ABSTRACT
Influenza A and B virus infections both cause a host innate immunity response. Here, we report that the robust production of type I and III interferons (IFNs), IFN-stimulated genes, and proinflammatory factors can be induced by influenza B virus rather than influenza A virus infection in alveolar epithelial (A549) cells during early infection. This response is mainly dependent on the retinoic acid-inducible gene I (RIG-I)-mediated signaling pathway. Infection by influenza B virus promotes intense Lys63-linked ubiquitination of RIG-I, resulting in cytokine eruption. It is known that the influenza A virus NS1 protein (NS1-A) interacts with RIG-I and TRIM25 to suppress the activation of RIG-I-mediated signaling. However, the present results indicate that the influenza B virus NS1 protein (NS1-B) is unable to interact with RIG-I but engages in the formation of a RIG-I/TRIM25/NS1-B ternary complex. Furthermore, we demonstrate that the N-terminal RNA-binding domain (RBD) of NS1-B is responsible for interaction with TRIM25 and that this interaction blocks the inhibitory effect of the NS1-B C-terminal effector domain (TED) on RIG-I ubiquitination. Our findings reveal a novel mechanism for the host cytokine response to influenza B virus infection through regulatory interplay between host and viral proteins.
IMPORTANCE Influenza B virus generally causes local mild epidemics but is occasionally lethal to individuals. Existing studies describe the broad characteristics of influenza B virus epidemiology and pathology. However, to develop better prevention and treatments for the disease, determining the concrete molecular mechanisms of pathogenesis becomes pivotal to understand how the host reacts to the challenge of influenza B virus. Thus, we aimed to characterize the host innate immune response to influenza B virus infection. Here, we show that vigorous Lys63-linked ubiquitination of RIG-I and cytokine eruption dependent on RIG-I-mediated signal transduction are induced by virus infection. Additionally, TRIM25 positively regulates RIG-I-mediated signaling by ablating the inhibitory function of NS1-B on RIG-I ubiquitination.
INTRODUCTION
Influenza viruses, members of the family Orthomyxoviridae, consist of negative-sense single-stranded RNA segments and include three different antigenic type viruses: influenza viruses A, B, and C. It is known that influenza A virus is an important threat to public health that has caused several appalling pandemics in human history, and it is categorized into different subtypes according to the types of hemagglutinin (HA) and neuraminidase (NA) proteins on the surface of the viral envelope (1, 2). Influenza B virus has no distinct subtypes because of homology but is separated into two lineages, Yamagata and Victoria, based on different antigenicities (3). The genome of influenza B virus is composed of eight negative-sense single-stranded RNA segments encoding 11 viral proteins: HA, NA, PA, PB1, PB2, NP, M1, M2, NEP, NB, and NS1 (4, 5). Their functions in viral infection cycles have been generally characterized. Influenza B virus has a limited host range, and its natural reservoir is mainly humans, though an influenza B virus was identified in a seal (6). Currently, the reason why influenza B virus has such a strictly limited host range remains unclear. One explanation is the species-specific binding of the influenza B virus NS1 protein (NS1-B) with human and nonhuman primate ISG15, not mouse or canine ISG15 (7–9). ISG15 is encoded by a representative interferon-induced antiviral gene involved in the innate immune system. This suggests the interactive behavior between influenza B virus and the innate immune system can imbue the virus with particular characteristics.
The innate immune response is a vital and formidable host defense against the invasion of pathogens. Influenza A virus infection triggers multiple innate immune pathway responses to counteract the replication and propagation of the virus. Retinoic acid-inducible gene I (RIG-I)-like receptors, Toll-like receptors (TLRs), and NOD-like receptors, three large classes of pattern recognition receptors (PRRs), recognize influenza A virus and induce downstream signaling. The host primes the initial antiviral response to deal with influenza A virus using the MyD88 or IPS-1 (MAVS) signal pathways. The former signaling pathway still functions in the adaptive immune response (10). In contrast to the fact that the TLR7 and RIG-I pathways restrict viral replication when lethal infection doses are used, physiologically low doses of influenza A virus induce a proinflammatory response through the TLR7 and RIG-I pathways that promotes viral replication (11). Additionally, TLR3, a receptor of double-stranded RNA (dsRNA), is involved in the recognition of influenza A virus and regulates the proinflammatory response process (12). RIG-I, a critical receptor that recognizes influenza virus RNA bearing a 5′-triphosphate group, is involved in the process of host cytokine production, including interferons (IFNs), IFN-stimulated genes (ISGs), and proinflammatory factors against influenza virus infection.
Remarkably, TRIM25 positively regulates the RIG-I-mediated signal pathway. TRIM25, which consists of a RING finger domain, a B box/coiled-coil domain, and a cluster of RFP-like domains (13), belongs to the tripartite motif (TRIM) family of proteins and plays an essential role in the innate immune response. TRIM25 functions as an E3 ligase by ubiquitinating the substrate, resulting in either degradation or functional regulation of the substrate. The ubiquitination of MAVS by TRIM25 leads to selective proteasomal degradation and primes dissociation of TBK1 and NEMO to promote IRF3 phosphorylation (14). Another known substrate of TRIM25 is RIG-I. TRIM25-mediated Lys63-linked ubiquitination of RIG-I upregulates RIG-I signal transduction (15).
Although the characteristics of influenza virus per se are important, the relationship between the virus and host is also a priority research subject. The interplay between influenza A virus and host innate immune signaling pathways has clearly been revealed to a great extent (16–18), but the regulatory mechanism of influenza B virus infection awaits further investigation. Currently, fundamental research has elucidated the interrelationship between influenza B virus and the host innate immune system. For instance, PKR, a key signal transducer involved in TLR signaling, is activated by influenza B virus ribonucleoprotein (19, 20). Further, ISG15 possesses the ability to block influenza virus infection. Elevated ISG15 modification without USP18 inhibition enhances resistance to influenza B virus infection in vivo (21). However, the overall circumstance of the innate immunological response and which pathway is dominantly in charge of innate immunity against influenza B virus infection are still unclear.
Here, we investigate the innate immunological reaction to influenza B virus infection in the host and the variation in the production of IFNs, ISGs, and proinflammatory factors. Using RIG-I−/− cell lines, we demonstrate that eruption of IFNs, ISGs, and proinflammatory factors depends on RIG-I signal transduction. Additionally, the host utilizes TRIM25 to block the inhibitory effect of the NS1-B C terminus on RIG-I regulative ubiquitination. These data provide new insights into the host innate immune response, especially the multifunctional TRIM25's positive regulatory mechanism against influenza B virus infection.
MATERIALS AND METHODS
Cells and viruses.
Human alveolar epithelial (A549) cells, human embryonic kidney 293 (293T) cells, and Madin-Darby canine kidney (MDCK) cells were grown in Dulbecco's modified Eagle medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) at 37°C in 5% CO2. B/Lee/1940 (B/Lee), influenza A virus A/California/04/2009 (A/CA04), and Sendai virus (SeV) were propagated in 9-day-old, specific-pathogen-free embryonated eggs (Merial, Beijing, China) at 37°C for 3 days. The titers of B/Lee and A/CA04 were 5 × 107 PFU/ml and 1 × 107 PFU/ml, respectively, which were determined by plaque assays on MDCK cells as described previously (22). The HA titer of SeV was 29, which was assessed with fresh chicken erythrocytes.
Reagents and antibodies.
Lipofectamine reagent was purchased from Invitrogen. Opti-MEM was purchased from Gibco. Rabbit anti-NP-B polyclonal antibody and rabbit anti-NS1-B polyclonal antibody were produced by immunizing a rabbit with purified His-NP-B and His-NS1-B, respectively, as described previously (23). Rabbit anti-NP-A/CA04 polyclonal antibody and rabbit anti-NS1-A/CA04 polyclonal antibody were also prepared in the same manner as before. Anti-β-actin antibody, anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody, goat anti-rabbit IgG-linked horseradish peroxidase (HRP) secondary antibody, and goat anti-mouse IgG-linked HRP secondary antibody were purchased from Boao Rui Jing Technology Development Co., Ltd. (Beijing, China). Rabbit anti-RIG-I (D14G6) was from Cell Signaling Technology. Anti-TRIM25 was obtained from Proteintech. Anti-NF-κB p65 (F6), anti-IRF3 (FC-425), anti-STAT1 (E-23), anti-P-STAT1 (Tyr 701), and mouse anti-c-Myc (9E10) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse anti-FLAG (M2) antibody and rabbit anti-Myc antibody were purchased from Sigma-Aldrich (St. Louis, MO). Anti-VDAC1 (ab14734) antibody was purchased from Abcam (Cambridge). Goat anti-rabbit IgG conjugated to TRITC and goat anti-mouse IgG conjugated to FITC secondary antibodies were purchased from Jackson ImmunoResearch, Inc.
