Abstract
Heterocyclic compounds are ubiquitous in natural products, pharmaceuticals, and agrochemicals. Therefore, the design of novel protocols to construct heterocycles more efficiently is a major area of focus in the organic chemistry. In the past several years, cyclization reactions based upon palladium-catalyzed C–H activation have received substantial attention due to their capacity for expediting heterocycle synthesis. This review discusses strategies for heterocycle synthesis via palladium-catalyzed C–H bond activation and highlights recent examples from the literature.
Keywords: Heterocycle, C–H activation, amination, carbonylation, olefination
1 Introduction
Due to the unique role of heterocycles in pharmaceuticals, agrochemicals and other biologically active molecular scaffolds, organic chemists have had a long-standing interest in developing reactions to construct heterocycles.1 In the past decade, transition metal–catalyzed C–H activation reactions for C–C and C–heteroatom bond formation have emerged as a class of promising new transformations.2 Applying this technology for the synthesis of heterocycles is appealing because it could improve the overall atom- and step-economy compared to traditional methods.3 Nevertheless, despite significant efforts in the past several years, these methods are still in their infancy.4 This short review will focus on new retrosynthetic disconnections based on C–H functionalization and will give an overview of examples from the literature illustrating these principles.
Broadly speaking, there are two types of the heterocyclic ring-forming reactions using C–H activation: (1) intramolecular cyclization and (2) intermolecular annulation. Within each of the general categories, there are several different categories of bond-forming reactions. As one representative example, consider the case of indoline motifs. There are three possible disconnections for indoline synthesis using C–H activation: N1–C2, N1–C8, and C3–C9 (Scheme 1, shown in red). The N1–C2 and C3–C9 bonds can also be disconnected simultaneously. Analogous disconnections exist for a range of N-, O-, and S-containing heterocycles of all different ring sizes.
Scheme 1.
Retrosynthetic disconnections of indoline based on C–H functionalization. Standard indole/indoline atom numbering is used.
This short review will focus on Pd-catalyzed C–H functionalization reactions for heterocycle synthesis. Due to space constraints, we are not able to discuss examples using other metals.
2 Intramolecular cyclization via C–C formation
Pd-catalyzed intramolecular C–H activation followed by C–C bond formation5 has found numerous applications in the construction of heterocycles, which will be discussed in the following order: aryl–aryl (Section 2.1), aryl-alkenyl (Section 2.2), and aryl–alkyl (Section 2.3) coupling reactions.6
2.1 Arene/arene coupling
Fused biaryl formation via Pd-catalyzed C–H functionalization can be grouped into two distinct categories: C–H/C–X cross-coupling (Type I), and C–H/C–H cross-coupling (Type II) (Scheme 2). It is important to note that the catalytically active species in these two types of reactions are different, Pd(0) in the former case, and Pd(II) in the latter case. The mechanism of the C–H activation step in these processes has been found to vary depending on the nature of the metal center, the substrate, and the reaction conditions. Electrophilic palladation, oxidative addition, as well as concerted metallation/deprotonation have all been invoked.7
Scheme 2. Two types of arene–arene coupling reactions.
C–H arylation with aryl halides
In 1982, Ames and coworkers reported a Pd(0)-catalyzed reaction to form benzofuran [3,2-c]cinnoline 2 and indolo[3,2-c]cinnoline 2a from aryl bromides (Scheme 3).8a The author further developed methodology to synthesize a variety of substituted dibenzofurans and carbozoles with moderate to good yields. The transformation was extended to the preparation of six-membered ring heterocycles (Scheme 4).8b,8c The low yields reported in the synthesis of six-membered ring heterocycles prompted several research groups to further investigate this transformation. For example, Rawal and coworkers developed an arylation reaction of phenols to produce six-membered heterocycles using Herrmann's catalyst (Scheme 5).9 Comparing the yields for 10 and 11, it is clear that the presence of a free hydroxyl group in the aryl ring is crucial; in situ deprotonation to generate the phenolate is thought to activate the ring for C–H cleavage
Scheme 3. Early examples of heterocycle formation via C–H arylation.
Scheme 4. Heterocycle formation via C–H arylation.
Scheme 5. Formation of six-membered heterocycles via C–H arylation.
Recently, Fagnou and co-workers developed a collection of practical protocols to obtain six-membered heterocycles by using biarylphosphine ligands. Both electron-donating and electron-withdrawing substituents gave excellent yields (Scheme 6).10a The authors further discovered that aryl chlorides could be used as substrates by employing electron-rich N-heterocyclic carbene (NHC) ligands.10b A general and efficient system to synthesize five- or six-membered heterocycles from aryl chlorides, bromides, or iodides was developed using HBF4-protected tricyclohexanyl phosphine as the ligand.10c It is important to point out that this aryl–aryl coupling reaction is widely used in total synthesis of natural product and other biologically active molecular scaffolds. Indeed, this topic has been extensively reviewed.6
Scheme 6. Formation of six-membered heterocycles via C–H arylation.
C(sp2)–H/C(sp2)–H coupling
Formation of heterocycles through Pd-catalyzed C–H/C–H coupling was first reported by Shiotani and Itatani in the 1974 (Scheme 7).11 However, the dibenzofuran product 3 was obtained in low yield with some dimerized by product.
Scheme 7.
Early example of C–H/C–H coupling for heterocycle synthesis.
Recently, DeBoef and coworkers reported an example of oxidative cyclization of N-benzoylindole by using stoichiometric amounts of Cu(OAc)2 under O2 at elevated pressure (Eq 1, Scheme 8).12 An electron-rich arene was found to be necessary for satisfactory yield, which suggests that the C–H activation step proceeds via an electrophilic palladation mechanism.13 Subsequently, Fagnou and co-workers reported a similar transformation with diarylethers and diarylanilines to construct carbazoles and dibenzofurans (Eq. 2).14 Interestingly, pivalic acid (PivOH) was found to be an effective solvent for achieving good yields. A variety of naturally occurring electron-rich tricyclic carbazoles could be accessed using this transformation.14,15 Interestingly Fujii and co-workers were able to design a one-pot procedure for carbazole synthesis by merging anilines and aryl triflates via tandem Buchwald–Hartwig amination/oxidative arylation (Eq. 3).16
Scheme 8. Formation of five-membered heterocycles via C–H/C–H coupling.
Recently, Liégault and Fagnou reported the first example of six-membered heterocycle formation via Pd-catalyzed C–H/C–H coupling, in which the presence of an electron-withdrawing group in the pyrrole ring appears to facilitate the cyclization process (Eq 1, Scheme 9).17 Dong and co-workers developed a similar method for lactam formation using persulfate as the oxidant (Eq. 2).18 Akermann and co-workers have reported a similar reaction between triazoles and arenes, which is thought to proceed via initial triazole palladation followed by arene C–H functionalization (Eq. 3).19 Interestingly, Pintori and Greaney demonstrated that seven- and eight-membered heterocycles could be prepared under similar conditions, by employing tether indoles in which the C-3 position was blocked (Eq. 4).20
Scheme 9. Formation of six- and seven-membered heterocycles via C–H/C–H coupling.
2.2 Arene/alkene coupling or arene/alkne coupling Arene/alkene coupling
In 1980, Kibayashi and co-workers disclosed a cyclization reaction of 3-(4-methoxyanilino)cyclohex-2-en-1-one 31 to give carbazole derivatives 32 in 31% yield (Scheme 10).21 The arylpalladium species generated from electrophilic palladation was proposed as the key intermediate, which subsequently undergoes intramolecular Heck-type cyclization to give the product.
Scheme 10. Carbazole formation via C–H olefination.
