ABSTRACT
The host intracellular antiviral restriction factors inhibit viral infection and replication. The 5′-AMP-activated protein kinase (AMPK) is a cellular energy sensor regulating metabolic homeostasis. Activated AMPK inhibits the replication of numerous RNA viruses but enhances the entry of vaccinia virus. However, the role of AMPK in herpesvirus infection is unclear. In this study, we showed that the constitutive AMPK activity restricted Kaposi's sarcoma-associated herpesvirus (KSHV) lytic replication in primary human umbilical vein endothelial cells while KSHV infection did not markedly affect the endogenous AMPK activity. Knockdown of the AMPKα1 considerably enhanced the expression of viral lytic genes and the production of infectious virions, while overexpression of a constitutively active AMPK had the opposite effects. Accordingly, an AMPK inhibitor, compound C, augmented viral lytic gene expressions and virion productions but an AMPK agonist, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), suppressed both. Furthermore, a common diabetes drug, metformin, which carries an AMPK-agonistic activity, drastically inhibited the expression of viral lytic genes and the production of infectious virions, suggesting the use of metformin as a therapeutic agent for KSHV infection and replication. Together, these results identify the host AMPK as a KSHV restriction factor that can serve as a potential therapeutic target.
IMPORTANCE Host cells encode specific proteins to restrict viral infection and replication. Kaposi's sarcoma-associated herpesvirus (KSHV) is a human tumor virus associated with several cancers. In this study, we have identified 5′-AMP-activated protein kinase (AMPK), a cellular energy sensor, as a restriction factor of KSHV lytic replication during primary infection. Activation of AMPK suppresses, while inhibition of AMPK enhances, KSHV lytic replication by regulating the expression of viral genes. AICAR and metformin, both of which are AMPK agonists currently used in clinics for the treatment of conditions associated with metabolic disorders, inhibit KSHV lytic replication. Thus, our work has identified AMPK as a potential therapeutic target and AICAR and metformin as potential therapeutic agents for KSHV-associated cancers.
INTRODUCTION
Mammalian cells encode numerous restriction factors that serve to defend against intrusions of viruses (1–4). Identification of novel host restriction factors and understanding how they control viral infections are essential for delineating the mechanisms of pathogenesis of viral infections and developing effective therapeutic approaches.
As a conserved cellular energy sensor, 5′-AMP-activated protein kinase (AMPK) maintains cellular energy homeostasis by regulating glucose and lipid metabolism (5, 6). AMPK is activated in response to an increased intracellular AMP/ATP ratio as a result of nutritional stress. AMPK signals the cell to stop the anabolic pathway and activates a catabolic state by inducing oxidative pathways to generate energy, thereby returning the cell to a state of energy homeostasis (6, 7). Thus, activated AMPK is important for cell survival during nutritional stress. Dysregulation of the AMPK pathway is implicated in type II diabetes, obesity, metabolic syndrome, decreased lifespan, and cancer (8–10).
AMPK is a heterotrimeric complex consisting of a catalytic alpha subunit, and one of each of regulatory beta and gamma subunits (6). Activation is triggered through binding of AMP or ADP to the Bateman domains of the gamma subunit, leading to increased phosphorylation at threonine 172 on the alpha subunit by inducing allosteric activation and inhibiting dephosphorylation (6). The canonical upstream activator catalyzing this phosphorylation event is the constitutively active tumor suppressor LKB1, but additional activators, including CaMKKβ and TAK1, have been identified (6, 11). Activated AMPK phosphorylates a number of substrates to regulate central carbon metabolism, lipid metabolism, physiological homeostasis, cell growth, apoptosis, and gene expression (6).
In recent years, several studies have suggested that AMPK can function as an antiviral restriction factor in addition to regulating cellular metabolic homeostasis (12). Activation of AMPK restricts infections of Bunyavirus and Rift Valley fever virus (RVFV) by decreasing cellular fatty acid synthesis (13). Several other RNA viruses, including Sindbis virus (SINV), vesicular stomatitis virus (VSV), and Kunjin virus (KUNV), which depend on cellular membrane modifications and fatty acid synthesis, are also restricted by AMPK (13). In contrast to RNA viruses, DNA viruses Zaire Ebolavirus and vaccinia virus rely on the AMPK activity for actin polymerization and the induction of macropinocytosis during entry (14, 15).
The roles of AMPK in the infection and replication of two members of herpesviruses, herpes simplex virus 1 (HSV-1) and human cytomegalovirus (HCMV), have been examined; however, the interactions of these viruses with the AMPK pathway appear to be complex (16–19). At the early stage of infection (2 h postinfection), the AMPK activity was inhibited by HSV-1 infection; however, it gradually recovered as the infection progressed. AMPK agonist inhibited HSV-1 gene expression and viral production (17, 19). Interestingly, both AMPK agonist and inhibitor impaired HCMV replication, suggesting that fine-tuning of AMPK activity might be essential for optimal HCMV replication (16, 18).
Kaposi's sarcoma-associated herpesvirus (KSHV) is a gammaherpesvirus etiologically associated with Kaposi's sarcoma (KS), a vascular tumor of endothelial cells commonly found in AIDS patients, and two B-cell lymphoproliferative diseases, including primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (20–22). KSHV infection of human telomerase reverse transcriptase (hTERT)-immortalized human umbilical endothelial cells (HUVEC) led to decreased phosphorylation of AMPK at 48 h postinfection as a result of activation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway (23). While the activation of the PI3K/AKT pathway is essential for the survival of KSHV-infected cells, it is unclear what role AMPK dephosphorylation might have during KSHV primary infection.
In this study, we focused on the role of AMPK in KSHV primary infection. We have found that KSHV infection does not alter the endogenous activity of AMPK. However, inhibition of constitutive endogenous AMPK activity increases virus yield by enhancing viral gene expression, while artificial activation of the AMPK activity significantly inhibits infectious virion production and viral gene expression. Significantly, a common diabetes drug, metformin, which is also an AMPK activator, inhibits infectious virion production and viral gene expression. Our results identify the AMPK pathway as a potential therapeutic target and metformin as a therapeutic agent for KSHV infection and replication.
MATERIALS AND METHODS
Antibodies and reagents.