Coimmunoprecipitation.
293T cells were grown in 60-mm dishes in DMEM containing 10% FBS. Plasmids and Lipofectamine reagent were mixed in a 1:2.5 ratio, and the mixture was added to the cell culture medium according to the manufacturer's instructions. The transfection system medium was replaced with DMEM supplemented with 10% FBS after 6 h, and the cells were cultured for 20 h at 37°C in 5% CO2. For infection, 293T cells were incubated with influenza viruses or SeV for 1 h at 37°C. The medium for influenza virus infection consisted of DMEM and 2 μg/ml l-1-tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin. The cells were infected at a multiplicity of infection (MOI) of 0.5 over 12 h and then lysed in immunoprecipitation buffer containing 1% Triton X-100, 20 mM HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, 1 mM EDTA, and Complete protease inhibitor cocktail. The lysates were centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant was collected and incubated with anti-FLAG M2 affinity gel (Sigma-Aldrich) for 4 h. The gel-protein complexes were washed with wash buffer containing 1% Triton X-100, 20 mM HEPES (pH 7.4), 300 mM NaCl, 10% glycerol, and 1 mM EDTA. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The blots were blocked overnight at 4°C with 5% (wt/vol) nonfat dry milk and 1% (wt/vol) bovine serum albumin in 0.5% Tween 20 in Tris-buffered saline (TBST). The membranes were then incubated with specific primary antibodies for 1 h at room temperature and subsequently washed with TBST three times for 10 min each time. Next, the membranes were incubated with goat anti-mouse or anti-rabbit IgG conjugated to HRP for 1 h at room temperature and washed three times. Finally, the membranes were developed using Immobilon Western Chemiluminescent HRP Substrate (Tiangen) and exposed using a chemiluminescence imaging system (ClinX Sciences instrument).
Real-time PCR.
A549 cells or 293T cells (1 × 106) were grown in 6-well plates and infected with influenza viruses for 1 h at an MOI of 0.5. The cells were harvested at the indicated times, and total cellular RNA was isolated as described previously (24, 25). Two micrograms of total cellular RNA was transcribed into cDNA with oligo(dT) primers (TaKaRa) using a murine leukemia virus (MLV) reverse transcriptase kit (Promega). Viral mRNA was transcribed using random primers (TaKaRa). Quantitative real-time PCR assays were performed using SYBR Premix Ex Taq (TaKaRa) with the following primers: NP-B forward, 5′-GATAAAGAAGAGCGTCTACAACAT-3′; NP-B reverse, 5′-TCATCTCTTACCATCTTATAAAAGG-3′; NS1-B forward, 5′-TGGGTCCGGGAGCAACCAAT-3′; NS1-B reverse, 5′-CACTCTTGTTGTGAGTCTTTATT-3′; NP-A forward, 5′-GCACTGAACTCAAACTAGT-3′; NP-A reverse, 5′-CTCTCCTTATTTCTTCTTTGTCAT-3′; NS1-A forward, 5′-CGATTTGCAGACAATGGATT-3′; NS1-A reverse, 5′-TCGAGGGTCATGTCAGAAAGGT-3′; IFN-β forward, 5′-TAGCACTGGCTGGAATGAGA-3′; IFN-β reverse, 5′-TCCTTGGCCTTCAGGTAATG-3′; IL28A forward, 5′-CTCAGGTTGCATGACTGGTGG-3′; IL28A reverse, 5′-GAGGCCTCTGTCACCTTCAAC-3′; IL28B forward, 5′-CAGCTGCAGGTGAGGGAGCGCCCCG-3′; IL28B reverse, 5′-GGTGGCCTCCAGAACCTT-3′; IL-29 forward, 5′-GGACGCCTTGGAAGAGTCACT-3′; IL-29 reverse, 5′-AGAAGCCTCAGGTCCCAATTC-3′; IL-6 forward, 5′-AGCCACTCACCTCTTCAGAACGAA-3′; IL-6 reverse, 5′-CAGTGCCTCTTTGCTGCTTTCACA-3′; IL-8 forward, 5′-AAGAAACCACCGGAACTGA. A-3′; IL-8 reverse, 5′-ACTTCTCCACAACCCTCTGC-3′; MxA forward, 5′-GGTGGCTGAGAACAACCTGT-3′; MxA reverse, 5′-GGTCCTGCTCCACACCTAGA-3′; ISG56 forward, 5′-CAGAACGGCTGCCTAATTTACA-3′; ISG56 reverse, 5′-CAGACTATCCTTGACCTGATGATCA-3′; RIG-I forward: 5′-GGACGTGGCAAAACAAATCAG-3′; RIG-I reverse: 5′-GCAATGTCAATGCCTTCATCA-3′; GAPDH forward, 5′-GGTGGTCTCCTCTGACTTCAAGA-3′; and GAPDH reverse, 5′-GTTGCTGTAGCCAAATTCGTTGT-3′. The GAPDH gene was regarded as the reference gene for internal standardization. All relative mRNA expression was calculated using the 2−ΔΔCT formula.
ELISA.
A549 cells (1 × 106) were grown in 6-well plates and infected with the influenza B/Lee strain for 1 h. The cell culture supernatants were collected at the indicated times, and the protein levels of beta interferon (IFN-β) and interleukin 29 (IL-29) were detected with enzyme-linked immunosorbent assay (ELISA) kits. The human IL-29 ELISA Ready-Set-Go kit was purchased from eBioscience, Affymetrix. The human IFN-β ELISA kit was purchased from PBL Assay Science.
Luciferase assay.
293T cells (1 × 105) were grown in 12-well plates and cotransfected with β-galactosidase (β-Gal) and either an NF-κB luciferase reporter plasmid or an interferon-stimulated response element (ISRE) luciferase reporter plasmid. After 20 h, the cells were infected with the influenza B/Lee or A/CA04 strain at an MOI of 0.5 and harvested at the indicated times. The luciferase activity was detected using a Luciferase Assay kit (Promega), and the data were normalized to the activity levels of β-Gal.
GST pulldown.
293T cells were grown in 60-mm dishes and transfected with 4 μg of pCMV-Myc-NS1-B and pCMV-Myc-NS1-A (including A/CA04 NS1 protein and A/WSN NS1 protein) plasmids. After 24 h, cell lysates were generated using cell lysis buffer containing 1% Triton X-100, 20 mM HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, 1 mM EDTA, and Complete protease inhibitor cocktail. Glutathione S-transferase (GST) and GST-tagged RIG-I proteins were expressed in Escherichia coli, followed by purification with glutathione-Sepharose (GE Healthcare). Because GST-tagged RIG-I was expressed in the form of inclusion bodies, we used the dilution method to obtain refolded RIG-I protein and then purified it. Modified GST pulldown assays were conducted as described previously (26). Briefly, the purified GST or GST-tagged RIG-I was incubated with cell lysates containing Myc-tagged NS1-B or NS1-A at 4°C for 2 h and precipitated with immobilized glutathione-Sepharose beads. Following four washes with wash buffer, the precipitates were analyzed by SDS-PAGE and blotted with anti-Myc antibody. The PVDF blotting membranes and the corresponding gels were stained with Coomassie brilliant blue.
Fluorescence microscopy.
293T cells were grown on coverslips in 24-well plates. When the cells reached 90% confluence, they were transfected with plasmids using Lipofectamine 2000 (0.8 μg plasmid per well). At the indicated time points, the cells were rinsed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min at room temperature, and then permeabilized with 0.5% Triton X-100 in PBS for 15 min. The cells were incubated with antibodies for 1 h at 37°C. After washing with PBST five times, the cells were incubated with the appropriate secondary antibodies for 1 h at 37°C. After five washes with PBST, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescence images were obtained using a Leica SP8 confocal laser scanning microscope.
Statistical analysis.
The data presented are the means and standard deviations (SD) of three independent experiments. A P value of <0.05 (t test) was considered statistically significant.
RESULTS
Influenza B virus infection blocks superinfection by influenza A virus in host cells and upregulates the production of IFNs, ISGs, and proinflammatory factors.
