ABSTRACT
Vaccinia virus (VACV) keratitis is a serious complication following smallpox vaccination and can lead to blindness. The pathological mechanisms involved in ocular VACV infection are poorly understood. Previous studies have used rabbits, but the lack of immune reagents and transgenic or knockout animals makes them less suitable for mechanistic studies. We report that infection of C57BL/6 mice with 1 × 107 PFU of vaccinia virus strain WR results in blepharitis, corneal neovascularization, and stromal keratitis. The DryVax strain of VACV was completely attenuated. Infection required corneal scarification and replication-competent virus, and the severity of ocular disease was similar in 4- to 6-week-old and 1-year-old mice. Viral titers peaked at approximately 1 × 106 PFU on day 5 postinfection, and virus had not cleared by day 13 postinfection. Neutrophils were found in the peripheral cornea on day 1 after infection and then declined, followed by infiltration of both CD4+ and CD8+ T cells, which remained peripheral throughout the infection. Blood vessel growth extended 2 to 5 mm into the cornea from the limbus. Infection of CD4−/−, CD8−/−, or antibody-depleted mice resulted in similar disease severity and corneal clouding, indicating that both T-cell subsets were involved in the immunopathological response. Depletion of both CD4+ and CD8+ T cells resulted in significantly more severe disease and failure to clear the virus. On the basis of our results, the pathology of VACV keratitis is significantly different from that of herpes simplex virus keratitis. Further studies are likely to reveal novel information regarding virulence and immune responses to viral ocular infection.
IMPORTANCE Potentially blinding eye infections can occur after vaccination for smallpox. Very little is known about the pathological mechanisms that are involved, and the information that is available was generated using rabbit models. The lack of immunological reagents for rabbits makes such studies difficult. We characterized a mouse model of vaccinia virus ocular disease using C57BL/6 mice and strain WR and show that both CD4+ and CD8+ T-cell subsets play a role in the blinding eye disease and in controlling virus replication. On the basis of these results, vaccinia virus keratitis is significantly different from herpes simplex virus keratitis, and further studies using this model should generate novel insights into immunopathological responses to viral ocular infection.
INTRODUCTION
In 1977, the World Health Organization reported the last known case of naturally acquired smallpox virus infection (1). The eradication of smallpox was accomplished by rigorous vaccination using vaccinia virus (VACV) and a contact tracing program. Shortly thereafter, the United States stopped vaccination of the general population. In response to increased terrorism incidents, including bioterror events, the U.S. government expanded the list of those who should be vaccinated to include first responders and stockpiled smallpox vaccine and vaccinia virus immune globulin (VIG) to treat adverse vaccine events in case of a deliberate release (2). In addition to the potential threat of a deliberate release, several animal poxviruses circulate naturally and can infect humans. For example, in 2003, there was a limited monkeypox virus outbreak in the upper Midwest of the United States that was due to imported infected African rodents (3). Thus, poxviruses remain a significant public health concern.
Ocular vaccinia virus infection is a side effect of smallpox vaccination and is usually the result of an accidental transfer of VACV from the vaccination site to the eye. Between 1963 and 1968, ocular VACV infections occurred in 348 people, 259 of which were primary vaccinees and 66 of which were contacts (4, 5). Keratitis occurred in 22 of these people, and 11 were blinded to some degree. In a group of 40,000 primary vaccinees, ocular vaccinia virus infection occurred 1 to 4 times (4, 5), and manifestations included conjunctival disease, iritis, and keratitis (6, 7). Accidental infection in the laboratory is also a potential means of acquiring vaccinia virus keratitis (VACVK) (8). In humans, VACVK begins as a finely granular opacification of the cornea and can progress to ulceration, deep stromal involvement (disciform keratitis), and diffuse interstitial keratitis (6). Corneal neovascularization and uveal involvement (aqueous flare) also commonly occur (6). VACVK was estimated to occur in up to 30% of all cases of ocular vaccinia virus infection (6).
The pathological mechanisms involved in VACVK are poorly understood. Recently, we developed a rabbit model for VACVK (8) and used this model to define the optimal therapy for treating these infections (9). In that study, we demonstrated that topical trifluridine (Viroptic) alone was the optimal therapy and that the inclusion of topical prednisolone with the antiviral resulted in a failure to clear the virus and the subsequent resumption of viral replication and increased stromal keratitis. These results suggest that the immune response to the virus, in addition to antiviral therapy, is critical for viral clearance. Since viral keratitis is an immunopathological disease, we also used the rabbit model to characterize the kinetics of immune cell infiltration into the corneas of the rabbits. We found that neutrophils were the predominant cell type early in infection, followed by infiltration of CD4+, CD8+, and, to a lesser extent, B cells. CD4+ T cells were the predominant cell type in the infected cornea later in the infection (10).
Immunopathological studies of the disease in rabbits are currently hampered by the lack of reagents and genetically modified animals. Therefore, we embarked on developing a mouse model using C57BL/6 mice to facilitate mechanistic studies of the disease, as many immunologically modified changes have been engineered in the C57BL/6 mouse background. We show that WR is a suitable strain of virus for such studies and that the DryVax strain is completely attenuated. Our data also show that C57BL/6 mice develop severe ocular disease and the virus replicates to high titers in the tear film. Finally, as we reported in the rabbit, both CD4+ and CD8+ cells infiltrate the cornea, appear to be involved in the pathology, and are necessary to control viral replication. This is different from herpes simplex virus (HSV) keratitis, where pathology is driven primarily by the infiltration of CD4+ cells (11). Thus, there are a number of fundamental differences between the keratitis caused by VACV and that caused by HSV, justifying further studies of VACV keratitis to identify critical factors in the immunopathological response that will reveal interesting immunological insights.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells (CCL-2; ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; catalog number S11150; Atlanta Biologicals, Atlanta, GA) and 10 ml/liter of penicillin-streptomycin (catalog number G1146; Sigma, St. Louis, MO). Cells used for determination of virus titers were cultured in DMEM supplemented with 10% FBS as described above and switched to DMEM supplemented with 2% FBS and 1% amphotericin B (catalog number 30-003-CF; Corning, Manassas, VA). All cell cultures were incubated at 37°C in a 5% CO2 atmosphere. High-titer virus stocks of the New York City Board of Health strain of vaccinia virus (DryVax; catalog number VR-1536; ATCC, Manassas, VA) or vaccinia virus strain WR were prepared by infecting HeLa cell monolayers in 10-cm plates at a multiplicity of infection (MOI) of 0.1 and harvesting the cells when the cytopathic effect (CPE) reached 90 to 100% (12). The cells were then pelleted by centrifugation at 400 × g for 10 min at 4°C, and the pellets were resuspended in phosphate-buffered saline (PBS). The suspension was then subjected to three freeze-thaw cycles in a dry ice-ethanol bath, vortexed at the maximum speed for 1 min, and then centrifuged at 750 × g for 10 min at 4°C. The supernatant was put on ice, while the pellet was resuspended in PBS, vortexed, and centrifuged as described above. The supernatants were combined, layered over a cushion of 36% sucrose in PBS, and centrifuged at 25,000 × g for 80 min at 4°C to pellet the virions. The purified virion pellet was resuspended in PBS, aliquoted, and stored at −80°C. Titers were determined by plaque assay on HeLa cell monolayers. Inactivation of the vaccinia virus was accomplished by a 30-min incubation at 65°C and then a 30-min exposure to UV light. No virus plaques were seen when the titer of the stock was determined.
Animal inoculation.