A monoclonal antibody isotype IgG2a (clone 6A) to KSHV small capsid protein (ORF65 [where ORF is open reading frame]) was used to stain KSHV particles (24). A rat anti-LANA monoclonal antibody was purchased from Abcam (Cambridge, MA). Anti-KSHV K-bZIP antibody was obtained from Santa Cruz Biotechnology (Dallas, TX). A monoclonal antibody was used to detect replication and transcriptional activator (RTA) (25). The primary antibodies against phospho-AMPKα (Thr172), AMPKα1, and AMPKα2 were purchased from Cell Signaling Technology (Danvers, MA). An antibody to β-tubulin was purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 568-conjugated goat anti-mouse immunoglobulin G (IgG), Alexa Fluor 568-conjugated goat anti-rat IgG, horseradish peroxidase (HRP)-conjugated goat anti-mouse, goat anti-rabbit, and goat anti-rat antibodies were obtained from Santa Cruz Biotechnology.
Kinase inhibitors and agonists were obtained from the following sources: compound C from VWR International, LLC (Radnor, PA), and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and metformin from Cayman Chemical (Ann Arbor, MI).
Cell culture.
Primary HUVEC were cultured in VascuLife VEGF complete medium (Lifeline Cell Technology, Frederick, MD). Recombinant KSHV bacterial artificial chromosome 16 (BAC16)-infected iSLK (iSLK-BAC16) cells were maintained in the presence of 1 μg/ml puromycin, 250 μg/ml G418 (Sigma-Aldrich), and 1.2 mg/ml hygromycin B (26). Human embryonic kidney 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1× penicillin-streptomycin solution (Genesee Scientific, San Diego, CA).
Virus preparation.
A volume of concentrated virus was prepared from iSLK-BAC16 as previously described (26). Briefly, iSLK-BAC16 cells were induced with both doxycycline (1 μg/ml) and sodium butyrate (1 mM) in the medium described above but without hygromycin, puromycin, and G418. Four days later, supernatant was collected, centrifuged at 5,000 × g for 10 min, and then filtered (0.45 μm) to eliminate cell debris. Virus particles were pelleted by ultracentrifugation (100,000 × g for 1 h with a 20% sucrose cushion at 4°C) using an SW32 Ti rotor. The final pellet was dissolved in culture medium overnight. Fresh virus preparations were titrated by infecting HUVEC. At day 2 postinfection, the green fluorescent protein (GFP)-positive cells were quantified, and the relative virus titers, termed infectious units (IUs), in the supernatants were calculated based on the number of GFP-positive cells observed with 1 ml of virus preparation.
Virus infection.
Fresh virus preparations with a titer of 2 × 106 IUs were used in the experiments. HUVEC were infected with virus as previously described (24, 27). For all experiments, cells were infected at a multiplicity of infection (MOI) of 2 per cell unless specified otherwise. To determine the effects of kinase inhibitor or agonists on KSHV infection, HUVEC grown to confluence in 36-mm dishes were first treated with the kinase inhibitor (compound C) or agonists (AICAR and metformin) for 1 h at 37°C prior to KSHV infection. Cells were then infected with KSHV in the presence of the inhibitor or agonists.
Immunofluorescence assay (IFA).
KSHV LANA protein was detected as previously described with minor modifications (28). At 48 h postinfection, KSHV-infected cells were fixed in methanol at room temperature for 10 min. Following three washes with phosphate-buffered saline (PBS), the cells were incubated with a rat anti-LANA monoclonal antibody (Abcam) at a 1:500 dilution for 60 min at room temperature. The cells were then washed three times with PBS, followed by incubation with an Alexa Fluor 568-conjugated goat anti-rat immunoglobulin G secondary antibody for 60 min at room temperature. The cells were again washed with PBS three times and stained with DAPI (4′,6-diamidino-2-phenylindole).
The method for examining virus entry and trafficking was previously described (29). Briefly, following small hairpin RNA (shRNA) knockdown of AMPKα1 or treatment of AMPK inhibitor or agonists, the cells were inoculated with virus for 6 h, fixed in 4% paraformaldehyde, and processed for immunostaining for KSHV particles using an anti-ORF65 antibody. The numbers of virus particles docked at the perinuclear regions were calculated.
Western blot analysis.
Cells infected with KSHV for specified times were washed once with ice-cold PBS, and total protein was extracted. Total protein preparations were separated in sodium dodecyl sulfate-polyacrylamide electrophoresis gels, transferred to nitrocellulose membranes, and detected with antibodies. Specific signals were revealed with chemiluminescence substrates and recorded using a UVP MultiSpectral Imaging System (UVP LLC, Upland, CA).
RT-qPCR.
The expression levels of viral genes were analyzed by reverse transcription quantitative real-time PCR (RT-qPCR) using procedures previously described (30). Briefly, total RNAs from KSHV-infected HUVEC were prepared with TRI reagent as recommended by the manufacturer (Sigma). The RNA was treated with RNase-free DNase (Thermo Fisher Scientific, Inc., Waltham, WA) and reverse transcribed to obtain the first-strand cDNA using a Maxima Reverse Transcriptase system (Thermo Fisher Scientific). For each sample, a control without reverse transcriptase was conducted in parallel. qPCR was then performed with the cDNA using gene-specific PCR primers as previously described (31). α-Tubulin was used as the internal control. Each sample was assayed with three repeats.
shRNA knockdown.
shRNA plasmids were constructed by inserting annealed oligonucleotides containing the shRNA sequences specific for the target genes into the EcoRI and AgeI sites downstream of the U6 promoter in the pLKO.1 vector (Sigma-Aldrich). Recombinant lentiviruses carrying shRNAs were produced by cotransfecting 293T cells with a mixture of plasmid DNA consisting of pMD-G (VSV-G envelope), pCMV-ψR8.91 (Gag/Pol/Rev), and the pLKO.1-shRNA vectors (Aldevron, Fargo, ND) using Lipofectamine 2000 reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. Virus-containing culture supernatants were collected 2 days after transfection and concentrated.
Knockdown with shRNAs was performed as previously described (32). Briefly, HUVEC were infected in the presence of 10 μg/ml Polybrene (Sigma-Aldrich). Stable cell lines were selected by culturing the cells in 1 μg/ml puromycin (Calbiochem, La Jolla, CA) for 3 days. Western blotting or RT-qPCR was used to determine the effect of shRNA knockdown. The AMPKα1 shRNA knockdown sequences were TGATTGATGATGAAGCCTTAA for shRNA1 and CCTGGAAGTCACACAATAGAA for shRNA2.
