Abstract
Objectives: This study examined the effects and molecular mechanisms of deep hypothermic low flow (DHLF) on brain tissue in three genotypes of 3-week-old C57BL/6 mice (N = 180).
Methods: Mice in the model condition were subjected to cerebral ischemia-reperfusion (I-R) while undergoing DHLF, then reperfused and rewarmed. Brain tissue damage was measured with 2,3,5-triphenyltetrazolium chloride (TTC) staining, and protein expression was measured by Western blot at 2 h, 24 h, and 72 h after treatment; messenger ribonucleic acid (mRNA) expressions were measured by real-time polymerase chain reaction (PCR) at 2 h, 24 h, and 72 h.
Results: The expressions of p-Akt1 and p-GSK-3β were significantly higher in the model condition than the condition across genotypes, but both were significantly lower in the Akt1 mice. The expressions of Akt1 mRNA and Akt3 mRNA, but not Akt2 mRNA, were significantly higher in the model condition across genotypes. Brain damage was significantly greater in the Akt1 knockout gene mice compared with Akt2 gene knockout and wild type mice at 24 h and 72 h.
Conclusion: These results suggest that the neuroprotective effects of DHLF reflect increased expression of p-GSK-3β induced through the PI3K/Akt signal pathway. Findings of real-time PCR imply that Akt1 mRNA and Akt3 mRNA may influence the expression of p-Akt1 and p-GSK-3β in mice undergoing DHLF.
Keywords: P13K/Akt signaling path way, deep hypothermic low flow, cerebral ischemia/reperfusion injury, glycogen synthase kinase-3β
Introduction
There is a high incidence of birth defects in our country, with some 800000 to 1200000 children being born with birth defects each year, including about 220000 cases of congenital heart disease. Cardiac surgery requiring cardiopulmonary bypass (CPB) and it leads to body ischemic and reperfusion injury.1,2) Deep hypothermic low-flow (DHLF) provides a clear surgical field for the surgeon. At the same time, it can provide sufficient oxygen and blood flow to the brain, enhance the hypoxia tolerance of brain cells to prolong tolerance of brain tissue to lack of oxygen, and reduce brain damage and the apoptosis of brain cells caused by lack of oxygen. However, because of the process of cerebral ischemia reperfusion, the nervous system will be subjected to a certain level of harm.3,4) Postoperative neurological dysfunction remains an important cause of perioperative death and postoperative disability. Therefore, research on the brain protective mechanisms of DHLF, using an animal model, is clinically important for understanding the mechanism by which DHLF protects the brain and for reducing brain injury.
Many scholars currently are focusing on the PI3K/Akt signaling pathway to study ischemia-reperfusion injury, and Hausenloy called the PI3K/Akt signaling pathway the Reperfusion Injury Salvage Kinase Pathway.5) The cumulative injuries of ischemia and reperfusion are complex, and reperfusion is known to exacerbate ischemia.6) As Akt is a major downstream target of PI3K, it is a key regulating factor after cerebral ischemic injury, enhancing the survival of nerve cells, activating a variety of protein kinases, and playing a role in the pathway of oxidative phosphorylation. The function of the Akt is complex and diverse, and its downstream target genes are numerous. Its functions involves nutrient metabolism, cell survival and cell growth, cell apoptosis, cell cycle regulation, and so on.7–9) Akt also is known as a protein kinase B (PKB), a kind of serine/threonine protein kinase, the virus oncogenes V-akt homologue, so named Akt. The Akt family includes the Akt1, Akt2, and Akt3 sub-types, which have similar structures, but their functions are not identical.10) Research shows that Akt1 is mainly distributed in the brain, thymus, and the lungs, the Akt2 gene is amplified in ovarian, breast, and pancreatic tumors, and Akt3 mainly is distributed in the brain, heart, and kidney.11) Akt1, Akt2 and Akt3 play different roles in cell growth and metabolism.12) Akt1 participates in the regulation of cell growth and survival, and Akt1 gene knockout mice have body growth defects and a higher rate of apoptosis.13) Akt2 play an important role in glucose metabolism, and Akt2 knockout mice have symptoms like type II diabetes.14) The lack of Akt3 can lead to brain neurons cells to change in size,15) and Akt3 knockout mice have altered brain development.16)