Four- to 6-week-old and 1-year-old female C57BL/6 mice (Harlan, Indianapolis, IN) were used for this study. The groups of mice used for all the studies consisted of 10 mice each. For all inoculations, examinations, treatments, and sample collections, the mice were anesthetized with isoflurane (catalog number 57319-47406; Phoenix Pharmaceutical, St. Joseph, MO). The right eyes of the mice were examined microscopically prior to infection for corneal defects, and those mice with defects were removed from the study. The remaining mice were then randomly assigned to groups. While the mice were under anesthesia, 6 to 10 scratches forming a crosshatch pattern were made on the cornea using a 30-gauge needle, taking care not to puncture the cornea. A 5-μl drop of DMEM containing the desired PFU of virus was applied to the scarified cornea, and the eyelids were closed twice over the cornea.
To provide analgesia, the mice were injected subcutaneously with 0.5 mg/kg of body weight of extended-release buprenorphine (kindly provided by Lisa Krugner-Higby, University of Wisconsin—Madison) just prior to the corneal scarification. This formulation provides adequate analgesia for 10 to 14 days. These studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research and NIH guidelines for the use of animals in research and were approved by the University of Wisconsin—Madison IACUC.
Disease scoring.
On 1, 3, 5, 7, 9, 11, 13, and 15 days postinfection, ocular disease severity was scored on a number scale, as previously described, on the basis of three disease parameters (13–15). Briefly, blepharitis, or swelling of the eyelid, was scored 1+ for puffy eyelids, 2+ for puffy eyelids with some crusting, 3+ for eye swollen shut with severe crusting, and 4+ for eye completely swollen shut and crusted over. Neovascularization, the growth of blood vessels into the cornea, was scored 1+ for <25% corneal involvement, 2+ for 25% to 50% corneal involvement, and 3+ for >50% corneal involvement. Stromal keratitis was scored 1+ for cloudiness with some iris detail visible, 2+ for iris detail obscured, 3+ for cornea totally opaque, and 4+ for corneal perforation.
Measurement of virus titers.
On days 1, 3, 5, 7, 9, 11, and 13 days postinfection, tear film samples were collected and the titers of infectious virus were determined. Mice were anesthetized with isoflurane, and the infected corneas were flushed with 10 μl of DMEM containing 2% FBS. The corneal wash was then added to 190 μl of DMEM containing 2% FBS and stored at −80°C. Each sample was thawed and then serially diluted 10-fold, and virus was quantified using a standard plaque assay on HeLa cells (14).
Animals.
In the first study, 4- to 6-week-old female C57BL/6 mice were inoculated with 101, 102, 103, 104, 105, 106, and 107 PFU of the DryVax vaccinia virus strain, eye disease was scored, and tear film virus titers were determined as described above. Since the mice in this study did not develop keratitis, we then tested the WR strain. Four- to 6-week-old female C57BL/6 mice were inoculated with 105, 106, and 107 PFU of the WR vaccinia virus strain, eye disease was scored, and tear film samples were taken as described above. To determine if susceptibility changed with age, 1-year-old female C57BL/6 mice were inoculated with 107 PFU of the WR vaccinia virus strain. To confirm that the scarification process alone did not trigger corneal clouding, that scarification was needed for efficient infection, and that replicating virus was required, we scored the corneal clouding in C57BL/6 mice that were not scarified but infected or that were infected with heat-inactivated virus. To test the role of CD4+ and CD8+ T-cell subsets in the pathology, 4- to 6-week-old female CD4−/− mice (B6.129S2-Cd4tm1Mak/J) and CD8−/− mice (B6.129S2-Cd8atm1Mak/J) (The Jackson Laboratory, Bar Harbor, ME) were infected with 4 × 107 PFU of WR. Ocular disease was scored and tear film samples were collected for determination of the virus titer as described above. The eyes were taken for histology at the end of the study. For the CD4+ and CD8+ depletion study, 4- to 6-week-old female C57BL/6 mice (Harlan) were injected intraperitoneally with 200 μg of anti-CD4 monoclonal antibody (MAb) clone GK1.5 (catalog number BE0003-1; BioXCell, West Lebanon, NH), anti-CD8α MAb clone 2.43 (catalog number BE0061; BioXCell), both antibodies (Abs), or a rat isotype control (clone LTF-2; catalog number BE0090; BioXCell) on days 4 and 2 prior to infection and on days 4 and 6 postinfection with 108 PFU of WR. Two additional groups were treated with 200 μg of either CD4+ or CD8+ Ab on days 4 and 6 postinfection only. An untreated, infected group served as a control. Ocular disease was scored, and tear film samples were collected for determination of virus titers as described above. The eyes were taken for histology at the end of the study. Additionally, blood from three mice per group was collected, and the red blood cells were lysed and counterstained with Ghost Dye violet 450 (1 μl; catalog number 13-0863-T100; Tonbo Biosciences, San Diego, CA). The lymphocytes were stained for 1 h for CD3+ (anti-mouse CD3ε-phycoerythrin [PE]; 10 μl of a 1/20 dilution of Ab in 100 μl of cells; clone 145-2C11; catalog number 553063), CD4+ (anti-mouse CD4-allophycocyanin [APC]; 10 μl of Ab in 100 μl of cells; clone RM4-5; catalog number 553051), CD8+ (anti-mouse CD8-peridinin chlorophyll protein [PerCP]; 10 μl of a 1/20 dilution of Ab in 100 μl of cells; clone 53-6.7; catalog number 553036), and CD45+ (anti-mouse CD45-fluorescein isothiocyanate [FITC]; 10 μl of a 1/20 dilution of Ab in 100 μl of cells; clone 30-F11; catalog number 553079) from BD Biosciences, San Jose, CA. The cells were then fixed in 2% paraformaldehyde, and the numbers of CD4+ and CD8+ cells were determined using flow cytometry.
Samples were analyzed on a 5-laser BD LSRII flow cytometer (Becton Dickinson, San Jose, CA) running Diva (v8.0) software. Ghost Dye 510 was excited by a 50-mW 405-nm laser and detected in a 525/50 band-pass filter. CD45-FITC and CD8-PerCP were excited by a 100-mW 488-nm laser and detected in 530/30 and 695/40 band-pass filters, respectively. CD3-PE was excited by a 150-mW 561-nm laser and detected in a 582/15 band-pass filter, and CD4-APC was excited by a 100-mW 642-nm laser and detected in a 675/20 band-pass filter. The compensated data were analyzed in FlowJo (v10.0.8r1) software (FlowJo LLC, Ashland, OR). Briefly, cells were gated on the basis of morphology in a plot of forward scatter versus side scatter. Doublets were eliminated using the forward scatter area versus the forward scatter height, and dead cells were excluded on the basis of Ghost Dye 510 fluorescence. From here, T cells were gated on the basis of the expression of both CD45+ and CD3+ and further divided into CD4+ and CD8+ populations.
Histology.
The eyes were fixed in 4% paraformaldehyde and then embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E), and examined microscopically.
Characterization of viral and immune cell infiltration.
Groups of three mice each infected with 107 PFU of VACV strain WR were sacrificed on each day of scoring, and the eyes were fixed in 4% paraformaldehyde for histopathology studies. The sections were stained using primary antibodies for neutrophils (catalog number sc-59338), CD4+ T cells (catalog number sc-1140), and CD8+ T cells (catalog number sc-7188) (all from Santa Cruz Biotechnology, Santa Cruz, CA) and anti-vaccinia virus antibody (catalog number ab35219; Abcam, Cambridge, MA). Secondary biotinylated antibody ABC kits were obtained from Santa Cruz (catalog numbers sc-2023 and sc-2018) or Vector Laboratories (catalog number PK-6104; Burlingame, CA). Nova Red or diaminobenzidine was used for development of the slides. Nuclei were counterstained with methyl green or Gill's hematoxylin. The number of positive cells in 2 to 3 sections of the central cornea and periphery of each eye was counted at a magnification of ×40, and the average for each group was calculated.
