Abstract
The current study examined blood oxygen level dependent (BOLD) signal underlying racial differences in threat detection. During fMRI, participants determined whether pictures of Black or White individuals held weapons. They were instructed to make shoot responses when the picture showed armed individuals but don’t shoot responses to unarmed individuals, with the cost of not shooting armed individuals being greater than that of shooting unarmed individuals. Participants were faster to shoot armed Blacks than Whites, but faster in making don’t shoot responses to unarmed Whites than Blacks. Brain activity differed to armed versus unarmed targets depending on target race, suggesting different mechanisms underlying threat versus safety decisions. Anterior cingulate cortex was preferentially engaged for unarmed Whites than Blacks. Parietal and visual cortical regions exhibited greater activity for armed Blacks than Whites. Seed-based functional connectivity of the amygdala revealed greater coherence with parietal and visual cortices for armed Blacks than Whites. Furthermore, greater implicit Black-danger associations were associated with increased amygdala activation to armed Blacks, compared to armed Whites. Our results suggest that different neural mechanisms may underlie racial differences in responses to armed versus unarmed targets.
Keywords: racial bias, fMRI, anterior cingulate cortex, amygdala, visual cortex
Racial category is perceived quickly, often within a quarter of a second (Ito & Urland, 2003, 2005), and once perceived, can activate stereotypes and prejudices (e.g., Bargh, 1984, 1999; Devine, 1989; Dovidio, Evans, & Tyler, 1986). While overt acts of explicit racial bias have diminished in recent decades (Dovidio & Gaertner, 2000; Dovidio & Gaertner, 2004), behavioral studies indicate that White Americans nevertheless hold attitudes and engage in behaviors that discriminate against racial minorities (Dovidio & Gaertner, 2004; Dovidio, Gaertner, Kawakami & Hodson, 2002; Sears, Sidanius, & Bobo, 2000). Cultural beliefs associated with young Black males may particularly facilitate implicit judgments of threat (Payne, 2001; Payne, Lambert, & Jacoby, 2002; Eberhardt, Goff, Purdie, & Davies, 2004). For example, Correll, Park, Judd, and Wittenbrink (2002) had participants perform a computer-based first-person-shooter task (FPST) involving decisions to shoot or don’t shoot Black or White males who were either holding a gun or another object. Participants were quicker and more accurate in correctly shooting when the armed individual was Black rather than White, but faster and more accurate in not shooting when the unarmed person was White rather than Black. While stereotypes have been shown to relate to the magnitude of racial bias displayed during the FPST (Correll et al., 2002; Correll, Urland, & Ito, 2006), more proximal mechanisms explaining how these stereotypical associations translate into differential behavior towards Blacks and Whites are not fully understood.
The purpose of our study was to examine brain activity that correlated with racial differences in the FPST. We examined a number of different but not mutually exclusive putative cognitive processes that have been implicated in past fMRI studies of race perception and stereotyping. Of note, many prior studies employed relatively simple tasks and stimulus arrays. This has the advantage of minimizing influence from other factors and has resulted in the identification of several potential brain regions associated with stereotyping. At the same time, it is also critical to study situations in which race is but one of many cues affecting behavior to facilitate generalization to more complex real-world situations. Factors such as a multifaceted stimulus array, numerous cues that could influence behavior, and a generally high level of subject engagement make the FPST task well suited for this assessment. The requirement of a response to both armed and unarmed targets also allows us to examine race effects on both the detection of threat (indicated by making a shoot response to armed targets) and safety (indicated by making a don’t shoot response to unarmed targets).
One focus of our investigation was on areas associated with response conflict detection and/or performance monitoring. Responding to threat tends to be the prepotent response in the FPST (e.g., participants are typically faster and more accurate in decisions to shoot than don’t shoot), suggesting that response conflict is higher for unarmed than armed targets. Regions of the ACC are associated with response-related selection (Milham et al., 2001), conflict detection (Nieuwenhuis, Yeung, Van Den Wildenberg, & Ridderinkhof, 2003; Van Veen & Carter, 2002), and inhibition of prepotent responses (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Swainson et al., 2003), leading to the prediction of greater ACC activity to unarmed than armed targets in the FPST. Of particular interest in the present investigation is whether this response varies as a function of target race. Past studies investigating event-related potentials (ERPs) associated with conflict detection and thought to derive from the ACC have shown sensitivity to target race (Amodio et al., 2006, 2008; Bartholow et al., 2012). One study in particular recorded ERPs during the FPST, finding increased N200 responses -- thought to be generated in the ACC (Niewenhuis et al., 2003) and reflective of conflict detection (Kopp et al., 1996; Liotti et al., 2000; Yeung et al., 2004) -- to unarmed Whites as compared to all other targets (i.e., armed Whites, unarmed and armed Blacks) (Correll et al., 2006). These findings suggest that race impacts behavior by modulating the degree of conflict signaled by Black and White targets. In this case, greater conflict was elicited by targets whose racial stereotype is most incongruent with the shoot response (i.e., unarmed Whites). A parallel result here would manifest as greater activation in the ACC to unarmed Whites than Blacks.
fMRI research on race perception also indicates a role for the amygdala -- an area broadly associated with the detection of emotionally relevant stimuli (Davis & Whalen, 2001; Phelps, 2006; Anderson, 2003) -- in the processing of threat based on racial stereotypes (Richeson et al., 2003; Phelps et al., 2000; Cunningham et al., 2004; Lieberman et al., 2005). However, race differences in amygdala activity have typically been observed with relatively simple stimuli and tasks. Thus, it remains to be seen how the amygdala responds to targets of different races within the context of a more complex task that is itself motivationally engaging.
