagated in endothelial cells.
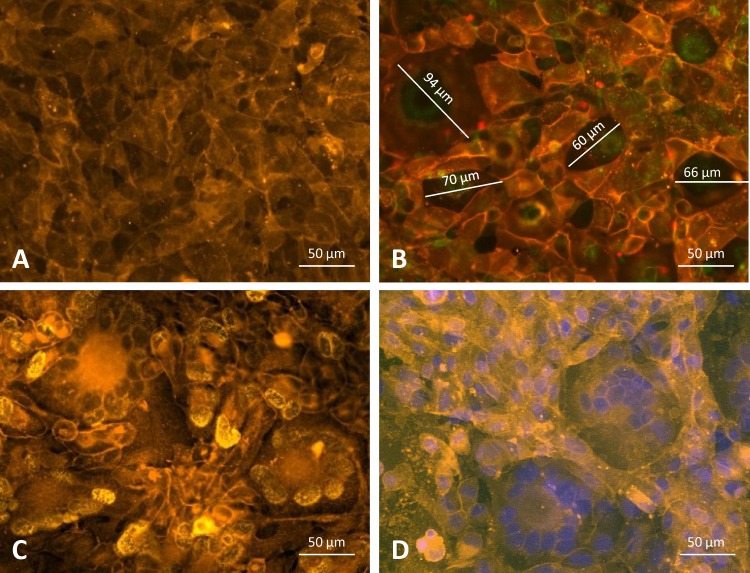
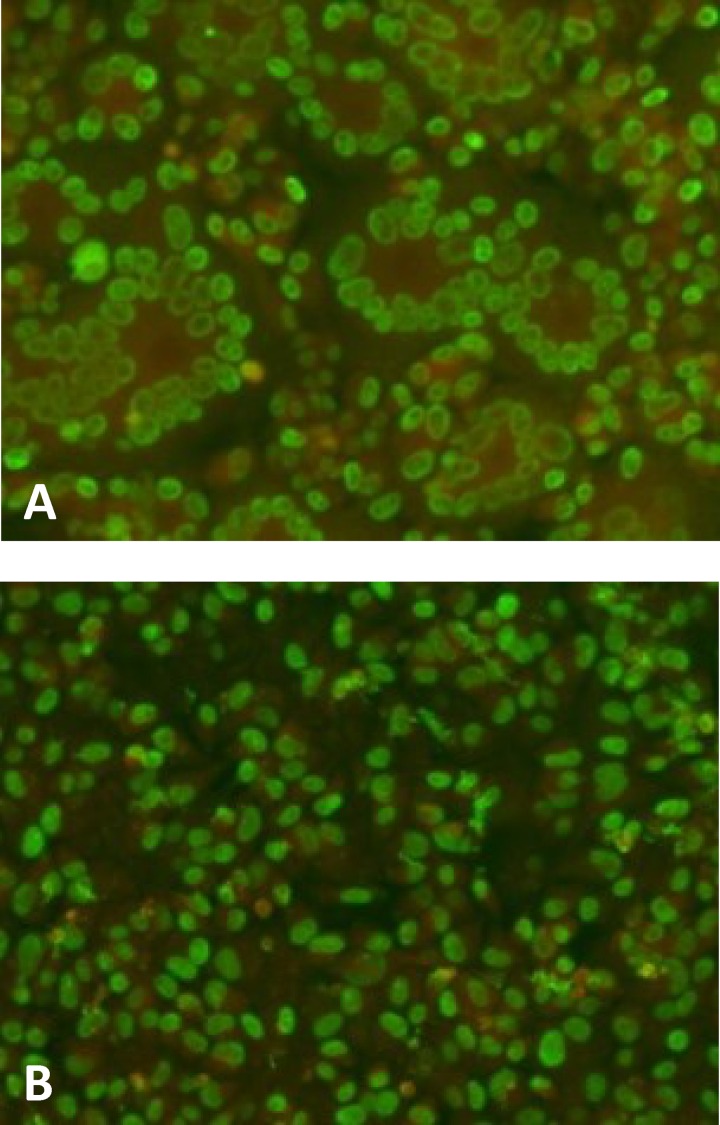
ARPE-19 epithelial cells, if uninfected, did not show the presence of SF (Fig. 2A), nor did they show SF if they were infected with AD169. Large syncytia were seen when cells were stained either with a membrane stain alone (Fig. 2B) or with both a membrane stain and a p72-specific MAb (Fig. 2C) or a membrane stain and DAPI (Fig. 2D). Microscopic pictures of p72 immunofluorescence staining of an infected cell culture, showing large syncytia, and of a culture with a block of SF by a human MAb directed to the pentamer, both taken at 96 h postinfection (p.i.), are given in Fig. 3A and B, respectively.