Statistical analysis.
Statistical analyses were conducted using SigmaPlot (v11.0) software (Systat Software, Chicago, IL). At the time points designated above, raw scores for each disease parameter were recorded for each mouse in a group. The mean disease scores for each group were calculated from the raw scores and analyzed for statistical significance. Mean peak disease scores (MPDS) were calculated as previously described (10, 11). Virus titers in tear films were averaged from the raw titer data for each mouse in a group, and the standard error was calculated. The Kruskal-Wallis one-way analysis of variance on ranks was used to compare the differences between groups. The Mann-Whitney rank sum test was used for pairwise comparisons of the average disease scores and MPDS of the groups. P values of <0.05 were deemed significant unless otherwise stated.
RESULTS
Corneal disease.
Our previous rabbit study used the DryVax strain of VACV, so we first tested the ability of DryVax to cause keratitis in C57BL/6 mice. Inocula ranging from 101 to 107 PFU failed to cause keratitis (data not shown), indicating that DryVax was highly attenuated in mice. We then tested inocula of 105, 106, and107 PFU of the WR strain and determined that the most severe and consistent disease was obtained with the highest inoculum (107 PFU); thus, we used 107 PFU for the remainder of the studies. Corneal clouding was evident within 24 h of infection and increased thereafter, peaking on day 9 at a score of 2.1 (Fig. 1A). The stromal disease scores then began to decline. Eyelid inflammation (blepharitis) was not observed until day 7 postinfection and peaked with a score of approximately 1.5, and then the incidence of blepharitis began to decline after day 9 (Fig. 1A). Vascularization was evident on day 1 through day 9 (Fig. 1A) but was limited to the peripheral cornea. Vascularization was difficult to score because the blood vessels extended into the infected corneas only approximately 2 to 5 mm. On the basis of these results, the most reliable indicator of disease was stromal keratitis. The MPDS are shown in Fig. 1B. The blepharitis and stromal keratitis scores were similar at a score of approximately 1.5, while the vascularization scores were only 0.5, reflecting the limited penetration into the cornea. Viral titers (Fig. 1C) increased after inoculation, peaking at approximately 1 × 106 on day 5 postinfection. The titers gradually declined thereafter, but infectious virus was still present at day 13, when we stopped collecting tear film samples.
FIG 1.

Disease scores of vaccinia virus strain WR-infected C57BL/6 mice. (A) Blepharitis, vascularization, and stromal keratitis scores. The scores are the mean ± SEM per group. (B) Mean peak disease scores for blepharitis, vascularization, and stromal keratitis. Scores are the means of the highest scores for each mouse in a group ± SEMs. (C) Virus titers in tear film measured by plaque assay for C57BL/6 mice infected with the vaccinia virus WR strain. Titers are the mean number of PFU of virus per milliliter per group ± SEM.
To determine if the age of the mice affected the disease severity, we infected 6-week-old and 1-year-old C57BL/6 mice with 107 PFU of strain WR, scored the ocular disease, and measured the titer of infectious virus. As shown in Fig. 2A, the stromal disease scores were similar between the two groups of mice. This is reflected in the MPDS, where we found no significant differences (Fig. 2B), and in viral replication, where the peak viral titers and the time to the peak titers (Fig. 2C) were essentially identical.
FIG 2.

Disease scores and viral titers for vaccinia virus strain WR-infected C57BL/6 mice at 6 weeks and 12 months of age. (A) Stromal keratitis scores, reported as the mean ± SEM per group. There were no significant differences between groups. (B) Mean peak disease scores for blepharitis, vascularization, and stromal keratitis. Scores are reported as the means of the highest scores for each mouse in a group ± SEMs. There were no significant differences between groups. (C) Virus titers in tear film measured by plaque assay for 6-week-old and 12-month-old infected mice. Titers are reported as the mean number of PFU of virus per milliliter for each group ± SEM. *, P < 0.05, day 1.
Corneal scarification alone did not induce clouding (data not shown). As shown in Fig. 3A, clouding was minimal in animals that were infected without scarification or that were infected with inactivated virus and significantly lower than that for the control mice (P < 0.05) at all times (days 1 through 15). This was also reflected in the titer data (Fig. 3B), where we saw replicating virus in the nonscarified group on day 1 (7 × 102 PFU) and day 3 (5 PFU). No infectious virus was detected in mice infected with inactivated virus. The titers of infectious virus in scarified animals were significantly different (P < 0.05) from those in the group infected with heat-inactivated virus and the no scarification group at all times (days 1 through 9). Thus, we conclude that replicating virus and corneal scarification are required for efficient infection.
FIG 3.

Disease scores and viral titers for C57BL/6 mice infected with vaccinia virus strain WR on unscarified corneas or infected using heat-inactivated virus and scarification. (A) Stromal keratitis scores, reported as the mean ± SEM per group. (B) Virus titers in tear films measured by plaque assay. Titers are reported as the mean number of PFU of virus per milliliter per group ± SEM. For days 1 through 9, the infected control mice had significantly higher stromal keratitis scores and titers than mice infected with inactivated virus or mice whose unscarified corneas were infected (P < 0.05).
Histology.
Figure 4 shows representative examples of H&E-stained infected and control corneal sections. On day 1, there was a mild mixed inflammatory infiltrate (neutrophils and lymphocytes) predominantly at the far periphery of the cornea and the substantia propria of the bulbar conjunctiva. The corneal epithelium was moderately to markedly hyperplastic. Most of the epithelialized cornea had a heavily keratinizing surface with disorganized dysplastic cells in the base. Paracentral zones of the cornea had a markedly attenuated epithelium. On day 3, the inflammation was restricted to the far periphery and the infiltrate was predominantly lymphocytic with some neutrophils. The corneal epithelium was irregular with hyperkeratosis and jumbled, poorly differentiated, dysplastic basal cells. At day 5, inflammatory infiltrate was restricted to the periphery and limbus and consisted of a mixture of lymphocytes, macrophages, and small numbers of neutrophils. The epithelium showed multifocal areas of epithelial thickening and epithelial thinning, the basal cells were large and sometimes dysplastic, and there was intermittent hyperkeratosis mostly at the far periphery and in the bulbar conjunctiva. The increasing severity of corneal clouding was evident at day 7, where there was general corneal stromal thickening and pronounced edema with an intense inflammatory cell infiltrate at the limbus and with the peripheral cornea consisting largely of mononuclear cells. There was also a robust infiltrate of neutrophils and macrophages. Neutrophilic inflammatory cells were seen extending deeply into the corneal stroma. By day 9, there was a moderate lymphoplasmacytic inflammatory infiltrate most prominently seen in the far periphery of the cornea and in the limbus. The corneal epithelium was uniformly edematous, and axial hyperkeratosis was present. After day 9, the amount of inflammatory infiltrate began to decline but was still present in the periphery and was predominantly mononuclear or lymphocytic. The corneas remained edematous, and the stroma was disorganized. Throughout the time course, blood vessels were seen only in the far periphery of the corneas, consistent with the clinical scores.
FIG 4.
(A to D) H&E staining of central and peripheral cornea. (A) Uninfected peripheral cornea, day 11; (B) infected peripheral cornea, day 7; (C) uninfected central cornea, day 11; (D) infected central cornea, day 11. (E to G) Immunostaining of infected peripheral cornea. Cells stained brown or red are positive. Nuclei counterstained with methyl green are negative. (E) Neutrophils in the peripheral cornea, day 1; (F) CD4+ cells in peripheral cornea, day 7; (G) CD8+ cells in peripheral cornea, day 9. (H) Staining with secondary antibody only. EP, corneal epithelium; S, stroma; EN, corneal endothelium.