The research indicates that injury from ischemia-reperfusion may be related to glycogen synthesis kinase-3 (GSK-3) and the mitochondrial permeability transition pore (MPTP). According to a 1980 report,17) GSK-3β was affected by molecules of the first found in the PI3K/Akt signaling pathways downstream. GSK-3β is a multifunctional protein kinase. Specifically, GSK-3β participates in a variety of biochemical reactions, including the activation of resting cells. Through the phosphorylation induced by glycogen synthetase and other signal molecules, beta serial proteins, such as transcription factors and structural proteins, are inactivated or suppressed.18) Phosphorylation of Akt1 can enhance GSK-3β Ser-9 site phosphorylation, whose inactivation can reduce apoptosis, inhibiting cell hypertrophy, and promoting angiogenesis; thus, it plays the role in antiapoptosis.19) How GSK- 3β plays a role in brain protection is not very clear.
Materials and Methods
Reagents
Dimethyl sulfoxide and 2,3,5-triphenyltetrazolium chloride were purchased from Sigma. The Akt, Akt3, p-Akt-ser473, and p-GSK-3β-ser9 antibodies were obtained from Cell Signaling Technology, and the Akt2 and p-Akt-ser474 antibodies were purchased from Epitomic. The Platinum resistance temperature sensor was obtained from Tianjin, the reverse transcription kits were obtained from Takara, and the Realtime PCR kits were obtained from (SYBR Premix Ex Taq™ – Perfect Real Time).
Grouping and the DHLF model
We used three-week-old 3-week old C57BL/6 mice, which were obtained from the Experimental Animals Center of Nanjing Medical University. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. All surgical procedures were carried out under sterile conditions. Healthy 3-week old C57BL/6 mice (N = 180) of three different genotypes were used in the study: a wild type (+/+), an Akt1 gene knockout type (+/–), and an Akt2 gene knockout (+/–). Mice of each genotype were randomly assigned to a control condition (sham group) and a DHLF model condition (model group), for a total of six groups consisting of 30 mice each. Each group was divided into subgroups for biochemical testing and brain biopsy at 24 h and 72 h after reperfusion.
The DHLF mice model was established based on our experience in our previous experiments.20) An intraperitoneal injection of 5% chloral hydrate was used to anesthesize the mouse, after which the neck shaved and disinfected, and midline incision made, to separate the tissue around the bilateral common carotid arteries. After the common carotid arteries were separated in the mice in the control condition (sham group), the wounds were left open for 120 min and then stitched closed. In the experimental condition (model group), each mouse was placed on a bed of ice and a platinum resistance temperature sensor was used to electronically measure the anal temperature of the mouse. When the temperature dropped to between 18°C and 19°C, a noninvasive fiber artery clamp was clipped bilaterally to the common carotid artery, and opened the artery after 120 min, while the mouse’s temperature was maintained between 18°C and 19°C. After the wound was stitched, the mouse was placed in a newborn incubator to recover its temperature. The temperature of the incubator was slowly increased at a rate of 0.5°C/min or less to restore the mouse’s body temperature to 32°C within 30 min.