FIG 2.
Monolayers of ARPE-19 epithelial cell cultures. (A) Uninfected ARPE-19 cells stained with the yellow-orange fluorescent dye PKH-26 and interacting with plasma membrane lipids. (B) VR1814-infected cell culture stained with PKH-26, showing large syncytia (a single diameter is reported for each syncytium) as well as single membrane-limited cells. (C) VR1814-infected cell culture stained with PKH-26 and an HCMV p72 MAb, showing true SF and limiting membranes. (D) VR1814-infected cell culture stained with PKH-26 and DAPI.
FIG 3.
Immunofluorescence staining of VR1814-infected ARPE-19 epithelial cells at 96 h p.i. HCMV fluorescent nuclei (p72) are shown in green, while Evans blue counterstaining is shown in red. (A) Monolayer of ARPE-19 cells. Several syncytia are seen throughout the cell monolayer. (B) One hundred percent SF blocking by a human MAb (4I22; 10 μg/ml) directed to pUL130-131 of the pentamer.
SFI by MAbs to the pentamer.
One hundred percent SFI was achieved with MAb 15D8, directed to pUL128, MAb 4I22, directed to the pUL130/131 dimer, MAb 6G4, directed to the pUL128/130/131 trimer, or MAb 8I21, directed to the gH/gL/pUL128-130 tetramer, for a concentration of 10 μg/ml, while at a lower concentration the activity decreased (Fig. 4). In addition, MAbs (reported above) directed to three different epitopes of the pUL130/131 dimer and two different epitopes of the pUL128/130/131 trimer yielded the same 100% blocking effect, regardless of the antigenic site to which they were reactive.
FIG 4.
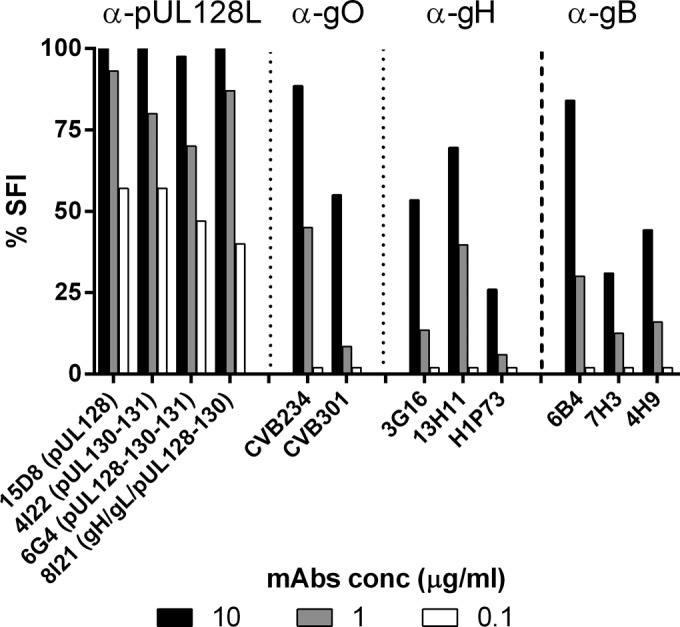
MAbs to the gH/gL/pUL128L pentamer and its pUL128L components completely blocked syncytium formation at the highest concentration tested (10 μg/ml), unlike MAbs to gO, gH, or gB, which only partially inhibited SF. All MAbs reported in this figure are human MAbs, with the exception of H1P73 (murine). The three columns relevant to each MAb indicate the three MAb concentrations tested.