Following this initial discovery, Akermark demonstrated that TBHP (tert-Butyl hydroperoxide) could function as an effective oxidant for this transformation, allowing for the synthesis of a variety of carbazoles in moderate yields (Eq. 1, Scheme 11).22 Recently, Stoltz and co-workers developed an efficient method to construct highly substituted benzofurans from electron-rich arenes (Eq. 2).23 The use of an pyridine-type ligand and BQ (1,4-benzoquinone) is crucial for catalytic turnover. Thereafter, Oestreich and coworkers applied a similar transformation to synthesize benzolactams. Importantly, the fact that O2 is the sole terminal oxidant makes this transformation highly atom economical (Eq. 3).24 Wang and co-workers reported an interesting example of benzofuran formation from simple arenes. It was found that the use of both PPh3 and silver trifluoroacetate is crucial (Eq. 4).25 It is important to note that Pd-catalyzed intramolecular alkenylations of nitrogen-containing heterocycles are well precedented26 and have been applied to total synthesis.27
Scheme 11. Heterocycle formation via intramolecular C–H olefination.
Arene/alkyne coupling
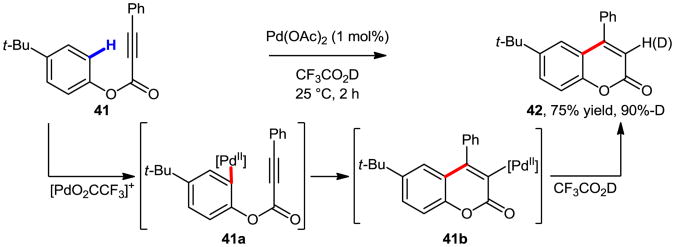
Fujiwara and co-workers demonstrated the first example of intramolecular arene/alkyne coupling.28 A possible mechanism is depicted in Scheme 12, in which electrophilic palladation, followed by a anti carbopalladation across the C–C triple bond, which gives the resulting alkenyl–Pd species 41b. Protodepalladation gives the product 42. Interestingly, deuterium labeling experiments revealed that the the alkenyl hydrogen atom of the product comes mainly from the protic solvent.
Scheme 12. Synthesis of coumarins from aryl alkynoates.
Later, Zhu and coworkers achieved a highly efficient Pd-catalyzed synthesis of unsymmetrically substituted 3-(diarylmethylenyl)indolinones from the corresponding aryl alkynes. The domino reaction involves oxidative addition of the aryl halide to Pd(0) to generate a [Pd(0)(Ar)(X)] species 43a, which undergoes intermolecular carbopalladation, followed by C–H activation, and C–C reductive elimination (Eq. 1, Scheme 13).29 Later, Li and coworkers were able to replace the aryl halide by a simple arene to perform a similar domino reaction.30 It is worth noting that the six member lactam product was obtained when the PivOH was used as an additive (Eq. 2).
Scheme 13. Synthesis of oxindoles from alkynanilides.
2.3 Arene/alkane coupling
C(sp2)-H alkylation with alkyl halides
In 2003, Buchwald and Hennessy reported a single pioneering example of Pd(0)-catalyzed alkylation of aryl C–H bonds using a tethered alkyl chloride to construct oxindoles (Scheme 14).31 Recently, Wipf and Petronijevic applied this method in the total synthesis of (±)-cycloclaven.32
Scheme 14. Synthesis of oxindole via C(sp2)–H alkylation.
C(sp3)–H arylation with aryl halides
In 1992, Dyker discovered an early example of Pd-catalyzed C(sp3)–H arylation, in which a polycyclic ether was formed from 2-iodoanisole via a cascade sequence involving multiple C–H actvation steps (Scheme 15).33
Scheme 15. Early example of Pd-catalyzed C(sp3)–H arylation.
Recently, Fagnou and co-workers reported formation of 2,2-dialkyldihydrobenzofurans from the corresponding aryl bromides via Pd-catalyzed C(sp3)–H arylation using phosphine ligands (Eq. 1, Scheme 16).34 It is worth noting that the authors also demonstrated that the aryl chlorides were also suitable substrates, although a lower yield (73%) was obtained. Subsequently, Fujii and co-worker developed a similar transformation to construct indolines from 2-chloroaniline-derived substrates.35 The protection of the aniline nitrogen atom is crucial, since the free amine is unreactive. Impressively methylene C–H activation was also found to be possible (Eq. 2). The asymmetric version of this reaction was recently achieved by Kündig and coworkers, in which chiral NHC ligand was used (Eq. 3).36a Concurrently, Kagan and co-workers reported the similar asymmetric cyclization by using chiral diphosphines ligand.36b Recently, Rousseaux and co-workers reported lactam and sulfonamide formation via Pd-catalyzed intramolecular arylation of C(sp3)–H bonds adjacent to amides and sulfonamides (Eq. 4).37
Scheme 16. Heterocycle formation via intramolecular Pd0-catalyzed C(sp3)–H arylation.
Interestingly, by employing a strongly binding auxiliary38 and using Pd(II)/Pd(IV) catalysis, Chen and co-workers achieved intramolecular C(sp3)–H arylation, and a variety of five- and six- membered heterocycles could be formed in good yield (Scheme 17).39
Scheme 17. Quinoline carboxamde directed C(sp3)–H arylation.
C(sp2)–H/C(sp3)–H coupling
Fagnou and co-workers reported a very rare example of C(sp3)–H/C(sp2)–H coupling.14 Electrophilic palladation of the pyrrole gives an aryl palladium(II) species, which performs C(sp3)–H bond activation. C–C reductive elimination gives the heterocyclic product (Scheme 18). Electron-withdrawing substituents are crucial in order to obtain synthetically useful yields.
Scheme 18. An example of heterocycle formation via C(sp2)–H/C(sp3)–H coupling.
3. Intramolecular cyclization via C–N bond formation
Heterocylic ring construction via Pd-catalyzed C–H amination is complementary approach to intramolecular Buchwald–Hartwig amination.40 Buchwald and co-workers reported an intramolecular ring-closing Pd-catalyzed C–H amination reactionof N-acetyl-2-phenylaniline 71 to form carbazole ring 72 via a presumed Pd(II)/Pd(0) catalytic cycle (Scheme 19).41 The authors proposed two possible pathways: syn- and anti-aminopalladation to give intermediates 71a and 71b, respectively. These species could undergo β-hydride elimination to provide carbazole 72. Both electron-rich and electron-deficient substrates gave excellent yields. The utility of the this transformation was further demonstrated by making several naturally occurring carbazoles, namely mukonine, mukonidine, and glycosinine.42 Subsequently, many protocol have been developed to effect this cyclization including metal-free examples.43
Scheme 19. Early example of carbozole formation via Pd-catalyzed C–H amidation.
Inamoto and Hiroya devised a protocol for making indazole rings 74 from highly conjugated hydrazones 73 via Pd-catalyzed C–H amination (Scheme 20).44 In order to achieve satisfactory results, stochiometric Ag(OAc)2 and Cu(OAc)2 were needed. The C–H cleavage step may proceed via electrophilic palladation, since electron-rich arenes were more reactive than electron-deficient arenes. The utility of this Pd(II)/Pd(0) catalytic manifold was further demonstrated in the synthesis of heterocycles such as indoles, benzothiazoles, imidazoles, quinolines, and phenanthridinone.45
Scheme 20. Indazole formation via Pd-catalyzed C–H amidation.