Characterization of infiltrating cells.
The histopathological analysis indicated that neutrophils appeared to infiltrate early, followed by lymphocytic cells. To confirm these results and identify the cell types, we stained tissue sections with antibodies specific for polymorphonuclear leukocytes (PMNs) and CD4+ and CD8+ cells. Representative examples are shown in Fig. 4E to H, and the results for the negative control are shown in Fig. 4H. To determine the kinetics of infiltration of each cell type, we quantified the number of infiltrating cells (Fig. 5) at various times postinfection. As expected from the H&E staining results, PMNs were most prominent at 24 h postinfection and declined thereafter, with few, if any, cells being evident on days 9 to 13. Low numbers of CD4+ and CD8+ cells were seen on day 1 and day 3, but the numbers began to increase on day 5. The numbers of CD4+ and CD8+ cells increased in parallel, peaking on day 7. The CD4+-cell numbers then declined through day 13. The number of CD8+ cells declined on day 9 but increased on day 11 and then declined on day 13. We repeated the CD8+ counts on day 9 with additional sections and again found a peak on day 11, but it is not clear if there were actually two peaks of CD8+T-cell infiltration.
FIG 5.

Kinetics of cellular infiltration into infected corneas. The mean number of cells staining positive with antibodies specific for PMNs, CD8+ cells, and CD4+ cells in vaccinia virus-infected C57BL/6 mice is shown. Eyes were taken from three mice per group and sectioned, the number of positive cells in each section was counted, and the average number per group was determined.
Vaccinia virus replication in corneal epithelium, stromal keratocytes, and infiltrating immune cells.
To determine which cells in the cornea were infected, sections obtained from infected corneas on days 5 and 7 postinfection were stained with anti-VACV antibody (Fig. 6). Both central and peripheral corneas are shown. No staining was seen in the absence of the primary antibody (Fig. 6A and B). In uninfected corneas, we saw background staining for the corneal epithelium and endothelium, despite extensive blocking and hydrogen peroxide pretreatment. In the presence of VACV antibody, there was more intense staining of corneal epithelial cells, particularly near Bowman's layer, suggesting that replication was occurring in epithelial cells. Extensive staining of stromal keratocytes and infiltrating immune cells was seen in the peripheral cornea. Because of pigmentation, we did not assess iris infection. Due to the high background, we could not determine if viral antigen was present in corneal endothelial cells.
FIG 6.
Immunostaining of infected peripheral cornea for VACV antigen. Cells stained brown or red are positive for VACV. Nuclei were counterstained with hematoxylin. (A, B) Infected central and peripheral cornea, no primary antibody; (C, D) uninfected central and peripheral cornea; (E, F) infected central and peripheral cornea, eye with ocular disease, day 7. EP, corneal epithelium; S, stroma; EN, corneal endothelium; arrows, cells staining positive for viral antigen.
CD4+ or CD8+ cells cause corneal clouding.
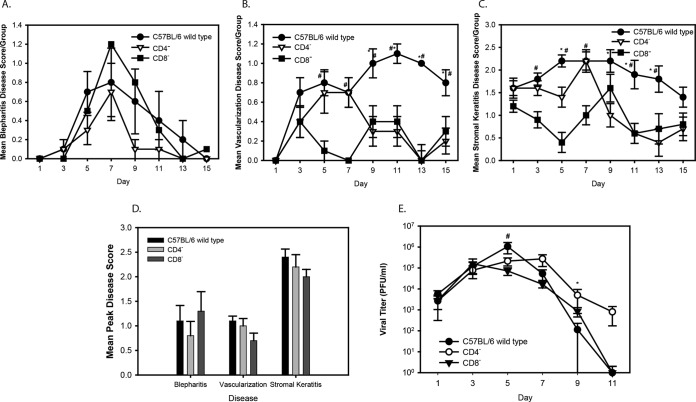
The histology and immunohistochemical staining data indicated that both CD4+ and CD8+ T cells infiltrated the infected corneas, suggesting that both cell types could contribute to the pathology. To confirm this, we infected CD4−/− mice (B6.129S2-Cd4tm1Mak/J) and CD8−/− mice (B6.129S2-Cd8atm1Mak/J) with 107 PFU of strain WR, scored the severity of ocular disease, and measured the viral titers. All of the mice developed blepharitis (Fig. 7A), although the peak score on day 7 was increased in the CD8−/− mice compared to that in the wild-type and CD4−/− mice. Blepharitis scores were not significantly different between the groups (P < 0.05). Vascularization (Fig. 7B) developed in all of the mice but was less severe in the CD8−/− mice at early times after infection. Vascularization in the CD4−/− mice peaked on day 7, similar to the findings for the wild-type mice, but declined thereafter, and the vascularization scores for the CD4−/− mice were similar to those for the CD8−/− mice. The degree of neovascularization continued to increase through the remainder of the study for the wild-type mice. All groups developed corneal clouding (Fig. 7C), but it appeared to be less severe in the CD8−/− mice. After day 7, the clouding decreased in the CD4−/− mice, with the scores being similar to those for the CD8−/− mice. Although the mean disease scores appeared to differ between the groups, the MPDS were not significantly different (Fig. 7D). Viral titers (Fig. 7E) were very similar for all groups through day 9 postinfection but were significantly higher on day 9 in the CD4−/− mice and on day 5 in the wild-type mice. These results are consistent with the findings of immunohistochemical staining and confirm that either CD4+ or CD8+ cells can contribute to the corneal clouding.
FIG 7.
(A to C) Disease scores for vaccinia virus strain WR-infected C57BL/6 wild-type, CD4−/−, and CD8−/− mice. (A to C) Mean ± SEM blepharitis (A), vascularization (B), and stromal keratitis (C) scores per group. *, P < 0.05 for CD4−/− versus wild-type mice; #, P < 0.05 for CD8−/− versus wild-type mice. (D) Mean peak disease scores for blepharitis, vascularization, and stromal keratitis. Scores are the means of the highest scores for each mouse in a group ± SEMs. There were no significant differences between groups. (E) Virus titers in tear films measured by plaque assay for C57BL/6 wild-type, CD4−/−, and CD8−/− mice infected with the vaccinia virus WR strain. Titers are reported as the mean number of PFU of virus per milliliter per group ± SEM. *, P < 0.05 for CD4−/− versus wild-type mice, day 9; #, P < 0.05 for CD8−/− versus wild-type mice, day 5.
Histologically, the amount of inflammatory infiltrate in the central cornea of control, CD4−/−, and CD8−/− mice was minimal (Fig. 8). Peripherally, there was extensive inflammatory infiltrate in the wild-type corneas. Inflammatory cells were also present in the CD4−/− and CD8−/− mice, but the amount was considerably reduced. Inflammation was not evident in the uninfected left eyes of the mice.
FIG 8.

Histological analysis of central and peripheral corneas in wild-type, CD4−/−, and CD8−/− mice. Representative sections are shown. Sections were stained with hematoxylin and eosin. The dark pigment in the iris and ciliary body is melanin. The text above each panel indicates the group of mice represented and whether the image is from the central cornea or the periphery. EN, corneal endothelium; S, stroma; EP, corneal epithelium; CB, ciliary body; I, iris.
Antibody depletion.