Blood-flow monitoring
After the mouse was anesthesized, its scalp was shaved, and disinfected. A 1.0 mm hole was drilled into the skull 2.0 mm to the right of the midline, and 1.0 mm posterior to bregma. A laser doppler microfiber probe, 0.5 mm in diameter, was passed through the hole in contact with the cerebral cortex, and the probe was fixed to the skull. Perimed software monitored blood flow to the brain (Perisoft for Windows, Version 2.50).21–24)
Tissue samples
After being anesthesized, the mouse was laid on its back; the chest was opened to expose the heart. To reduce blood vessel damage, micro forceps were used to separate the surrounding tissues from the ascending aorta, and a lavage needle was inserted into the ascending aorta. A cut was made to open the right auricle and the lavage needle slowly injected saline into the ascending aorta until the outflow of liquid from the right auricle was colorless. Then, the mouse was beheaded and the whole brain removed.
Five mice from each of the six groups were randomly selected for 2,3,5-triphenyltetrazolium chloride (TTC) staining of their brain tissue. They were sacrificed at reperfusion 24 h and 72 h after treatment and their whole brains were cryopreserved at –20°C. The remaining mice from each group were used to measure messenger ribonucleic acid (mRNA) expressions by real-time polymerase chain reaction (PCR) at 24 h and 72 h, and Western blot to measure proteins at 24 h and 72 h after treatment. The whole brain of these mice was removed after reperfusion, quick frozen in liquid nitrogen, and stored at –80°C in a freezer.
Measures
The Western blot method was used to detect total Akt, Akt1 and p-Akt1, Akt3, p-GSK-3β protein expression in brain tissue. The bicinchoninic acid (BCA) method was used to detect protein concentration at 95°C modified about 5 min. The proteins in a 30 µg sample were separated by sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE), at a voltage of 70 and 120 v, respectively. The protein bands were transferred to a polyvinylidene fluoride (PVDF) membrane (25 v, 35 min), placed in 5% skimmed milk for 2 h at the room temperature, and incubated in the antibody for the night in 4°C. The membranes were washed in tris buffered saline with tween-20 (TBST) 3 times for 5 min, incubated with the antibody for about 2 h at room temperature, and washed in TBST again 3 times for 5 min. Enhanced chemiluminescence (ECL) chemiluminescence images were captured and saved for statistical analysis.
Real-time PCR
Real-time PCR was used to detect the expression of Akt1 mRNA, Akt2 mRNA, and Akt3 mRNA in the brain tissue: At each time-point, mice were scarified to extract the total ribonucleic acid (RNA) from the brain tissue. Nanodrop software was used detect the total RNA concentration, and reverse transcription was used to obtain complementary deoxyribonucleic acid (cDNA). The cDNA template was analyzed by real-time PCR, using the Dalian Biological Treasure Company’s SYBR Premix Ex Taq™ (Perfect Real Time) kit. PCR amplification was performed with StepOnePlus PCR™ (US). The reaction conditions were as follows: 95°C pre-modified, approximately 30 s; 95°C modified, approximately 5 s; 60°C annealing, approximately 34 s, for a total of 40 cycles. The procedures were conducted in accordance with the Delta-delta Ct analytical method for the relative quantification of the expression of housekeeping genes.
TTC staining
TTC staining was used to detect brain damage: after saline infusion the brain tissue was put into –20°C refrigerator for about 10 min. A 2 mm thick portion of brain tissue was taken from 1 mm behind the optic chiasm and cut into 5 pieces. Brain slices were soaked in 0.8% 2 3 5-triphenyltetrazolium chloride in darkness at 37°C to incubate, (turned after 5 min), and removed after 20 min, after which they were fixed in 4% paraformaldehyde for about 24 h for photography. Image-Pro Plus 6.0 software was used to calculate the area of brain damage and assess the volume of damage as a percentage of the slices: volume = (the sum of the slice’s double-sided area/2) × slice thickness; cerebral infarction volume = (the sum of the slice’s double-sided infarction area/2) × slice thickness. The infarction area (white area) of the slices and a thickness of 2 mm were multiplied to obtain the product, which gave an approximation of the infarction volume. The same method was used to calculate the brain volume: cerebral infarction volume = cerebral infarction volume/total volume × 100%.