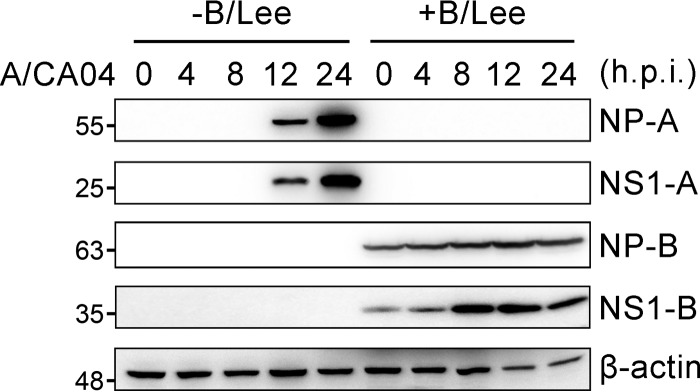
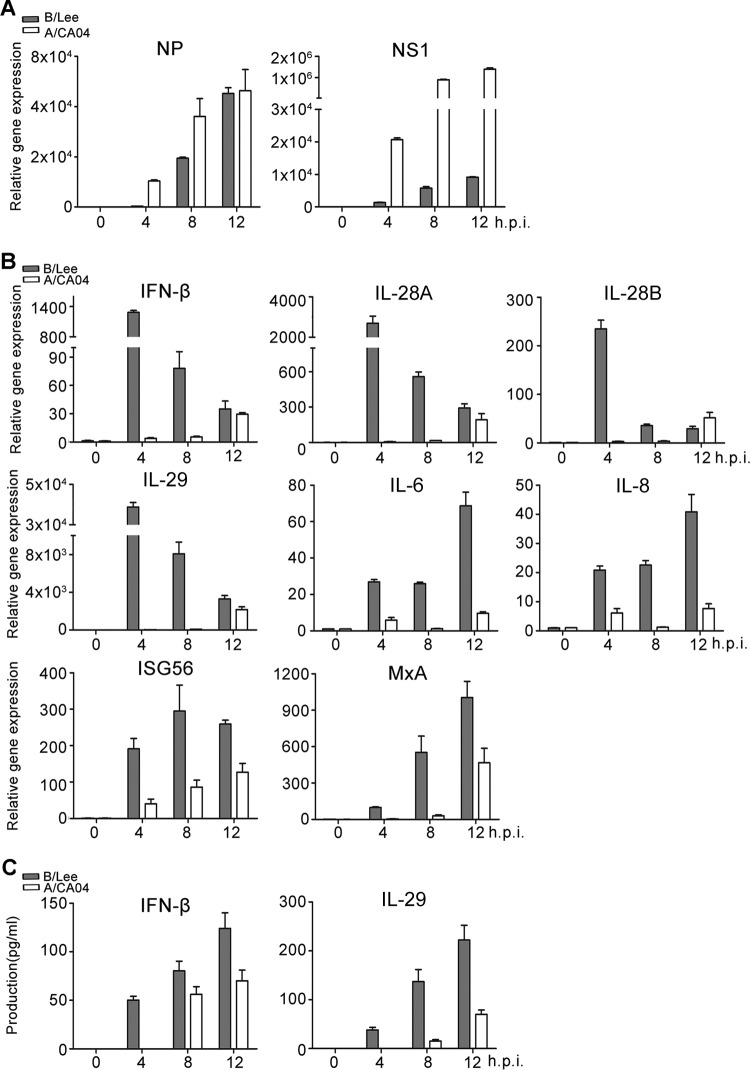
In early experiments, a very intriguing phenomenon was found, i.e., human alveolar epithelial (A549) cells could not be infected with A/CA04 at an MOI of 0.5 again if infected with B/Lee at an MOI of 0.5 in advance (Fig. 1). NP-A and NS1-A were not detected during A/CA04 infection in cells pretreated with B/Lee for 8 h. The experiment was also performed in 293T cells, yielding the same results (data not shown). This prompted us to question why infection with B/Lee prevented superinfection with A/CA04. We postulated that the activation of the innate immune response by influenza B virus infection protected the host cells from superinfection. Given that viral infections elicit the activation of IFNs and proinflammatory factors and that influenza B virus induces IFNs directly upon entry into human primary monocyte-derived dendritic cells (27–29), we tested the cytokine expression levels induced by influenza B virus in A549 cells, and the transcriptional levels of viral NP and NS1 protein were also analyzed. As shown in Fig. 2A, the amount of NP and NS1 mRNA was increased as time progressed, and the transcriptional efficiency of influenza A virus NP and NS1 was higher than that of influenza B virus NP and NS1. To analyze cytokine production, the gene expression levels of type I and III IFNs (i.e., IFN-β, IL-28A, IL-28B, and IL-29) and proinflammatory factors (i.e., IL-6 and IL-8) were monitored. The transcriptional levels of these genes were calculated by real-time PCR in A549 cells infected with B/Lee virus at an MOI of 0.5 over 12 h (Fig. 2B). IFN has no direct antiviral activity, but it binds its receptor to activate the downstream transcription factor STAT, which induces the expression of antiviral proteins (27). Consistent with the former results, IFN-induced genes, such as ISG56 and MxA genes, that are transcribed by STAT1 were also strongly induced during early influenza B virus infection (Fig. 2B). With infection of B/Lee, most expression levels peaked at 4 h postinfection. Moreover, the cell culture supernatants were collected to analyze the protein levels by ELISA (Fig. 2C). B/Lee virus induced rapid IFN-β and IL-29 production at 4 h postinfection, and the production increased as time progressed. At the same time, we chose the A/CA04 virus strain as a control to estimate the relative induction activity of B/Lee. Both the relative mRNA expression and protein production levels of cytokines induced by A/CA04 were lower than those induced by B/Lee. These results indicate that influenza B virus induces robust cytokine responses during the early stage of infection.
FIG 1.
Influenza B virus infection blocks superinfection. A549 cells were infected with B/Lee (MOI = 0.5). After 8 h, the cells were washed once with PBS and infected with A/CA04 (MOI = 0.5). Cell lysates were extracted at the indicated times and quantified by bicinchoninic acid (BCA) protein assays. The same amounts of samples were loaded on the gel to perform immunoblotting (IB) assays with anti-NP-A/CA04, anti-NS1-A/CA04, or anti-NP-B antibody. h.p.i., hours postinfection.
FIG 2.
Production of IFNs, ISGs, and proinflammatory factors is activated by influenza B virus infection. (A and B) A549 cells were infected with B/Lee or A/CA04 at an MOI of 0.5 for the indicated times. Expression of NP and NS1 (A) and IFN-β, IL-28A, IL-28B, IL-29, IL-6, IL-8, ISG56, and MxA (B) in cells was measured by real-time PCR. (C) Supernatants were collected and assessed for IFN-β and IL-29 by ELISA. The error bars indicate the SD.
Influenza B virus infection induces the activation of IFN signaling via the RIG-I-dependent pathway.
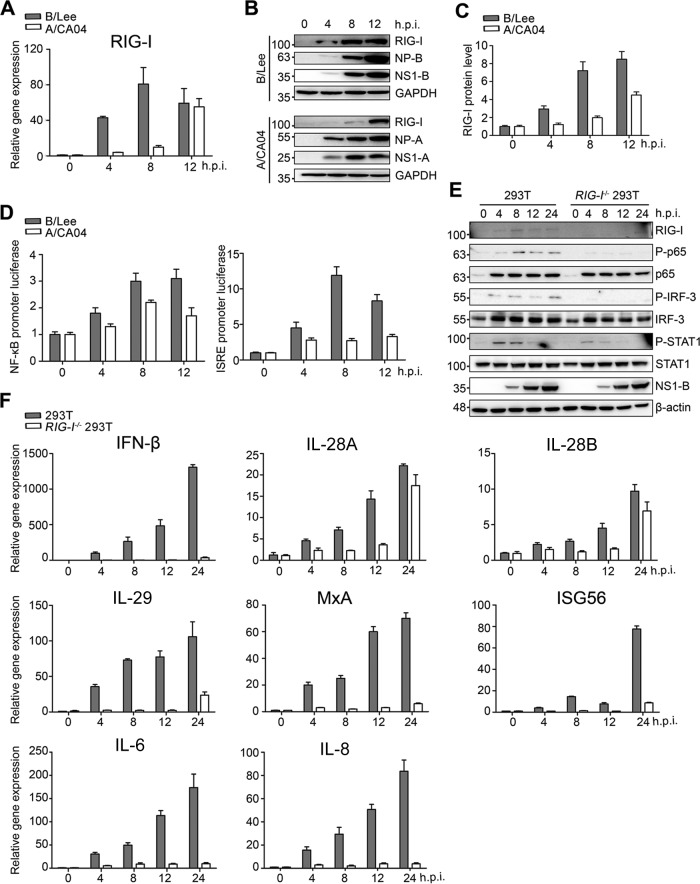
Influenza virus infection can activate multiple innate immune signaling pathways (30). To determine which pathway plays the most important role in the host response to influenza B virus infection, RIG-I was first analyzed at the transcriptional and translational levels (Fig. 3A to C). Both mRNA and protein levels were increased earlier and more strongly upon B/Lee virus infection than those induced by A/CA04 virus. Additionally, the ISRE and NF-κB promoter luciferase activities were examined utilizing the two diverse influenza virus strains (Fig. 3D). Consistently, more efficient induction of ISRE and NF-κB promoter luciferase activity was observed in 293T cells infected with B/Lee.
