Abstract
Background
The prediction of infection and its severity remains difficult in the critically ill. A novel, simple biomarker derived from five blood-cell derived parameters that characterize the innate immune response in routine blood samples, the intensive care infection score (ICIS), could be helpful in this respect. We therefore compared the predictive value of the ICIS with that of the white blood cell count (WBC), C-reactive protein (CRP) and procalcitonin (PCT) for infection and its severity in critically ill patients.
Methods
We performed a multicenter, cluster-randomized, crossover study in critically ill patients between January 2013 and September 2014. Patients with a suspected infection for which blood cultures were taken by the attending intensivist were included. Blood was taken at the same time for WBC, ICIS, CRP and PCT measurements in the control study periods. Results of imaging and cultures were collected. Patients were divided into groups of increasing likelihood of infection and invasiveness: group 1 without infection or with possible infection irrespective of cultures, group 2 with probable or microbiologically proven local infection without blood stream infection (BSI) and group 3 with BSI irrespective of local infection. Septic shock was assessed.
Results
In total, 301 patients were enrolled. CRP, PCT and ICIS were higher in groups 2 and 3 than group 1. The area under the receiver operating characteristic curve (AUROC) for the prediction of infection was 0.70 for CRP, 0.71 for PCT and 0.73 for ICIS (P < 0.001). For the prediction of septic shock the AUROC was 0.73 for CRP, 0.85 for PCT and 0.76 for ICIS. These AUROC did not differ from each other.
Conclusion
The data suggest that the ICIS is potentially useful for the prediction of infection and its severity in critically ill patients, non-inferiorly to CRP and PCT. In contrast to CRP and PCT, the ICIS can be determined routinely without extra blood sampling and lower costs, yielding results within 15 minutes.
Trial registration
ClinicalTrials.gov identifier: ID NCT01847079. Registered on 24 April 2013.
Keyword: Critically ill, Infection, Septic shock, Biomarkers, PCT, ICIS
Background
Infection is probable or can be proven in approximately 50 % of critically ill patients with suspected infection [1, 2]. This could lead potentially to overtreatment with empiric antibiotics [1, 2]. Moreover, infection diagnostics are often delayed because it takes 48 h at the minimum for cultures to become positive and thereby to prove a clinically suspected infection. Hence, there is a continuing need for fast and accurate biomarkers of infection, which may help to predict infection, its invasiveness and severity, and may guide empiric antibiotic treatment in the future [3]. We believe that prediction of infection is more helpful in patient management than prediction of sepsis, because the majority of critically ill patients have two or more criteria of the systemic inflammatory response syndrome (SIRS) as criteria for sepsis, irrespective of infection.
Commonly applied biomarkers include C-reactive protein (CRP) and procalcitonin (PCT), but predictive values vary among studies [3–11]. A new biomarker is the intensive care infection score (ICIS), which is composed of five blood-cell-derived parameters characterizing the early innate immune response and is routinely obtainable in blood samples sent to the laboratory for cell counts. The ICIS has been retrospectively evaluated in two pilot studies of 70 and 172 patients, respectively, suggesting it has potential predictive value for infection [12, 13].
We therefore performed a prospective study on the predictive value of ICIS for probable or proven infection in critically ill patients with suspected infection, and compared its performance with that of the white blood cell count (WBC), CRP and PCT levels. We hypothesized that in the critically ill patient with suspected infection the diagnostic accuracy of the simply obtainable ICIS is at least equivalent in this respect to WBC, CRP and PCT, without requiring extra blood sampling.
Methods
Study design and patients
The ICIS study is an add-on non-interventional study of patients who had been enrolled into a prospective, cluster-randomized, crossover trial, involving both intensive care units (ICUs) of the Erasmus Medical Center Rotterdam and both ICUs of the Maasstad hospital Rotterdam. The ICUs were stratified and randomized by treatment regimen into a control group (standard of care) and an intervention group. In the intervention arm, blood culturing for a suspected infection was guided by PCT measurements. The acronym for PCT-guided blood culturing in the intensive care, ProBIC, was used for this study and results will be reported later. The trial was conducted between January 2013 and September 2014. The ICU of the Erasmus Medical Center is a tertiary care mixed medical-surgical ICU with approximately 2000 admissions per year. The ICU of the Maasstad hospital is a secondary care mixed medical-surgical ICU with 1200 admissions per year.