SFI by MAbs to gH, gO, and gB.
Human MAbs recognizing two distinct epitopes of the gH component of the pentamer both yielded partial (∼50%) inhibition, while a murine MAb to a different gH epitope (H1P73) showed the weakest activity. Similarly, three human MAbs to three distinct antigenic sites of gB displayed only partial inhibitory effects (Fig. 4), as did three additional MAbs reactive to the same antigenic sites. The neutralizing human MAb directed against gO (CVB234) showed complete SFI activity at 10 μg/ml and partial or null activity at lower concentrations, while the nonneutralizing MAb (CVB301) showed only partial SFI activity at the highest concentration tested (10 μg/ml). Unlike MAbs to the pentamer, MAbs to gO, gH, and gB were totally ineffective at 0.1 μg/ml. Anti-gH MAbs have been shown to bind to both gH/gL complexes (gH/gL/gO and gH/gL/pUL128L) and to possess the same binding affinity as anti-pUL128L MAbs (23).
SFI by human serum samples from primary HCMV infections.
In parallel, SFI activities were determined for sequential sera from seven pregnant women with primary HCMV infection (Table 1). On average, SFI antibodies were detected later than neutralizing (Nt) and ELISA IgG antibodies (15). In general, the SFI antibody titer was 10 to 100 times lower than the titers of Nt and ELISA IgG antibodies to gH/gL/gO during the entire follow-up period (Table 1). This finding may be explained by the fact that SFI requires an accumulation of neutralizing antibodies to a high enough titer to block cell-cell fusion (which requires a large amount of virus). However, the gross correlation of Nt and SFI antibody titers suggests that SFI antibodies are mostly neutralizing. In addition, the titers of antibodies to gH/gL/gO and gB (Table 1) show that while gH/gL/gO antibody titers grossly parallel those of antibodies to the pentamer, antibodies to gB appear very early (20 to 30 days p.i.) and at high titers, reach a plateau that persists for months, and follow totally independent kinetics. No significant difference in the SFI antibody response was detected between the three women who transmitted the infection to the fetus and the four who did not.
TABLE 1.
SFI by convalescent-phase human sera from primary HCMV infections during pregnancy
| Patient no., transmission status, age (yr)a | Days after infection onset | 50% SFI titer of human serum | ARPE-19 cell Nt antibody titer | ELISA titer |
||
|---|---|---|---|---|---|---|
| Pentamer IgG | gH/gL/gO IgG | gB IgG | ||||
| 1, NT, 29 | 30 | <50 | 1,280 | 400 | 200 | 6,400 |
| 40 | 50 | 1,280 | 400 | 200 | 12,800 | |
| 110 | 200 | 1,280 | 1,600 | 1,600 | 12,800 | |
| 202 | 200 | 2,560 | 3,200 | 3,200 | 25,600 | |
| 266 | 200 | 2,560 | 3,200 | 3,200 | 25,600 | |
| 2, T, 30 | 20 | <50 | 160 | 1,600 | <50 | 25,600 |
| 90 | 200 | 1,280 | 6,400 | 400 | 51,200 | |
| 238 | 200 | 5,120 | 12,800 | 3,200 | 102,400 | |
| 3, NT, 30 | 21 | <50 | <50 | <50 | <50 | 25,600 |
| 98 | 50 | 640 | 3,200 | 800 | 25,600 | |
| 240 | 50 | 10,240 | 12,800 | 3,200 | 25,600 | |
| 4, T, 37 | 28 | 50 | 2,560 | 6,400 | 200 | 51,200 |
| 91 | 50 | 10,240 | 12,800 | 800 | 51,200 | |
| 363 | 200 | 20,480 | 25,600 | 6,400 | 51,200 | |
| 5, T, 32 | 28 | 50 | 1,280 | <50 | 100 | 12,800 |
| 125 | 100 | 5,120 | 12,800 | 6,400 | 25,600 | |
| 385 | 200 | 20,480 | 25,600 | 6,400 | 25,600 | |
| 6, NT, 37 | 22 | 50 | 2,560 | 6,400 | 200 | 51,200 |
| 85 | 200 | 2,560 | 6,400 | 1,600 | 51,200 | |
| 248 | 200 | 5,120 | 12,800 | 6,400 | 102,400 | |
| 7, NT, 39 | 19 | <50 | 2,560 | 1,600 | 200 | 25,600 |
| 76 | ≤50 | 10,240 | 6,400 | 3,200 | 25,600 | |
| 216 | 200 | 20,480 | 12,800 | 6,400 | 25,600 | |
All patients were pregnant women. T, transmitter; NT, nontransmitter.