In addition to areas associated with behavioral control and motivation, areas involved in basic perceptual processes may be related to race perception. Past studies show biases in visual attention both to negative stimuli (Brosch & Sharma, 2005; Blanchette, 2006; Koster, Crombez, Van Damme, Verschuere, & De Houwer, 2004; Ohman, Flykt, & Esteves, 2001; LoBue, 2010) and stereotype-congruent stimuli (Eberhardt et al., 2004; von Hippel, Sekaquaptewa, & Vargas, 1995), suggesting that threatening, racially stereotypical targets may preferentially attract visual attention (Correll, Wittenbrink, Crawford, & Sadler, 2015). Additionally, prior research (Richeson & Trawalter, 2008; Bean et al., 2012); has shown that those high in intergroup anxiety show heightened attention to race, as assessed with a dot-probe task or via eye-tracking measures. And critically, recent research (Ofan, Rubin, & Amodio, 2013) manipulated intergroup anxiety, and found that those with greater intergroup social anxiety showed heightened racial outgroup face processing as indexed by the face-sensitive N170 ERP component. As such, it is possible that areas associated with visual attention will increase to armed Blacks as compared to Whites.
As can be seen, past research suggests a number of brain areas that may be affected by racial cues. In addition to the hypotheses about specific brain areas derived from extant research, we also examined relationships between brain activation and a behavioral measure of implicit racial bias. Prior studies show that racial bias in the FPST reflects operation of the racial stereotype linking young Black males to danger (Correll et al., 2002, 2007). Because fMRI studies on race perception have shown differential amygdala activation due to implicit racial bias (Cunningham et al., 2004; Phelps et al., 2000), and because individuals high in implicit racial bias visually perceive Blacks as especially dangerous and threatening (Hugenberg & Bodenhausen, 2003), we expected variability in amygdala activity to the most stereotypically threatening stimuli (armed Black targets) to relate to perceivers’ implicit Black-danger associations. Finally, we also conducted exploratory functional connectivity analyses between brain areas showing significant racial modulation in our task to explore functional relationships among our obtained effects.
Method
Participants
Seventeen right-handed undergraduates (13 White, 1 Asian, 1 Hispanic, 2 unknown; 9 female, mean age = 18.86) were recruited through fliers or web-based psychology participation programs available at the University of Colorado at Boulder. Informed consent was obtained and participants were screened for no history of neurological insult, attention related difficulties and past psychiatric diagnoses or medication (all self-reported questionnaires). Individuals received monetary compensation for participation. Data from one participant (male) was omitted because of artifact due to a non-removable earring, leaving a final sample of 16.
Procedure
First-Person Shooter Task
Trials began with a 500 ms presentation of a background scene (e.g., office park, city street). A Black or White man holding either a handgun or similarly shaped object (e.g., wallet, cell phone) was then superimposed on the scene for 850 ms. From the participant’s perspective, the individual seemed to appear on an unchanged background. The individual was then removed and the scene alone was shown for an additional 650 ms, for a total trial length of 2000 ms. Following past research (Correll et al., 2002), participants had to respond within 850 ms of target onset and received rewards/penalties for correct/incorrect performance. Not shooting an unarmed target earned 5 points, but erroneous shooting cost 20. Shooting an armed target earned 10 points, but erroneously not shooting cost 40. The reward structure attempts to mirror the contingencies of a police officer wanting to avoid shooting innocent people, but suffering most by failing to neutralize a threat. Failure to respond within 850 ms resulted in a 10-point penalty. Experimental trials were preceded by 16 practice trials during which feedback was presented after every trial. During scanning, feedback indicating the cumulative point total and number of correct, incorrect, and too slow responses was presented for 3000 ms every 36 trials. Participants made shoot and don’t-shoot responses with response pads below each index finger (response mapping varied across participants). Thirty trials of each of the four experimental trial types were presented in random order during scanning.
Implicit Association Test (IAT)
After scanning, participants completed a computerized measure to assess implicit Black-danger associations known as the IAT (Dasgupta & Greenwald, 2001; Greenwald, McGhee, & Schwartz, 1998). The task involves making speeded judgments about Black and White faces, as well as words associated with safety and danger. Participants completed multiple blocks of trials involving both Black/White faces and safety/danger words, making Black/White judgments whenever they saw a picture and safety/danger judgments whenever they saw a word. Differences in RT between blocks with a stereotypically-congruent response mapping (e.g., Black and danger made with the same hand) versus a stereotypically-incongruent response mapping (e.g., Black and safety made with the same hand) provided an IAT bias score, or an indication of the degree to which they implicitly associate danger with Blacks versus Whites. IAT bias scores (D) were computed according to Greenwald, Nosek, and Banaji (2003) (see Supplementary Materials for a fuller description of the IAT procedure).