In 2008, our group developed a method for oxindole synthesis using intramolecular C–H amination (Scheme 21).46 Diverse δ- and γ-lactams were prepared by this method and alkenyl C(sp2)–H and allyllic C(sp3)–H bonds could also be aminated using this protocol.47 Subsequently, Murakami and coworkers reported a similar transformation, in which the N-OMe group was replaced by an N-Ts group.48 One of the fundamental challenges in intramolecular C–H amination is developing reactions that employ readily available substrates and that generate broadly useful product classes. To our delight, we were able to carry out the desired intramolecular C–H amination of phenethyltriflimide 81 in the presence of an F+ oxidant 82,49 enabling a highly expedient route to functionalized indoline 83 (Scheme 22).50 This work represents the first example in which intramolecular C–H amidation was observed with simple non-substituted phenethylamine substrates—though substituted starting materials give excellent results as well. We also demonstrated that C–N reductive elimination could be promoted by a one-electron oxidant, Ce(SO4)2.51 Recently, many groups have studied this transformation with a variety of phenethylamine derivatives and developed efficient protocols to construct indoline heterocycles (Scheme 23).52
Scheme 21. Oxindole formation via Pd-catalyzed C–H activation.
Scheme 22. Indoline formation via PdII/PdIV catalysis.
Scheme 23. Indoline formation via PdII/PdIV catalysis.
Hartwig and coworkers reported a novel method for indole formation via Pd(0)/Pd(II) catalysis. Oxidative addition of the Pd(0) species to N-acetyl-oxime substrate 88 gives intermediate 88a, which undergoes C–H activation to give palladacyle 88b. After reductive elimination and tautomerization, the indole 89 is obtained in high yield. Interestingly low Pd loading was used and no exernal co-oxidant was needed. α,α-Diaryl substitution was necessary for the reaction to take place (Scheme 24).53
Scheme 24. Indole formation via Pd0/PdII catalysis.
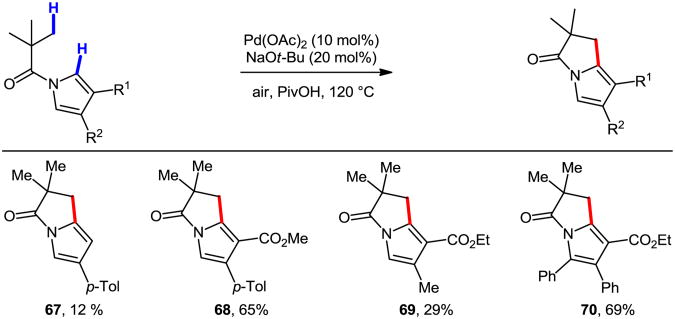
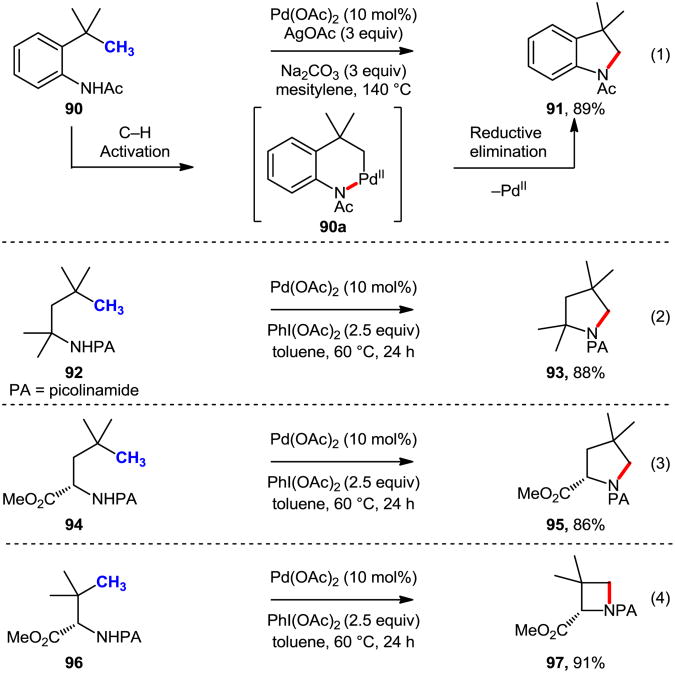
While Pd-catalyzed intramolecular C(sp2)–H amination reactions have become more and more established, C(sp3)–H amination remains a challenge where progress has been far slower until recently.54 Glorius and coworkers reported indoline formation via Pd-catalyzed C(sp3)–H bond amination (Eq. 1, Scheme 25).55 The reaction is believed to proceed by amide directed C(sp3)–H activation, giving palladacycle 90a, which subsequently undergoes reductive elimination to give the product 91. Subsequently, Daugulis and Chen independently developed a similar transformation with picolinamide (PA) -protected amine derivatives and PhI(OAc)2 as the oxidant (Eq. 2 and Eq. 3).52b,52c Interestingly, Chen and coworkers demonstrated that a four-membered ring could be achieved through this cyclization protocol (Eq. 4).52b
Scheme 25.
Indoline formation via Pd-catalyzed C(sp3)–H amination.
4 Intramolecular cyclization via C–O or C–S bond formation
Oxygen-containing heterocyles are another key class of ubiquitous structural motifs. However, Pd-catalyzed intramolecular C–H activation/C–O cyclization is less developed compared to C–N bond formation. In the context of our group's research on new disconnection for heterocycle sythesis via C–H activation, we demonstrated the first example of Pd-catalyzed aliphatic alcohol-directed C–H activation/C–O cyclization for the synthesis of dihydrobenzofuran 100 (Scheme 26).56 The reaction presumably proceeds via Pd(II)/Pd(IV) catalysis by way of intermediate 98a. Both NFSI or hypervalent iodine reagents were found to be suitable bystanding oxidants.51 Importantly, this transformation could be applied in the synthesis of natural products and drug molecules containing spirocyclic dihydrobenzofurans.
Scheme 26. Dihydrobenzofuran formation via PdII/PdIV catalysis.
Recently, Liu and coworkers reported a phenol-directed C–H activation/C–O cyclization reaction as an efficient route to dibenzofurans 103 (Scheme 27) via Pd(II)/Pd(0) catalysis.57 The use of IPr carbene ligand 102 was crucial for C–O reductive elimination from Pd(II), which was believed to be the rate-determining step. Consistent with this hypothesis, a KIE study revealed kH/kD = 1.
Scheme 27. Benzofuran formation via Pd-catalyzed C–H activation/C–O bond formation.
Similar to Pd-catalyzed C–H activation/C–N or C–O bond formation, analogous methodology for C–S bond formation has also been developed. For instance, Inamoto and co-workers described a novel Pd-catalyzed cyclization of thiobenzanilides 104 through a Pd(II)/Pd(0) catalysis.58 The combination of NMP and Bu4NBr is believed to facilitate reoxidation of Pd(0) to Pd(II) (Eq.1, Scheme 28). Later, Joyce and Batey developped an efficient protocol to construct thiazole 107 by using O2 as the terminal oxidant (Eq. 2, Scheme 27).59 The presence of MnO2, which serves a cooxidant, was found to be crucial. Recently, Antonchick and co-workers reported an interesting route to thiophene 109 via Pd-catalyzed C–H activation (Eq. 3, Scheme 27).60
Scheme 28. Early example of Pd-catalyzed C–H activation/C–S bond formation.