The trial was conducted in accordance with the ethical principles decreed by the Declaration of Helsinki and in compliance with International Conference on Harmonization Good Clinical Practice Guidelines. The institutional review board (IRB) or the independent medical ethical committee at each of the investigational centers (Medisch Ethische commissie Maasstad ziekenhuis, Rotterdam, Nederland and Medisch Ethische commissie Erasmus Medisch Centrum, Rotterdam, Nederland) reviewed and approved the protocol, amendments and informed consent document. The medical ethical committee of the Erasmus Medical Center finally approved the study (MEC 2011-505). The trial was registered at ClinicalTrial.gov (protocol ID NCT01847079) on 24 April 2013. All patients or their proxy provided written informed consent prior to study inclusion, at ICU admission.
Inclusion criteria were age above 18 and below 80 years and the clinical suspicion of infection, for which the attending intensivist established a medical need for blood culture. Suspicion of infection included but was not limited to increased body temperature above 38.3 °C (tympanic temperature), chills, progressive leukocytosis, increased CRP, increasing consolidation on chest radiography or other imaging of potential infection sources. It was possible for each patient to be included more than once, but in the current study we only analyzed the first time that blood was sampled for culture. Patients were excluded if they were pregnant, had neutropenia (defined as leukocyte count less than 0.5 × 109/L), used immunosuppressive or immunostimulatory therapy, or had a predetermined illness with death expected within 24 h. Patients were not included if blood cultures were performed as part of a standard protocol (such as patients with veno-venous or veno-arterial extracorporeal membrane oxygenation (ECMO)) or were performed to check the effectiveness of treatment (such as in endocarditis), unless the blood culture was done because of suspicion of infection. The ICUs switched the allocated regimen every 3 months, so that there were six 3-month episodes of standard care in which 774 patients were eligible for inclusion, and 473 patients were excluded (5 patients who were ≤18 years of age; 63 with neutropenia (<0.5 × 104/L); 35 with uncontrolled malignancy; 256 on immunosuppressive medication; 22 who were expected to die within 24 h; and 92 without informed consent). Data for the ICIS study were thus collected in 301 patients in the control arm (six 3-month episodes) of the ProBIC study.
Study protocol, data collection and assays
Baseline demographic data and clinical variables were recorded on the day of inclusion, and included age, sex, comorbidity, reasons for admission, use of antibiotics including selective decontamination of the digestive tract (SDD), antifungal treatment, steroids, immunosuppressive medication, immune status and recent surgery. The treatment received during ICU stay was also recorded and included mechanical ventilation, renal replacement therapy, total parenteral nutrition, arterial and central venous catheters, and the use of vasopressor or inotropic medication. The acute physiology and chronic health evaluation II (APACHE II) and the sequential organ failure assessment (SOFA) score were recorded at admission. The length of ICU and hospital stay and vital outcomes were recorded for up to 90 days after inclusion.
At the same time that blood was taken for culture, blood samples were taken for determination of WBC, CRP, PCT, and ICIS (day 0). Blood for similar measurements (except for PCT) was taken in the morning on the two following days (days 1 and 2). Treating physicians and investigators were blinded to the PCT and ICIS measurement results. Also the outcome adjudicators that decided presence or absence of infection were blinded to the biomarker results. Two sets of blood cultures were taken and directly sent to the department of medical microbiology. The set taken for blood culture consisted of one aerobic and one anaerobic bottle (BD Bactec™, Franklin Lakes, NJ, USA), which contain resin to enhance recovery of organisms. The samples were incubated for a 7-day period in an automatic analyzer (BD Bactec™) that automatically demonstrates the time to positive blood culture in the case of positive bacterial or fungal growth. Gram strains were performed, and the organisms were cultured on agar plates after identification of growth using the VITEK® 2 (Biomerieux, Marcy l’Etoile, France).