Image Acquisition
Functional MRI was performed on a General Electric 3.0 Tesla whole-body MR scanner to acquire blood oxygenation level–dependent (BOLD) contrast using gradient echo T2*-weighted echo-planar imaging; (repetition time (TR)=3000 ms; echo time = 32ms; 220 mm field of view, 64x64 matrix, 32 slices, 4-mm slice thickness, 0-mm slice gap; flip angle=77°). Slices were oriented obliquely along the AC-PC line. A total to 200 volumes were acquire in 10 minutes. The first four volumes from each run were discarded to allow the MRI signal to reach steady state. Additionally, two separate T1-weighted structural scans were acquired in each participant for subsequent anatomic localization, a T1-FLAIR scan with a spatial resolution of 0.86x1.14x4mm3 using the same slice orientation and thickness as the EPI images, and another 3D image acquired coronally with a spatial resolution of 0.86x0.86x1.7 mm3. The display of the visual tasks was triggered by the TTL pulses generated in each TR on the MRI scanner to synchronize the timing of the tasks and fMRI data acquisition. Head movement was minimized using a custom-fitted head holder, consisting of polyurethane foam beads inflated to tightly mold around the head and neck.
Image Analysis
Image processing and data analysis were performed using the FMRIB software library package FSL (Analysis group, FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/). Standard pre-processing was applied; MCFLIRT – slice time correction/motion correction, BET – brain extraction, time-series prewhitening, registration and spatial normalization to the Montreal Neurological Institute (MNI) high-resolution 152-T1 template. Images were resampled into this space with 3-mm isotropic voxels and smoothed with a Gaussian kernel of 8-mm full-width at half-maximum to minimize noise and residual differences in gyral anatomy, resulting in an effective spatial resolution of 10.2x10.7x11.5 mm. Each normalized image was band-pass filtered (high-pass filter=40 sec) to remove high frequency noise. FMRIB’s improved linear model (FILM) was then applied from which statistical inferences were based on the theory of random Gaussian fields, and changes relative to the experimental conditions were modeled by convolution of single trial epochs with the canonical HRF to approximate the activation patterns (Friston et al., 1994). Using multiple regression analysis, statistical maps representing the association between the observed time series (i.e., BOLD signal) and one or a linear combination of regressors for each subject were constructed.
Functional Connectivity
Exploratory seed-based functional connectivity was performed to examine coherence among all areas identified as showing significant race effects in our initial analyses. These were performed in the following manner: functional data were trimmed to remove 7 initial volumes and a first FSL FEAT model was run applying motion correction and high-pass filtering. Then the first model’s res4d and mean_func output were added together. From that output, FSL’s fslmeants utility extracted the average time course over three brain masks: ventricles, white matter, and subject space whole brain, thresholded by fslmaths at 0.5 demeaned. A second FSL FEAT model was then run on the combined first model’s res4d and mean_func ouput with the three demeaned time courses as explanatory variables (EVs). Subsequently, the res4d and mean_func output of the second model were added together, and FSL’s fslmeants utility extracted the average demeaned time course over each of the contrasts of interest in subject space. Seed regions for whole brain functional connectivity were selected from peak activation of overall whole-brain analyses (see Supplementary Table 1), with the addition of two masks defined by the Harvard-Oxford atlas: 1) bilateral amygdala (no activation in any contrast) and 2) intra-calcarine cortex (activation in both Unarmed greater than Armed and Black Armed greater than White Armed contrasts). Finally, a third FSL FEAT model was run on the combined res4d and mean_func output of the second model, with input EVs containing the masked demeaned condition time courses. After these three models were run per subject at the lower level, a higher-level group model was then run in FSL FEAT.
Results
Following past research (e.g., Correll et al., 2002), participants made relatively few errors (M=1.28, 2%). Nonetheless, trials with incorrect responses were dropped from all behavioral and brain activation analyses.
Behavioral Results
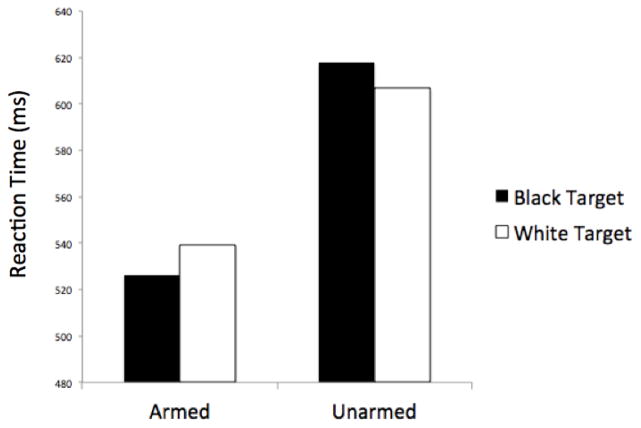
A 2 (Race: Black/White) x 2 (Weapon Presence: armed/unarmed) ANOVA on log-transformed response latencies revealed the expected pattern of racial bias during the FPST. There was a main effect of Weapon Presence, F(1,15)=272.45, p<.0001, indicating that participants were faster to make shoot (M=532.82 ms) than don’t shoot responses (M=612.35 ms). This both replicates past research and supports the argument that, in this task, shooting is the prepotent response. More critically, the Race x Weapon Presence interaction was significant, F(1,15)=18.44, p=.001, ηp2=.55, indicating that participants were faster to shoot armed Black (M=526.37 ms) than White targets (M =539.27 ms), t(15)=2.95, p=.01, ηp2=.37, but faster to not shoot unarmed White (M=606.66 ms) than Black targets (M=618.04 ms), t(15)=2.57, p<.03, ηp2=.31 (see Figure 1).