AMPK-CA construction.
Residues 1 to 314 of AMPKα1 were amplified from the construct pWZL-Neo-Myr-Flag-PRKAA1 (Addgene, Cambridge, MA) (33) and then cloned to pCMR2 lentiviral vector to express the constitutively activated AMPKα1 (AMPK-CA). Recombinant lentiviruses of the empty vector (pWZL-Neo-Myr-Flag-DEST) or AMPK-CA were obtained as described above.
RESULTS
KSHV infection does not alter AMPK expression and activation during primary infection.
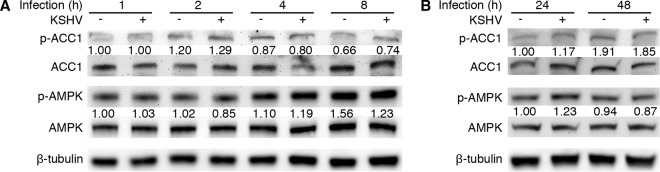
Previous research has reported that AMPK activity is essential for infection by several viruses (13, 16, 17). We investigated the status of AMPK activity and its role in primary KSHV infection with a focus on viral lytic replication. We used primary HUVEC as the target cells because they are the relevant cell type for KS tumor cells and KSHV infection of HUVEC is productive at the early stage of infection (24, 30). This system allows the examination of virus entry, trafficking, infectivity, expression of viral genes, and production of infectious virions (24, 29, 30, 34). We infected primary HUVEC with KSHV for 1, 2, 4, 8, 24, and 48 h and examined the status of AMPKα, the catalytic subunit of the complex by Western blotting. We did not detect any obvious change of total AMPKα and phosphorylated AMPKα (p-AMPKα) between KSHV- and mock-infected HUVEC for all the time points examined (Fig. 1A and B). There was also no change in the total acetyl coenzyme A (acetyl-CoA) carboxylase 1 (ACC1), an AMPK downstream effector, and its phosphorylated form (p-ACC1) (35). These results indicated that KSHV infection did not alter AMPK activity during primary infection of primary HUVEC.
FIG 1.
KSHV infection does not affect the AMPK activity during primary infection. (A and B) Time kinetics of the levels of phosphorylated AMPK (p-AMPK) and phosphorylated ACC1 (p-ACC1) during KSHV primary infection. HUVEC were mock infected or KSHV infected for the indicated times. Whole-cell lysates were collected for Western blot analysis for p-AMPK (T172), total AMPK, p-ACC1 (S79), and total ACC1. An anti-β-tubulin antibody was used to normalize the sample loading. Numbers under p-ACC1 and p-AMPK are the relative levels following calibration with β-tubulin.
Knockdown of AMPK enhances KSHV lytic replication at the postentry stage.
Although KSHV infection did not alter AMPK activity, the constitutive endogenous activated AMPK could still regulate KSHV infection and replication. Thus, we performed knockdown of AMPK and tested the effect on KSHV infection. In humans, there are two isoforms of the catalytic subunit AMPKα: AMPKα1 and AMPKα2. These two isoforms have distinct tissue- and cell type-specific distributions (36). First, we examined the expression of the two AMPKα isoforms in HUVEC by Western blotting using 293T cells as a positive control, as these two isoforms are expressed in these cells. As expected, we detected both AMPKα1 and AMPKα2 isoforms in 293T cells; however, only AMPKα1 was detected in HUVEC (Fig. 2A and B). Next, we performed shRNA knockdown of AMPKα1 in HUVEC to determine its role in viral lytic replication during KSHV primary infection. The Western blotting results showed that the AMPKα1 protein level was reduced by over 80% in cells transduced with lentiviruses expressing AMPKα1-specific shRNAs compared to the nontargeting control (Fig. 2C). To confirm the loss of function following knockdown of AMPKα1, we treated cells transduced with lentiviruses of both AMPKα1 and scrambled control shRNAs with AICAR, a potent AMPK agonist. In cells transduced with the control shRNA, AICAR potently enhanced the phosphorylation levels of AMPKα1 at site Thr172 and its downstream target ACC1 at site Ser79 (Fig. 2D). Knockdown of AMPKα1 completely abrogated AICAR induction of AMPK phosphorylation and significantly reduced ACC1 phosphorylation (Fig. 2D), indicating that knockdown of AMPK had disabled its function.
FIG 2.
Knockdown of AMPKα1 increases the production of infectious virions but not virus entry, trafficking, and infectivity during KSHV primary infection. (A and B) Expression profiles of two AMPKα isoforms: AMPKα1 (A) and AMPKα2 (B) in HUVEC. HEK293T cells were used as positive controls for AMPKα1 and AMPKα2. (C) shRNA knockdown of AMPKα1. HUVEC were infected with lentiviral viruses expressing shRNAs (shRNA 1 or shRNA 2) targeting AMPKα1 or the scrambled control shRNA sequences (Ctrl). Cells were selected in the presence of puromycin (1 μg/ml) for 3 days and analyzed by Western blotting at day 4 after transduction. (D) Knockdown of AMPKα1 inhibits its functions. HUVEC with AMPKα1 or control knockdown were untreated or treated with AICAR for 8 h. Cell lysates were analyzed by Western blotting for p-AMPK (T172), total AMPK, p-ACC1 (S79), and total ACC1. (E and F) Knockdown of AMPKα1 increases the production of KSHV infectious virions. HUVEC with knockdown of AMPKα1 were infected with KSHV for 24 h. Cells were extensively washed, and medium was replaced with new medium. At day 4 postinfection, the supernatants were collected and infectious virions were titrated by infecting fresh HUVEC. GFP-positive cells are shown (E) and quantified (F). (G and H) AMPKα1 knockdown has no effect on virus entry and trafficking. HUVEC with knockdown of AMPKα1 were infected with KSHV for 6 h, fixed, and stained for nuclei with DAPI and KSHV particles with an antibody to ORF65 (G). The total number of KSHV particles successfully docked at each nucleus was determined and analyzed (H). The results are shown as box and whisker plots with the open boxes representing the 75th and 25th percentiles, respectively; top and bottom short lines representing the 90th and 10th percentiles, respectively; middle thick lines in the boxes representing the medians; and the open circles outside the 90th and 10th percentiles representing the outliers. “N” represents the total number of cells analyzed per sample. A P value of <0.05 signifies statistical significance. (I and J) Knockdown of AMPKα1 has no effect on the expression level and pattern of KSHV latent protein LANA and infectivity. HUVEC with knockdown of AMPKα1 were infected with KSHV, fixed at day 2 postinfection, and stained for LANA. The expression level and pattern of LANA are shown (I), and the results of quantification of LANA-positive cells are shown (J). NS, not significant.