DISCUSSION
The results of our study showing that antibodies to the pentamer are required for SFI in infected epithelial cells (ARPE-19) may explain some discrepancies reported in the literature. Findings reported by Vanarsdall et al. (24) showed that overexpression of pentamer subunits causes cells to fuse with cells not expressing the pentamer. However, the same group reported that gH/gL and gB overexpression was enough to cause the same level of fusion. These data are in contrast with recent findings from Ciferri et al. (9), who reported that SF occurred only when the pentamer was overexpressed with gB, whereas SF was not observed when gB was overexpressed with gH/gL. Unlike the previous two studies, in the current study we investigated syncytium formation in the context of authentic HCMV infection of epithelial cells. We observed that syncytium formation occurs at late times after infection (72 to 96 h p.i.) and is thus likely to require a high level of de novo expression of viral glycoproteins in infected cells. Moreover, syncytium formation can be potently inhibited by using anti-pentamer antibodies. These findings show that the pentamer has a critical role in the cell fusion process in epithelial cells. Therefore, these results suggest that during authentic HCMV infection, syncytium formation in epithelial cells is regulated differently from the case under experimental conditions where gH/gL plus gB is overexpressed by use of ectopic vectors. However, it should be kept in mind that the fusion event of HCMV entry into all cell types is mediated by gB and gH/gL/gO. The neutralizing properties of anti-gO MAbs (i.e., virus neutralization potency and cellular specificity similar to those of gH MAbs) are consistent with the idea that the gH/gL/gO complex is involved in entry in all cell types, probably by promoting gB fusion activity. On the other hand, for entry into many cell types, such as epithelial/endothelial and myeloid cells, efficient fusion must be preceded by an as yet unidentified process (cell receptor binding?) mediated by the pentameric complex (11). However, while gH/gL/gO is important for infection of all cell types by cell-free virus, cell-to-cell spread does not require gH/gL/gO (10). This was already documented for murine CMV (MCMV) (25), although HCMV and MCMV are distinct viruses and the relevant roles of gO may be very different. In addition, gB activation in the case of cell-free HCMV infection might be different from gB activation for viral spreading and cell-cell fusion.
A major finding derived from this study is that only antibodies elicited by the UL128L gene products, which have potent neutralizing activity (in the picomolar range), are capable of blocking SF, while antibodies to gO, gH, and gB, displaying 100- to 1,000-fold lower neutralizing activities (in the nanomolar range), exhibit only partial or null SFI activity at the concentrations tested. The differential Nt activity has been attributed hypothetically to differences in the amounts of gH/gL/gO and the pentamer on the viral envelope or to differences in entry mechanisms, epitope exposure, or glycan networks (11). This variable effect, at least for anti-gH antibodies, cannot be attributed to differences in binding affinity, as recently shown (23). We also found that the neutralizing anti-gO MAb CVB234 showed a binding affinity similar to that reported for an anti-gH (13H11) and two anti-pUL128L (15D8 and 10F7) MAbs. It was previously shown by our group that absorption of highly neutralizing human sera with the purified pentamer nearly abolished the neutralizing antibody titer, while absorption with gH/gL/gO significantly reduced the titer and absorption with purified gB did not affect the neutralizing titer (15). This finding further supports the conclusion that the potency of neutralizing activity is the major factor responsible for SFI.