Figure 1.
Mean reaction times as a function of Target Race (Black vs. White) and Weapon Presence (armed vs. unarmed). Participants exhibited racial bias in reaction times: shoot responses were faster for armed Black than armed White targets, whereas don’t shoot responses were faster for unarmed White than unarmed Black targets.
Neuroimaging Results
fMRI effects were assessed with theoretically motivated contrasts that mirror previous behavioral research on the FPST (Correll et al., 2002) to best gain insight into the neural and psychological mechanisms underlying race bias in decisions regarding threat. Statistically defined clusters were considered significant if they exceeded the specific voxel-wise threshold (p<0.005) and consisted of at least the required number of contiguous voxels to correct for a whole-brain error rate at p<0.05 as determined by FSL’s cluster-based correction (see Supplementary Table 1).
Effect of race on responses to unarmed targets
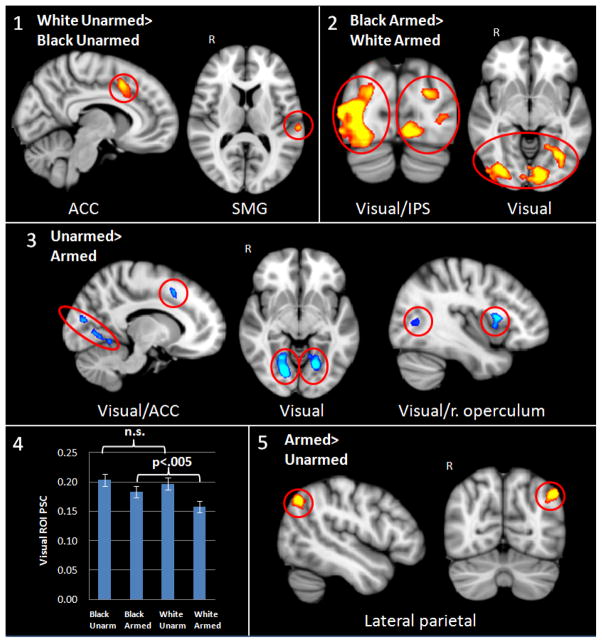
Past ERP research has yielded larger N200 ERP responses, thought to be generated by the ACC, to targets in which both the object being held and the cultural stereotype associated with the individual’s race indicate low threat (i.e., unarmed Whites) (Correll, et al. 2006). Based on this past result and our a priori hypotheses that conflict detection and behavior regulation may differ as a function of race even when the targets are objectively equally harmless (i.e., they are unarmed), we first examined a contrast reflecting the effect of race on unarmed targets. Consistent with past ERP results, regions that yielded significantly greater activity to unarmed Whites than unarmed Blacks included a large medial frontal region spanning the bilateral ACC and preSMA, and activation in the posterior cortex in the supramarginal gyrus (SMG) (see Figure 2, panel 1).
Figure 2.
fMRI results. Panel 1 shows greater activation in the bilateral anterior cingulate cortex (ACC) and supramarginal gyrus (SMG) to unarmed White than unarmed Black targets; Panel 2 shows greater activation in visual cortex and intraparietal sulcus (IPS) to armed Black than armed White targets; Panel 3 shows greater activation in bilateral visual cortex, ACC, and right operculum to unarmed than armed targets; Panel 4 shows percent signal change (PSC) from the visual cortex ROI for each condition versus baseline; Panel 5 shows greater activation in the right lateral parietal cortex to armed than unarmed targets. R = right hemisphere.
Analysis of the inverse contrast of greater activity for unarmed targets who were Black versus White yielded no significant findings.
Effect of race on responses to armed targets
We similarly evaluated the effects of race on responses to targets that were threatening. Regions yielding greater activity for armed targets who were Black versus White included bilateral visual cortex (intra/supra-calcarine cortex, lingual gyrus), precuneus, and lateral occipital cortex along the intra-parietal sulcus (IPS) (see Figure 2, panel 2).
Analysis of the inverse contrast, which would indicate greater activity during decision-making for armed targets who were White versus Black, yielded no significant differences.
Effects of weapon presence
In addition to the more focused contrasts just reported – which isolate the effects of race within targets who are holding the same objects -- we also computed contrasts that assess main effects of weapon presence and race. Starting first with the weapon presence contrast, regions showing significantly greater activity to unarmed than armed targets included bilateral visual cortex (intra/supra-calcarine cortex, lingual gyrus), a region spanning the bilateral anterior cingulate cortex (ACC), and the pre-supplementary motor area (preSMA) and right opercular cortex (see Figure 2, panel 3). Differences in visual cortex were also found in the armed Black versus armed White contrast, as noted earlier. To clarify the pattern of results, Figure 2 panel 4 shows the percent signal change in the visual cortex ROI for each condition against baseline, showing greater activation to unarmed targets of both races compared to armed targets of both races, but greater activation to Black armed than White armed targets.