Blood for the WBC and ICIS measurement was obtained in a K3EDTA tube. Both the WBC and ICIS parameters were measured on a modified fluorescence flow hematology analyzer with fully automated gating (Sysmex, Kobe, Japan) [14]. The ICIS was measured promptly after collection but within a maximum of 24 h. The ICIS score is composed of five blood-cell-derived parameters that characterize the innate immune response [15–19]. The five parameters include the mean fluorescence intensity of mature (segmented) neutrophils, the difference in hemoglobin concentration between newly formed and mature red blood cells, the total segmented neutrophil count, the antibody secreting lymphocytes, and the accurate immature granulocytes count, as previously described [12]. Each parameter is available from a standard routine method and can be measured within 1 minute without sample preparation on a modified fluorescence flow hematology analyzer with fully automated gating (Symex) [12]. The methodology is based on routine hematology fluorescence flow cytometry using different fluorescence reagents for mainly nucleic acids, and specifically designed blood cell membrane surfactant reagents generating information about cell shape and the formation of bioactive lipids from cell membranes [12]. Side and forward scatter light are used to determine the intracellular structure and size of blood cells [12]. By adding all weighting values for all five parameter components, the maximum possible ICIS is 20. Serum CRP (turbidimetric assay) and PCT (electrochemiluminescence BRAHMS immunoassay) measurements were routinely performed using a Cobas 8000 platform (Roche, Almere, Netherlands). Blood for PCT measurement was sampled in a z serum clot activator tube.
Definitions
After completion of the study, the investigators decided whether an infection was present from days 0–2, on the basis of the available imaging and culture results. The outcome adjudicators were blinded to all biomarkers. Source and likelihood of infection were based on criteria defined at the International Sepsis Forum Consensus Conference [20]. Culture results were analyzed within a 48-h window from before and after taking blood cultures. The causative microorganisms were recorded. BSI was defined as a positive blood culture with a recognized pathogen except for skin contaminants [20, 21]. In the case of skin contaminants, BSI was identified if at least two blood cultures drawn on separate locations were positive [20, 21]. Patients were divided into groups according to increasing likelihood of infection and invasiveness of associated microorganisms that was suggestive of increasing severity: group 1 without infection or with possible infection irrespective of cultures; group 2 with probable (irrespective of cultures) or proven local infection (with positive cultures of a causative microorganism) without BSI; and group 3 with BSI irrespective of local infection. SIRS was defined as two or more of the following criteria: (1) body temperature >38 °C or <36 °C; (2) WBC (>10,000/μL), leukopenia (<4,000/μL), or >10 % bands; (3) heart rate >90 beats/minute; and (4) respiratory rate >20 breaths/minute or mechanical ventilation, for values at day 0. When SIRS and a probable/proven infection (groups 2 or 3) were present, patients were classified as having sepsis. Shock was defined as acute circulatory failure characterized by persistent systolic arterial pressure <90 mm Hg or mean arterial pressure (MAP) <70 mm Hg for at least 1 h despite adequate fluid resuscitation or requirement of vasopressor support to maintain MAP, at day 0. In the presence of sepsis, shock was defined as septic shock.
Statistical analysis
This was performed using SPSS version 23 (SPSS inc., Chicago IL, USA) and using R package. Data are expressed as median (interquartile range) or as number of patients (percentage) where appropriate. Most data were distributed non-normally (Kolmogorov-Smirnov test P < 0.05). Group (>2) differences were evaluated using the Kruskal-Wallis test or chi-square (X2) test, for continuous and categorical data, respectively. The Mann-Whitney U test and Fisher exact test were used to compare two groups. To evaluate predictive values we calculated the areas under the receiver operating characteristic curves (AUROC) for day 0 values. For the predictive values of sepsis and septic shock we used the values for day 0. We consider an AUROC >0.70 as clinically relevant [22]. The optimum cutoff value was calculated on the basis of the highest sensitivity and specificity combined (Youden index). Positive and negative predictive values were calculated. To correct for multiple testing we set the level of statistical evidence at P ≤ 0.01. Exact P values >0.001 are given.
Results
Patient characteristics
Table 1 describes the baseline characteristics of the 301 patients enrolled: 149 patients (group 1) had no infection and 152 patients (groups 2 + 3) had a probable or proven infection. Patients with a probable or proven infection were older and more often had a history of cancer, cardiac disease or gastrointestinal problems. Mechanical ventilation or renal replacement therapy was more often used in patients with a probable or proven infection. All patients with a probable or proven infection were on antibiotics. No difference was seen in 28-day or 90-day mortality or in the length of ICU or hospital stay.
Table 1.