To further confirm that both CD4+ and CD8+ cells were involved in the pathogenesis, we carried out antibody depletion studies. This also allowed us to test mice depleted of both CD4+ and CD8+ cells, as well as the effect of delayed depletion. The groups included mice that were infected but in which CD4+ and CD8+ cells were not depleted, mice in which CD4+ or CD8+ cells were depleted pre- and postinfection, mice in which CD4+ or CD8+ cells were depleted postinfection, mice in which both CD4+ and CD8+ cells were depleted pre- and postinfection, and an isotype control group. Depletion was confirmed by collecting blood at the time of sacrifice and quantifying the CD4+ and CD8+ cells by fluorescence-activated cell sorting (FACS). Representative examples of the results of FACS analysis and the percentages of CD4+ and CD8+ cells are shown in Fig. 9. The level of depletion ranged from 97% to 100%. No depletion was seen in the infected control or the isotype control groups; thus, the appropriate T-cell subsets were depleted from the mice. The disease scores for blepharitis, neovascularization, and corneal clouding are shown in Fig. 10. For blepharitis, the scores were similar for the mice in which CD4+ cells were depleted pre- and postinfection, mice in which CD8+ cells were depleted pre- and postinfection, mice in which CD8+ cells were depleted postinfection, isotype control mice, and mice in which CD4+ and CD8+ cells were not depleted, with the severity peaking on day 7 and declining thereafter. The blepharitis score (Fig. 10A) on days 7 and 9 in the group in which CD4+ cells were depleted postinfection was significantly lower (P < 0.05) than that in the control group in which CD4+ and CD8+ cells were not depleted. In mice in which both CD4+ and CD8+ cells were depleted pre- and postinfection, blepharitis continued to increase in severity, reaching a score of 3.5 on day 13, when the study was terminated. For corneal neovascularization (Fig. 10B), the scores were more variable, but the group in which CD4+ cells were depleted postinfection again had significantly lower scores than the control group in which CD4+ and CD8+ cells were not depleted on days 7 and 9. On day 9, the group in which both CD4+ and CD8+ cells were depleted had the highest score (1.9), but this was not significantly different from that for the mice in the other groups, with the exception of the mice in which CD4+ cells were depleted postinfection. Stromal disease (Fig. 10C) scores overlapped between the groups on most of the days. However, as with blepharitis, the group in which both CD4+ and CD8+ cells were depleted pre- and postinfection developed significantly more severe disease than the other groups (P < 0.05, days 9, 11, and 13). The mean peak disease scores are shown in Fig. 10D, and the scores for the group in which CD4+ and CD8+ cells were depleted and the isotype control group were compared to those for the infected control group. For blepharitis only, the scores for the group in which both CD4+ and CD8+ cells were depleted pre- and postinfection (severe disease) and the group in which CD4+ cells were depleted postinfection (milder disease) were significantly different from those of the nondepleted control group. The MDPS for vascularization were significantly different only for the group in which CD4+ cells were depleted postinfection and the isotype control group. Opacity MPDS were significantly different for the group in which CD4+ cells were depleted pre- and postinfection. As shown in Fig. 10E, viral titers were similar among all the groups on days 1 and 3, with the titers peaking on day 5 postinfection. The mice began clearing virus after day 5, with the titers declining thereafter. Clearance was delayed in the group in which CD4+ cells were depleted pre- and postinfection and in the group in which CD4+ cells were depleted postinfection. The one exception was the group in which both CD4+ and CD8+ cells were depleted pre- and postinfection, where the titers remained significantly elevated through the end of the experiment (day 13). These results indicate that either CD4+ or CD8+ cells can contribute to controlling replication in the eye and that removal of both cell types abrogates control.
FIG 9.
Flow cytometry analysis of T-cell subsets in infected mice in which CD4+ and CD8+ cells were depleted and not depleted. (A) Percentage of CD4+ and CD8+ cells in vaccinia virus WR-infected mice in which CD4+ cells, CD8+ cells, or both CD4+ and CD8+ cells were depleted, isotype control mice, and control C57BL/6 wild-type mice. Values are means ± SEMs. (B to H) Gating graphs for mice in which CD4+, CD8+, or CD4+ and CD8+ cells were depleted and isotype control and wild-type control mice in which CD4+ and CD8+ cells were not depleted. Pre Post, pre- and postinfection; Post, postinfection.
FIG 10.
Disease scores of vaccinia virus strain WR-infected mice in which CD4+ cells were depleted (CD4 pre/post inf), CD8+ cells were depleted (CD8 pre/post inf), or both CD4+ and CD8+ cells were depleted (CD4/CD8 pre/post inf), in which CD4 cells were depleted postinfection (CD4 post inf), or in which CD8 cells were depleted postinfection (CD8 post inf), isotype control mice, and C57BL/6 mice in which CD4+ and CD8+ cells were not depleted (control). (A to C) Blepharitis (A), vascularization (B), and stromal keratitis (C) scores (mean ± SEM per group). *, P < 0.05 versus control group. (D) Mean peak disease scores for blepharitis, vascularization, and stromal keratitis. Scores are the means of the highest scores for each mouse in a group ± SEMs. *, P < 0.05 versus the control group. (E) Virus titers in tear films measured by plaque assay for vaccinia virus WR-infected mice in which CD4+ cells, CD8+ cells, or both CD4+ and CD8+ cells were depleted, isotype control mice, and C57BL/6 mice in which CD4+ and CD8+ cells were not depleted. Titers are reported as the mean number of PFU of virus per milliliter per group ± SEM. *, P < 0.05 versus the control group.
Histologically, the isotype and infected control groups had severe infiltration in the peripheral cornea. Infiltrating cells were also seen in the peripheral corneas of the groups in which CD4+ or CD8+ cells were depleted, but the amount was reduced compared to that in the controls. The central cornea in all groups had little, if any, infiltration (Fig. 11). The cell types infiltrating the cornea in the mice in which CD4+ or CD8+ cells were depleted were characterized by staining with CD4+ and CD8+ antibodies. No staining was seen in the absence of primary antibody, and little, if any, staining was seen in uninfected corneas. CD4+-cell infiltration was seen in the eyes of infected mice in which CD4+ and CD8+ cells were not depleted (Fig. 12). There was little, if any, CD4+ staining in the corneas of mice in which CD4+ cells were depleted pre- and postinfection. We saw some CD4+ inflammatory infiltrate in the corneas of mice in which CD4+ cells were depleted postinfection. Fewer CD4+ cells were also seen in the group in which both CD4+ and CD8+ cells were depleted. Extensive CD4+-cell infiltration was seen in all of the groups in which CD8+ cells were depleted.
FIG 11.
Histological analysis of central and peripheral corneas of antibody-depleted mice. Representative sections are shown. Sections were stained with hematoxylin and eosin. The dark pigment in the iris and ciliary body is melanin. The text above each panel indicates the group of mice represented and whether the image is from the central cornea or the periphery. EN, corneal endothelium; S, stroma; EP, corneal epithelium; CB, ciliary body; I, iris; depl pre/post, depletion of the indicated cell type pre- and postinfection; depl post, depletion of the indicated cell type postinfection; isotype, isotype control group.
FIG 12.
Immunohistochemical staining of corneas from mice depleted of CD4+ T cells. Positive staining is indicated by the reddish brown color. The dark pigment in the iris and ciliary body is melanin. The text above each image indicates the location and the group. Note that the reddish brown color in the corneal epithelium and endothelium is background staining even with extensive blocking and peroxide treatment. EN, corneal endothelium; S, stroma; EP, corneal epithelium; CB, ciliary body; I, iris; arrows, cells positive for CD4+ cells.
For CD8+, no staining was seen in the absence of the primary antibody (Fig. 13). Some CD8+ cells were seen in the uninfected corneas, whereas infiltration of CD8+ cells was seen in the corneas of infected control mice in which CD4+ and CD8+ cells were not depleted. Some CD8+ T-cell infiltration was seen in the groups in which CD4+ cells were depleted pre- and postinfection and in which CD4+ cells were depleted postinfection. The peripheral corneas of the mice in which CD8+ cells were depleted were similar to those of mice in which CD4+ cells were depleted in having a few positive cells. Positive staining for CD8+ cells in the group in which both CD4+ and CD8+ cells were depleted was more extensive than that in the groups in which only CD4+ or CD8+ cells were depleted. The presence of some CD4+ and CD8+ cells in the corneas of mice in which CD4+ or CD8+ cells were depleted is not unexpected since depletion was not 100%.