We then identified brain regions yielding statistically greater activity to armed than unarmed targets. The only region passing significance in this contrast was localized to the right lateral parietal cortex (see Figure 2, panel 5).
Effects of race
Neither contrast examining greater activity for Black than White targets, or for White than Black targets yielded any significant effects.
Functional connectivity analyses
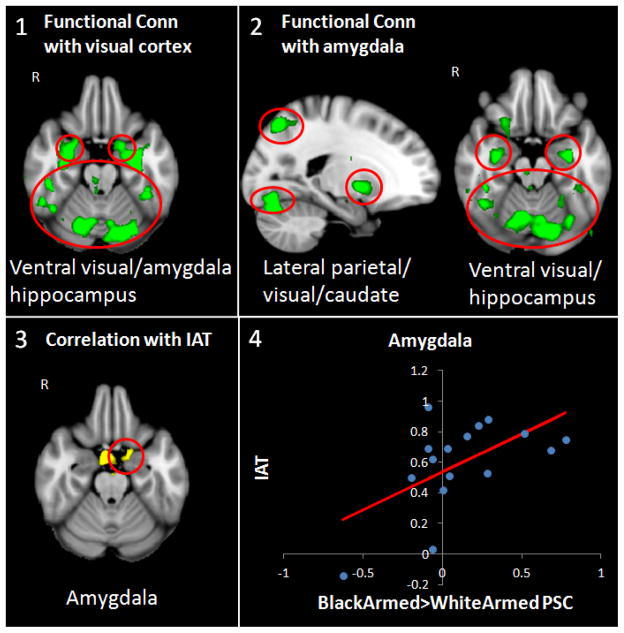
To assess whether any of the brain regions in the previous analyses elicited functional connectivity with other regions of the brain, we used seed-based functional connectivity. Results indicated that the visual cortex exhibited significant coherence with the amygdala, hippocampus, and ventral visual processing stream, but exclusively for the contrast of armed targets who were Black versus White (see Figure 3, panel 1), and not for the reverse armed contrast or for either of the unarmed contrasts.
Figure 3.
Seed-based functional connectivity and implicit racial bias results. Panel 1 shows functional connectivity with bilateral visual cortex ROI indicating coherence with the amygdala, hippocampus, and ventral visual processing stream for the contrast of Black armed versus White armed; Panel 2 shows functional connectivity with bilateral amygdala ROI indicating coherence with the hippocampus, ventral visual processing stream, visual cortex, lateral parietal cortex and caudate nucleus for the contrast of Black armed versus White armed; Panels 3 shows the correlation between implicit Black-danger associations from the Implicit Association Task and the left amygdala for the contrast of Black armed versus White armed; Panel 4 shows the scatter plot regressing implicit Black-danger associations from the Implicit Association Task on amygdala percent signal change (PSC). R = right hemisphere.
Next, because prior research has shown the amygdala to cohere with areas involved in motivational salience (e.g., Vuilleumier & Brosch, 2009; Cunningham & Brosch, 2012), we examined functional connections between the amygdala and other regions performing the same analysis as above for all contrasts using the bilateral amygdala as a seed ROI. Analyses revealed that the amygdala exhibited significant functional coherence with the hippocampus, ventral visual processing stream, visual cortex (lingual gyrus), lateral parietal cortex, and caudate nucleus. Similar to the previous functional connectivity analysis, these relations were only significant for the contrast of armed targets who were Black versus White (see Figure 3, panel 2).
Individual differences in implicit black-danger racial bias
Finally, we investigated whether individual differences in racial bias affected brain activity by including implicit Black-danger associations, as measured by the IAT, as a regressor in the general linear model. The regression analysis indicated that individuals who displayed greater implicit biases associating Blacks with violence and danger showed greater left amygdala activation. Again, this finding was only significant for the contrast of Black versus White armed targets (see Figure 3, panel 3, 4).
Discussion
The current study adopted a combination of behavioral and neuroscience approaches to study mechanisms underlying racial bias. Our goal was to build on past fMRI studies of race perception by employing a more complex decision-making task in which race was only one of several cues that could affect judgments, much like many real-world situations. Given the presence of stereotypes linking Black males and aggression and violence, and the potential negative implications for such associations (Payne, 2006), we selected a task in which participants must differentiate between threatening (armed) and non-threatening (unarmed) Black and White men. Behavioral analyses revealed a pattern of racial bias replicating past research (Correll et al., 2002, 2006, 2007; Sadler et al., 2012): participants were faster to shoot armed Black than White targets, but faster in making don’t shoot responses to unarmed White than Black targets. Most crucially, our fMRI results suggest that different brain regions or networks may underlie racial differences in responses to armed versus unarmed targets. In some cases, the activation patterns converge with past research, but in instances in which they diverge, we suggest that differences are informative about how race-related responses can deviate subtly depending on the nature of the task.