FIG 3.
The RIG-I-mediated signaling pathway determines the cytokine response to influenza B virus infection. (A) A549 cells were infected with B/Lee (MOI = 0.5) or A/CA04 (MOI = 0.5), and the cell solutions in TRIzol or the cell lysates in lysis buffer were collected at the indicated times. The gene expression levels of RIG-I were calculated by real-time PCR. (B) A549 cells were infected with B/Lee (MOI = 0.5) or A/CA04 (MOI = 0.5), and the cell lysates were collected at the indicated times. BCA protein assays were performed, followed by IB assays to analyze the protein levels of RIG-I, NP-B, NS1-B, NP-A, NS1-A, and GAPDH. (C) The RIG-I protein levels shown in panel B were quantified by densitometry and normalized to GAPDH. (D) 293T cells were transfected with ISRE-luciferase or NF-κB–luciferase plasmids, together with β-Gal. Luciferase and β-Gal values in the case of B/Lee infection or A/CA04 infection at the indicated times were determined. The data represent means and SD (n = 3). (E) 293T cells or RIG-I−/− 293T cells were infected with B/Lee at an MOI of 0.5, and cell lysates were harvested at the indicated times. All samples were tested with BCA protein quantitation assays, followed by IB assays with anti-RIG-I, anti-P-p65, anti-p65, anti-P-IRF3, anti-IRF3, anti-P-STAT1, anti-STAT1, anti-NP-B, and anti-β-actin antibodies. (F) Real-time PCR assays were performed to detect the relative gene expression levels of IFN-β, IL-28A, IL-28B, IL-29, MxA, and ISG56 in 293T cells and RIG-I−/− 293T cells. The data represent the means and SD (n = 3).
To determine whether RIG-I is a main RNA sensor to recognize influenza B virus and mediates downstream signaling, RIG-I knockout (RIG-I−/−) 293T cells were generated using the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) technique to validate the importance of RIG-I. We observed the expression levels of the vital transcription factors in RIG-I−/− 293T cells infected with B/Lee, including p65, IRF3, and STAT1, compared to those in wild-type 293T cells (Fig. 3E). Although the total protein levels of p65, IRF3, and STAT1 did not change between 293T cells and RIG-I−/− 293T cells, the phosphorylated forms of these three transcription factors decreased in influenza B virus-infected RIG-I−/− 293T cells.
To test the effect of RIG-I on cytokine expression, real-time PCR assays were performed to examine the transcriptional levels of type I and III IFNs (i.e., IFN-β, IL-28A, IL-28B, and IL-29), IFN-induced genes (i.e., MxA and ISG56 genes), and proinflammatory factors (i.e., IL-6 and IL-8) (Fig. 3F). In line with the earlier results, these cytokine transcripts were dramatically reduced in RIG-I−/− 293T cells after B/Lee infection. These results indicate that RIG-I is essential for the activation of transcriptional factors and cytokine production during the early stage of influenza B virus infection. Previous studies reported similar results (29), suggesting that RIG-I signaling plays crucial roles against influenza B virus infection.
Influenza B virus infection promotes TRIM25-mediated RIG-I Lys63-linked ubiquitination.
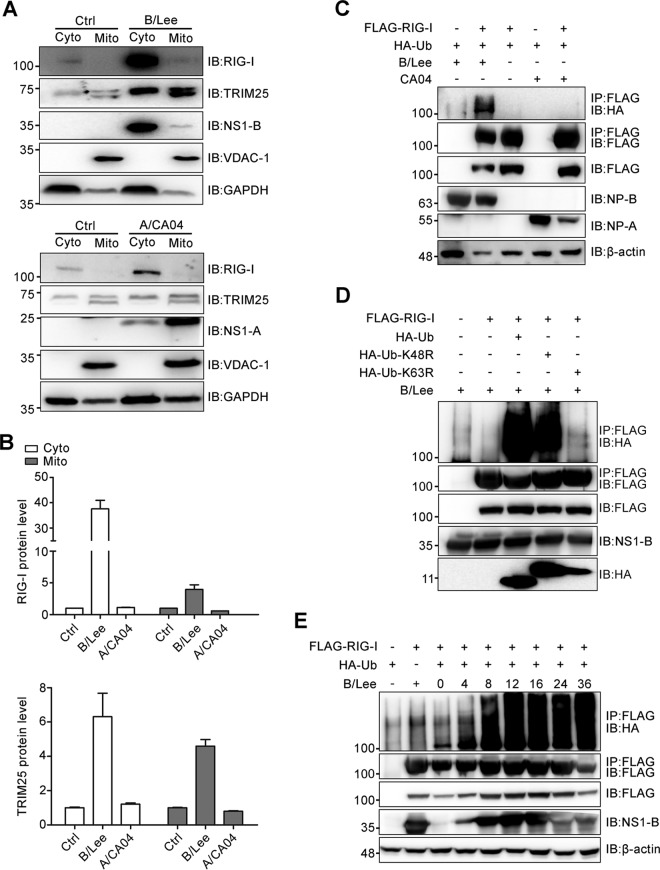
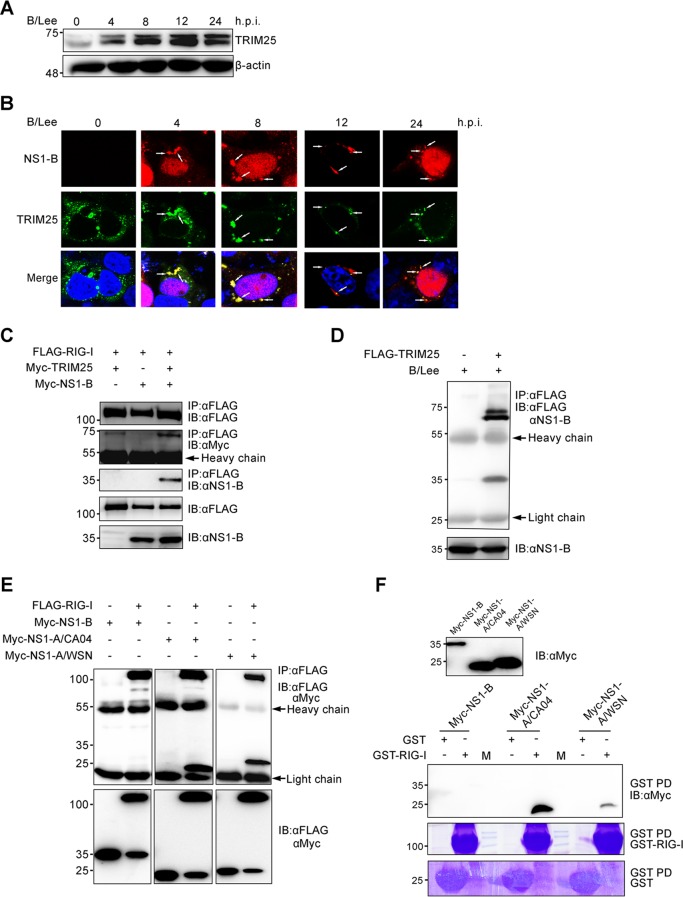
From the above-described experiments, we concluded that influenza B virus infection stimulates the robust production of antiviral cytokines during the early stage of infection and that this was mainly dependent on RIG-I-mediated signaling. We next wondered why influenza B virus causes such prompt activation of the RIG-I-mediated signaling pathway. A series of studies verified that TRIM25-mediated RIG-I ubiquitination facilitates RIG-I binding with MAVS, which localizes to the mitochondria to mediate the activation of NF-κB and IRF3 (15, 31). To corroborate that influenza B virus infection stimulates RIG-I-mediated signaling starting from the initial stage, mitochondrial isolation assays were performed to analyze the levels of endogenous RIG-I and TRIM25 in mitochondria. RIG-I and TRIM25 mitochondrial localization was increased upon influenza B virus infection (Fig. 4A and B). A portion of NS1-B also localized to the mitochondria. Compared to influenza A virus infection, RIG-I and TRIM25 localized more to the mitochondria during influenza B virus infection.