FIG 13.
Immunohistochemical staining of corneas of mice depleted of CD8+ T cells. Positive staining is indicated by the reddish brown color. The dark pigment in the iris and ciliary body is melanin. The text above each image indicates the location and the group. Note that the reddish brown color in the corneal epithelium and endothelium is background staining even with extensive blocking and peroxide treatment. EN, corneal endothelium; S, stroma; EP, corneal epithelium; CB, ciliary body; I, iris; arrows, cells positive for CD8+.
DISCUSSION
Vaccinia virus ocular infection in individuals who are vaccinated against smallpox or who have laboratory-acquired infections, although rare, can be devastating and lead to blindness resulting from corneal clouding. Very little is known about the pathological mechanisms involved in VACV ocular disease. In order to further studies on the pathology of VACV keratitis, we describe a mouse model and present several novel findings. A comparison between our previous rabbit study (9, 16) and this study supports previous conclusions indicating that mice are more resistant to vaccinia virus infection than rabbits (2), as 1 × 104 PFU of DryVax caused keratitis in rabbits but was attenuated in our mouse model (16) even at an inoculum of 107 PFU. Strain WR was suitable for use in the C57BL/6 mice, and there was a rapid onset of corneal clouding. Neutrophils peaked very early postinfection, followed by CD4+ and CD8+ T cells. Using mice deficient for CD4+ or CD8+ cells and antibody depletion, we show that both T-cell subsets contribute to corneal pathology. In addition, depletion of both T-cell subsets resulted in the failure to control viral replication and the development of more severe ocular disease. These findings set the stage for further studies on the genetics of virulence in viral strains to study key pathological events and the role of the immune system in corneal pathology.
The eye is typically considered immunoprivileged. Although most of the studies on immunoprivilege have focused on the anterior chamber (anterior chamber-associated immune deviation [ACAID]) or the retina (17–19), the cornea also has some features that suppress inflammation. Most notably, these are reduced constitutive major histocompatibility complex expression and a lack of vascularization and lymphatic vessels in the cornea (20, 21). Despite these protective mechanisms, inflammatory responses do occur. Previous work has shown that infection of corneal epithelial cells by HSV results in the synthesis of several proinflammatory cytokines and chemokines that result in the recruitment of neutrophils and then various types of T cells into the cornea (21–24). For some infections, like HSV infections, blood vessels and lymphatics infiltrate into the cornea, amplifying the response. One of the primary barriers to infection is the mucin layer of the ocular surface, which prevents pathogen attachment, and the tear film, which has numerous antimicrobial molecules (25). It is generally believed that mechanical damage to the cornea that breaches the infection barrier is required in most cases of keratitis; thus, scarification is frequently used in animal models.
Two types of murine VACV infection models have been widely used. Vaccinia virus strain WR has been the most commonly used virus for these studies. The first model involves dermal inoculation on either the footpad, the ear pinna, or the flank skin of mice and is thought to mimic the routes of inoculation used in vaccination protocols (26–30). In dermal models using immunocompetent mice, systemic spread does not occur and the skin lesions are limited. The second model involves either inhalation or intranasal inoculation, which results in a lung infection followed by systemic dissemination and death (31–33). These models are thought to mimic naturally acquired variola virus infection. In addition to different syndromes, these two routes of infection have been shown to generate different immune responses (33), with some investigators reporting that antibodies (CD4+-cell dependent) are more important for protection, while others report that CD8+ cells are more important for viral clearance (34–36). One potential explanation for the differential protective responses may be the use of different routes of infection.
On the basis of these observations, it might be predicted that the immune response generated following ocular infection may be different from that generated following infection by the other two routes. We found that mice developed corneal clouding when only CD8+ or CD4+ T cells were present, suggesting that in wild-type mice, both cell types are involved in the immunopathological response that causes corneal clouding. There were subtle differences in the disease between the CD4−/− and CD8−/− mice. For example, in the CD4−/− mice, vascularization was more severe than that in the CD8−/− mice and similar to that in the wild-type mice on days 5 and 7 postinfection. A similar pattern was seen for corneal clouding, where the disease severity was similar for the wild-type and CD4−/− mice but significantly reduced in the CD8−/− mice on days 3 to 7 postinfection. The potential significance of these differences in terms of pathological mechanisms requires additional studies.
Ocular infection results in shedding of infectious virus in the tear film, which drains via the lacrimal duct into the nasal cavity; thus, it is possible that in our ocular infection model, infectious virus reaches the nose and perhaps the respiratory tract. Whether infection of the respiratory mucosa occurs will be a subject of future studies, but if it does occur, it does not result in systemic signs or symptoms or death, as the infected mice in our study exhibited little, if any, weight loss (data not shown), and all mice survived the infection.
Domenico et al. (29) reported that younger mice are more susceptible to VACV infection, developing more severe skin lesions and higher viral titers. That study compared mice at age 4 weeks and mice at age 10 weeks and used the WR strain and C57BL/6 mice. When we compared the severity of VACV keratitis in 4- to 5-week-old mice with that in 1-year-old C57BL/6 mice, we found no significant differences in the severity of the ocular infection or in viral titers. The reason for the difference in the findings of these studies is not clear, but the route of infection is the most significant difference, suggesting that the ocular environment is different from the skin environment.
Given our previous findings that DryVax was efficient in causing corneal clouding in rabbits (16), it was somewhat surprising that DryVax was completely attenuated in mice. It was not necessarily surprising that the WR strain was virulent, as this strain was derived by brain passage in mice (37). We previously showed that the ocular virulence phenotype of a given HSV strain depends on the contributions of multiple viral genes (38–40), and it is likely that VACV is similar. The availability of two strains of virus with different virulence properties will allow us to use our recently reported virulence quantitative trait locus mapping procedure (40) to identify and characterize virulence genes in ocular infections.
Although we detected corneal neovascularization in VACV-infected C57BL/6 mice, it was restricted to within 2 to 5 mm of the corneal limbus, even though the virus was replicating in the central cornea. In HSV keratitis in C57BL/6 mice, blood vessels invade the entire cornea (41), usually in concert with corneal clouding; thus, VACV and HSV are significantly different in this aspect of the pathology. With HSV keratitis, immune cell infiltration occurs throughout the cornea, most likely aided by the ingrowth of new blood vessels. In VACV keratitis, histologically, immune cell infiltration was restricted to the limbus, where we saw blood vessel growth. Thus, one potential explanation for restricted cell infiltration may be the lack of blood vessels growing into the central cornea of the VACV-infected mice. The explanation for why blood vessel infiltration is restricted is not clear, but it is known that only cowpox and rabbitpox viruses induce hemorrhagic pocks on chorioallantoic membranes and that this is due to the expression of specific viral genes (42). The lack of this capability in strain WR could explain the restricted vascularization of the cornea. Another possibility is that VACV and poxviruses in general produce a number of secreted proteins that modify host responses (reviewed in references 11 and 43), and these factors may suppress blood vessel formation. Whether the limited neovascularization is due to the lack of inducing factors in the virus or active suppression by viral proteins will require further studies. For HSV keratitis, the virus activates vascular endothelial growth factor (VEGF) expression (44), and this has been shown to be required for new blood vessel ingrowth into the cornea. There are two sources of VEGF in HSV-infected corneas: neutrophils that infiltrate the tissue and corneal epithelial cells (24, 45). One potential explanation for the restricted corneal neovascularization in VACV keratitis is the presence of neutrophils only at the earliest times and their absence in the central cornea. The VACV genome may also encode viral factors that inhibit VEGF; thus, it will be interesting to determine if VACV alters VEGF expression in the infected tissue.