Baseline demographic and clinical characteristics
| Group 1 | Groups 2 + 3 | P | |
|---|---|---|---|
| (n = 149) | (n = 152) | ||
| Age (years) | 57 (24) | 62 (19) | 0.01 |
| Gender (male) | 100 (67) | 105 (69) | 0.72 |
| APACHE II score | 22 (10) | 22 (8) | 0.92 |
| APACHE IV score | 63 (38) | 60 (34) | 0.49 |
| SOFA score | 7 (7) | 8 (6) | 0.06 |
| Comorbidity | |||
| Neurologic | 39 (26) | 41 (27) | 0.88 |
| Cardiac | 40 (27) | 58 (38) | 0.04 |
| Pulmonary | 28 (19) | 38 (25) | 0.25 |
| Gastrointestinal | 39 (26) | 55 (36) | 0.05 |
| Renal | 15 (10) | 24 (16) | 0.14 |
| DM II | 22 (15) | 33 (22) | 0.13 |
| Cancer | 22 (15) | 46 (30) | 0.002 |
| Autoimmune | 7 (5) | 8 (5) | 0.97 |
| Reasons for ICU admission | <0.001 | ||
| Suspected infection | 23 (15) | 69 (45) | |
| Respiratory failure | 24 (16) | 33 (22) | |
| Renal failure | 0 (0) | 1 (1) | |
| Liver failure | 5 (3) | 3 (2) | |
| Neurology | 31 (21) | 8 (5) | |
| CPR | 9 (6) | 5 (3) | |
| Shock | 10 (7) | 3 (2) | |
| Trauma | 13 (9) | 5 (3) | |
| Postoperative | 34 (23) | 25 (17) | |
| Treatment on ICU | |||
| Antibiotics | 140 (94) | 152 (100) | 0.02 |
| Norepinephrine | 109 (73) | 128 (84) | 0.03 |
| Dobutamine | 18 (12) | 17 (11) | 0.80 |
| TPN | 39 (26) | 50 (33) | 0.20 |
| Mechanical ventilation | 133 (89) | 125 (82) | 0.06 |
| Renal replacement therapy | 15 (10) | 52 (34) | <0.001 |
| Length of ICU stay (days) | 9 (17) | 11 (17) | 0.49 |
| Length of hospital stay (days) | 22 (34) | 26 (36) | 0.13 |
| Mortality day 28 | 42 (28) | 58 (38) | 0.09 |
| Mortality day 90 | 98 (36) | 68 (45) | 0.13 |
Numbers (percentage) or median (interquartile range), where appropriate. Abbreviations: APACHE II acute physiology and chronic health evaluation II, CPR cardiopulmonary resuscitation, DM II diabetes mellitus type II, ECMO extracorporeal membrane oxygenation, SOFA sequential organ failure assessment score, TPN total parenteral nutrition
Source of infection and microbial species
The abdomen and lungs were the most frequent source of infection (Table 2). Gram-positive pathogens were mostly cultured, followed by Gram-negative pathogens, fungi and viruses (Table 2).
Table 2.
Infection characteristics
| Group 1 | Group 2 | Group 3 | P | |
|---|---|---|---|---|
| (n = 149) | (n = 91) | (n = 61) | ||
| Source of infection | 0.01 | |||
| Pulmonary | - | 44 (48) | 15 (25) | |
| Abdominal | - | 30 (33) | 29 (47) | |
| Urogenital | - | 8 (9) | 2 (3) | |
| Neurologic | - | 2 (2) | 2 (3) | |
| Soft tissue/bones | - | 7 (8) | 10 (17) | |
| Blood and catheter | - | 0 | 3 (5) | |
| Gram strain | 0.01 | |||
| Gram-negative | - | 30 (33) | 16 (26) | |
| Gram-positive | - | 27 (30) | 36 (59) | |
| Type of microorganism | 0.15 | |||
| Staphylococci | - | 15 (16) | 17 (28) | |
| Streptococci | - | 12 (13) | 19 (31) | |
| Enterobacteriaceae | - | 26 (29) | 15 (25) | |
| Pseudomonas | - | 4 (4) | 1 (2) | |
| Fungi | - | 12 (13) | 6 (10) | |
| Viral | - | 5 (5) | 3 (5) | |
| Biomarkers | ||||
| SIRS | 146 (98) | 90 (99) | 58 (95) | 0.94 |
| Septic shock | - | 12 (13) | 9 (15) | 0.78 |
| Temperature (°C) | 38.1 (1.5) | 38.3 (1.7) | 38.0 (1.5) | 0.94 |