4 Incorporation with carbon monoxide/isonitrile
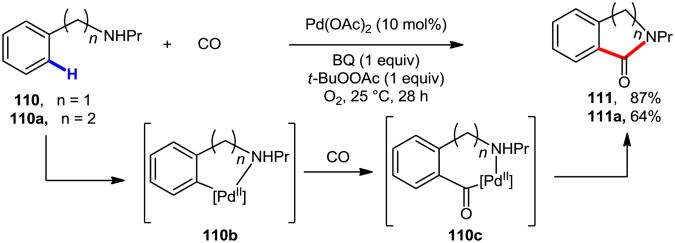
Pd(II)-catalyzed C–H carbonylation is another attractive synthetic tool for the construction of a variety of biologically active heterocycles.61 For instance, in 2004, Orito and co-workers developed a novel and efficient process for the preparation of benzolactams via C–H activation/carbonylation process (Scheme 29).62 Both five and six membered lactams could be prepared, although six-membered lactam formation was found to be favored. Electron-rich substrates gave better yields. Regarding the mechanism, after ortho-palladation, key intermediate 110b is formed. Coordination of CO, followed by 1,1-migratory insertion leads to formation of acylpalladium complex 110c. After nucleophilic attack of the internal amino group on the carbonyl group, the benzolactam product is formed. The same group also reported the synthesis of N-protected staurosporinones and quinolizidine alkaloids by using Pd-catalyzed C–H carbonylation as a key step.63
Scheme 29. Lactam formation via Pd-catalyzed C–H carbonylation.
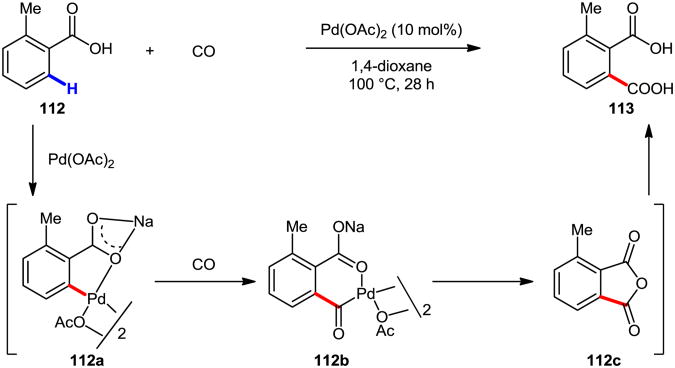
In context of our work in developing C–H functionalization enabled by weakly coordinating functional groups,64 our group developed a Pd(II)-catalyzed C–H carbonylation protocol to convert benzoic acids, such as o-toluic acid 112, to dicarboxylic acids 113 (Scheme 30).65 Mechanistically, the substrate undergoes C–H activation to give palladacyle 112a; CO coordination, followed by 1,1-migratory insertion, and reductive elimination lead the anhydride 112c. Under the reaction conditions, the anhydride is hydrolyzed in situ in the presence of base and adventitious water to give dicarboxylic acid 113 (Scheme 30). Subsequently, the Lloyd-Jones group and our group independently developed C–H carbonylation of anilide-type substrates to construct benzolactams (Eq. 1 and Eq. 2, Scheme 31).66 We were further able to isolate and characterize a monomeric palladacycle containing p-toluenesulfonate as a ligand.66b Another example of C–H carbonylation under mild conditions was reported by Gaunt (Eq. 3).52a cis-Substitution on the side chain in arylethyl amine substrate 118 was necessary. Garcia and Granell demonstrated that unprotected tertiary arylethylamine 120 could undergo C–H carbonylation (Eq. 4).67 Zhu and coworkers reported the synthesis of quinazolin-4(3H)-one 123 from N-arylamidine 122 by employing a similar transformation (Eq. 5).68 Aryl lactones are another important class of heterocycles that we have targeted as desirable targets for synthesis. After extensive optimization, we developed a Pd-catalyzed C–H carbonylation reaction of phenethyl alcohol substrate (Eq. 6).69 The use of (+)-Men-Leu-OH, which presumably facilitates C–H cleavage and stabilizes Pd(0) (preventing precipitation of inactive Pd black), 124a was found to be crucial for high yields.70
Scheme 30. Pd-catalyzed C–H carbonylation of o-toluic acid.
Scheme 31. Annulation with carbon monoxide.
Despite extensive progress made in Pd-catalyzed C(sp2)–H carbonylation, until recently no examples of C(sp3)–H carbonylation had been reported. Our group reported the first example of such a reaction, enabling a new method for the synthesis of succinimide 127 (Scheme 32).71
Scheme 32. Lone example of Pd-catalyzed C(sp3)–H carbonylation.
Incorporation with isonitrile
Analagous to the CO chemistry described above, Zhu and co-workers reported a rare example of heterocycle formation with isonitrile insertion to give the product 130 in good yield (Scheme 33)72
Scheme 33. Synthesis of quinazoline derivatives via C–H activation.
5 Incorporation with olefin/alkyne
PdII-catalyzed C(aryl)–H olefination, one of the oldest classes of PdII-catalyzed C–H activation reactions, provides an alternative approach to C–C bond formation compared to the traditional Pd(0)-catalyzed Mizoroki–Heck reaction, which couples aryl halides with olefins.73 An attractive approach for heterocycle formation would thus be to merge this chemistry with a subsequent reaction in which the olefinated product would undergo intramolecular 1,4-conjugate addition or Wacker-type oxidative cyclization.74 For instance, Miura and coworkers developed such a sequence to generate phenanthridine derivative 133 (Scheme 34).75 The acidity of the sulfonamide N–H bond is quite crucial, since other amides proved to be ineffective. The authors expandws these powerful reactions to benzoic and naphthoic acids, which are structurally related acidic compounds, though low yields were obtained due to the existence of multiple possible pathways.
Scheme 34. An early example of Pd-catalyzed C–H olefination followed by nucleophilic cyclization.
More recently, the group of Lloyd-Jones and Booker-Milburn reported a similar transformation to construct indoline 136 via Pd-catalyzed C–H olefination, followed by intramlecular Tsuji–Trost-type allylic amination (Scheme 35).76 It is worth noting that both the use of Pd(OTs)2(CH3CN)2 and anhydrous conditions were crucial to obtain the observed results.
Scheme 35. Indoline formation via C–H olefination.
By tuning the acidity of amides through the introduction of electron-withdrawing groups, our group was able to achieve C–H iodination of simple phenethyl amine derivatives.77 We envisioned that important heterocyles such as tetrahydroisoquinolines could be formed via C–H olefination followed by aza-Michael addition. Indeed, when vinyl ketone 138 was used as the olefin coupling partner, the desired tetrahydroquinolines were achieved with excellent diastereoselectivity (Scheme 36).78 Our group also demonstrated alcohol-directed C–H olefination followed by oxidative Wacker-type cyclization to give the cyclic ether 142 (Scheme 37).78 It should be pointed out the addition of amino acid ligand70 was found to be crucial for high yields. Secondary and primary alcohols were also effective, though they gave lower yields.
Scheme 36. Tetrahydroquinoline formation via Pd-catalyzed C-H olefination followed by nucleophilic cyclization.
Scheme 37.
cyclic ether formation via Pd-catalyzed C–H olefination.
Zhu and Falck reported lactam formation via a similar transformation using N-tosylbenzamide substrate 143 (Scheme 38).79 Importantly, a simple alkene could also be used to give annulations products via C–H olefination and oxidative Wacker-type cyclization. Phenanthroline ligands were found to be effective in this transformation. Electrophilc palladation was suggested based on the observed relative rates, though a KIE = 3.0 was also observed.
Scheme 38. Lactam formation via Pd-catalyzed C–H olefination/cyclization.
Similar to the case of Pd(II)-catalyzed C(sp3)–H carbonylation, no examples of C(sp3)–H olefination had been reported until recently. Using an analogous approach for reaction development, our group was able to achieve a Pd(II)-catalyzed reaction protocol for the direct olefination of sp3 C–H bonds (Scheme 39). After C–H olefination, the amide product was observed to undergo 1,4-conjugate addition to give the corresponding lactam compound 149 (Scheme 39).80 The reaction conditions could also be applied to effect olefination of cyclopropyl methylene C–H bonds and substrates containing α-hydrogen atoms.