We infected AMPKα1 knockdown cells with a recombinant KSHV BAC16. Extensive wash of the cells with culture medium was carried out to remove any residual input viruses. At day 4 postinfection, we collected the culture supernatants and titrated the numbers of infectious virions produced by the cultures. Based on the expression of the GFP cassette in the BAC16 genome, we quantified the infected cells (26). Knockdown of AMPKα1 significantly increased the production of infectious virions 2.5- to 6.5-fold compared to cells transduced with the scrambled shRNA control (Fig. 2E and F). We concluded that constitutive endogenous AMPKα1 restricted viral lytic replication during KSHV primary infection.
The early phase of KSHV primary infection is highly regulated at multiple steps consisting of virus entry, trafficking, and viral gene expression, among others (37). To identify the step(s) restricted by AMPK, we first examined KSHV entry and trafficking to the cell nucleus. Following AMPKα1 knockdown, HUVEC were infected with KSHV for 6 h, extensively washed, fixed, and stained for ORF65 to visualize the viral capsids that had successfully trafficked to the perinuclear region (Fig. 2G). By counting viral capsids docking at the nucleus, we found that similar numbers of virus particles had successfully reached the cell nucleus in the cells with or without AMPK knockdown (Fig. 2G and H), indicating that AMPKα1 did not regulate KSHV entry and intracellular trafficking.
We further determined if knockdown of AMPKα1 might affect KSHV infectivity. Because LANA is the major KSHV latent protein that is required for episome persistence, detection of LANA protein, which manifested as a punctate staining pattern, would indicate successful viral infection. HUVEC transduced with lentiviruses expressing AMPKα1 or the scrambled control shRNAs were infected with KSHV and stained for LANA protein at day 2 postinfection. We found that AMPKα1 knockdown changed neither the expression and staining pattern of LANA nor KSHV infectivity (Fig. 2I and J). Taken together, these results indicated that AMPKα1 might regulate the production of infectious virions at the postentry stage.
Knockdown of AMPK enhances KSHV lytic replication by increasing viral gene expression.
Based on distinct gene expression features, KSHV genes can be classified into latent and lytic genes. Latent genes, which are mainly those encoding LANA, vFLIP, vCyclin, and a cluster of microRNAs, are essential for maintaining viral latency and promoting cell proliferation and survival (38). Lytic genes, which are further divided into immediate early (IE), early (E), and late (L) genes, are expressed in a coordinated fashion during KSHV lytic replication (24, 39). The expression of IE gene, the replication and transcriptional activator (RTA), is necessary and sufficient for activating KSHV into full lytic replication (38). Early gene ORF-K8 encodes another important regulator of lytic replication, K-bZIP. Late genes are mostly those encoding viral structural proteins and those required for viral lytic replication. The expression of these genes, such as ORF65, encoding a small capsid protein, and ORF39, encoding glycoprotein M, often depends on viral genome replication (38). During KSHV primary infection of HUVEC, both latent and lytic genes are actively transcribed, with most of them peaking at 48 to 72 h postinfection (30).
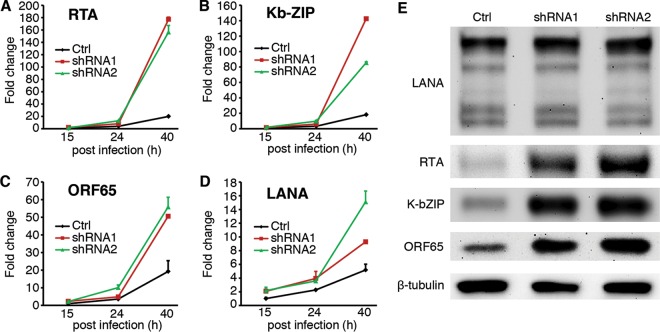
To determine if AMPK might regulate the expression of KSHV genes during primary infection, we monitored the kinetics of several representative KSHV lytic genes by RT-qPCR. Knockdown of AMPKα1 dramatically increased the expression of lytic genes encoding RTA, K-bZip, and ORF65 and caused a moderate increase in the expression of latent LANA gene (Fig. 3A to D). Western blotting confirmed the increased expression of RTA, K-bZip, and ORF65, while the change of LANA protein was not obvious in cells with knockdown of AMPKα1 (Fig. 3E). Taken together, AMPKα1 knockdown enhanced KSHV lytic replication by increasing the expression of viral lytic genes.
FIG 3.
Knockdown of AMPKα1 increases the expression of viral lytic transcripts and proteins during KSHV primary infection. (A to D) shRNA knockdown of AMPKα1 promotes the expression of KSHV lytic transcripts of RTA (A), K-bZIP (B), ORF65 (C), and to a lesser extent, latent LANA gene (D). HUVEC with knockdown of AMPKα1 were infected with KSHV for 15, 24, and 40 h and examined for the expression of KSHV lytic transcripts by RT-qPCR. (E) Knockdown of AMPKα1 increases the expression of KSHV lytic proteins RTA, K-bZIP, and ORF65 but not that of LANA. HUVEC with knockdown of AMPKα1 were infected with KSHV for 48 h and analyzed for the expression of viral proteins by Western blotting.
Overexpression of a constitutively active AMPKα1 construct (AMPK-CA) inhibits KSHV lytic replication.