Statistical analyses
The data were analyzed using the SPSS 13.0 statistical software. The results are presented as the mean ± the standard deviation. One-way analysis of variance was performed to compare mean differences between groups, and the least significant difference (LSD)-test was used to perform pairwise comparisons between groups.
Results
Protein test results
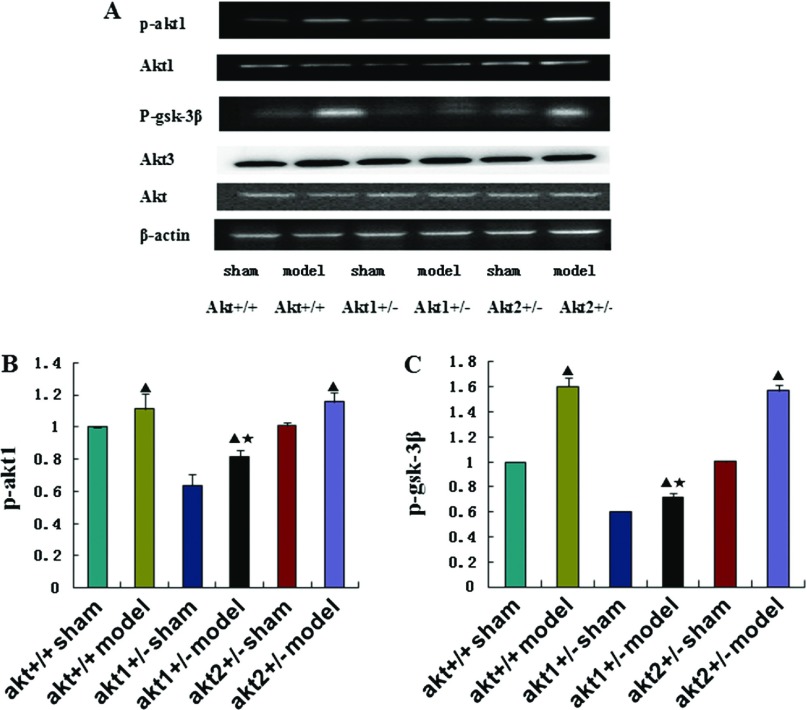
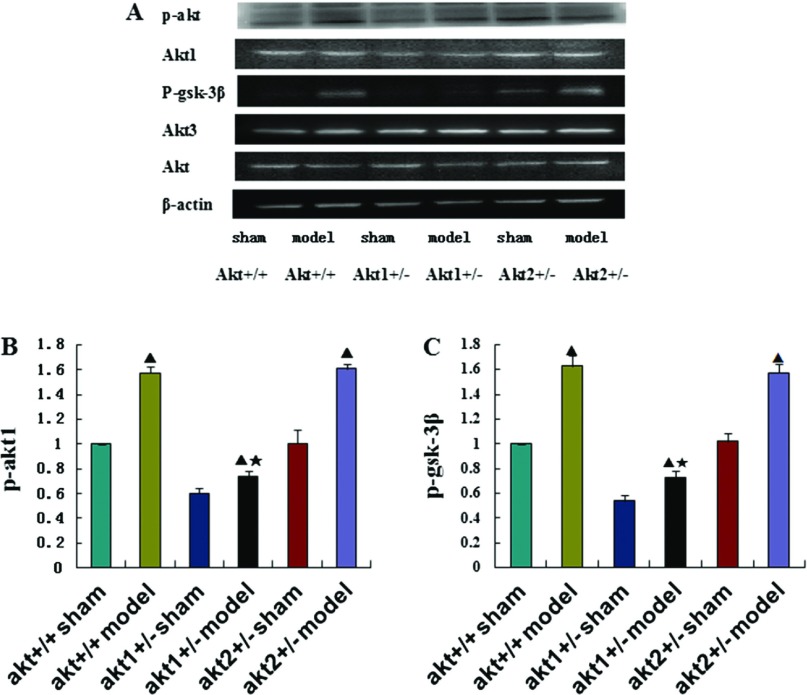
There was no significant difference in the expression of total Akt protein and Akt3 protein in any of the groups (p >0.05). However, p-Akt1 and p-GSK-3β protein expression was significantly higher in the DHLF model condition than in the sham condition in all three genotypes (p <0.05). The difference in p-Akt1 and p-GSK-3β protein expression between the control and model conditions was smaller in the Akt1 gene knockout mice than in the wild type and the Akt2 gene knockout mice, and this difference was statistically significant (p <0.05). However, the difference in p-Akt1 and p-GSK-3β protein expression between the sham and model conditions in the Akt2 gene knockout mice was not significantly different compared with wild type mice (p >0.05) (Figs. 1, 2). The Akt1 protein expression was lower in the Akt1 gene knockout mice than in the wild type mice and the Akt2 gene knockout mice, and this difference was statistically significant (p <0.05). There was no statistically significant difference between the Akt2 gene knockout mice and the wild type mice (p >0.05). Nor was there a statistically significant difference between the sham condition and the model condition among various kinds of genotypes (p >0.05) (Figs. 1A, 2A).