Scheme 39. Pd-catalyzed C(sp3)–H olefination.
Sanford and coworkers later found that pyridine-directed C(sp3)–H olefination/cyclization could be achieved (Scheme 40).81
Scheme 40. Pd-catalyzed C(sp3)–H olefination.
Incorporation with alkyne
In addition to olefins, other unsaturated carbon units can be used for C–H functionalization/annulation sequences. Zhu and coworkers reported a highly efficient Pd-catalyzed synthesis of unsymmetrically substituted 3-(diarylmethylenyl)indolinones from the corresponding aryl alkyne.82 The domino reaction involves oxidative addition of an aryl halide to generate an aryl palladium species, which undergoes intermolecular carbopalladation, followed by C–H activation, and C–C bond formation (Scheme 41).
Scheme 41. Pd-catalyzed C–H carbonylation of analide.
Jiao and coworker recently developed a tandem reaction to construct indoles by reacting anilines with alkynes. The author extended this method to 2-substitued indole substrates to give six-membered lactams or pyridines (Scheme 42).83
Scheme 42. Synthesis of indole via tandem reaction.
6. Conclusions
Due to the importance of heterocycles in all aspects of chemistry, the development of efficient and straightforward methods for their synthesis is of paramout importance. In this short review, we have sought to highlight recent advances in construction of heterocycles via Pd-catalyzed C–H activation followed by C–C or C–heteroatom bond formation. This technology not only provides more atom- and step-economical methodology but also provides alternative retrosynthetic disconnections that map bulk chemicals onto valuable heterocylic building blocks. The development of more reliable and efficient catalysis will make C–H functionalization ever more practical and useful in the heterocycle synthesis.
Acknowledgments
We are indebted to all present and former group members for their invaluable contributions to the work described herein. We acknowledge TSRI, the NSF (CHE-1011898), the NIH (NIGMS, 1 R01 GM084019-02), and Pfizer for financial assistance. Additional support was provided through the NSF Center for Stereoselective C–H Functionalization (CHE-0943980). Individual awards and fellowships were granted by TSRI, the NSF GRFP, the NDSEG Fellowship program, and the Skaggs-Oxford Scholarship program (K.M.E.); the Chinese Government Scholarship Council (T.-S.M.): TSRI Manuscript no. xxxxx.
References
- 1.For selected reviews of methodology for constructing heterocycles using traditional cross-coupling reactions, see: Diederich F, Stang PJ. Metal-Catalyzed Cross-Coupling Reactions. Eds. Wiley-VCH; New York: 1998. Nakamura I, Yamamoto Y. Chem Rev. 2004;104:2127. doi: 10.1021/cr020095i.Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873. doi: 10.1021/cr040639b.Zeni G, Larock RC. Chem Rev. 2006;106:4644. doi: 10.1021/cr0683966.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem, Int Ed. 2008;47:6338.
- 2.For selected reviews on transition metal–catalyzed C–H activation reactions, see: Crabtree RH. Chem Rev. 1985;85:245.Shilov AE, Shul'pin GB. Chem Rev. 1997;97:2879. doi: 10.1021/cr9411886.Jia C, Kitamura T, Fujiwara Y. Acc Chem Res. 2001;34:633. doi: 10.1021/ar000209h.Ritleng V, Sirlin C, Pfeffer M. Chem Rev. 2002;102:1731. doi: 10.1021/cr0104330.Labinger JA, Bercaw JE. Nature. 2002;417:507. doi: 10.1038/417507a.Kakiuchi F, Chatani N. Adv Synth Catal. 2003;345:1077.Yu JQ, Giri R, Chen X. Org Biomol Chem. 2006;4:4041. doi: 10.1039/b611094k.Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.Bergman RG. Nature. 2007;446:391. doi: 10.1038/446391a.Hartwig JF. Nature. 2008;455:314. doi: 10.1038/nature07369.Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.
- 3.For perspectives on atom-, step-, and redox economy, respectively, see: Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40. doi: 10.1021/ar700155p.Burns NZ, Baran PS, Hoffmann RW. Angew Chem, Int Ed. 2009;48:2854. doi: 10.1002/anie.200806086.
- 4.For selected reviews of methodology for constructing heterocycles by transition metal–catalyzed C–H functionalization, see: Thansandote P, Lautens M. Chem Eur J. 2009;15:5874. doi: 10.1002/chem.200900281.Zhang M. Adv Synth Catal. 2009;351:2243.Beccalli EM, Broggini G, Fasana A, Rigamonti M. J Organomet Chem. 2011;696:277.Stokes BJ, Driver TG. Eur J Org Chem. 2011:4071.. For selected reviews on synthetic application of C–H bond functionalization, see: Gutekunst WR, Baran PS. Chem Soc Rev. 2011;40:1976. doi: 10.1039/c0cs00182a.Engle KM, Yu JQ. Transition Metal–Catalyzed C–H Functionalization: Synthetically Enabling Reactions for Building Molecular Complexity. In: Ding K, Dai LX, editors. Organic Chemistry – Breakthroughs and Perspectives. Wiley-VCH; Weinheim: 2012.
- 5.For selected reviews of Pd-catalyzed C–H activation/C–C bond formation, see: Campeau LC, Stuart DR, Fagnou K. Aldrichimica Acta. 2007;40:35.Li BJ, Yang SD, Shi ZJ. Synlett. 2008:949.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem, Int Ed. 2009;48:5094. doi: 10.1002/anie.200806273.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058.
- 6.For reviews of methodology for constructing heterocycles via Pd-catalyzed C–H activation arene/arene coupling, see: Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem Rev. 2002;102:1359. doi: 10.1021/cr000664r.Harayama T. Heterocycles. 2005;65:697.Campeau LC, Fagnou K. Chem Commun. 2006:1253. doi: 10.1039/b515481m.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173. doi: 10.1039/b606984n.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174. doi: 10.1021/cr0509760.Ackermann L, Vicente R, Kapdi AR. Angew Chem, Int Ed. 2009;48:9792. doi: 10.1002/anie.200902996.
- 7.For mechanistic studies of C–H cleavage by Pd(II), see: Ryabov AD, Sakodinskaya IK, Yatsimirsky AK. J Chem Soc Dalton Trans. 1985;2629Canty AJ, van Koten G. Acc Chem Res. 1995;28:406.Gómez M, Granell J, Martinez M. J Chem Soc Dalton Trans. 1998;37Davies DL, Donald SMA, Macgregor SA. J Am Chem Soc. 2005;127:13754. doi: 10.1021/ja052047w.. For a recent review, see: Lapointe D, Fagnou K. Chem Lett. 2010;39:1118.
- 8.For early examples of heterocycle formation via Pd-catalyzed C–H arylation, see: Ames DE, Bull D. Tetrahedron. 1982;38:383.Ames DE, Opalko A. Synthesis. 1983;39:234.Ames DE, Opalko A. Tetrahedron. 1984;40:1919.