It can reasonably be hypothesized that for SF, binding of the relevant receptor by the pentamer should occur on the cell membranes of contiguous cells, thus activating gB. According to recent studies, the role of gB in the membrane fusion machinery should follow its interaction with gH/gL/gO after binding the cell receptor at the receptor binding site (22). In our experiments using the VR1814 strain produced in HUVECs, the high MOI allowed multiple contiguous cells to be infected simultaneously and to fuse into multinucleated giant cells at about the same time, thus resulting in the presence of multiple syncytia at 96 h p.i. However, we did not observe syncytium formation occurring at an earlier time point before viral replication, as observed by Wang and coworkers using the BADrUL131 strain (UL131-repaired AD169) produced in epithelial cells (26).
The high virus inoculum required to generate syncytia in cell cultures likely mirrors what occurs in vivo in HCMV end-organ diseases associated with high viral loads, such as cerebral astrocytoma in AIDS patients (27), cutaneous HCMV infection in liver transplant recipients (28), or HCMV congenital infection (29) or neonatal hepatitis (30). In addition, congenitally infected infants often excrete large amounts of infectious virus in urine, thus generating multiple syncytia in fibroblasts inoculated for virus isolation (our unpublished observations).
The SFI antibody response observed in the convalescent-phase serum samples from all subjects with primary HCMV infection tested and its gross correlation with the Nt antibody response confirm that the Nt component of the serum antibody response plays a major role in SFI activity. In recent years, we had the opportunity to verify that a similar inhibiting activity was exhibited by MAbs to the pentamer pUL128L components in both PFI and LTI assays (14–16). Thus, it can be hypothesized that antibodies that mediate SFI as well as PFI and LTI in vitro may prevent in vivo virus spreading.
It is currently believed that syncytia are not a major source of virus spreading. However, the finding that both in vivo (when syncytia are detected in patients with AIDS or congenital infection or following transplantation) and in vitro (as shown here) SF is associated with high viral loads suggests that multiple cells are infected simultaneously and then fuse to each other. Along this line of thought, SFI antibodies may limit virus dissemination. A recent report documented that cell-to-cell infection by HIV-1 contributes to over half of the virus infection compared to cell-free virus infection and suggested that solely blocking cell-free virus infection in the absence of a cell-to-cell block would only partially limit virus dissemination (31). This conclusion may be applicable to viruses other than HIV, such as herpesviruses (e.g., HCMV and herpes simplex virus [HSV]). This result would make the activity of antibodies mediating SFI as well as PFI and LTI indispensable for virus infection control. It must be emphasized that anti-pentamer antibodies showed the most potent activity in all these assays.
In keeping with the data reported herein, in the herpes simplex viral system it was shown that SFI by an anti-gD MAb was displayed at a concentration about 10 times higher than that for Nt antibody activity (32), whereas anti-gB MAbs were only minimally inhibitory at the highest concentration tested (33). This strengthens and adds further evidence (with another herpesvirus) to the hypothesis of a lack or minimal role of anti-gB antibodies in limiting SF.
In conclusion, Nt antibodies to the pUL128L portion of the pentamer appear to prevent both virus infection and SF in epithelial cells, and the Nt antibody response in serum samples from subjects with primary HCMV infection follows, although at a higher titer, a kinetics similar to that of SFI antibodies. These results have implications for the HCMV entry mechanism, as they suggest a potential role for the pentamer in membrane fusion activation, and perhaps in the development of antibodies and vaccines against HCMV.