FIG 4.
Influenza B virus infection promotes RIG-I ubiquitination with K63-linked polyubiquitin chains, largely during the early stage of infection. (A) A549 cells were infected with B/Lee and A/CA04 at an MOI of 0.5 for 4 h and harvested to isolate mitochondria. The cytoplasm without mitochondria (Cyto) and mitochondrial (Mito) fractions were tested with BCA protein quantitation assays followed by IB assays with anti-RIG-I, anti-TRIM25, anti-NS1-B, anti-NS1-A, anti-VDAC1, and anti-GAPDH antibodies. (B) The RIG-I and TRIM25 protein levels in the Cyto and Mito fractions shown in panel A were quantitated by densitometry and normalized to GAPDH or VDAC1. The error bars represent the standard errors (SE). Ctrl, control. (C) 293T cells were transfected with FLAG-RIG-I or FLAG, as well as HA-Ub. After 24 h, the cells were infected with B/Lee or A/CA04 at an MOI of 0.5 for 4 h. Cell lysates were collected for immunoprecipitation (IP) with an anti-FLAG antibody, followed by IB with anti-HA, anti-FLAG, anti-NS1-B, anti-NS1-A/CA04, or anti-β-actin antibody. (D) 293T cells were transfected with FLAG-RIG-I and HA-Ub, HA-Ub-K48R, or HA-Ub-K63R for 24 h and then infected with B/Lee (MOI = 0.5) for 4 h. Cell lysates were harvested for immunoprecipitation with an anti-FLAG antibody, followed by IB with anti-HA, anti-FLAG, or anti-NS1-B antibody. (E) At 24 h posttransfection with HA-Ub and FLAG-RIG-I or FLAG, 293T cells were infected with B/Lee (MOI = 0.5). Whole-cell lysates were harvested at the indicated times and used for immunoprecipitation with an anti-FLAG antibody, followed by IB with anti-HA, anti-FLAG, anti-NS1-B, or anti-β-actin antibody.
Considering that TRIM25 positively regulates RIG-I signal transduction, immunoprecipitation assays and Western blotting were also performed to analyze the influence on ubiquitination on RIG-I by influenza B virus infection. Thus, FLAG-RIG-I and HA-ubiquitin (Ub) were coexpressed in 293T cells, followed by influenza B virus infection (Fig. 4C). Here, A/CA04 was again used as a control. After 4 h of infection with influenza viruses, cell lysates were extracted for immunoprecipitation. The Western blotting results indicated that influenza B virus infection induced the ubiquitination of RIG-I to a great extent.
Next, we wondered which ubiquitination mode was selected during influenza B virus infection. FLAG-RIG-I was coexpressed with HA-Ub, HA-Ub-Lys48Arg (HA-Ub-K48R), or HA-Ub-Lys63Arg (HA-Ub-K63R) in 293T cells, followed by influenza B virus infection at an MOI of 0.5 for 4 h (Fig. 4D). As shown in the figure, the presence of polyubiquitin chains on RIG-I was lost when coexpressed with HA-Ub-K63R. To further delineate the time of RIG-I ubiquitination in response to influenza B virus infection, immunoprecipitation assays were again performed at the indicated times (Fig. 4E). These assays revealed that the ubiquitination of RIG-I is largely promoted after 4 h postinfection. In sum, these results confirm that RIG-I can be ubiquitinated with K63-linked polyubiquitin chains and that its signaling can be transduced promptly and strongly upon influenza B virus infection.
NS1-B interacts with TRIM25, but not RIG-I, to form a RIG-I/TRIM25/NS1-B ternary complex.
The influenza B virus nonstructural NS1 protein (NS1-B) is capable of antagonizing IFN-β induction (32), but the detailed mechanism remains unknown. To determine the phase at which NS1-B functions to suppress IFN-β induction, we emphasized the upstream signaling pathway of IFN production. Influenza A virus NS1 protein binds to TRIM25 to inhibit the ubiquitination of RIG-I (16, 33) and also interacts with RIG-I to suppress the induction of IFN-β (34). Thus, we hypothesized that NS1-B may bind to TRIM25 and/or RIG-I. The expression level of endogenous TRIM25 was detected in A549 cells during influenza B virus infection. We found that TRIM25 expression increased during the time course of influenza B virus infection (Fig. 5A). The colocalization of TRIM25 and NS1-B was assessed by fluorescence microscopy. As shown in Fig. 5B, TRIM25 and NS1-B colocalized in the cytoplasm during influenza B virus infection, assembling in granule-like aggregates. Using coimmunoprecipitation assays, we observed that RIG-I, TRIM25, and NS1-B form a ternary complex when coexpressed in 293T cell lines (Fig. 5C). Strangely, NS1-B protein bound to TRIM25 (Fig. 5D) but did not interact with RIG-I when coexpressed in 293T cells (Fig. 5E). To further verify these results, we performed GST pulldown assays and found that overexpressed NS1-B was unable to interact with RIG-I (Fig. 5F). In contrast to NS1-B, NS1-A maintained its interaction with RIG-I (Fig. 5E and F). These data collectively demonstrate a relationship among the three proteins (RIG-I, TRIM25, and NS1-B) in which TRIM25 interacts with RIG-I and NS1-B but RIG-I has no direct interaction with NS1-B.
FIG 5.
NS1-B is involved in a RIG-I/TRIM25/NS1-B ternary complex by interacting with TRIM25. (A) A549 cells were infected at an MOI of 0.5 with B/Lee. At the indicated time points, the cell lysates were harvested and quantified with a BCA protein assay kit. The amount of TRIM25 was tested by immunoblotting with anti-TRIM25 antibody. (B) 293T cells were transfected with FLAG-TRIM25 for 24 h, followed by infection with B/Lee at an MOI of 0.5 for the indicated times. The localization of FLAG-TRIM25 (green) and NS1-B (red) was determined using fluorescence microscopy with an anti-FLAG monoclonal antibody and anti-NS1-B polyclonal antibody. The nuclei were stained with DAPI. White arrows indicate the granule-like aggregates. (C) 293T cells were transfected with FLAG-RIG-I, together with Myc-TRIM25, Myc-NS1-B, or both, for 36 h. Whole-cell lysates of all the samples were subjected to immunoprecipitation with anti-FLAG, followed by IB with anti-FLAG, anti-Myc, and anti-NS1-B antibodies. (D) 293T cells were transfected with FLAG-TRIM25 for 24 h, followed by infection with B/Lee at an MOI of 0.5 for 4 h. The cell lysates were harvested and subjected to immunoprecipitation with an anti-FLAG antibody, followed by IB with anti-FLAG and anti-NS1-B antibodies. (E) Whole-cell lysates of 293T transfected with FLAG-RIG-I or FLAG, together with Myc-NS1-B or Myc-NS1-A (NS1-A/CA04 and NS1-A/WSN), for 24 h were subjected to immunoprecipitation with an anti-FLAG antibody, followed by IB with anti-FLAG and anti-Myc antibodies. (F) Whole-cell lysates of 293T cells were transfected with Myc-NS1-B or Myc-NS1-A (NS1-A/CA04 and NS1-A/WSN) for 48 h mixed with GST–RIG-I or GST for GST pulldowns (GST-PD), followed by IB with an anti-Myc antibody or Coomassie staining. M, protein marker.
TRIM25 blocks the inhibitory effect of NS1-B on RIG-I ubiquitination.