- 9.Hennings DD, Iwasa S, Rawal VH. J Org Chem. 1997;62:2. doi: 10.1021/jo961876k. [DOI] [PubMed] [Google Scholar]
- 10.(a) Campeau LC, Parisien M, Leblanc M, Fagnou K. J Am Chem Soc. 2004;126:9186. doi: 10.1021/ja049017y. [DOI] [PubMed] [Google Scholar]; (b) Campeau LC, Thansandote P, Fagnou K. Org Lett. 2005;7:1857. doi: 10.1021/ol050501v. [DOI] [PubMed] [Google Scholar]; (c) Campeau LC, Parisien M, Jean A, Fagnou K. J Am Chem Soc. 2006;128:581. doi: 10.1021/ja055819x. [DOI] [PubMed] [Google Scholar]
- 11.Shiotani A, Itatani H. Angew Chem, Int Ed. 1974;13:471. [Google Scholar]
- 12.Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org Lett. 2007;9:3137. doi: 10.1021/ol071308z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For early examples of biaryl formations via Pd-catalyzed intermolecular C–H/C–H cross-coupling, see: Li R, Jiang L, Lu W. Organometallics. 2006;25:5973.Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956.Stuart DR, Villemure E, Fagnou K. J Am Chem Soc. 2007;129:12072. doi: 10.1021/ja0745862.Hull KL, Sanford MS. J Am Chem Soc. 2007;129:11904. doi: 10.1021/ja074395z.
- 14.Liégault B, Lee D, Huestis MP, Stuart DR, Fagnou K. J Org Chem. 2008;73:5022. doi: 10.1021/jo800596m. [DOI] [PubMed] [Google Scholar]
- 15.Sridharan V, Martín MA, Menéndez JC. Eur J Org Chem. 2009:4614. [Google Scholar]
- 16.(a) Watanabe T, Ueda S, Inuki S, Oishi S, Fujii N, Ohno H. Chem Commun. 2007:4516. doi: 10.1039/b707899d. [DOI] [PubMed] [Google Scholar]; (b) Watanabe T, Oishi S, Fujii N, Ohno H. J Org Chem. 2009;74:4720. doi: 10.1021/jo9003376. [DOI] [PubMed] [Google Scholar]
- 17.Liégault B, Fagnou K. Organometallics. 2008;27:4841. [Google Scholar]
- 18.Yeung CS, Borduas N, Zhao X, Dong VM. Chem Sci. 2010;1:331. [Google Scholar]
- 19.Ackermann L, Jeyachandran R, Potukuchi HK, Novák P, Büttner L. Org Lett. 2010;12:2056. doi: 10.1021/ol1005517. [DOI] [PubMed] [Google Scholar]
- 20.Pintori DG, Greaney MF. J Am Chem Soc. 2011;133:1209. doi: 10.1021/ja1090854. [DOI] [PubMed] [Google Scholar]
- 21.Iida H, Yuasa Y, Kibayashi C. J Org Chem. 1980;45:2938. [Google Scholar]
- 22.Åkermark B, Oslob JD, Heuschert U. Tetrahedron Lett. 1995;36:1325. [Google Scholar]
- 23.Zhang H, Ferreira EM, Stoltz BM. Angew Chem, Int Ed. 2004;43:6144. doi: 10.1002/anie.200461294. [DOI] [PubMed] [Google Scholar]
- 24.Schiffner JA, Oestreich M. Eur J Org Chem. 2011:1148. [Google Scholar]
- 25.Li C, Zhang Y, Li P, Wang L. J Org Chem. 2011;76:4692. doi: 10.1021/jo200317f. [DOI] [PubMed] [Google Scholar]
- 26.For examples of Pd(II)-catalyzed intramolecular C–H olefination with nitrogen-containing heterocycles, see: Ferreira EM, Stoltz BM. J Am Chem Soc. 2003;125:9578. doi: 10.1021/ja035054y.Beccalli EM, Broggini G. Tetrahedron Lett. 2003;44:1919.Liu C, Widenhoefer RA. J Am Chem Soc. 2004;126:10250. doi: 10.1021/ja046810i.. For examples of C–H activation followed by enantioselective addition to a tethered olefin, see: Schiffner JA, Machotta AB, Oestreich M. Synlett. 2008:2271.Schiffner JA, Wöste TH, Oestreich M. Eur J Org Chem. 2010:174.
- 27.For applications of Pd(II)-mediated intramolecular C–H olefination with nitrogen-containing heterocycles in total synthesis, see: Trost BM, Godleski SA, Genêt JP. J Am Chem Soc. 1978;100:3930.Cushing TD, Sanz-Cervera JF, Williams RM. J Am Chem Soc. 1993;115:9323.Baran PS, Corey EJ. J Am Chem Soc. 2002;124:7904. doi: 10.1021/ja026663t.Garg NK, Caspi DD, Stoltz BM. J Am Chem Soc. 2004;126:9552. doi: 10.1021/ja046695b.Beck EM, Hatley R, Gaunt MJ. Angew Chem, Int Ed. 2008;47:3004. doi: 10.1002/anie.200705005.Bowie AL, Jr, Trauner D. J Org Chem. 2009;74:1581. doi: 10.1021/jo801791j.
- 28.(a) Jia C, Piao D, Oyamada J, Lu W, Kitamura T, Fujiwara Y. Science. 2000;287:1992. doi: 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]; (b) Jia C, Piao D, Oyamada J, Lu W, Kitamura T, Fujiwara Y. J Org Chem. 2000;65:7516. doi: 10.1021/jo000861q. [DOI] [PubMed] [Google Scholar]
- 29.Pinto A, Neuville L, Retailleau P, Zhu J. Org Lett. 2006;8:4927. doi: 10.1021/ol062022h. [DOI] [PubMed] [Google Scholar]
- 30.Tang DJ, Tang BX, Li JH. J Org Chem. 2009;74:6749. doi: 10.1021/jo901314t. [DOI] [PubMed] [Google Scholar]
- 31.Hennessy EJ, Buchwald SL. J Am Chem Soc. 2003;125:12084. doi: 10.1021/ja037546g. [DOI] [PubMed] [Google Scholar]
- 32.Petronijevic FR, Wipf P. J Am Chem Soc. 2011;133:7704. doi: 10.1021/ja2026882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.For early examples of C(sp3)–H activation/arylation, see: Dyker G. Angew Chem, Int Ed. 1992;31:1023.Dyker G. J Org Chem. 1993;58:6426.Dyker G. Angew Chem, Int Ed. 1999;38:1698. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1698::AID-ANIE1698>3.0.CO;2-6.. For reviews of C(sp3)–H activation/arylation, see Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur, J. 2010;16:2654. doi: 10.1002/chem.200902374.Baudoin O. Chem Soc, Rev. 2011;40:4902. doi: 10.1039/c1cs15058h.
- 34.Lafrance M, Gorelsky SI, Fagnou K. J Am Chem Soc. 2007;129:14570. doi: 10.1021/ja076588s.. For mechanistic studies, see: Rousseaux S, Davi M, Sofack-Kreutzer J, Pierre C, Kefalidis CE, Clot E, Fagnou K, Baudoin O. J Am Chem Soc. 2010;132:10706. doi: 10.1021/ja1048847.
- 35.Watanabe T, Oishi S, Fujii N, Ohno H. Org Lett. 2008;10:1759. doi: 10.1021/ol800425z. [DOI] [PubMed] [Google Scholar]
- 36.(a) Nakanishi M, Katayev D, Besnard C, Kündig EP. Angew Chem, Int Ed. 2011;50:7438. doi: 10.1002/anie.201102639. [DOI] [PubMed] [Google Scholar]; (b) Anas S, Cordi A, Kagan HB. Chem Comm. 2011;47:11483. doi: 10.1039/c1cc14292e. [DOI] [PubMed] [Google Scholar]
- 37.Rousseaux S, Gorelsky SI, Chung BKW, Fagnou K. J Am Chem Soc. 2010;132:10692. doi: 10.1021/ja103081n. [DOI] [PubMed] [Google Scholar]
- 38.For selected examples of intermolecular C(sp3)–H arylation, see: Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154. doi: 10.1021/ja054549f.Reddy BVS, Reddy LR, Corey EJ. Org Lett. 2006;8:3391. doi: 10.1021/ol061389j.Giri R, Maugel N, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J Am Chem Soc. 2007;129:3510. doi: 10.1021/ja0701614.Wasa M, Engle KM, Yu JQ. J Am Chem Soc. 2009;131:9886. doi: 10.1021/ja903573p.Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965. doi: 10.1021/ja910900p.Feng Y, Chen G. Angew Chem, Int Ed. 2010;49:958. doi: 10.1002/anie.200905134.He G, Chen G. Angew Chem, Int Ed. 2011;50:5192. doi: 10.1002/anie.201100984.Gutekunst WR, Baran PS. J Am Chem Soc. 2011;133:19076. doi: 10.1021/ja209205x.