Fig. 1.
(A) Western blot analysis of p-Akt1, p-GSK-3β, Akt1, Akt3 and Akt protein expression in different treatment groups at 24 h after reperfusion. β-actin was used as an equal loading control. (B) and (C) Representative Western blot of p-Akt1 and p-GSK-3β in the brain tissue at 24 h post-reperfusion in Akt +/+ sham groups, Akt +/+ model groups, Akt1 +/– sham groups, Akt1 +/– model groups, Akt2 +/– sham groups, Akt2 +/– model groups respectively. ▲p <0.05, compared with the sham group in the same genotype. ★p <0.05, compared with the model group in different genotypes.
Fig. 2.
(A) Western blot analysis of p-Akt1, p-GSK-3β, Akt1, Akt3 and Akt protein expression in different treatment groups at 72 h after reperfusion. β-actin was used as an equal loading control. (B) and (C) Representative Western blot of p-Akt1 and p-GSK-3β in the brain tissue at 72 h post-reperfusion in Akt +/+ sham groups, Akt +/+ model groups, Akt1 +/– sham groups, Akt1 +/– model groups, Akt2 +/– sham groups, Akt2 +/– model groups respectively. ▲p <0.05, compared with the sham group in the same genotype. ★p <0.05, compared with the model group in different genotypes.
Real time PCR results
The expressions of Akt1 mRNA in the model condition were higher than in the sham condition in each of the genotypes, and they were significantly lower in the Akt1 gene knockout mice than in the Akt2 knockout gene and wild type mice (p <0.05). Their expression was not significantly different between the Akt2 gene knockout mice and the wild type mice in model groups and the result was not significantly different in sham groups (p >0.05) (Table 1). The expressions of Akt3 mRNA in the model condition were higher than in the sham condition in each of the genotypes. Their expression was not significantly different in model groups and the result was not significantly different in sham groups (p >0.05) (Table 1). The expression of Akt2 mRNA was not statistically different between the model condition and the sham condition in each of the genotypes (p >0.05). However, the expression of Akt2 mRNA was significantly lower in the Akt2 gene knockout mice than in the Akt1 gene knockout and the wild type mice (p <0.05) (Table 1).
Table 1.