| Heart rate (beats/minute) | 105 (29) | 109 (37) | 112 (39) | 0.15 |
| Respiratory rate (breaths/minute) | 28 (13) | 29 (17) | 29 (17) | 0.61 |
| WBC day 0 (109/L) | 12.4 (7.8) | 14.4 (12.9) | 13.9 (12.2) | 0.63 |
| WBC day 1 (109/L) | 11.7 (8.1) | 13.9 (13.1) | 13.4 (11.3) | 0.47 |
| WBC day 2 (109/L) | 12.1 (7.2) | 14.4 (9.8) | 14.8 (15.0) | 0.19 |
| CRP day 0 (mg/L) | 84 (109) | 163 (156) | 167 (161) | <0.001 |
| CRP day 1 (mg/L) | 88 (131) | 156 (156) | 197 (154) | <0.001 |
| CRP day 2 (mg/L) | 82 (141) | 131 (136) | 180 (172) | <0.001 |
| PCT day 0 (μg/L) | 0.65 (2.30) | 2.71 (9.88) | 4.13 (38.0) | <0.001 |
| ICIS day 0 | 3 (3) | 6 (5) | 6 (5) | <0.001 |
| ICIS day 1 | 3 (3) | 6 (4) | 6 (4) | <0.001 |
| ICIS day 2 | 4 (4) | 6 (4) | 6 (4) | <0.001 |
Numbers (percentage) or median (interquartile range), where appropriate. Group 1: no infection; Group 2: local infection without blood stream infection; Group3: blood stream infection. Abbreviations: CRP C-reactive protein, ICIS intensive care infection score, PCT procalcitonin, SIRS systemic inflammatory response syndrome, WBC white blood cells
Biomarkers
Table 2 shows the infection markers according to invasiveness of infection. Most patients had SIRS on day 0, so that the patients with infection in groups 2 and 3 had mostly sepsis. CRP, PCT and ICIS were increased on days 0–2 in patients with infection as compared to those without infection. In contrast to PCT, there was no difference in CRP and ICIS between groups 2 and 3. The CRP, PCT and ICIS were increased in patients with septic shock (Table 3).
Table 3.
Septic shock
| No (n = 280) | Yes (n = 21) | P | |
|---|---|---|---|
| Temperature (°C) | 38.2 (1.5) | 38.2 (1.6) | 0.75 |
| Heart rate (beats/minute) | 108 (33) | 119 (59) | 0.04 |
| Respiratory rate (breaths/minute) | 28 (15) | 29 (13) | 0.85 |
| WBC day 0 (109/L) | 12.8 (9.5) | 12.5 (17.6) | 0.68 |
| WBC day 1 (109/L) | 12.4 (9.5) | 14.1 (20.4) | 0.60 |
| WBC day 2 (109/L) | 12.5 (9.4) | 14.6 (14.4) | 0.41 |
| CRP day 0 (mg/L) | 107 (144) | 234 (182) | <0.001 |
| CRP day 1 (mg/L) | 124 (147) | 327 (160) | <0.001 |
| CRP day 2 (mg/L) | 107 (144) | 244 (205) | <0.001 |
| PCT day 0 (μg/L) | 1.15 (6.1) | 32.2 (94.0) | <0.001 |
| ICIS day 0 | 4 (5) | 9 (6) | <0.001 |
| ICIS day 1 | 4 (4) | 8 (7) | <0.001 |
| ICIS day 2 | 5 (5) | 7 (6) | 0.03 |
Median (interquartile range). Abbreviations: CRP C-reactive protein, ICIS intensive care infection score, PCT procalcitonin, WBC white blood cells
Predictive values
The AUROC for the prediction of infection (groups 2 + 3 vs group 1) on day 0 was similar for CRP, PCT and ICIS (Table 4, Fig. 1). At a cutoff ≥7, the positive predictive value of ICIS was >80 % and at a cutoff ≤1 the negative predictive value of ICIS was >80 %. Otherwise, the AUROC for ICIS did not differ from that of any other biomarkers, including PCT, expect for that of WBC (P < 0.001). The highest AUROC for the prediction of septic shock was for PCT (AUROC 0.85, P < 0.001), but this was not significantly different to the AUROC for ICIS (AUROC 0.76, P < 0.001), whereas the AUROC for CRP was 0.73 (P < 0.001) and the AUROC for WBC was 0.53 (P = 0.68) (Fig. 2).