- 39.Feng Y, Wang Y, Landgraf B, Liu S, Chen G. Org Lett. 2010;12:3414. doi: 10.1021/ol101220x. [DOI] [PubMed] [Google Scholar]
- 40.For initial reports of the Buchwald–Hartwig amination reaction, see: Paul F, Patt J, Hartwig JF. J Am Chem Soc. 1994;116:5969.Guram AS, Buchwald SL. J Am Chem Soc. 1994;116:7901.. For early studies concerning C–N bond formation using Pd(0), see: Kosugi M, Kameyama M, Migita T. Chem Lett. 1983:927.Boger DL, Panek JS. Tetrahedron Lett. 1984;25:3175.
- 41.For pioneering work in Pd-catalyzed C–H amination, see: Tsang WCP, Zheng N, Buchwald SL. J Am Chem Soc. 2005;127:14560. doi: 10.1021/ja055353i.
- 42.For mechanistic studies and applications of the transformation, see: Tsang WCP, Munday RH, Brasche G, Zheng N, Buchwald SL. J Org Chem. 2008;73:7603. doi: 10.1021/jo801273q.. For another application of this transformation, see: Li BJ, Tian SL, Fang Z, Shi ZJ. Angew Chem, Int Ed. 2008;47:1115. doi: 10.1002/anie.200704092.
- 43.For carbazole formation via Pd-catalyzed C–H amination, see: Jorden-Hore JA, Johansson CCC, Gulias M, Beck EM, Gaunt MJ. J Am Chem Soc. 2008;130:16184. doi: 10.1021/ja806543s.Youn SW, Bihn JH, Kim BS. Org Lett. 2011;13:3738. doi: 10.1021/ol201416u.. For carbazole formation via a radical cyclization approach, see: Cho SH, Yoon J, Chang S. J Am Chem Soc. 2011;133:5996. doi: 10.1021/ja111652v.Antonchick AP, Samanta R, Kulikov K, Lategahn J. Angew Chem, Int Ed. 2011;50:8605. doi: 10.1002/anie.201102984.. For an early example of hypervalent iodine–mediated radical cyclization, see: Togo H, Hoshina Y, Yokoyama M. Tetrahedron Lett. 1996;37:6129.
- 44.Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Org Lett. 2007;9:2931. doi: 10.1021/ol0711117. [DOI] [PubMed] [Google Scholar]
- 45.(a) Inamoto K, Saito T, Hiroya K, Doi T. Synlett. 2008;20:3157. [Google Scholar]; (b) Inamoto K, Saito T, Hiroya K, Doi T. J Org Chem. 2010;75:3900. doi: 10.1021/jo100557s. [DOI] [PubMed] [Google Scholar]; (c) Xiao Q, Wang WH, Liu G, Meng FK, Chen JH, Yang Z, Shi ZJ. Chem Eur J. 2009;15:7292. doi: 10.1002/chem.200900154. [DOI] [PubMed] [Google Scholar]; (d) Inamoto K, Hasegawa C, Kawasaki J, Hiroya K, Doi T. Adv Syn Cat. 2010;352:2643. [Google Scholar]; (e) Wang GW, Yuan TT, Li DD. Angew Chem, Int Ed. 2011;50:1380. doi: 10.1002/anie.201005874. [DOI] [PubMed] [Google Scholar]
- 46.Wasa M, Yu JQ. J Am Chem Soc. 2008;130:14058. doi: 10.1021/ja807129e. [DOI] [PubMed] [Google Scholar]
- 47.For selected examples for allylic amination, see: Fraunhoffer KJ, White MC. J Am Chem Soc. 2007;129:7274. doi: 10.1021/ja071905g.Reed SA, White MC. J Am Chem Soc. 2008;130:3316. doi: 10.1021/ja710206u.Liu G, Yin G, Wu L. Angew Chem, Int Ed. 2008;47:4733. doi: 10.1002/anie.200801009.
- 48.Miura T, Ito Y, Murakami M. Chem Lett. 2009;38:328. [Google Scholar]
- 49.For selected examples of Pd-catalyzed fluorination using F+, see: Hull KL, Anani WQ, Sanford MS. J Am Chem Soc. 2006;128:7134. doi: 10.1021/ja061943k.Wang X, Mei TS, Yu JQ. J Am Chem Soc. 2009;131:7520. doi: 10.1021/ja901352k.Chan KSL, Wasa M, Wang X, Yu JQ. Angew Chem, Int Ed. 2011;50:9081. doi: 10.1002/anie.201102985.. For studies on C–F reductive elimination from Pd(IV) complexes, see: Yahav A, Goldberg I, Vigalok A. J Am Chem Soc. 2003;125:13634. doi: 10.1021/ja0377753.Kaspi AW, Yahav-Levi A, Goldberg I, Vigalok A. Inorg Chem. 2008;47:5. doi: 10.1021/ic701722f.Furuya T, Ritter T. J Am Chem Soc. 2008;130:10060. doi: 10.1021/ja803187x.Ball ND, Sanford MS. J Am Chem Soc. 2009;131:3796. doi: 10.1021/ja8054595.Furuya T, Benitez D, Tkatchouk E, Strom AE, Tang P, Goddard WA, III, Ritter T. J Am Chem Soc. 2010;132:3793. doi: 10.1021/ja909371t.
- 50.Mei TS, Wang X, Yu JQ. J Am Chem Soc. 2009;131:10806. doi: 10.1021/ja904709b. [DOI] [PubMed] [Google Scholar]
- 51.For a review of bystanding F+ oxidants, see: Engle KM, Mei TS, Wang X, Yu JQ. Angew Chem, Int Ed. 2011;50:1478. doi: 10.1002/anie.201005142.
- 52.(a) Haffemayer B, Gulias M, Gaunt MJ. Chem Sci. 2011;2:312. [Google Scholar]; (b) He G, Zhao Y, Zhang S, Lu C, Chen G. J Am Chem Soc. 2012;134:3. doi: 10.1021/ja210660g. [DOI] [PubMed] [Google Scholar]; (c) Nadres ET, Daugulis O. J Am Chem Soc. 2012;134:7. doi: 10.1021/ja210959p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.For C–H amination via Pd(0)/Pd(II) catalysis, see: Tan Y, Hartwig JF. J Am Chem Soc. 2010;132:3676. doi: 10.1021/ja100676r.
- 54.For examples of Pd-catalyzed C(sp3)–H amination, see: Thu HY, Yu WY, Che CM. J Am Soc Chem. 2006;128:9048. doi: 10.1021/ja062856v.Pan J, Su M, Buchwald SL. Angew Chem, Int Ed. 2011;50:8647. doi: 10.1002/anie.201102880.