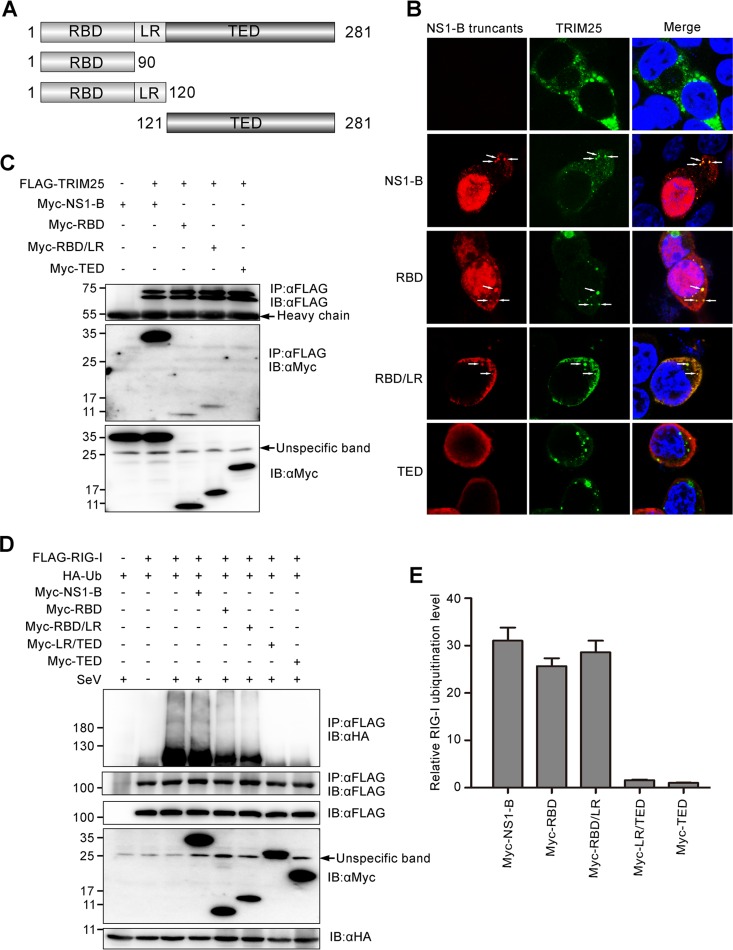
To determine the domain of NS1-B involved in binding to TRIM25, we generated Myc-tagged NS1-B truncations corresponding to the N-terminal RNA-binding domain (RBD) (amino acids [aa] 1 to 90), the N-terminal RNA-binding domain plus linker region (RBD/LR; aa 1 to 120), and the C-terminal effector domain (TED) (aa 121 to 281) (Fig. 6A). Fluorescence microscopy was performed to analyze the colocalization of TRIM25 and NS1-B truncations (Fig. 6B). The full-length NS1-B, NS1-B RBD, and NS1-B RBD/LR colocalized with FLAG-TRIM25, assembling in granule-like aggregates. The NS1-B TED showed no granule formation in the cytoplasm. Coimmunoprecipitation assays were performed to further determine the TRIM25-binding domain of NS1-B. As shown in Fig. 6C, only the TED of NS1-B had no capacity to bind to TRIM25. The other three proteins or truncations (full-length NS1-B, RBD, and RBD/LR) all bound to TRIM25, indicating that the RBD is indispensable for interaction with TRIM25.
FIG 6.
The N terminus of NS1-B is responsible for interaction with TRIM25 in the RIG-I/TRIM25/NS1-B ternary complex, and this interaction negates the inhibitory ability of the C terminus of NS1-B on RIG-I ubiquitination. (A) Schematic representation of the constructs encoding Myc fused to the full-length NS1-B or NS1-B truncations. (B) Colocalization of TRIM25 and NS1-B or NS1-B truncations, as assessed by fluorescence microscopy. FLAG-TRIM25 and Myc-NS1-B or Myc-NS1-B truncations were cotransfected into 293T cells for 24 h, followed by staining with anti-FLAG (green) and anti-Myc (red) antibodies. The nucleus was stained with DAPI (blue). White arrows indicate the granule-like aggregates. (C) FLAG-TRIM25 was cotransfected with constructs encoding full-length NS1-B or NS1-B truncations into 293T cells for 24 h. Whole-cell lysates were subjected to immunoprecipitation with an anti-FLAG antibody, followed by IB with anti-FLAG and anti-Myc antibodies. (D) The influence of the constructs encoding full-length NS1-B or NS1-B truncations on the ubiquitination of RIG-I was confirmed by immunoprecipitation and immunoblotting assays with anti-HA, anti-FLAG, and anti-Myc antibodies. (E) The relative RIG-I ubiquitination levels with overexpression of full-length NS1-B and NS1-B truncations (lanes 4 to 8 from left in panel D) were quantitated by densitometry and normalized to the corresponding full-length NS1-B or NS1-B truncations. The error bars represent the SE.
To further elucidate what effect the interaction between NS1-B and TRIM25 has on TRIM25-mediated RIG-I ubiquitination, immunoprecipitation assays were performed to examine RIG-I ubiquitination (Fig. 6C). To investigate if the short peptide LR is dispensable for RIG-I ubiquitination, we generated another NS1-B truncation, LR/TED (aa 91 to 281). Significantly, LR/TED and TED inhibited the ubiquitination of RIG-I, whereas RBD and RBD/LR minimally blocked ubiquitination. Interestingly, full-length NS1-B acted like the last two truncations, having no effect on the ubiquitination of RIG-I. These results indicate that the isolated TED can suppress RIG-I ubiquitination but that this can be overcome by the RBD in the context of full-length NS1-B. Overall, we conclude that TRIM25 binds to the N-terminal RBD of NS1-B and inactivates the inhibitory effect of the C-terminal TED on RIG-I ubiquitination. These data indicate that the robust cytokine production during the early stage of infection results from NS1-B failing to inhibit the RIG-I-mediated pathway. In Fig. 7, we present a proposed model of the detailed mechanism of the host defense response to influenza B virus infection based on our experiments.
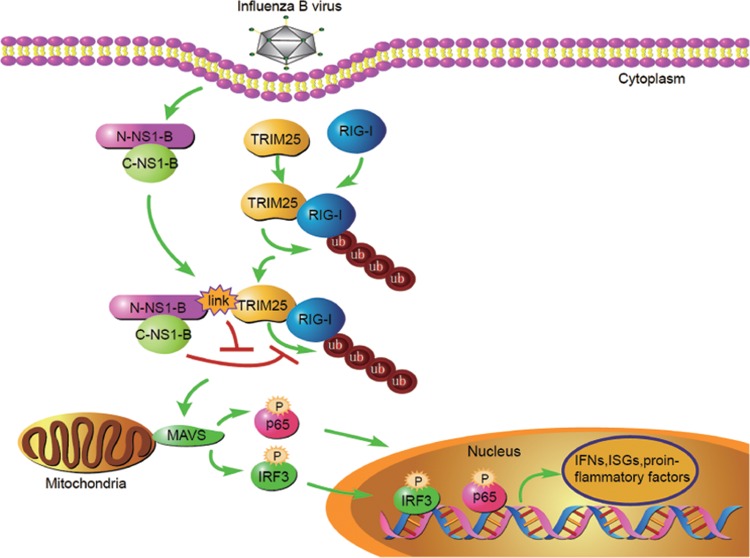
FIG 7.
Proposed model for the interplay between TRIM25, RIG-I, and NS1-B. After influenza B virus infection, TRIM25-mediated RIG-I ubiquitination promotes the eruption of IFNs, ISGs, and proinflammatory factors. Host cells utilize TRIM25 to interact with the N-terminal RBD of NS1-B (N-NS1-B) and to relieve the inhibitory effect of the NS1-B C-terminal TED (C-NS1-B) on RIG-I ubiquitination.
DISCUSSION
Influenza B virus, which has strict host range limits and initiates local epidemics, has a lower evolutionary rate and causes milder clinical manifestations than influenza A virus (35, 36). The dissimilar intensity of innate immune responses provoked by influenza B virus infection may provide a clue to the differences between the two viruses. We showed that influenza B virus infection results in rapid and strong production of representative cytokines. Type I and III IFNs, ISGs, and proinflammatory factors, such as IL-6 and IL-8, are conspicuously upregulated during the early stage of influenza B virus infection. Among the cytokines, type I IFN possesses the dominant ability to antagonize influenza virus infection (37), inducing the expression of a number of antiviral proteins, including MxA and ISG56, and activating the adaptive immune system (38). Further, proinflammatory factors play a vital role in activating protective adaptive immune responses (39). Here, we directly demonstrated that the expression of these cytokines induced by influenza B virus chiefly depends on the RIG-I-mediated signaling pathway by using RIG-I−/− 293T cell lines. Indeed, phosphorylated NF-κB, IRF3, and STAT1, the transcription factors of greatest importance in RIG-I-mediated and IFN-mediated signaling, are barely activated in RIG-I−/− 293T cells infected by influenza B virus, as expected. RIG-I is a key intracellular RNA sensor that recognizes the negative-stranded RNA of influenza A virus and initiates the antiviral response (40), and it also determines the cytokine eruption in response to influenza B virus infection.