We observed greater ACC activity to unarmed Whites than Blacks, which suggests that racial differences in behavior toward unarmed individuals may be linked to this brain region. Without falling victim to reverse inference, we suggest that activation of the ACC could be due to a number of different processes that are suggested in the literature associated with the ACC. These processes include top-down attentional control (MacDonald et al., 2000), error detection (Ullsperger & Von Cramon, 2004), response conflict (Milham et al., 2001), and over-riding pre-potent responses (Depue et al., 2015). While researchers have theorized that racial bias can derive from a failure to correct for the undesirable influences of negative and/or stereotypical associations (e.g., Payne, Shimizu, & Jacoby, 2005), carefully designed future studies need to be conducted that parse out different processes associated with the ACC in racial bias contexts.
While race differences in response to unarmed targets manifested in the ACC, racial differences in responses to armed targets occurred within areas associated with basic visual perception. Specifically, activity was greater for armed Blacks than armed Whites in parietal and visual cortical regions. It is difficult to determine specifically what coactivation of these regions is reflecting in the current context. However, connections between the parietal and visual cortices are thought to direct attention to visual processing (Fink et al., 1996). Indeed, Correll et al. (2015), suggests that attentional differences may drive racial differences to armed targets, with stereotypically dangerous Black men influencing visual processing resources to a greater extent than comparably dangerous White men. Furthermore, these results are in line with previous research showing that heightened intergroup anxiety facilitates visual attention to and processing of racial outgroup members (Ofan et al., 2013; Richeson & Trawalter, 2008; Bean et al., 2012). More research is needed to more fully understand parietal-occipital interactions as they relate to attention and visual processes in the context of racial bias.
Other brain regions associated with racial differences of armed targets are suggested by the current connectivity analyses. We obtained functional relationships between the amygdala, and parietal and visual cortices, as well as the hippocampus and caudate nucleus, but only for decisions involving shoot responses to armed Black versus armed White targets. Upon first glance, it is somewhat surprising that we did not also observe heightened amygdala activity in the contrast assessing greater activation to armed Blacks than Whites. However, given that the task is attentionally demanding and each target stimulus is presumably high in motivational relevance, we observed significant amygdala activation in all conditions relative to baseline. This is not unlike previous fMRI studies on race perception that showed an absence of mean level differences between Black and White targets (e.g., Phelps et al., 2000; Richeson et al., 2002). This suggests that race may not show invariant effects on brain activation, but must instead be understood within the context of ongoing behavior. These findings indicate that the amygdala, which previous research has shown to be sensitive to social goals (Van Bavel et al., 2008; Cunningham et al., 2008), played a critical role in processing targets that are the most stereotypically threatening (armed Blacks).
In addition to showing differential race effects associated with armed and unarmed targets, our results show that activation was greater to unarmed versus armed targets in areas of bilateral visual cortex. This result may seem at odds with the greater visual activity to armed Blacks than Whites discussed earlier, but are sensible when considering that greater visual processing is typically required to detect the absence of a feature as opposed to its presence (Treisman & Souther, 1985). The greater visual activity overall to unarmed than armed targets, regardless of race could reflect the greater difficulty in detecting the absence of a gun when the target is unarmed. At the same time, even in the presence of heightened visual activity associated with the absence of a feature, race impacts how armed targets are visually discriminated, with greater visual processing in the most stereotypically congruent condition (i.e., armed Blacks).
Finally, we observed a correlation between amygdalar activity to armed Black relative to White targets and a measure of implicit racial bias associating Blacks with danger and violence: higher levels of implicit racial bias associating Blacks with danger and violence were associated with greater amygdala activity for armed Black, relative to White, targets. These findings are compatible with other studies that showed amygdala responses indicative of threat associated with automatic racial bias (Richeson et al., 2003; Phelps et al., 2000; Cunningham et al., 2004; Lieberman et al., 2005).
Despite what we believe to be strong findings reported herein, we must also acknowledge certain limitations in the current study. First, our experimental design did not allow for the direct comparison of White perceivers to other racial groups. In the future it will be important to understand whether similar effects are modulated in participants of other racial groups. Despite this shortcoming, we have reason to predict that these results would generalize to Black participants as well. Firstly, Black participants cognitively encode skin town differences in a similar manner as White participants (Maddox & Gray, 2002), and further characterize darker-skinned Black targets in more stereotypic terms than lighter-skinned Black targets. Similarly, because the shooter bias is based on the knowledge of cultural stereotypes, Black participants show equivalent levels of shooter bias to Black targets as White participants during the FPST (Correll et al., 2002; Kahn & Davies, 2015). Thus, pre-existing cultural associations present in Black and White participants in the US likely shape neural processes that underlie these types of biases in a similar fashion. An additional shortcoming of the current study is that despite the FPST’s complex nature in relation to typical experimental tasks that are intended to assess racial bias, it may not sufficiently represent the complexity of decision-making processes associated with life-threatening circumstances. Lastly, while fMRI provides the best combination of spatial and temporal resolution of the brain in-vivo, interpretation of the BOLD response is largely correlational and does not suggest a brain region is causal for any behavior or process. Therefore, more research is needed to understand the neural mechanisms that contribute to racial bias and stereotypical behavior in real-world contexts involving threat.