AMPKα is composed of several functional domains: a kinase domain, an autoinhibitory domain (AID), and a C-terminal domain. Deletion of the C terminus at residue 312 results in a polypeptide that retains kinase activity but is no longer associated with the β and γ subunits and hence is constitutively active (33, 40). We cloned and expressed this truncated AMPKα1 (AMPK-CA) by lentiviral transduction. Expression of AMPK-CA significantly increased the phosphorylation level of the AMPK downstream effector ACC1, indicating that the AMPK-CA construct was functional (Fig. 4A). Interestingly, expression of AMPK-CA slightly increased the expression of the wild-type endogenous AMPKα1, but there was no obvious change in AMPKα1 phosphorylation at T172 (Fig. 4A).
FIG 4.
Expression of a constitutively active construct of AMPKα1 (AMPK-CA) inhibits KSHV lytic replication during primary infection. (A) Expression of the AMPK-CA construct in HUVEC. HUVEC were infected with lentiviral viruses expressing vector control (Vec) or a constitutively active construct of AMPKα1 (AMPK-CA). Cells were selected in the presence of puromycin (1 μg/ml) for 2 days and analyzed by Western blotting at day 3 after transduction. (B) Expression of the AMPK-CA construct inhibits the production of KSHV infectious virions. HUVEC expressing AMPK-CA were infected with KSHV for 24 h. Cells were extensively washed, and old medium was replaced with new. At day 4 postinfection, the supernatants were collected and subjected to titration of infectious virions as described for Fig. 2E and F. (C) Expression of the AMPK-CA construct has no effect on virus entry and trafficking. HUVEC expressing AMPK-CA were infected with KSHV for 6 h. The number of KSHV particles successfully docked at the nuclei was analyzed as described for Fig. 2G and H. (D) Expression of the AMPK-CA construct has no effect on KSHV infectivity. HUVEC expressing AMPK-CA were infected with KSHV for 48 h and analyzed for the numbers of LANA-positive cells. (E to H) Expression of the AMPK-CA construct significantly decreases the expression of KSHV lytic transcripts of RTA (E), K-bZIP (F), and ORF65 (G) but has minimal effect on latent LANA transcript (H). HUVEC expressing AMPK-CA were infected with KSHV for 15, 24, and 40 h and analyzed for the expression of viral transcripts by RT-qPCR. (I) Expression of the AMPK-CA construct decreases the expression of KSHV lytic proteins RTA, K-bZIP, and ORF65 but to a lesser extent the expression of latent protein LANA. HUVEC expressing AMPK-CA were infected with KSHV for 48 h and analyzed for the expression of viral proteins by Western blotting.
We then examined the effect of AMPK-CA expression on KSHV infection and replication during primary infection. Compared to cells transduced with a vector control, expression of AMPK-CA significantly reduced the production of infectious virions by 80% (Fig. 4B). Consistent with the results of AMPK knockdown, expression of AMPK-CA neither affected virus entry and trafficking (Fig. 4C) nor altered KSHV infectivity (Fig. 4D). However, expression of AMPK-CA significantly decreased the expression of viral lytic genes encoding RTA, K-bZIP, and ORF65 (Fig. 4E to G). We did not observe any significant change of latent LANA gene (Fig. 4H). As expected, the expression of viral lytic proteins RTA, K-bZIP, and ORF65 was significantly decreased while LANA was slightly increased following the expression of AMPK-CA (Fig. 4I). Taken together, enhanced constitutive activation of AMPK with AMPK-CA inhibited KSHV lytic replication by suppressing the expression of viral lytic genes.
Inhibitor of AMPK enhances, while agonists of AMPK suppress, KSHV lytic replication.
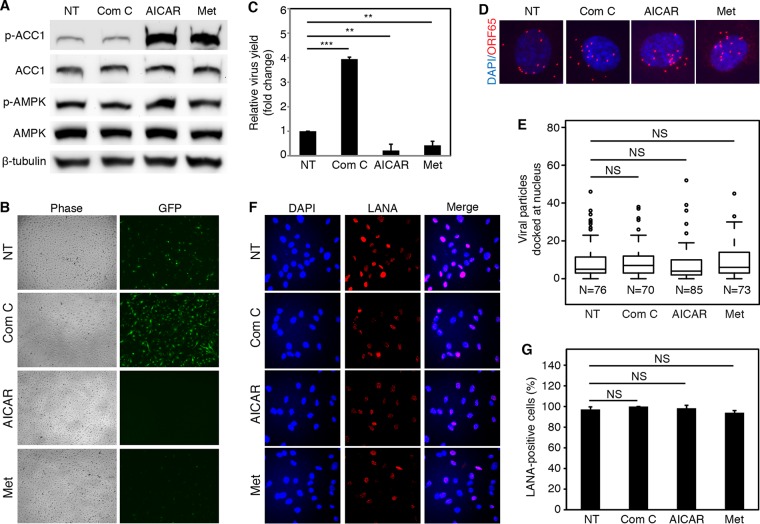
Our results so far indicated that activated AMPK robustly restricted KSHV lytic replication during primary infection. We wished to further confirm these results using AMPK inhibitor and agonists as well as explore the potential therapeutic applications of these chemical compounds. We selected compound C to inhibit the AMPK activity, as it is the only agent that is available as a cell-permeable AMPK inhibitor, although it also inhibits several other kinases besides AMPK (41). We selected AICAR and metformin to activate the AMPK activity. AICAR is an analog of AMP, an intermediate for generating IMP. AICAR has been used in clinics to treat and protect against cardiac ischemic injury (42) and has also shown promising results for treating patients with diabetes (43). Metformin, which is a front-line drug for treating type 2 diabetes, is a potent agonist for AMPK, though its mechanism of action remains elusive (44, 45). We first tested the cytotoxicity of different concentrations of these compounds on HUVEC. No obvious toxicity was observed for compound C at up to 10 μM, AICAR at up to 5 mM, and metformin at up to 1.5 mM (data not shown). Hence, compound C at 5 μM, AICAR at 1 mM, and metformin at 1 mM were chosen for the experiments. Treatment with AICAR or metformin robustly increased the phosphorylation levels of AMPK and ACC1, reflecting the activation of AMPK activity (Fig. 5A). However, treatment with compound C did not cause any obvious change in the phosphorylation levels of AMPK and ACC1, which might be due to the relatively low AMPK activity in cells with normal metabolism when they were grown in the normal medium (Fig. 5A). Since ACC1 can also be phosphorylated by protein kinase A (PKA) (5, 6, 35), it is possible that ACC1 is phosphorylated primarily by PKA under this experimental condition.