RIG-I ubiquitination is critical for binding to MAVS to induce cytokine production. TRIM25 functions as an E3 ubiquitin ligase, and its C-terminal SPRY domain is responsible for the interaction with the CARD domain of RIG-I and robust K63-linked ubiquitination of RIG-I, increasing the activation of downstream signaling (15). Influenza B virus infection causes a marked increase in the ubiquitination of RIG-I (Fig. 4C), and this type of modification happened during the early stage of infection (Fig. 4E). Furthermore, we demonstrated that the type of ubiquitination is a K63 ubiquitin linkage (Fig. 4D). These results indicate that the explosion of cytokines in the early stage of influenza B virus infection is due to dramatic activation of the RIG-I-mediated signaling pathway.
The influenza A virus NS1 protein is an antagonist of TRIM25-mediated RIG-I ubiquitination, directly binding to the center coiled-coil domain of TRIM25 (16). Moreover, influenza A virus NS1 interacts with RIG-I to sequester dsRNA from recognition by RIG-I and blocks the activation of RIG-I signaling (34). However, we found that NS1-B interacts with TRIM25 but not RIG-I. Thus, there are inherent differences between the influenza A and influenza B virus NS1 proteins. Specifically, the N-terminal RBD of NS1-B is responsible for the interaction with TRIM25. It was also surprising that the NS1-B construct comprising only the C-terminal effector domain inhibited TRIM25-mediated RIG-I ubiquitination, whereas other constructs did not if they contained the N-terminal domain. The dsRNA-binding domain (aa 15 to 93) of NS1-B is also localized in the N terminus (41) and is needed for efficient replication by the influenza B virus (42). Thus, the interaction between TRIM25 and the N-terminal RBD of NS1-B both blocks the RNA-binding function of NS1-B and antagonizes the inhibitory effect of the NS1-B C-terminal TED on RIG-I ubiquitination. We showed that the N-terminal RBD of NS1-B is indispensable for interaction with TRIM25, while the C-terminal TED of NS1-B is not required. However, the isolated C-terminal TED of NS1-B is capable of inhibiting RIG-I ubiquitination, indicating that the NS1-B TED inhibits RIG-I ubiquitination independently of TRIM25 binding.
Protein ubiquitination is accomplished via a cascade of catalytic reactions with three classes of enzymes: E1, E2, and E3. E1 activates ubiquitin and transfers ubiquitin to E2. E3 interacts with E2-Ub and substrates, catalyzing the transfer of ubiquitin to the substrate (43, 44). The step at which the NS1-B TED functions to inhibit RIG-I ubiquitination is an intriguing and complicated scientific question. TRIM25 is a primary E3 ubiquitin ligase of RIG-I, and the Lys172 of RIG-I is the critical site for Lys63-linked ubiquitination involved in positive signaling transduction (15). However, the E1 and E2 enzymes involved in RIG-I ubiquitination remain unknown. It is possible that the NS1-B TED functions by interaction with E1, E2, or ubiquitin to block RIG-I ubiquitination, but these speculations remain to be confirmed. Although it has been reported that NS1-B is an inhibitor of the activation of IRF-3 signaling and the IFN-β promoter (32, 45), the host adopts measures to deal with the invasion of influenza B virus, targeting the NS1 protein to eliminate its inhibitory effects, like TRIM25 functions.
Compared to influenza B virus, influenza A virus evolves to evade host innate immune responses, especially the IFN system. NS1-A functions as a virulence factor to inhibit IFN responses by targeting multiple sites in signaling pathways. In RIG-I-mediated signal transduction, NS1-A interacts with TRIM25, RIG-I, and IKK to directly suppress signal activation through distinct mechanisms (16, 46, 47). Thus, influenza A virus establishes effective infection by counteracting host antiviral signaling, resulting in apparent and severe clinical symptoms.
In contrast, influenza B virus provokes cytokine eruption patterns resembling those provoked by some viruses belonging to the family Paramyxovirus, such as Newcastle disease virus (NDV) and SeV. NDV infection causes the activation of multiple transcription factors and induces robust type I IFN expression at an early phase of infection dependent on the RIG-I-mediated signaling pathway (48–50). The immediate genes whose expression is stimulated by SeV infection are mainly dependent on the activation of IRF3 and NF-κB (51). Currently, NDV and SeV are utilized as common vaccine vectors against diseases.
Here, we demonstrated that influenza B virus is a sensitive activator to stimulate the RIG-I-mediated antiviral signaling pathway. In terms of highly efficient innate immune responses, influenza B virus has the potential to become an immunopotentiator if convincing application safety limits are established. However, there is still the puzzling problem of why influenza B virus protein expression increases whereas host antiviral cytokines gradually increase during the late stage of infection. We aim to obtain a clear understanding of how influenza B virus can evade the innate immune response or, in other words, how the balance is maintained between influenza B virus and host cells. This question will be the subject of our further investigations to address the interplay between influenza B virus and the host.
ACKNOWLEDGMENTS
We thank Shuang Zhang, Yun Li, and Xi Liang for technical support, as well as Xiaolan Zhang for assistance with confocal microscopy analysis.
This work was supported by grants from China Ministry of Science and Technology (MOST) Project 973 (grant no. 2012CB955501) and the National Natural Science Foundation of China (NSFC) (grant no. 31402216, 81271849, and 31472178), an intramural special grant for influenza virus research from the Chinese Academy of Sciences (KJZD-EW-L09-2), and a grant from the Key Research Program of the Chinese Academy of Sciences (KSZD-EW-Z-005-001). W.L. is the principal investigator of the NSFC Innovative Research Group (grant no. 81321063).
W.L. and J.L. designed the experiments. J.J., W.L., and J.L. analyzed data and wrote the paper. J.J., W.F., W.Z., M.Y., and C.C. collected data. J.J., L.S., Y.B., C.D., and G.F.G. modified the paper.
We declare no conflicts of interest.