In sum, these results highlight a number of different processes that may contribute to both a greater likelihood of treating Blacks as more dangerous and Whites as more safe among White perceivers. Decisions to shoot armed Black targets were associated with biased visual attention, involving the cooperation of the amygdala and the parietal and visual cortices, to a greater extent than for armed White targets, and sensitivity of the amygdala to implicit stereotyping. By contrast, decisions to not shoot unarmed White targets were associated with greater cognitive control than for unarmed Black targets. We also note that while there were differences in activation comparing unarmed to armed targets in areas of visual cortex (collapsing across target race), there were no significant effects for contrasts comparing Blacks and Whites (collapsing across object). Instead, race effects manifested within the context of comparisons of just armed or just unarmed targets. This suggests to us the importance of understanding how race impacts processes such as attention, response conflict, over-riding prepotent responses and motivational salience within the context of a perceiver’s on-going goals and intentions. While engagement of these specific brain areas might be expected to vary with features of the particular task, we think the results highlight different ways in which stereotypical content can be translated into racial differences in behavior of relevance across contexts.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health under Grant R21-MH66739; and National Science Foundation under Grant RL-0910373.
References
- Amodio DM. Intergroup anxiety effects on the control of racial stereotypes: A psychoneuroendocrine analysis. Journal of Experimental Social Psychology. 2009;45:60–67. [Google Scholar]
- Amodio DM, Devine PG, Harmon-Jones E. Individual differences in the regulation of intergroup bias: The role of conflict monitoring and neural signals for control. Journal of Personality and Social Psychology. 2008;94(1):60–74. doi: 10.1037/0022-3514.94.1.60. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink response and self-report. Journal of Personality and Social Psychology. 2003;84(4):738–753. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Kubota JT, Harmon-Jones E, Devine PG. Alternative mechanisms for regulating racial responses according to internal vs external cues. Social Cognitive and Affective Neuroscience. 2006;1(1):26–36. doi: 10.1093/scan/nsl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. The Journal of Neuroscience. 2003;23(13):5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh JA. Automatic and conscious processing of social information. Handbook of Social Cognition. 1984;3:1–43. [Google Scholar]
- Bargh JA, Chartrand TL. The unbearable automaticity of being. American Psychologist. 1999;54(7):462–479. [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK. Alcohol effects on performance monitoring and adjustment: Affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology. 2012;121(1):173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean MG, Slaten DG, Horton WS, Murphy MC, Todd AR, Richeson JA. Prejudice concerns and race-based attentional bias: new evidence from eyetracking. Social Psychological and Personality Science. 2012;3:722–729. [Google Scholar]
- Beer JS, Stallen M, Lombardo MV, Gonsalkorale K, Cunningham WA, Sherman JW. The Quadruple Process model approach to examining the neural underpinnings of prejudice. NeuroImage. 2008;43(4):775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Blanchette I. Snakes, spiders, guns, and syringes: How specific are evolutionary constraints on the detection of threatening stimuli? The Quarterly Journal of Experimental Psychology. 2006;59(8):1484–1504. doi: 10.1080/02724980543000204. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brosch T, Sharma D. The role of fear-relevant stimuli in visual search: A comparison of phylogenetic and ontogenetic stimuli. Emotion. 2005;5(3):360–364. doi: 10.1037/1528-3542.5.3.360. [DOI] [PubMed] [Google Scholar]
- Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Correll J, Park B, Judd CM, Wittenbrink B. The police officer’s dilemma: Using ethnicity to disambiguate potentially threatening individuals. Journal of Personality and Social Psychology. 2002;83(6):1314–1329. [PubMed] [Google Scholar]
- Correll J, Park B, Judd CM, Wittenbrink B. The influence of stereotypes on decisions to shoot. European Journal of Social Psychology. 2007;37(6):1102–1117. [Google Scholar]
- Correll J, Urland GR, Ito TA. Shooting straight from the brain: Early attention to race promotes bias in the decision to shoot. Journal of Experimental and Social Psychology. 2006;42:120–128. [Google Scholar]
- Correll J, Wittenbrink P, Crawford M, Sadler MS. Stereotypic vision: How stereotypes disambiguate complex visual stimuli. Journal of Personality and Social Psychology. 2015;108(2):219–233. doi: 10.1037/pspa0000015. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21(1):54–59. [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychological Science. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Dafny N, Dauth G, Gilman S. A direct input from amygdaloid complex to caudate nucleus of the rat. Experimental Brain Research. 1975;23(2):203–210. doi: 10.1007/BF00235461. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Greenwald AG. On the malleability of automatic attitudes: Combating automatic prejudice with images of admired and disliked individuals. Journal of Personality and Social Psychology. 2001;81(5):800–814. doi: 10.1037//0022-3514.81.5.800. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Devine PG. Stereotypes and prejudice: Their automatic and controlled components. Journal of Personality and Social Psychology. 1989;56(1):5–18. [Google Scholar]
- Dovidio JF, Evans N, Tyler RB. Racial stereotypes: The contents of their cognitive representations. Journal of Experimental Social Psychology. 1986;22(1):22–37. [Google Scholar]
- Dovidio JF, Gaertner SL. Aversive racism and selection decisions: 1989 and 1999. Psychological Science. 2000;11(4):315–319. doi: 10.1111/1467-9280.00262. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Gaertner SL. Aversive racism. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 36. San Diego, CA: Academic Press; 2004. pp. 1–51. [Google Scholar]