ACKNOWLEDGMENTS
We thank Daniela Sartori for manuscript editing and Laurene Kelly for revision of the English.
This work was supported by the Fondazione CARIPLO (grants 93043/A and 2012-0626) and the Ministero della Salute, Ricerca Finalizzata (grant RF-2010-GR-2010) and Ricerca Corrente (grant 08069614).
REFERENCES
- 1.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R, National Vaccine Advisory Committee. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis 39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 2.Revello MG, Percivalle E, Arbustini E, Pardi R, Sozzani S, Gerna G. 1998. In vitro generation of human cytomegalovirus pp65 antigenemia, viremia, and leukoDNAemia. J Clin Invest 101:2686–2692. doi: 10.1172/JCI1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerna G, Percivalle E, Lilleri D, Lozza L, Fornara C, Hahn G, Baldanti F, Revello MG. 2005. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL128-131 genes and mediates efficient viral antigen presentation to CD8+ T cells. J Gen Virol 86:275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 4.Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 325:63–83. [DOI] [PubMed] [Google Scholar]
- 5.Hahn G, Revello MG, Patrone M, Percivalle E, Campanini G, Sarasini A, Wagner M, Gallina A, Milanesi G, Koszinowski U, Baldanti F, Gerna G. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Shenk T. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A 102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryckman BJ, Chase MC, Johnson DC. 2008. HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for type-specific receptors. Proc Natl Acad Sci U S A 105:14118–14123. doi: 10.1073/pnas.0804365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciferri C, Chandramouli S, Donnarumma D, Nikitin PA, Cianfrocco MA, Gerrein R, Feire AL, Barnett SW, Lilja AE, Rappuoli R, Norais N, Settembre EC, Carfi A. 2015. Structural and biochemical studies of HCMV gH/gL/gO and pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A 112:1767–1772. doi: 10.1073/pnas.1424818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wille PT, Knoche AJ, Nelson JA, Jarvis MA, Johnson DC. 2010. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J Virol 84:2585–2596. doi: 10.1128/JVI.02249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M, Lanchy J-M, Ryckman BJ. 2015. Human cytomegalovirus gH/gL/gO promotes the fusion step of entry into all cell types whereas gH/gL/UL128-131 broadens virus tropism through a distinct mechanism. J Virol 89:8999–9009. doi: 10.1128/JVI.01325-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bughio F, Umashankar M, Wilson J, Goodrum F. 2015. Human cytomegalovirus UL135 and UL136 genes are required for postentry tropism in endothelial cells. J Virol 89:6536–6550. doi: 10.1128/JVI.00284-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G, Nguyen CC, Ryckman BJ, Britt WJ, Kamil JP. 2015. A viral regulator of glycoprotein complexes contributes to human cytomegalovirus cell tropism. Proc Natl Acad Sci U S A 112:4471–4476. doi: 10.1073/pnas.1419875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerna G, Sarasini A, Patrone M, Percivelle E, Fiorina L, Campanini G, Gallina A, Baldanti F, Revello MG. 2008. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol 89:853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 15.Lilleri D, Kabanova A, Revello MG, Percivalle E, Sarasini A, Genini E, Sallusto F, Lanzavecchia A, Corti D, Gerna G. 2013. Fetal cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131. PLoS One 8:e59863. doi: 10.1371/journal.pone.0059863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabanova A, Perez L, Lilleri D, Marcandalli J, Agatic G, Becattini S, Preite S, Fuschillo D, Percivalle E, Sallusto F, Gerna G, Corti D, Lanzavecchia A. 2014. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A 111:17965–17970. doi: 10.1073/pnas.1415310111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 84:1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouts AE, Chan P, Stefan JP, Vandler R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 86:7444–7447. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freed DC, Tang Q, Tang A, Li F, He X, Huang Z, Meng W, Xia L, Finnefrock AC, Durr E, Espeseth AS, Casimiro DR, Zhang N, Shiver JW, Wang D, An Z, Fu TM. 2013. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc Natl Acad Sci U S A 110:E4997–E5005. doi: 10.1073/pnas.1316517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wussow F, Yue Y, Martinez J, Deere JD, Longmate J, Herrmann A, Barry PA, Diamond DJ. 2013. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol 87:1322–1332. doi: 10.1128/JVI.01669-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen Y, Monroe J, Linton C, Archer J, Beard CW, Barnett SW, Palladino G, Mason PW, Carfi A, Lilja AE. 2014. Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potent neutralizing antibodies in mice. Vaccine 32:3796–3804. doi: 10.1016/j.vaccine.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Wille PT, Wisner TW, Ryckman B, Johnson DC. 2013. Human cytomegalovirus (HCMV) glycoprotein B promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein. mBio 4:e00332–13. doi: 10.1128/mBio.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciferri C, Chandramouli S, Leitner A, Donnarumma D, Cianfrocco MA, Gerrein R, Friedrich K, Aggarwal Y, Palladino G, Aebersold R, Norais N, Settembre EC, Carfi A. 2015. Antigenic characterization of the HCMV gH/gL/gO and pentamer cell entry complexes reveals binding sites for potently neutralizing human antibodies. PLoS Pathog 11:e1005230. doi: 10.1371/journal.ppat.1005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J Virol 82:11837–11850. doi: 10.1128/JVI.01623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemmermann NA, Krmpotic A, Podlech J, Brisic I, Prager A, Adler H, Karbach A, Wu Y, Jonjic S, Reddehase MJ, Adler B. 2015. Non-redundant and redundant roles of cytomegalovirus gH/gL complexes in host organ entry and intra-tissue spread. PLoS Pathog 11:e1004640. doi: 10.1371/journal.ppat.1004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Yu QC, Schröer J, Murphy E, Shenk T. 2007. Human cytomegalovirus uses two distinct pathways to enter retinal pigmented epithelial cells. Proc Natl Acad Sci U S A 104:20037–20042. doi: 10.1073/pnas.0709704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho KL, Gottlieb C, Zarbo RJ. 1991. Cytomegalovirus infection of cerebral astrocytoma in an AIDS patient. Clin Neuropathol 10:127–133. [PubMed] [Google Scholar]
- 28.Patterson JW, Broecker AH, Kornstein MJ, Mills AS. 1988. Cutaneous cytomegalovirus infection in a liver transplant patient. Diagnosis by in situ DNA hybridization. Am J Dermatopathol 10:524–530. [DOI] [PubMed] [Google Scholar]
- 29.Dancygier H. 2009. Clinical hepatology: principles and practice of hepatobiliary disease, vol 2, p 825 Springer Science & Business Media, Berlin, Germany. [Google Scholar]
- 30.Podolski DK, Camilleri M, Fitz JG, Kalloo AN, Shanahan F, Wang TC. 2015. Yamada's textbook of gastroenterology, vol 2, p 1914 John Wiley & Sons, Hoboken, NJ. [Google Scholar]
- 31.Iwami S, Takeuchi JS, Nakaoka S, Mammano F, Clavel F, Inaba H, Kobayashi T, Misawa N, Aihara K, Koyanagi Y, Sato K. 2015. Cell-to-cell infection by HIV contributes over half of virus infection. eLife 4:e08150. doi: 10.7554/eLife.08150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Logu A, Williamson RA, Rozenshteyn R, Ramiro-Ibanez F, Simpson CD, Burton DR, Sanna PP. 1998. Characterization of a type-common human recombinant monoclonal antibody to herpes simplex virus with high therapeutic potential. J Clin Microbiol 36:3198–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noble AG, Lee GT, Sprague R, Parish ML, Spear PG. 1983. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology 129:218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]