The Akt1 mRNA, Akt2 mRNA and Akt3 mRNA expression by real time PCR in different treatment group at 24 h and 72 h after reperfusion
| Group Akt1 +/– | Akt1 mRNA | Akt2 mRNA | Akt3 mRNA | |||
|---|---|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | |
| Sham | 0.36 ± 0.03 | 0.34 ± 0.03 | 0.98 ± 0.04 | 1.00 ± 0.01 | 1.02 ± 0.05 | 1.04 ± 0.07 |
| Model | 0.73 ± 0.04▲★ | 0.59 ± 0.03▲★ | 0.98 ± 0.03 | 1.03 ± 0.03 | 1.42 ± 0.07▲★ | 1.58 ± 0.04▲★ |
| Group Akt2 +/– | ||||||
| Sham | 1.02 ± 0.07 | 0.99 ± 0.01 | 0.65 ± 0.04 | 0.64 ± 0.03 | 1.01 ± 0.03 | 0.96 ± 0.05 |
| Model | 1.60 ± 0.06▲★ | 1.56 ± 0.05▲★ | 0.64 ± 0.04 | 0.64 ± 0.05 | 1.46 ± 0.07▲★ | 1.55 ± 0.09▲★ |
▲p <0.05, compared with sham group in the same genotype. ★p <0.05, compared with sham group in the different genotype. PCR: polymerase chain reaction
TTC staining results
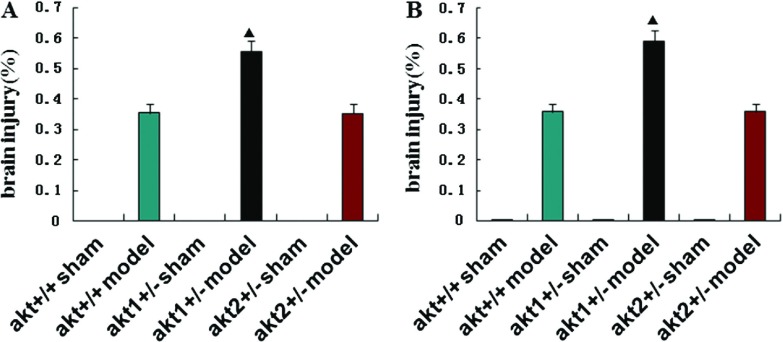
Brain damage was greater in model group compared with sham group in each of the genotypes at 24 h and 72 h after reperfusion (p <0.05). Brain damage was significantly greater in the Akt1 knockout gene mice compared with Akt2 knockout gene and wild type mice at 24 h and 72 h after reperfusion (p <0.05). There was no statistically significant difference in brain damage between the Akt2 knockout gene and wild type mice (p >0.05) (Table 2, Fig. 3).
Table 2.
The percentage of brain injury in different treatment group at 24 h after reperfusion
| Group | Akt +/+ | Akt1 +/– | Akt2 +/– | |||
|---|---|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | |
| Sham | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Model | 0.35 ± 0.03▲ | 0.36 ± 0.03▲ | 0.56 ± 0.03▲★ | 0.59 ± 0.04▲★ | 0.35 ± 0.03▲ | 0.36 ± 0.02▲ |
▲p <0.05, compared with sham group in the same genotype. ★p <0.05, compared with model group in the different genotype.
Fig. 3.
(A) and (B) TTC staining analysis of brain injury in different treatment groups at 24 h and 72 h after reperfusion in Akt +/+ sham groups, Akt +/+ model groups, Akt1 +/– sham groups, Akt1 +/– model groups, Akt2 +/– sham groups, Akt2 +/– model groups respectively. ▲p <0.05, compared with the sham group in the same genotype. ★p <0.05, compared with the model group in different genotypes.
Discussion
The current experiment shows that the expression of Akt1 protein in the DHLF model condition was significantly higher than in the control condition in each genotype. The Akt1 protein expression was lower in the Akt1 gene knockout mice than in the wild type mice and the Akt2 gene knockout mice, and this difference was statistically significant (p <0.05). There was no statistically significant difference between the Akt2 gene knockout mice and the wild type mice in model groups and in sham groups (p >0.05). Akt1 gene knockout maybe affect the expression of akt1 protein. Akt3 protein expression did not differ significantly (Figs. 1, 2). The experiment indicates that the DHLF model affects Akt1 protein expression, and may have a smaller influence on Akt3 protein expression.