REFERENCES
- 1.Babakir-Mina M, Dimonte S, Perno CF, Ciotti M. 2009. Origin of the 2009 Mexico influenza virus: a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch Virol 154:1349–1352. doi: 10.1007/s00705-009-0438-1. [DOI] [PubMed] [Google Scholar]
- 2.Taubenberger JK, Morens DM. 2006. 1918 influenza: the mother of all pandemics. Emerg Infect Dis 12:15–22. doi: 10.3201/eid1209.05-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biere B, Bauer B, Schweiger B. 2010. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J Clin Microbiol 48:1425–1427. doi: 10.1128/JCM.02116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann E, Mahmood K, Yang CF, Webster RG, Greenberg HB, Kemble G. 2002. Rescue of influenza B virus from eight plasmids. Proc Natl Acad Sci U S A 99:11411–11416. doi: 10.1073/pnas.172393399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almond JW, Haymerle HA, Felsenreich VD, Reeve P. 1979. The structural and infected cell polypeptides of influenza B virus. J Gen Virol 45:611–621. doi: 10.1099/0022-1317-45-3-611. [DOI] [PubMed] [Google Scholar]
- 6.Osterhaus ADME, Rimmelzwaan GF, Martina BEE, Bestebroer TM, Fouchier RAM. 2000. Influenza B virus in seals. Science 288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- 7.Sridharan H, Zhao C, Krug RM. 2010. Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. J Biol Chem 285:7852–7856. doi: 10.1074/jbc.C109.095703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan R, Ma LC, Leonard PG, Amer BR, Sridharan H, Zhao C, Krug RM, Montelione GT. 2011. Structural basis for the sequence-specific recognition of human ISG15 by the NS1 protein of influenza B virus. Proc Natl Acad Sci U S A 108:13468–13473. doi: 10.1073/pnas.1107032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Wang D, Jiang Y, Sun J, Zhang S, Chen Y, Wang X. 2011. Crystal structure of human ISG15 protein in complex with influenza B virus NS1B. J Biol Chem 286:30258–30262. doi: 10.1074/jbc.C111.257899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. 2007. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol 179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 11.Pang IK, Pillai PS, Iwasaki A. 2013. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. Proc Natl Acad Sci U S A 110:13910–13915. doi: 10.1073/pnas.1303275110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. 2007. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol 178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 13.Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. 2001. The tripartite motif family identifies cell compartments. EMBO J 20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castanier C, Zemirli N, Portier A, Garcin D, Bidère N, Vazquez A, Arnoult D. 2012. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol 10:44. doi: 10.1186/1741-7007-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 16.Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, Kawakami E, Shoemaker JE, Lopes TJ, Matsuoka Y, Tomita Y, Kozuka-Hata H, Gorai T, Kuwahara T, Takeda E, Nagata A, Takano R, Kiso M, Yamashita M, Sakai-Tagawa Y, Katsura H, Nonaka N, Fujii H, Fujii K, Sugita Y, Noda T, Goto H, Fukuyama S, Watanabe S, Neumann G, Oyama M, Kitano H, Kawaoka Y. 2014. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe 16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei H, Wang S, Chen Q, Chen Y, Chi X, Zhang L, Huang S, Gao GF, Chen JL. 2014. Suppression of interferon lambda signaling by SOCS-1 results in their excessive production during influenza virus infection. PLoS Pathog 10:e1003845. doi: 10.1371/journal.ppat.1003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dauber B, Martinez-Sobrido L, Schneider J, Hai R, Waibler Z, Kalinke U, Garcia-Sastre A, Wolff T. 2009. Influenza B virus ribonucleoprotein is a potent activator of the antiviral kinase PKR. PLoS Pathog 5:e1000473. doi: 10.1371/journal.ppat.1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ketscher L, Hannss R, Morales DJ, Basters A, Guerra S, Goldmann T, Hausmann A, Prinz M, Naumann R, Pekosz A, Utermohlen O, Lenschow DJ, Knobeloch KP. 2015. Selective inactivation of USP18 isopeptidase activity in vivo enhances ISG15 conjugation and viral resistance. Proc Natl Acad Sci U S A 112:1577–1582. doi: 10.1073/pnas.1412881112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Zhao Z, Bi Y, Sun L, Liu X, Liu W. 2013. Tyrosine 132 phosphorylation of influenza A virus M1 protein is crucial for virus replication by controlling the nuclear import of M1. J Virol 87:6182–6191. doi: 10.1128/JVI.03024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Sun L, Yu M, Wang Z, Xu C, Xue Q, Zhang K, Ye X, Kitamura Y, Liu W. 2009. Cyclophilin A interacts with influenza A virus M1 protein and impairs the early stage of the viral replication. Cell Microbiol 11:730–741. doi: 10.1111/j.1462-5822.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 24.Qu H, Yang L, Meng S, Xu L, Bi Y, Jia X, Li J, Sun L, Liu W. 2013. The differential antiviral activities of chicken interferon alpha (ChIFN-alpha) and ChIFN-beta are related to distinct interferon-stimulated gene expression. PLoS One 8:e59307. doi: 10.1371/journal.pone.0059307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zhao Z, Xu C, Sun L, Chen J, Zhang L, Liu W. 2012. Cyclophilin A restricts influenza A virus replication through degradation of the M1 protein. PLoS One 7:e31063. doi: 10.1371/journal.pone.0031063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao S, Jiang J, Li J, Li Y, Yang L, Wang S, Yan J, Gao GF, Liu W. 2014. Characterization of the nucleocytoplasmic shuttle of the matrix protein of influenza B virus. J Virol 88:7464–7473. doi: 10.1128/JVI.00794-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. 2007. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem 282:7576–7581. [DOI] [PubMed] [Google Scholar]
- 28.Österlund P, Strengell M, Sarin LP, Poranen MM, Fagerlund R, Melén K, Julkunen I. 2012. Incoming influenza A virus evades early host recognition, while influenza B virus induces interferon expression directly upon entry. J Virol 86:11183–11193. doi: 10.1128/JVI.01050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mäkelä SM, Österlund P, Westenius V, Latvala S, Diamond MS, Gale M, Julkunen I, Lyles DS. 2015. RIG-I signaling is essential for influenza B virus-induced rapid interferon gene expression. J Virol 89:12014–12025. doi: 10.1128/JVI.01576-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasaki A, Pillai PS. 2014. Innate immunity to influenza virus infection. Nat Rev Immunol 14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seth RB, Sun L, Ea CK, Chen ZJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Dauber B, Heins G, Wolff T. 2004. The influenza B virus nonstructural NS1 protein is essential for efficient viral growth and antagonizes beta interferon induction. J Virol 78:1865–1872. doi: 10.1128/JVI.78.4.1865-1872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU. 2012. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog 8:e1003059. doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M Jr, Garcia-Sastre A. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol 81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson KG, Wood JM, Zambon M. 2003. Influenza. Lancet 362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobusawa E, Sato K. 2006. Comparison of the mutation rates of human influenza A and B viruses. J Virol 80:3675–3678. doi: 10.1128/JVI.80.7.3675-3678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrhardt C, Seyer R, Hrincius ER, Eierhoff T, Wolff T, Ludwig S. 2010. Interplay between influenza A virus and the innate immune signaling. Microbes Infect 12:81–87. doi: 10.1016/j.micinf.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Sastre A, Biron CA. 2006. Type I interferons and the virus-host relationship: a lesson in detente. Science 312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 39.Pulendran B, Maddur MS. 2015. Innate immune sensing and response to influenza. Curr Top Microbiol Immunol 386:23–71. doi: 10.1007/82_2014_405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Sousa CR. 2006. RIG-I–mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001. [DOI] [PubMed] [Google Scholar]
- 41.Yin C, Khan JA, Swapna GV, Ertekin A, Krug RM, Tong L, Montelione GT. 2007. Conserved surface features form the double-stranded RNA binding site of non-structural protein 1 (NS1) from influenza A and B viruses. J Biol Chem 282:20584–20592. doi: 10.1074/jbc.M611619200. [DOI] [PubMed] [Google Scholar]
- 42.Dauber B, Schneider J, Wolff T. 2006. Double-stranded RNA binding of influenza B virus nonstructural NS1 protein inhibits protein kinase R but is not essential to antagonize production of alpha/beta interferon. J Virol 80:11667–11677. doi: 10.1128/JVI.01142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berndsen CE, Wolberger C. 2014. New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol 21:301–307. doi: 10.1038/nsmb.2780. [DOI] [PubMed] [Google Scholar]
- 44.Deshaies RJ, Joazeiro CA. 2009. RING domain E3 ubiquitin ligases. Annu Rev Biochem 78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 45.Donelan NR, Dauber B, Wang X, Basler CF, Wolff T, Garcia-Sastre A. 2004. The N- and C-terminal domains of the NS1 protein of influenza B virus can independently inhibit IRF-3 and beta interferon promoter activation. J Virol 78:11574–11582. doi: 10.1128/JVI.78.21.11574-11582.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jureka AS, Kleinpeter AB, Cornilescu G, Cornilescu CC, Petit CM. 2015. Structural basis for a novel interaction between the NS1 protein derived from the 1918 influenza virus and RIG-I. Structure 23:2001–2010. doi: 10.1016/j.str.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao S, Song L, Li J, Zhang Z, Peng H, Jiang W, Wang Q, Kang T, Chen S, Huang W. 2012. Influenza A virus-encoded NS1 virulence factor protein inhibits innate immune response by targeting IKK. Cell Microbiol 14:1849–1866. doi: 10.1111/cmi.12005. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond S, Flano E, Durbin RK, Garcia-Sastre A, Durbin JE. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol 80:1130–1139. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schirrmacher V. 2015. Signaling through RIG-I and type I interferon receptor: immune activation by Newcastle disease virus in man versus immune evasion by Ebola virus. Int J Mol Med 36:3–10. doi: 10.3892/ijmm.2015.2213. [DOI] [PubMed] [Google Scholar]
- 50.Zaslavsky E, Hershberg U, Seto J, Pham AM, Marquez S, Duke JL, Wetmur JG, Tenoever BR, Sealfon SC, Kleinstein SH. 2010. Antiviral response dictated by choreographed cascade of transcription factors. J Immunol 184:2908–2917. doi: 10.4049/jimmunol.0903453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elco CP, Guenther JM, Williams BR, Sen GC. 2005. Analysis of genes induced by Sendai virus infection of mutant cell lines reveals essential roles of interferon regulatory factor 3, NF-kappaB, and interferon but not Toll-like receptor 3. J Virol 79:3920–3929. doi: 10.1128/JVI.79.7.3920-3929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]