FIG 5.
AMPK inhibitor and agonists affect viral lytic replication but have no effect on virus entry, trafficking, and infectivity during KSHV primary infection. (A) Effects of AMPK inhibitor and agonists on AMPK activity. HUVEC were treated with compound C (Com C, 5 μM), AICAR (1 mM), or metformin (Met, 1 mM) for 8 h and examined for phosphorylated AMPK (T172) (p-AMPK), total AMPK, phosphorylated ACC1 (S79) (p-ACC1), and total ACC1. NT, no treatment. (B and C) AMPK inhibitor increases, while agonists inhibit the production of infectious virions. HUVEC pretreated with AMPK inhibitor or agonists for 1 h were infected with KSHV in the presence of the chemicals for 24 h. Cells were extensively washed, and medium was replaced. At day 4 postinfection, the supernatants were collected and subjected to titration of infectious virions as described for Fig. 2E and F. (D and E) AMPK inhibitor and agonists have no effect on virus entry and trafficking. HUVEC pretreated with AMPK inhibitor or agonists for 1 h were infected with KSHV for 6 h in the presence of the chemicals. The number of KSHV particles successfully docked at the nuclei was analyzed as described for Fig. 2G and H. (F and G) AMPK inhibitor and agonists have no effect on the expression level and pattern of KSHV latent protein LANA or infectivity. HUVEC pretreated with AMPK inhibitor or agonists for 1 h were infected with KSHV in the presence of these chemicals for 48 h and analyzed for the expression level and pattern of LANA (F) and the numbers of LANA-positive cells (G).
We first determined the effects of the AMPK inhibitor or agonists on the production of infectious virions during KSHV primary infection. Inhibition of AMPK activity with compound C significantly increased the production of infectious virions 4-fold, while activation of AMPK activity with agonists AICAR and metformin decreased the virus yields by 80% and 60%, respectively (Fig. 5B and C). These results indicated that exogenous activation of AMPK can potently inhibit KSHV lytic replication and virion production while exogenous inhibition of AMPK activity can enhance KSHV lytic replication.
Next, we examined the effects of the AMPK inhibitor or agonists on virus entry and trafficking. Neither AMPK inhibitor nor agonists affected the numbers of virus particles docking at the perinuclear regions, indicating that they had no effect on virus entry and intracellular trafficking and hence might regulate the production of infectious virions at the postentry stage (Fig. 5D and E). The AMPK inhibitor or agonists also did not have any effect on LANA staining pattern and intensity, the number of punctate dots per cell, and the percentage of LANA-positive cells (Fig. 5F and G), indicating that AMPK affects neither the expression and localization of LANA protein nor KSHV infectivity.
Lastly, we checked the expression of viral genes. Inhibition of AMPK activity with compound C increased the expression of KSHV lytic genes approximately 10-fold and latent LANA gene 5-fold (Fig. 6A to D). Western blotting confirmed that compound C significantly enhanced the expression of lytic proteins RTA, K-bZIP, and ORF65 and more moderately that of LANA (Fig. 6E). In contrast, AMPK agonists AICAR and metformin strongly inhibited the expression of lytic transcripts and proteins at both transcription level and translational level (Fig. 6F, G, H, and J). On the other hand, the effects of AICAR and metformin on the expression of LANA transcript were inconsistent (Fig. 6I). AICAR decreased, while metformin increased, the expression of LANA transcript. However, both AICAR and metformin inhibited the expression of LANA protein (Fig. 6J). These results indicated that AICAR and metformin might regulate the expression of viral genes by different mechanisms albeit both strongly induced AMPK activation. Taken together, these results confirmed that AMPK can robustly restrict KSHV productive lytic replication by inhibiting the viral lytic transcriptional program.
FIG 6.
AMPK inhibitor and agonists mediate KSHV lytic replication by altering the expression of viral lytic genes. (A to D) Inhibition of AMPK activity with compound C significantly increases the expression of KSHV lytic transcripts RTA (A), K-bZIP (B), and ORF65 (C) and latent transcript LANA (D). HUVEC pretreated with compound C (Com C) for 1 h were infected with KSHV for 15, 24, and 40 h and analyzed for the expression of KSHV transcripts by RT-qPCR. (E) Inhibition of AMPK activity with compound C increases the expression of KSHV lytic proteins RTA, K-bZIP, and ORF65 but to a lesser extent latent protein LANA. (F to H) Activation of AMPK activity by either AICAR or metformin (Met) significantly decreases the expression of KSHV lytic transcripts RTA (F), K-bZIP (G), and ORF65 (H). (I) Activation of AMPK by AICAR decreases, while metformin increases, latent LANA transcript. (J) Activation of AMPK by either AICAR or metformin decreases the expression of KSHV lytic proteins RTA, K-bZIP, and ORF65 but to a lesser extent that of LANA protein.
DISCUSSION
AMPK is an intracellular energy sensor conserved in eukaryotes ranging from yeast to humans. AMPK plays a pivotal role in cellular metabolism, particularly in lipid and glucose metabolism. Since efficient infection and replication of viruses often depend on the optimal cell metabolism and physiology, it is not surprising that AMPK has recently been found to regulate viral infection and replication (12).
The role and mechanism of regulation of viral infection by the AMPK pathway appear to be virus specific and might even vary depending on the stages of viral infection. AMPK is activated immediately following vaccinia virus infection, and such activation promotes virus entry by regulating macropinocytosis and actin-dependent membrane ruffling (or lamellipodia) (15). In AMPK-deficient cells, Rac1 relocalization and actin mobilization, both of which are essential for vaccinia virus infection, are defective. The pathways of KSHV entry into cells are cell type specific. KSHV entry into cells is primarily through clathrin-mediated endocytosis in HUVEC and fibroblasts (34, 46) and macropinocytosis in dermal microvascular endothelial cells (DMVEC) (47). Because other reports showed that AMPK activation enhanced macropinocytosis (14, 15), we examined the role of AMPK activation on KSHV entry and infection. We found that inhibition of AMPK activation by either AMPKα1 knockdown or the use of inhibitor compound C did not affect KSHV entry and trafficking. Similarly, activation of AMPK by expressing a constitutively active AMPKα construct and the use of AICAR and metformin had no effect on KSHV entry and trafficking. Thus, AMPK does not regulate KSHV entry and trafficking in HUVEC. Consistent with previous studies, these results further indicate that KSHV entry into HUVEC is unlikely to be mediated by the macropinocytosis pathway (29, 34).