- Dovidio JF, Gaertner SL. On the nature of contemporary prejudice: The causes, consequences, and challenges of aversive racism. In: Eberhardt J, Fiske ST, editors. Confronting racism: The problem and the response. Newbury Park, CA: Sage; 1998. pp. 3–32. [Google Scholar]
- Dovidio JF, Gaertner SE, Kawakami K, Hodson G. Why can’t we just get along? Interpersonal biases and interracial distrust. Cultural Diversity and Ethnic Minority Psychology. 2002;8(2):88–102. doi: 10.1037/1099-9809.8.2.88. [DOI] [PubMed] [Google Scholar]
- Eberhardt JL, Goff PA, Purdie VJ, Davies PG. Seeing Black: Race, crime, and visual processing. Journal of Personality and Social Psychology. 2004;87(6):876–893. doi: 10.1037/0022-3514.87.6.876. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382:626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Cox CL, Schmader T, Ryan L. Negative stereotype activation alters interaction between neural correlates of arousal, inhibition and cognitive control. Social Cognitive and Affective Neuroscience. 2012;7(7):771–781. doi: 10.1093/scan/nsr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1994;2(4):189–210. [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology. 1998;74(6):1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85(2):197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Hugenberg K, Bodenhausen GV. Facing prejudice: Implicit prejudice and the perception of facial threat. Psychological Science. 2003;14(6):640–643. doi: 10.1046/j.0956-7976.2003.psci_1478.x. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. Race and gender on the brain: Electrocortical measures of attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology. 2003;85(4):616–626. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(1):21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33(3):282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Van Damme S, Verschuere B, De Houwer J. Does imminent threat capture and hold attention? Emotion. 2004;4(3):312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Human Brain Mapping. 2007;28(10):915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonards U, Sunaert S, Van Hecke P, Orban GA. Attention mechanisms in visual search—an fMRI study. Journal of Cognitive Neuroscience. 2000;12:61–75. doi: 10.1162/089892900564073. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience. 2005;8(6):720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, III, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38(5):701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- LoBue V. And along came a spider: An attentional bias for the detection of spiders in young children and adults. Journal of Experimental Child Psychology. 2010;107(1):59–66. doi: 10.1016/j.jecp.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Walsh V, Frith CD. Brain activations during visual search: Contributions of search efficiency versus feature binding. Neuroimage. 2003;18(1):91–103. doi: 10.1006/nimg.2002.1329. [DOI] [PubMed] [Google Scholar]
- Ofan RH, Rubin N, Amodio DM. Situation-based social anxiety enhances the neural processing of faces: evidence from an intergroup context. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst087. nst087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences. 1994;91(18):8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BK. Prejudice and perception: The role of automatic and controlled processes in misperceiving a weapon. Journal of Personality and Social Psychology. 2001;81(2):181–192. doi: 10.1037//0022-3514.81.2.181. [DOI] [PubMed] [Google Scholar]
- Payne BK. Weapon Bias Split-Second Decisions and Unintended Stereotyping. Current Directions in Psychological Science. 2006;15(6):287–291. [Google Scholar]
- Payne BK, Lambert AJ, Jacoby LL. Best laid plans: Effects of goals on accessibility bias and cognitive control in race-based misperceptions of weapons. Journal of Experimental Social Psychology. 2002;38(4):384–396. [Google Scholar]
- Payne BK, Shimizu Y, Jacoby LL. Mental control and visual illusions: Toward explaining race-biased weapon misidentifications. Journal of Experimental Social Psychology. 2005;41:36–47. [Google Scholar]
- Pessoa L, Japee S, Sturman D, Ungerleider LG. Target visibility and visual awareness modulate amygdala responses to fearful faces. Cerebral Cortex. 2006;16(3):366–375. doi: 10.1093/cercor/bhi115. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, Banaji M. Performance on indirect measures of race evaluative predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, Shelton JN. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Trawalter S. The threat of appearing prejudiced and race-based attentional biases. Psychological Science. 2008;19:98–102. doi: 10.1111/j.1467-9280.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- Richter W, Ugurbil K, Georgopoulos A, Kim SG. Time-resolved fMRI of mental rotation. NeuroReport. 1997;8(17):3697–3702. doi: 10.1097/00001756-199712010-00008. [DOI] [PubMed] [Google Scholar]
- Sadler MS, Correll J, Park B, Judd CM. The world is not Black and White: Racial bias in the decision to shoot in a multiethnic context. Journal of Social Issues. 2012;68(2):286–313. [Google Scholar]
- Sears DO, Sidanius J, Bobo L, editors. Racialized politics: The debate about racism in America. Chicago: University of Chicago Press; 2000. [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: Evidence from repeated task-switching. Journal of Cognitive Neuroscience. 2003;15(6):785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Treisman A, Souther J. Search asymmetry: A diagnostic for preattentive processing of separable features. Journal of Experimental Psychology: General. 1985;114(3):285. doi: 10.1037//0096-3445.114.3.285. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40(4):593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- von Hippel W, Sekaquaptewa D, Vargas P. On the role of encoding processes in stereotype maintenance. Advances in Experimental Social Psychology. 1995;27:177–254. [Google Scholar]
- Vuilleumier P, Brosch T. Interactions of emotion and attention. In: Gazzaniga MS, editor. The cognitive neurosciences IV. Cambridge: MIT Press; 2009. pp. 925–934. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.