The very early neutrophil infiltration into the VACV-infected cornea is similar to the timing of infiltration in other viral infection models, including HSV keratitis (24), but there are significant differences. Neutrophils are detected very early with both viral infections, but the neutrophil numbers in the VACV-infected corneas dropped quickly and were much lower on day 2 postinfection. With HSV keratitis, neutrophil counts in the cornea remain elevated for several days, and there are two waves of neutrophil infiltration in HSV keratitis (46, 47). Neutrophils are critical for the development of HSV keratitis because depletion of these cells abrogates the development of keratitis (48). Cytokines and chemokines secreted by the neutrophils are chemotactic for the T cells that infiltrate the cornea later in infection in HSV keratitis, and T cells are involved in the second wave of cellular infiltration (24). It will be interesting to determine if neutrophils are essential for clouding in VACV keratitis and whether the cytokine and chemokine profiles are similar between HSV and VACV. There are likely to be functional differences, since VACV secretes decoy cytokine receptors that interfere with the immune response (43), while HSV does not rely heavily on this strategy. This may be one explanation for the limbal restriction of cellular infiltration in VACV-infected corneas.
Significant differences also exist regarding the role of T-cell subsets in HSV and VACV keratitis. In VACV keratitis, we found that both CD8+ and CD4+ cells infiltrated into the cornea, suggesting that both T-cell subsets play a role in infection. We confirmed the hypothesis that both subsets were involved in the pathology, as infection of either CD4+- or CD8+-deficient mice resulted in corneal clouding. Antibody depletion of CD4+ and CD8+ T cells confirmed the findings obtained with the knockout mice and showed that depletion postinfection did not significantly alter corneal clouding. There may be subtle differences in the pathological role of each subset, but it is clear that either subset can cause stromal keratitis. This is in contrast to HSV keratitis, where it has been shown that corneal clouding is a type IV hypersensitivity response mediated by CD4+ Th1 cells (11). Thus, there are profound differences in the immunopathology of the two viral infections, and further characterization of the role of T cells in VACV keratitis will reveal novel insights into immunopathologic mechanisms.
In wild-type mice, viral titers peaked between days 3 and 7 postinfection and then began to decline. When we analyzed the kinetics of immune cell infiltration, we found that neutrophils were present only very early in infection, suggesting that they play roles other than the direct suppression of viral replication. The decrease in viral titer after day 7 postinfection would be consistent with a major role for the adaptive immune response in viral clearance. When we measured viral titers in the CD4+ and CD8+ T-cell-knockout mice, we found that the kinetics of viral replication were essentially identical when wild-type mice and CD8− T-cell-knockout mice were compared. Viral clearance was delayed in the CD4− T-cell-knockout mice, supporting the suggestion that CD4+ cells play an important role. This finding would be consistent with CD4+ T-helper-cell functions. A similar result was seen in the antibody depletion studies, where viral clearance seemed to be delayed in the CD4+ depletion group. However, at least one subset, either CD8+ or CD4+ cells, is required for clearance, as viral titers remained elevated even out to day 13 in the group in which both CD4+ and CD8+ cells were depleted. This result also suggests that the different mechanisms used by either CD4+ or CD8+ cells are sufficient to control virus replication to at least some degree.
In summary, we have characterized a mouse model of VACV keratitis and shown that there are significant differences between VACV keratitis and HSV keratitis. We also have shown that C57BL/6 mice are susceptible to VACV infection and that DryVax is highly attenuated in mice. We have also provided evidence that both CD4+ and CD8+ cells play a role in the immunopathological corneal clouding that leads to blindness. This model will be useful for studying the role of host genes in VACV keratitis and the role of viral genes in the infection and pathology. In addition, novel insights into immunopathologic mechanisms in the cornea will be able to be identified with further study. It will also be useful for testing potential antiviral agents.
ACKNOWLEDGMENT
We thank Richard Dubielzig for pathological analysis of the sections stained with H&E.
REFERENCES
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Quenelle DC, Kern ER. 2010. Treatment of vaccinia and cowpox virus infections in mice with CMX001 and ST-246. Viruses 2:2681–2695. doi: 10.3390/v2122681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft DR, Sotir MJ, Williams CJ, Kazmierczak JJ, Wegner MV, Rausch D, Graham MB, Foldy SL, Wolters M, Damon IK, Karem KL, Davis JO. 2007. Occupational risks during a monkeypox outbreak, Wisconsin, 2003. Emerg Infect Dis 13:1150–1157. doi: 10.3201/eid1308.061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane JM, Ruben FL, Neff JM. 1969. Complications of smallpox vaccination. N Engl J Med 281:1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- 5.Ruben FL, Lane JM. 1970. Ocular vaccinia. An epidemiologic analysis of 348 cases. Arch Ophthalmol 84:45–48. [DOI] [PubMed] [Google Scholar]
- 6.Duke-Elder S. 1965. Diseases of the outer eye. In Duke-Elder S. (ed), System of ophthalmology, vol. 8 C. V. Mosby, St. Louis, MO. [Google Scholar]
- 7.Pepose JS, Margolis TP, LaRussa P, Pavan-Langston D. 2003. Ocular complications of smallpox vaccination. Am J Ophthalmol 136:343–352. doi: 10.1016/S0002-9394(03)00293-9. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs S. 2012. Working safely with vaccinia virus: laboratory techniques and review of published cases of accidental laboratory infections. Methods Mol Biol 890:1–22. doi: 10.1007/978-1-61779-876-4_1. [DOI] [PubMed] [Google Scholar]
- 9.Altmann S, Brandt CR, Murphy CJ, Patnaikuni R, Takla T, Toomey M, Nesbit B, McIntyre K, Covert J, Dubielzig R, Leatherberry G, Adkins E, Kodihalli S. 2011. Evaluation of therapeutic interventions for vaccinia keratitis. J Infect Dis 203:683–690. doi: 10.1093/infdis/jiq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmann S, Toomey M, Nesbit B, McIntyre K, Covert J, Dubielzig R, Leatherberry G, Adkins E, Murphy CJ, Brandt CR. 2010. Kinetics of immune cell infiltration in vaccinia virus keratitis. Invest Ophthalmol Vis Sci 51:4541–4548. doi: 10.1167/iovs.09-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahar MW, Graham SC, Chen RA-J, Cooray S, Smith GL, Stuart DI, Grimes JM. 2011. How vaccinia virus has evolved to subvert the host immune response. J Struct Biol 175:127–134. doi: 10.1016/j.jsb.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grau DR, Visalli RJ, Brandt CR. 1989. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Invest Ophthalmol Vis Sci 30:2474–2480. [PubMed] [Google Scholar]
- 13.Brandt CR, Spencer B, Imesch P, Garneau M, Deziel R. 1996. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob Agents Chemother 40:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt CR, Akkarawongsa R, Altmann S, Jose G, Kolb AW, Waring AJ, Lehrer RI. 2007. Evaluation of a theta-defensin in a murine model of herpes simplex virus type 1 keratitis. Invest Ophthalmol Vis Sci 48:5118–5124. doi: 10.1167/iovs.07-0302. [DOI] [PubMed] [Google Scholar]