The role of AMPK in viral replication at the postentry stage is complex depending on the virus type. A variety of RNA viruses, including RVFV, KUNV, SINV, and VSV, manipulate cellular membranes to generate new complex membrane structures, which are essential for viral replication. As this process is dependent on fatty acid biosynthesis, it is tightly regulated by AMPK. As a result, activated AMPK restricts infection by several RNA viruses by decreasing levels of fatty acid synthesis (13). Several studies have indicated that HCMV replication is regulated by a precise level or specific subcellular localization of activated AMPK (16, 18). Hence, either activation or inhibition of the AMPK activity could significantly impair HCMV replication (16, 18). In this study, we have found that KSHV primary infection does not significantly alter the phosphorylation level of AMPK in HUVEC. However, inhibition of endogenous activated AMPK can dramatically enhance KSHV lytic replication during primary infection, thereby increasing the production of infectious virions. On the other hand, activation of the AMPK pathway with a constitutively active construct or agonists severely impaired KSHV lytic replication and the production of infectious virions. These results are similar to those observed with the RNA viruses but are in sharp contrast to those observed with HCMV. Together, these studies point to the complexity of the regulatory role of AMPK in virus infections.
To determine the mechanism of AMPK regulation of KSHV lytic transcriptional program, we have investigated several known transcriptional factors that are essential for the expression of viral lytic genes during KSHV primary infection of HUVEC, including cFos, cJun, and CREB1 (31, 48). However, we have not observed any correlations of these transcriptional factors with the AMPK activity (data not shown), indicating that they are not regulated by AMPK. Examination of AMPK downstream targets, including peroxisome proliferator-activated receptor α (PPARα) and PPARγ coactivator 1-alpha (PGC-1α) (5), has also failed to identify any correlation of these targets with KSHV lytic replication (data not shown), indicating that they are not involved in AMPK regulation of KSHV lytic replication.
Because of the essential role of AMPK in cellular metabolism, both inhibitors and activators (agonists) have been developed, some of which are used for treating diseases related to metabolism. AICAR, an analog of AMP, was first used to preserve blood flow to the heart during surgery (49) and protect against cardiac ischemic injury (42). Our results have shown that AICAR can significantly suppress KSHV lytic replication by inhibiting the expression of viral lytic genes.
Metformin is the first line of drug widely prescribed for treating type 2 diabetes, with over 120 million users worldwide (50). Long-term uptake of metformin has been associated with reduction of several types of cancer, though the mechanism is unclear (51, 52) and is one of the “provocative questions” raised by the National Cancer Institute that has attracted significant attention in the last few years (53). It has been reported that activation of AMPK is one of the mechanisms associated with the pleiotropic actions of metformin (44). Phenotypically, metformin robustly upregulates the AMPK activity through a mild and specific inhibition of the mitochondrial respiratory-chain complex 1, though the exact mechanism(s) remains unknown (50). In this study, we have identified an antiviral effect of metformin. Metformin is able to effectively suppress KSHV lytic replication during primary infection. While metformin inhibits the expression of viral lytic transcripts, it enhances the expression of latent LANA transcript. However, metformin inhibits the expression of both latent and lytic proteins. These inconsistent results might reflect the complexity of the metformin effects on metabolism in vivo. Indeed, activation of AMPK can inhibit the mTORC pathway, resulting in reduced initiation of protein translation (3, 8, 54). Nevertheless, it is unclear why AICAR does not have a similar effect. Further investigations on their distinct effects on the expression of KSHV genes might lead to better understanding of the regulation of expression of KSHV genes and the underlying mechanisms. Nevertheless, our results have shown that AMPK is a potential therapeutic target and metformin and AICAR are potential therapeutic agents for inhibiting KSHV replication.
ACKNOWLEDGMENTS
We thank members of S.-J.G.'s laboratory for technical assistance and helpful discussions.
This work was supported by grants from NIH (CA096512, CA124332, CA132637, CA177377, DE025465, and CA197153) to S.-J.G. and from NIH (CA200422, CA082057, CA180779, HL110609, DE023926, AI073099, AI116585), the Hastings Foundation, and the Fletcher Jones Foundation to J.U.J.