The p-Akt1 and p-GSK 3β protein expression in the model condition were significantly higher than in the sham condition at 24 h and 72 h after reperfusion in each genotype. The p-Akt1 and p-GSK-3β protein expression in Akt1 gene knockout mice was significantly lower than the in wild type and Akt2 gene knockout mice. However, the p-Akt1 and p-GSK-3β protein expression in the Akt2 gene knockout mice was not significantly different from the wild type mice (Figs. 1, 2). This indicates that the DHLF model increases p-Akt1 and p-GSK-3β protein expression in mice. The DHLF model may activate the PI3K/Akt signal pathway that makes the Akt1 protein, and this activation would increase protein expression. Akt1 protein or its activated form could alter the GSK-3β phosphorylation level, which would be the main active factor. The finding that Akt3 protein did not change significantly suggests that this process may have nothing to do with the Akt3 protein or its activated form. The brain damage in the Akt1 gene knockout mice was significantly greater than in Akt2 gene knockout mice and wild type mice, but there was no significant difference in brain damage between the Akt2 gene knockout and the wild type mice (Table 2, Fig. 3). This indicates that Akt1 protein phosphorylation may lead to GSK-3β phosphorylation, which plays a role in protecting the brain of mice. Combined with our team’s previous experimental results that indicate the DHLF model has cerebral protective effects, we draw the conclusion that the DHLF model plays a role in brain protection, and its mechanism of action may be the activation of the PI3K/Akt signaling pathway. It increases the phosphorylation of the Akt1 protein, which then alters the phosphorylation of GSK-3β and leads to further biological effects. The research indicates that injury from ischemia-reperfusion may be related to glycogen synthesis kinase-3 (GSK-3) and the mitochondrial permeability transition pore (MPTP). Intrinsic apoptosis is regulated by mitochondria, which integrate lethal and pro-survival signals, to eventually reach a decision on the cell’s fate.25) If the death sentence is pronounced, mitochondrial membrane permeabilization takes place and cells trespass the frontier between life and death, the “point-of-no-return” in the cascade of events that delineates this lethal routine. In some settings, matrix metalloproteinase (MMP) results from protein-permeable pores formed across the outer mitochondrial membrane by proapoptotic members of the Bcl-2 protein family.26) Such pores are responsible for the early release of IMSproteins, followed by blockade of the respiratory chain, reactive oxygen species (ROS) overgeneration and functional breakdown of mitochondria.27) Currently, however, many scholars find the relationship between the GSK-3β with MPTP and MPTP in mitochondria-related cell death to be debatable.28)
In the current experiment, the expressions of Akt1 mRNA and Akt3 mRNA in the DHLF model condition were significantly higher than in the sham condition in each of the genotypes, and they were significantly lower in the Akt1 knockout gene mice than in the Akt2 knockout gene and wild type mice. Moreover, there was no significant difference between the Akt2 gene knockout mice and the wild type mice; nor was the expression of Akt2 mRNA significantly differed between the model condition and the sham condition. Finally, the expressions of Akt1 mRNA and Akt3 mRNA in the Akt2 gene knockout mice were significantly lower than in the Akt1 knockout gene and wild type mice. This suggests that the Akt1 protein changes with the change in the Akt1 mRNA. As Akt2 protein changes were not tested in the experiment, the relationship between Akt2 protein changes and Akt2 mRNA changes still need to be explored. The finding that Akt3 protein changes with the change of the Akt3 mRNA is inconsistent. This indicates that Akt3 protein may play a role regulating the process of transcription, and this specific process still needs further explored.
Disclosure Statement
All authors do not have any possible conflicts of interest.
Acknowledgment
Funding: Natural Science Foundation of China (81370279).