- 15.Brandt CR, Coakley LM, Grau DR. 1992. A murine model of herpes simplex virus-induced ocular disease for antiviral drug testing. J Virol Methods 36:209–222. [DOI] [PubMed] [Google Scholar]
- 16.Altmann S, Emanuel A, Toomey M, McIntyre K, Covert J, Dubielzig RR, Leatherberry G, Murphy CJ, Kodihalli S, Brandt CR. 2010. A quantitative rabbit model of vaccinia keratitis. Invest Ophthalmol Vis Sci 51:4531–4540. doi: 10.1167/iovs.09-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biros D. 2008. Anterior chamber associated immune deviation. Vet Clin Small Anim 38:309–321. doi: 10.1016/j.cvsm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Streilein JW. 1990. Anterior chamber associated immune deviation: the privilege of immunity in the eye. Surv Ophthalmol 35:67–73. doi: 10.1016/0039-6257(90)90048-Z. [DOI] [PubMed] [Google Scholar]
- 19.de Smet MD, Chan CC. 2001. Regulation of ocular inflammation—what experimental and human studies have taught us. Prog Retin Eye Res 20:761–797. doi: 10.1016/S1350-9462(01)00011-8. [DOI] [PubMed] [Google Scholar]
- 20.Brandt CR, Knupfer PB, Boush GA, Gausas RE, Chandler JW. 1990. In vivo induction of Ia expression in murine cornea after intravitreal injection of interferon-γ. Invest Ophthalmol Vis Sci 31:2248–2253. [PubMed] [Google Scholar]
- 21.Park PJ, Chang M, Garg N, Zhu J, Chang J-H, Shukla D. 2015. Corneal lymphangiogenesis in herpetic stromal keratitis. Surv Ophthalmol 60:60–71. doi: 10.1016/j.survophthal.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babu JS, Kanagat S, Rouse BT. 1995. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J Immunol 154:4822–4829. [PubMed] [Google Scholar]
- 23.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. 2002. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci 43:737–743. [PubMed] [Google Scholar]
- 24.Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. 2013. Herpes keratitis. Prog Retin Eye Res 32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott AM. 2013. Antimicrobial compounds in tears. Exp Eye Res 117:53–61. doi: 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tscharke DC, Smith GL. 1999. A model for vaccinia virus pathogenesis and immunity based on intradermal injection of mouse ear pinnae. J Gen Virol 80:2751–2755. doi: 10.1099/0022-1317-80-10-2751. [DOI] [PubMed] [Google Scholar]
- 27.Tscharke DC, Reading PC, Smith GL. 2002. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J Gen Virol 83:1977–1986. doi: 10.1099/0022-1317-83-8-1977. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs N, Bartlett NW, Clark RH, Smith GL. 2006. Vaccinia virus lacking the Bcl-2-like protein N1 induces a stronger natural killer cell response to infection. J Gen Virol 87:1157–1161. doi: 10.1099/vir.0.81556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domenico J, Lucas JJ, Fujita M, Gelfand EW. 2012. Susceptibility to vaccinia virus infection and spread in mice is determined by age at infection, allergen sensitization, and mast cell status. Int Arch Allergy Immunol 158:196–205. doi: 10.1159/000330647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siciliano NA, Hersperger AR, Lacuanan AM, Xu R-H, Sidney J, Sette A, Sigal LJ, Eisenlohr LC. 2014. Impact of distinct poxvirus infections on the specificities and functionalities of CD4+ T cell responses. J Virol 88:10078–10091. doi: 10.1128/JVI.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson JD, Reith RW, Jeffrey LJ, Arrand JR, Mackett M. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J Gen Virol 71:2761–2767. doi: 10.1099/0022-1317-71-11-2761. [DOI] [PubMed] [Google Scholar]
- 32.Alcami A, Smith GL. 1992. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell 71:153–167. doi: 10.1016/0092-8674(92)90274-G. [DOI] [PubMed] [Google Scholar]
- 33.Reading PC, Smith GL. 2003. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J Gen Virol 84:1973–1983. doi: 10.1099/vir.0.19285-0. [DOI] [PubMed] [Google Scholar]
- 34.Goulding J, Bogue R, Tahiliani V, Croft M, Salek-Ardakani S. 2012. CD8 T cells are essential for recovery from a respiratory vaccinia virus infection. J Immunol 189:2432–2440. doi: 10.4049/jimmunol.1200799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goulding J, Abboud G, Tahiliani V, Desai P, Hutchinson TE, Salek-Ardakani S. 2014. CD8 T cells use IFN-γ to protect against the lethal effects of a respiratory poxvirus infection. J Immunol 192:5415–5425. doi: 10.4049/jimmunol.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kremer M, Suezer Y, Volz A, Frenz T, Majzoub M, Hanschmann K-M, Lehmann MH, Kalinke U, Sutter G. 2012. Critical role of perforin-dependent CD8+ T cell immunity for rapid protective vaccination in a murine model of human smallpox. PLoS Pathol 8:e1002557. doi: 10.1371/journal.ppat.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker RF, Bronson LH, Green RH. 1941. Further studies of the infectious unit of vaccinia. J Exp Med 74:263–281. doi: 10.1084/jem.74.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandt CR, Kolb AW, Shah DD, Pumfery AM, Kintner RL, Jaehnig E, Van Gompel JJ. 2003. Multiple determinants contribute to the virulence of HSV ocular and CNS infection and identification of serine 34 of the US1 gene as an ocular disease determinant. Invest Ophthalmol Vis Sci 44:2657–2668. doi: 10.1167/iovs.02-1105. [DOI] [PubMed] [Google Scholar]
- 39.Brandt CR. 2005. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp Eye Res 80:607–621. doi: 10.1016/j.exer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Kolb AW, Lee K, Larsen IV, Craven M, Brandt CR. 2016. Quantitative trait locus based virulence determinant mapping of the HSV-1 genome in murine ocular infection: genes involved in viral regulatory and innate immune networks contribute to virulence. PLoS Pathog 12:e1005499. doi: 10.1371/journal.ppat.1005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajasagi NK, Reddy PB, Mulik S, Gjorstrup P, Rouse BT. 2013. Neuroprotectin D1 reduces the severity of herpes simplex virus-induced corneal immunopathology. Invest Ophthalmol Vis Sci 54:6269–6279. doi: 10.1167/iovs.13-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Zikos D, Tamosiunaite A, Klopfleisch R, Osterrieder N, Tischer BK. 2014. Identification of 10 cowpox virus proteins that are necessary for induction of hemorrhagic lesions (red pocks) on chorioallantoic membranes. J Virol 88:8615–8628. doi: 10.1128/JVI.00901-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith GL, Benfield CTO, de Motes CM, Mazzon M, Ember SWJ, Ferguson BJ, Sumner RP. 2013. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol 94:2367–2392. doi: 10.1099/vir.0.055921-0. [DOI] [PubMed] [Google Scholar]
- 44.Gimenez F, Suryawanshi A, Rouse BT. 2013. Pathogenesis of herpes stromal keratitis—a focus on corneal neovascularization. Prog Ret Eye Res 33:1–9. doi: 10.1016/j.preteyeres.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuest TR, Carr DJ. 2010. VEGF-A expression by HSV-1 infected cells drives corneal lymphangiogenesis. J Exp Med 207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Tang Q, Hendricks RL. 1996. Ex vivo model of leukocyte migration into herpes simplex virus-infected mouse corneas. J Leukoc Biol 60:167–173. [DOI] [PubMed] [Google Scholar]
- 47.Osorio Y, Wechsler SL, Nesburn AB, Ghiasi H. 2002. Reduced severity of HSV-1 induced corneal scarring in IL-12 deficient mice. Virus Res 90:317–326. doi: 10.1016/S0168-1702(02)00249-6. [DOI] [PubMed] [Google Scholar]
- 48.Thomas J, Gangappa S, Kanangat S, Rouse BT. 1997. On the essential involvement of neutrophils in the immunopathologic disease. Herpetic stromal keratitis. J Immunol 158:1383–1391. [PubMed] [Google Scholar]