REFERENCES
- 1.Johnson WE. 2013. Rapid adversarial co-evolution of viruses and cellular restriction factors. Curr Top Microbiol Immunol 371:123–151. doi: 10.1007/978-3-642-37765-5_5. [DOI] [PubMed] [Google Scholar]
- 2.Goubau D, Deddouche S, Reis e Sousa C. 2013. Cytosolic sensing of viruses. Immunity 38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon V, Bloch N, Landau NR. 2015. Intrinsic host restrictions to HIV-1 and mechanisms of viral escape. Nat Immunol 16:546–553. doi: 10.1038/ni.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou LY, Zhang LL. 2016. Host restriction factors for hepatitis C virus. World J Gastroenterol 22:1477–1486. doi: 10.3748/wjg.v22.i4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mihaylova MM, Shaw RJ. 2011. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie DG, Ross FA, Hawley SA. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crozet P, Margalha L, Confraria A, Rodrigues A, Martinho C, Adamo M, Elias CA, Baena-Gonzalez E. 2014. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front Plant Sci 5:190. doi: 10.3389/fpls.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoki K, Kim J, Guan KL. 2012. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 9.Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. 2011. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motoshima H, Goldstein BJ, Igata M, Araki E. 2006. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Momcilovic M, Hong SP, Carlson M. 2006. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 12.Mankouri J, Harris M. 2011. Viruses and the fuel sensor: the emerging link between AMPK and virus replication. Rev Med Virol 21:205–212. doi: 10.1002/rmv.687. [DOI] [PubMed] [Google Scholar]
- 13.Moser TS, Schieffer D, Cherry S. 2012. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog 8:e1002661. doi: 10.1371/journal.ppat.1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondratowicz AS, Hunt CL, Davey RA, Cherry S, Maury WJ. 2013. AMP-activated protein kinase is required for the macropinocytic internalization of ebolavirus. J Virol 87:746–755. doi: 10.1128/JVI.01634-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. 2010. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog 6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry LJ, Vastag L, Rabinowitz JD, Shenk T. 2012. Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. Proc Natl Acad Sci U S A 109:3071–3076. doi: 10.1073/pnas.1200494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leyton L, Hott M, Acuna F, Caroca J, Nunez M, Martin C, Zambrano A, Concha MI, Otth C. 2015. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res 205:63–72. doi: 10.1016/j.virusres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Hutterer C, Wandinger SK, Wagner S, Muller R, Stamminger T, Zeittrager I, Godl K, Baumgartner R, Strobl S, Marschall M. 2013. Profiling of the kinome of cytomegalovirus-infected cells reveals the functional importance of host kinases Aurora A, ABL and AMPK. Antiviral Res 99:139–148. doi: 10.1016/j.antiviral.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Martin C, Leyton L, Arancibia Y, Cuevas A, Zambrano A, Concha MI, Otth C. 2014. Modulation of the AMPK/Sirt1 axis during neuronal infection by herpes simplex virus type 1. J Alzheimers Dis 42:301–312. doi: 10.3233/JAD-140237. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 21.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 22.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280. [PubMed] [Google Scholar]
- 23.Wang L, Damania B. 2008. Kaposi's sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res 68:4640–4648. doi: 10.1158/0008-5472.CAN-07-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao SJ, Deng JH, Zhou FC. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J Virol 77:9738–9749. doi: 10.1128/JVI.77.18.9738-9749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Q, Liang D, Sun R, Jia B, Xia T, Xiao H, Lan K. 2015. Kaposi's sarcoma-associated herpesvirus-encoded replication and transcription activator impairs innate immunity via ubiquitin-mediated degradation of myeloid differentiation factor 88. J Virol 89:415–427. doi: 10.1128/JVI.02591-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brulois KF, Chang H, Lee AS, Ensser A, Wong LY, Toth Z, Lee SH, Lee HR, Myoung J, Ganem D, Oh TK, Kim JF, Gao SJ, Jung JU. 2012. Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone. J Virol 86:9708–9720. doi: 10.1128/JVI.01019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou FC, Zhang YJ, Deng JH, Wang XP, Pan HY, Hettler E, Gao SJ. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J Virol 76:6185–6196. doi: 10.1128/JVI.76.12.6185-6196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo CR, Saah A, Phair J, Detels R, Chang Y, Moore PS. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med 2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 29.Greene W, Zhang W, He M, Witt C, Ye F, Gao SJ. 2012. The ubiquitin/proteasome system mediates entry and endosomal trafficking of Kaposi's sarcoma-associated herpesvirus in endothelial cells. PLoS Pathog 8:e1002703. doi: 10.1371/journal.ppat.1002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo SM, Zhou FC, Ye FC, Pan HY, Gao SJ. 2005. Early and sustained expression of latent and host modulating genes in coordinated transcriptional program of KSHV productive primary infection of human primary endothelial cells. Virology 343:47–64. doi: 10.1016/j.virol.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng F, Sawant TV, Lan K, Lu C, Jung JU, Gao SJ. 2015. Screening of the human kinome identifies MSK1/2-CREB1 as an essential pathway mediating Kaposi's sarcoma-associated herpesvirus lytic replication during primary infection. J Virol 89:9262–9280. doi: 10.1128/JVI.01098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He M, Zhang W, Bakken T, Schutten M, Toth Z, Jung JU, Gill P, Cannon M, Gao SJ. 2012. Cancer angiogenesis induced by Kaposi's sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res 72:3582–3592. doi: 10.1158/0008-5472.CAN-11-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL, Greulich H, Stewart CJ, Mulvey LA, Shen RR, Ambrogio L, Hirozane-Kishikawa T, Hill DE, Vidal M, Meyerson M, Grenier JK, Hinkle G, Root DE, Roberts TM, Lander ES, Polyak K, Hahn WC. 2007. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 34.Greene W, Gao SJ. 2009. Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog 5:e1000512. doi: 10.1371/journal.ppat.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. 2002. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol (1985) 92:2475–2482. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- 36.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. 1996. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Gao SJ. 2012. Exploitation of cellular cytoskeletons and signaling pathways for cell entry by Kaposi's sarcoma-associated herpesvirus and the closely related rhesus rhadinovirus. Pathogens 1:102–127. doi: 10.3390/pathogens1020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye F, Lei X, Gao SJ. 2011. Mechanisms of Kaposi's sarcoma-associated herpesvirus latency and reactivation. Adv Virol 2011:193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene W, Kuhne K, Ye F, Chen J, Zhou F, Lei X, Gao SJ. 2007. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat Res 133:69–127. doi: 10.1007/978-0-387-46816-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. 1998. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem 273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 41.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. 2007. The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 1995. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565. [DOI] [PubMed] [Google Scholar]
- 43.Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. 2005. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- 44.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. 2002. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 46.Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol 77:7978–7990. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. 2009. Kaposi's sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J Virol 83:4895–4911. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye FC, Blackbourn DJ, Mengel M, Xie JP, Qian LW, Greene W, Yeh IT, Graham D, Gao SJ. 2007. Kaposi's sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J Virol 81:3980–3991. doi: 10.1128/JVI.02089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galinanes M, Bullough D, Mullane KM, Hearse DJ. 1992. Sustained protection by acadesine against ischemia- and reperfusion-induced injury. Studies in the transplanted rat heart. Circulation 86:589–597. [DOI] [PubMed] [Google Scholar]
- 50.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. 2012. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasgupta B, Chhipa RR. 2016. Evolving lessons on the complex role of AMPK in normal physiology and cancer. Trends Pharmacol Sci 37:192–206. doi: 10.1016/j.tips.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cazzaniga M, Bonanni B. 2015. Relationship between metabolic reprogramming and mitochondrial activity in cancer cells. Understanding the anticancer effect of metformin and its clinical implications. Anticancer Res 35:5789–5796. [PubMed] [Google Scholar]
- 53.Blagosklonny MV. 2011. NCI's provocative questions on cancer: some answers to ignite discussion. Oncotarget 2:1352–1367. doi: 10.18632/oncotarget.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Ji J, Yan XH. 2012. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr 52:373–381. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]