References
- 1).Checchia PA, Bronicki RA, Muenzer JT, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children—a randomized trial. J Thorac Cardiovasc Surg 2013; 146: 530-6. [DOI] [PubMed] [Google Scholar]
- 2).Inafuku H, Kuniyoshi Y, Yamashiro S, et al. Determination of oxidative stress and cardiac dysfunction after ischemia/reperfusion injury in isolated rat hearts. Ann Thorac Cardiovasc Surg 2013; 19: 186-94. [DOI] [PubMed] [Google Scholar]
- 3).Gaynor JW, Kim DS, Arrington CB, et al. Validation of association of the apolipoprotein E ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg 2014; 148: 2560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Holinski S, Claus B, Alaaraj N, et al. Cerebroprotective effect of piracetam in patients undergoing open heart surgery. Ann Thorac Cardiovasc Surg 2011; 17: 137-42. [DOI] [PubMed] [Google Scholar]
- 5).Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 2004; 61: 448-60. [DOI] [PubMed] [Google Scholar]
- 6).Bell MT, Puskas F, Bennett DT, et al. Dexmedetomidine, an a-2a adrenergic agonist, promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion. J Thorac Cardiovasc Surg 2014; 147: 500-6. [DOI] [PubMed] [Google Scholar]
- 7).Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans 2007; 35: 231-5. [DOI] [PubMed] [Google Scholar]
- 8).Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal 2009; 21: 470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets 2008; 8: 19-26. [DOI] [PubMed] [Google Scholar]
- 10).Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 2005; 9: 59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Le Page C, Koumakpayi IH, Alam-Fahmy M, et al. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer 2006; 94: 1906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 2009; 8: 2502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Chen WS, Xu PZ, Gottlob K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 2001; 15: 2203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 2003; 112: 197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol 2006; 16: 461-6. [DOI] [PubMed] [Google Scholar]
- 16).Tschopp O, Yang ZZ, Brodbeck D, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 2005; 132: 2943-54. [DOI] [PubMed] [Google Scholar]
- 17).Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 1980; 107: 519-27. [PubMed] [Google Scholar]
- 18).Wada A. Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J Pharmacol Sci 2009; 110: 14-28. [DOI] [PubMed] [Google Scholar]
- 19).Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 2004; 113: 1535-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Ma ZF, Mo XM, Yang ZZ, et al. Construction of Akt1+/– transgenic animal model of deep hypothermic low flow andphenotype analysis. Jiangsu Medical Journal 2010; 36: 909-12. (in Chinese) [Google Scholar]
- 21).Ma ZF, Mo XM, Yang ZZ, et al. Phenotype and molecular mechanism analysis of Akt1+/– mice undergoing deep hypothermia low flow. Chinese Journal of Experimental Surgery 2010; 27: 933-5. (in Chinese) [Google Scholar]
- 22).He X, Mo X, Gu Q, et al. Effect of diazoxide on oxygen free radicals and cell apoptosis in brain tissue after deep hypothermia cerebral ischemia reperfusion injury in young rats. Chinese Journal of Surgery 2010; 48: 142-5. (in Chinese) [PubMed] [Google Scholar]
- 23).Kim CS, Park JB, Kim KJ, et al. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin 2002; 23: 1152-6. [PubMed] [Google Scholar]
- 24).Hoyte LC, Papadakis M, Barber PA, et al. Improved regional cerebral blood flow is important for the protection seen in a mouse model of late phase ischemic preconditioning. Brain Res 2006; 1121: 231-7. [DOI] [PubMed] [Google Scholar]
- 25).Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol 1998; 8: 267-71. [DOI] [PubMed] [Google Scholar]
- 26).Lalier L, Cartron PF, Juin P, et al. Bax activation and mitochondrial insertion during apoptosis. Apoptosis 2007; 12: 887-96. [DOI] [PubMed] [Google Scholar]
- 27).Tajeddine N, Galluzzi L, Kepp O, et al. Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene 2008; 27: 4221-32. [DOI] [PubMed] [Google Scholar]
- 28).Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2010; 87: 406-23. [DOI] [PubMed] [Google Scholar]