Table 4.
Receiver operating characteristic curve analysis to determine the optimum cutoff value of the different biomarkers on day 0 for the prediction of infection (groups 2 + 3)
| Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Biomarkers | AUROC (95 % CI) | P | Cutoff | Sensitivity | Specificity | PPV | NPV |
| WBC (109/L) | 0.53 (0.46, 0.60) | 0.38 | 12.9 | 0.54 | 0.54 | 0.55 | 0.54 |
| CRP (mg/L) | 0.70 (0.64, 0.76) | <0.001 | 111 | 0.65 | 0.64 | 0.65 | 0.64 |
| PCT (μg/L) | 0.71 (0.66, 0.77) | <0.001 | 1.41 | 0.65 | 0.66 | 0.66 | 0.65 |
| ICIS | 0.73 (0.67, 0.79) | <0.001 | 5 | 0.66 | 0.71 | 0.70 | 0.67 |
Abbreviations: AUROC area under the receiver operating characteristic curve, CI confidence interval, CRP C-reactive protein, ICIS intensive care infection score, PCT procalcitonin, PPV positive predictive value, NPV negative predictive value, WBC white blood cells. The AUROC for ICIS differed from that for WBC (P < 0.001)
Fig. 1.
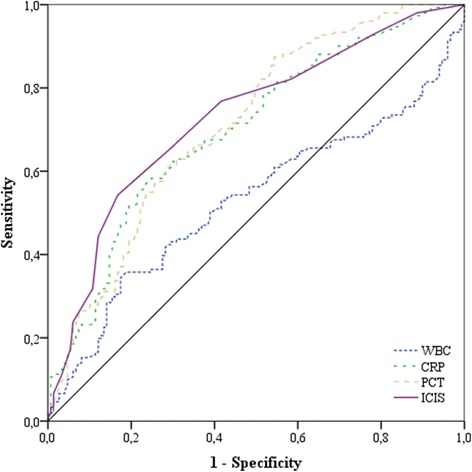
Area under the receiver operating characteristic curve for the four biomarkers for the prediction of infection: for white blood cell count (WBC) 0.53, for C-reactive protein (CRP) 0.70, for procalcitonin (PCT) 0.71, and for intensive care infection score (ICIS) 0.73
Fig. 2.
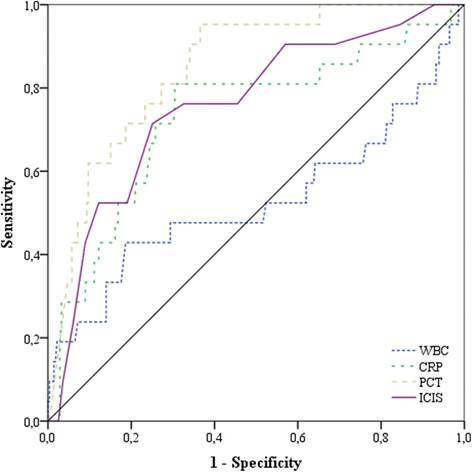
Area under the receiver operating characteristic curve for the four biomarkers for the prediction of septic shock: for white blood cell count (WBC) 0.53, for C-reactive protein (CRP) 0.73, for procalcitonin (PCT) 0.85 and for intensive care infection score (ICIS) 0.76
Discussion
This study evaluated the predictive values of ICIS to discriminate between non-infectious systemic inflammation and infection (mostly sepsis) in critically ill patients with a suspicion of infection. The data suggest that ICIS is a useful marker to predict probable or proven infection and its severity and is non-inferior in this respect to CRP and PCT.
In the current study the frequency of probable or proven infection was 56 % of patients when an infection was suspected, which is comparable with the reported frequency of 51–58 % in large studies on the epidemiology of sepsis in the ICU [23]. The lung and abdomen were the most common origin of sepsis, followed by infections of soft tissues, as described before [24]. A large recently performed study showed that Gram-negative bacteria were isolated in 62 % of patients with sepsis who had positive cultures, Gram-positive bacteria in 47 %, and fungi in 19 % [23]. The results are in contrast with our study, which suggests that Gram-positive isolates are most likely to cause infection. The difference can be explained by the fact that we use SDD in our ICUs, which is known to eliminate Gram-negative bacteria and fungi from the digestive tract [25]. Blood cultures are typically positive in approximately one third of the patients with sepsis, in line with the incidence of 20 % in this study [24]. The overall ICU and hospital mortality rates were 28 and 37 %, respectively. The results are comparable with the reported rates in a European multicenter study of critically ill patients with sepsis [26].