- 55.Neumann JJ, Rakshit S, Dröge T, Glorius F. Angew Chem, Int Ed. 2009;48:6892. doi: 10.1002/anie.200903035. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Lu Y, Dai HX, Yu JQ. J Am Chem Soc. 2010;132:12203. doi: 10.1021/ja105366u. [DOI] [PubMed] [Google Scholar]
- 57.Xiao B, Gong TJ, Liu ZJ, Liu JH, Luo DF, Xu J, Liu L. J Am Chem Soc. 2011;133:9250. doi: 10.1021/ja203335u. [DOI] [PubMed] [Google Scholar]
- 58.Inamoto K, Hasegawa C, Hiroya K, Doi T. Org Lett. 2008;10:5147. doi: 10.1021/ol802033p. [DOI] [PubMed] [Google Scholar]
- 59.Joyce LL, Batey RA. Org Lett. 2009;11:2792. doi: 10.1021/ol900958z. [DOI] [PubMed] [Google Scholar]
- 60.Samanta R, Antonchick AP. Angew Chem, Int Ed. 2011;50:5217. doi: 10.1002/anie.201100775. [DOI] [PubMed] [Google Scholar]
- 61.For selected reviews of palladacycle carbonylation, see: Ryabov AD. Synthesis. 1985:233. (b) Ref 6b. For recent examples, see: Vicente J, Saura-Llamas I, García-López JA, Calmuschi-Cula B. Organometallic. 2007;26:2768.Vicente J, Saura-Llamas I, García-López JA, Bautista D. Organometallic. 2009;28:448.Vicente J, González-Herrero P, Frutos-Pedreño R, Chicote MT, Jones PG, Bautista D. Organometallic. 2011;30:1079.. For selected reviews of Pd(II)-catalyzed C–H carbonylation of benzene, see: Fujiwara Y, Takaki K, Taniguchi Y. Synlett. 1996:591. (g) Ref. 2c.
- 62.For the first report of lactam formation via Pd-catalyzed C–H carbonylation, see: Orito K, Horibata A, Nakamura T, Ushito H, Nagasaki H, Yuguchi M, Yamashita S, Tokuda M. J Am Chem Soc. 2004;126:14342. doi: 10.1021/ja045342+.
- 63.(a) Wada Y, Nagasaki H, Tokuda M, Orito K. J Org Chem. 2007;72:2008. doi: 10.1021/jo062184r. [DOI] [PubMed] [Google Scholar]; (b) Yamashita S, Kurono N, Senboku H, Tokuda M, Orito K. Eur J Org Chem. 2009:1173. doi: 10.1021/jo900311g. [DOI] [PubMed] [Google Scholar]
- 64.Mei TS, Giri R, Maugel N, Yu JQ. Angew Chem, Int Ed. 2008;47:5215. doi: 10.1002/anie.200705613.Mei TS, Wang D, Yu JQ. Org Lett. 2010;12:3140. doi: 10.1021/ol1010483.. For a review of directed Pd-catalyzed C–H via weak coordination, see: Engle KM, Mei TS, Yu JQ. Acc Chem Res. 2012 doi: 10.1021/ar200185g.
- 65.Giri R, Yu JQ. J Am Chem Soc. 2008;130:14082. doi: 10.1021/ja8063827. [DOI] [PubMed] [Google Scholar]
- 66.(a) Houlden CE, Hutchby M, Bailey CD, Ford JG, Tyler SNG, Gagné MR, Lloyd-Jones GC, Booker-Milburn KI. Angew Chem, Int Ed. 2009;48:1830. doi: 10.1002/anie.200805842. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Giri R, Lam JK, Yu JQ. J Am Chem Soc. 2010;132:686. doi: 10.1021/ja9077705. [DOI] [PubMed] [Google Scholar]
- 67.López B, Rodriguez A, Santos D, Albert J, Ariza X, Garcia J, Granell J. Chem Commun. 2011;47:1054. doi: 10.1039/c0cc03478a. [DOI] [PubMed] [Google Scholar]
- 68.Ma B, Wang Y, Peng J, Zhu Q. J Org Chem. 2011;76:6362. doi: 10.1021/jo2007362. [DOI] [PubMed] [Google Scholar]
- 69.Lu Y, Leow D, Wang X, Engle KM, Yu JQ. Chem Sci. 2011;2:967. [Google Scholar]
- 70.For selected examples highlighting the effects of amino acid ligands in Pd-catalyzed C–H activation, see: Shi BF, Maugel N, Zhang YH, Yu JQ. Angew Chem, Int Ed. 2008;47:4882. doi: 10.1002/anie.200801030.Shi BF, Zhang YH, Lam JK, Wang DH, Yu JQ. J Am Chem Soc. 2010;132:460. doi: 10.1021/ja909571z.Engle KM, Wang DH, Yu JQ. J Am Chem Soc. 2010;132:14137. doi: 10.1021/ja105044s.
- 71.For the sole example of Pd(II)-catalyzed C(sp3)–H carbonylation reported to date, see: Yoo EJ, Wasa M, Yu JQ. J Am Chem Soc. 2010;132:17378. doi: 10.1021/ja108754f.
- 72.Wang Y, Wang H, Peng J, Zhu Q. Org Lett. 2011;13:4604. doi: 10.1021/ol201807n. [DOI] [PubMed] [Google Scholar]
- 73.For early reports of Pd(II)-mediated C(aryl)–H olefination, see: Moritani I, Fujiwara Y. Tetrahedron Lett. 1967;8:1119.Fujiwara Y, Moritani I, Matsuda M, Teranishi S. Tetrahedron Lett. 1968;9:633.Fujiwara Y, Noritani I, Danno S, Asano R, Teranishi S. J Am Chem Soc. 1969;91:7166. doi: 10.1021/ja01053a047.. For selected examples of Pd-catalyzed C–H olefination using directing groups, see: Diamond SE, Szalkiewicz A, Mares F. J Am Chem Soc. 1979;101:490.Miura M, Tsuda T, Satoh T, Nomura M. Chem Lett. 1997;26:1103.Boele MDK, van Strijdonck GPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J Am Chem Soc. 2002;124:1586. doi: 10.1021/ja0176907.
- 74.Larock RC, Varaprath S, Lau HH, Fellows CA. J Am Chem Soc. 1984;106:5274. [Google Scholar]
- 75.Miura M, Tsuda T, Satoh T, Pivsa-Art S, Nomura M. J Org Chem. 1998;63:5211. [Google Scholar]
- 76.Houlden CE, Bailey CD, Ford JG, Gagné MR, Lloyd-Jones GC, Booker-Milburn KI. J Am Chem Soc. 2008;130:10066. doi: 10.1021/ja803397y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li JJ, Mei TS, Yu JQ. Angew Chem, Int Ed. 2008;47:6452. doi: 10.1002/anie.200802187. [DOI] [PubMed] [Google Scholar]
- 78.Lu Y, Wang DH, Engle KM, Yu JQ. J Am Chem Soc. 2010;132:5916. doi: 10.1021/ja101909t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu C, Falck JR. Org Lett. 2011;13:1214. doi: 10.1021/ol200093f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.For the first example of Pd(II)-catalyzed C(sp3)–H olefination, see: Wasa M, Engle KM, Yu JQ. J Am Chem Soc. 2010;132:3680. doi: 10.1021/ja1010866.
- 81.Stowers KJ, Fortner KC, Sanford MS. J Am Chem Soc. 2011;133:6541. doi: 10.1021/ja2015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pinto A, Neuville L, Retailleau P, Zhu J. Org Lett. 2006;8:4927. doi: 10.1021/ol062022h. [DOI] [PubMed] [Google Scholar]
- 83.Zhou W, Xu J, Zhang L, Jiao N. Org Lett. 2010;12:2888. doi: 10.1021/ol101094u. [DOI] [PubMed] [Google Scholar]