In the current study the predictive values of WBC and CRP for infection and sepsis are comparable with those identified in previous studies, in which a low AUROC of 0.55–0.66 for WBC (sensitivity 65–91 %; specificity 35–54 %) and an intermediate AUROC of 0.64–0.77 for CRP (sensitivity 82–100 %; specificity 40–64 %) was reported [4, 5, 10, 11]. Large reviews report an AUROC of 0.78–0.81 for PCT (sensitivity 42–100 %; specificity 48–100 %), comparable to our study [7, 9]. The reported predictive value of ICIS in this study is lower compared with two previous studies, in which AUROC of 0.79 (sensitivity 70 %; specificity 79 %) and 0.85 (sensitivity 80 %; specificity 75 %) were reported, respectively [12, 13]. Both studies investigated a relatively small number of patients or investigated postoperative critically ill patients only [12, 13]. They were pilot studies to define the cutoff values of ICIS as a marker of infection in critically ill patients and recommended determination of the suitability and effectiveness of this score in a prospective trial [12, 13].
Using ICIS has several advantages over using CRP or PCT. First, no extra blood needs to be taken since the ICIS can be measured from the same K3EDTA tube that is used for the WBC measurement, thereby allowing routine daily measurements. Second, lower costs are involved because the ICIS measurement is performed on the same machine used for a WBC measurement. The major limitation is that in our study the predictive values of biomarkers including ICIS was not very high. Nevertheless, a high ICIS increases the likelihood of infection when suspected and a low ICIS decreases it. This may help the clinician in ordering extra tests or starting empiric antibiotics. The predictive value of ICIS for infection and septic shock is comparable with that of the percentage of immature granulocytes as assessed in a relatively small study in critically ill patients [27]. Both the percentage of immature granulocytes and ICIS can be obtained routinely without extra blood sampling or cost, though the current study focused on the diagnostic accuracy of ICIS and not its feasibility or cost-effectiveness. For future use the ICIS is expected to prove more robust.
Conclusions
In conclusion, the present study suggests that ICIS is a novel and potentially useful predictor of infection and sepsis in critically ill patients with a suspected infection. The ICIS score can be collected routinely without extra blood sampling and with lower costs, yielding results within 15 minutes.
Key messages
The ICIS score is composed of five blood-cell-derived parameters that characterize the innate immune response
The ICIS score is elevated in patients with probable or proven infection in the critically ill, similar to CRP and PCT
The ICIS score is a novel and potentially useful predictor of infection and sepsis in critically ill patients
The ICIS score can be collected routinely without extra blood sampling and at a lower cost as compared to CRP and PCT determinations
Abbreviations
APACHE II, acute physiology and chronic health evaluation II; AUROC, area under the receiver operating characteristic curve; BSI, blood stream infection; CI, confidence interval; CPR, cardiopulmonary resuscitation; CRP, C-reactive protein; DM II, diabetes mellitus type II; ECMO, extracorporeal membrane oxygenation; ICIS, intensive care infection score; ICU, intensive care unit; IRB, institutional review board; MAP, mean arterial pressure; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value; SDD, selective decontamination of the digestive tract; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment score; TPN, total parenteral nutrition; WBC, white blood cells
Acknowledgements
We thank the staff of Sysmex Europe GmbH for an unrestricted research grant. We thank the staff of participating intensive care units for their support.
Authors’ contributions
PJG, ABJG, JL, and RdJ designed the study. PJG, JL, and ABJG performed the analyses. PJG, MM, JL, and SD collected the data. PJG and ABJG drafted the manuscript. All authors reviewed the manuscript. All Authors read and approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Circiumaru B, Baldock G, Cohen J. A prospective study of fever in the intensive care unit. Intensive Care Med. 1999;25:668–73. doi: 10.1007/s001340050928. [DOI] [PubMed] [Google Scholar]
- 2.Peres Bota D, Lopes Ferreira F, Mélot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med. 2004;30:811–6. doi: 10.1007/s00134-004-2166-z. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Beumier M. Diagnostic and prognostic markers in sepsis. Expert Rev Anti Infect Ther. 2013;11:265–75. doi: 10.1586/eri.13.9. [DOI] [PubMed] [Google Scholar]
- 4.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31:1737–41. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 5.Charles PE, Kus E, AHO S, Prrin S, Doise J-M, Olsson N-O, Blettery B, Quenot J-P. Serum procalcitonin for the early recognition of nosocomial infection in the critically patients: a preliminary report. BMC Infect Dis. 2009;9:49. [DOI] [PMC free article] [PubMed]
- 6.Cid J, Garcia-Pardo G, Aguinaco R, Sánchez R, LIorente A. Neutrophil CD64: diagnostic accuracy and prognostic value in patients presenting to the emergency department. Eur J Clin Microbiol Infect Dis. 2011;30:845–52. doi: 10.1007/s10096-011-1164-7. [DOI] [PubMed] [Google Scholar]
- 7.Kibe S, Adams K, Borlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66:i33–ii40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Donadello K, Schmit X. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin. 2011;27:241–51. doi: 10.1016/j.ccc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:426–35. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 10.Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence. 2014;5:154–60. doi: 10.4161/viru.27393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnacho-Montero J, Huici-Moreno MJ, Gutiérrer-Pizarraya A, López I, Márquez-Vacaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18:R116. doi: 10.1186/cc13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nierhaus A, Linssen J, Wichmann D, Braune S, Kluge S. Use of a weighted, automated analysis of the differential blood count to differentiate sepsis from non-infectious systemic inflammation: the intensive care infection score (ICIS) Inflamm Allergy Drug Targets. 2012;11:109–15. doi: 10.2174/187152812800392841. [DOI] [PubMed] [Google Scholar]
- 13.Weimann K, Zimmermann M, Spies CD, Wernecke KD, Vicherek O, Nachtigall I, et al. Intensive care infection score – a new approach to distinguish between infectious and noninfectious processes in intensive care and medicosurgical patients. J Int Med Res. 2015;43:435–51. doi: 10.1177/0300060514557711. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka C, Nagai T, Nakamura M, Yamauchi Y, Noguchi K, Takimoto Y, et al. Automated hematology analyzer XE-5000 – overview and basic performance. Sysmex J Int. 2007;17:1–6. [Google Scholar]
- 15.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–86. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 16.Franck S, Linssen J, Messinger M, Thomas L. Potential utility of Ret-Y in the diagnosis of iron-restricted erythropoiesis. Clin Chem. 2004;50:1240–2. doi: 10.1373/clinchem.2004.030254. [DOI] [PubMed] [Google Scholar]
- 17.Linssen J, Jennissen V, Hildmann J, Reisinger E, Schindler J, Malchau G, et al. Identification and quantification of high fluorescence-stained lymphocytes as antibody synthesizing/secreting cells using the automated routine hematology analyzer XE-2100. Cytometry B Clin Cytom. 2007;72:157–66. doi: 10.1002/cyto.b.20150. [DOI] [PubMed] [Google Scholar]
- 18.Bender L, Thaarup J, Varming K, Krarup H, Ellermann- Eriksen S, Ebbesen F. Early and late markers for the detection of early-onset neonatal sepsis. Dan Med Bull. 2008;55:219–23. [PubMed] [Google Scholar]
- 19.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–86. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 20.Calandra T, Cohen J. International sepsis forum definition of infection in the ICU consensus conference. Crit Care Med. 2005;33:1538–48. doi: 10.1097/01.CCM.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 21.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 22.Tape TG. Interpreting diagnostic tests. http://gim.unmc.edu/dxtests/. Accessed 14 April 2016.
- 23.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. J Am Med Ass. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 24.Angus DC, Van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 25.Bonten MJ, Kullberg BJ, Van Dalen R, Girbes AR, Hoepelman IM, Hustinx W, et al. Selective digestive decontamination in patients in intensive care. The Dutch working group on antibiotic policy. J Antimicrob Chemother. 2000;46:351–62. doi: 10.1093/jac/46.3.351. [DOI] [PubMed] [Google Scholar]
- 26.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 27.Van der Geest PJ, Mohseni M, Brouwer R, van der Hoven B, Steyerberg EW, Groeneveld AB. Immature granulocytes predict microbial infection and its adverse sequelae in the intensive care unit. J Crit Care. 2014;29:523–7. doi: 10.1016/j.jcrc.2014.03.033. [DOI] [PubMed] [Google Scholar]


