Abstract
Nocturnal enuresis often causes considerable distress or functional impairment to patient and their parents necessitating a multidisciplinary approach from paediatrician, paediatric nephrologist, urologists and psychiatrist. Mechanisms of monosymptomatic nocturnal enuresis are mainly nocturnal polyuria, bladder overactivity and failure to awaken from sleep in response to bladder sensations. Goal oriented and etiology wise treatment includes simple behavioral intervention, conditioning alarm regimen and pharmacotherapy with desmopressin, imipramine and anticholinergic drugs. Symptoms often recurs requiring change over or combination of different modes of
treatment.
Keywords: Nocturnal enuresis, Monosymptomatic, Conditioning alarm, Desmopressin, Imipramine
Core tip: Nocturnal enuresis often causes considerable distress to patient and their parents’ lifestyle necessitating a multidisciplinary management. Simple behavioral interventions, conditioning alarm regimen and pharmacotherapy as desmopressin, imipramine and anticholinergic drugs are the mainstay of therapy used as per underlying etiology or parents’ concern. Therapy should be structured and goal directed to reduce recurrence.
INTRODUCTION
Enuresis though often conceived as a simple problem can have multiple hidden etiologies necessitating a multidisciplinary approach involving paediatrician, paediatric nephrologist, urologists and child and adolescent psychiatrist. The complexity in both assessment and treatment underscores the need for practice parameters for clinicians confronting this problem.
DEFINITIONS
As per DSM-IV-TR, enuresis is defined as repeated voiding of urine into the bed or clothes at least twice per week for at least three consecutive months in a child who is ≥ 5 years of age[1]. A child may also be considered to be enuretic if the frequency or duration is less, but there is associated distress or functional impairment. As per International Children’s Continence Society (ICCS), enuresis can be defined as urinary incontinence while asleep in a child aged at least 5 years[2]. The DSM-III and ICD-10 define a bed-wetting frequency of twice per month in the past 3 mo for children ages 5 and 6 years and once per month in the past 3 mo for children ages 7 years or older. The DSM-IV-TR includes voluntary as well as involuntary voiding, although most studies exclude children who voluntarily or intentionally wet their bed or clothes. Nocturnal enuresis refers to voiding during sleep; diurnal enuresis defines wetting while awake.
TYPES
Enuresis may be of monosymptomatic or non-monosymptomatic forms.
Monosymptomatic enuresis (MNE) denotes enuresis in children without any other lower urinary tract symptoms and without a history of bladder dysfunction[2]. Non-monosymptomatic (NMNE) enuresis is defined as enuresis in children with other lower urinary tract symptoms (Table 1). It can also be classified as primary enuresis occurring in children who have never been consistently dry throughout the night, or secondary enuresis which refers to the resumption of wetting after at least 6 mo of dryness[3].
Table 1.
Lower urinary tract symptoms
| Consistently increased (≥ 8 times/d) or decreased (≤ 3 times/d) voiding frequency |
| Daytime incontinence |
| Urgency |
| Hesitancy |
| Straining (application of abdominal pressure to initiate and maintain voiding) |
| A weak stream |
| Intermittency (micturition occurs in several discrete spurts) |
| Holding maneuvers (strategies used to postpone voiding) |
| A feeling of incomplete emptying |
| Post-micturition dribbling |
| Genital or lower urinary tract pain |
EPIDEMIOLOGY
The reported prevalence of enuresis at different ages varies considerably because of inconsistencies in its definition as stated earlier, differences in the method of data collection, and differences in the characteristics of the population sampled. Nocturnal incontinence occurs in 12% to 25% of 4-year-old children, 7% to 10% of 8-year-old children, and 2% to 3% of 12-year-old children[4]. It may be problematic even in late teenage years (1% to 3%)[5] and if untreated enuresis (especially if severe) can persist indefinitely with prevalence rates of 2%-3% in adulthood[6,7]. Primary enuresis is twice as common as secondary enuresis. Enuresis seems to be more common among boys (2:1) in whom the problem is often more difficult to treat[8,9]. Enuresis is more common at all ages in lower socioeconomic groups and in institutionalized children. Majority of children have primary nocturnal enuresis whereas children with secondary enuresis may have precipitating factor such as an unusually stressful event (e.g., parental divorce, birth of a sibling, school trauma and sexual abuse). The spontaneous cure rate of night time enuresis is 14% to 16% annually[10].
ETIOLOGY
Factors that are believed to contribute to enuresis include genetics, sleep disturbances, maturational delay and abnormal secretion of antidiuretic hormone (ADH, vasopressin). Psychological and behavioral abnormalities although common are likely to be a result of enuresis rather than the cause.
Genetics
Bakwin showed that compared with a 15% incidence of enuresis in children from non-enuretic families, 44% and 77% of children were enuretic when one or both parents, respectively, were themselves enuretic. Scandinavian linkage studies depicted a locus for enuresis on chromosome 13 (ENUR 1) and another (ENUR 2) on chromosome 12[11,12].
Sleep aspects
Whether sleep disturbances are a result of the enuresis or contributes to the pathogenesis of enuresis is still debatable. Attempts at arousal were more often successful in control subjects than in boys with enuresis (40% vs 9%)[13]. In contrast another sleep study found that children with severe enuresis were actually “light sleeper” but they did not wake before voiding[14]. The arousal centre may be suppressed in these children. Persistently overactive bladder may lead to the abnormal arousal response just like the analogy of someone constantly knocking at the door leading to one either ignoring the knock or even installing an extra lock. Enuresis has been associated with snoring or sleep apneas due to adenotonsillar hypertrophy. This may be due to paradoxical rising of the arousal threshold due to constant stimuli from the obstructed airways or polyuria secondary to increased anti natriuretic peptide due to persistent negative intra-thoracic pressure found in sleep apnea syndrome.
Maturational delay
Since most cases of MNE resolves spontaneously a delayed maturation of a normal developmental process has been explored. Increased incidence of delayed language and slowed motor performances has been identified in some studies among children with enuresis[15]. Urodynamic and EEG findings have shown progressive maturation in bladder stability along with EEG changes suggesting increased central nervous system recognition of bladder fullness and the ultimate ability to suppress the onset of bladder contraction. Bladder capacity at birth is only around 60 mL and thereafter increases with age[16]. Children with nocturnal enuresis have been noted to have a smaller bladder capacity (functional rather than anatomical) even when there are no day time concerns[17]. There are reports of lower average height and lower mean bone age and late sexual maturation in enuretic than in non-enuretic children and adolescent. There is a greater incidence of enuresis in children who were delayed in the attainment of motor and language milestones as well.
Nocturnal polyuria
Increased urinary output overnight might also play an important role in MNE[18]. The cause may include increased fluid intake before bedtime, reduced response to antidiuretic hormone, and or decreased secretion of ADH.
Role of ADH
Despite the utility of desmopressin in the treatment of MNE the relationship between ADH secretion and night time urinary output remains controversial.
Initial studies did suggest presence of a blunted response to vasopressin in enuretic children compared with age-matched controls but subsequent studies failed to reproduce this observation[19].
Some studies have also demonstrated decreased nocturnal secretion of ADH but whether this is primary or secondary to the small bladder capacity (ADH secretion is thought to be stimulated with bladder distension) is not clear[20].
Additionally it needs to be emphasized that abnormalities in ADH secretion does not explain as to why these children do not wake to void.
Psychosocial factors
Psychiatric disorders in children with enuresis are higher than the rate found in non-enuretic groups but the relationship may be of etiologic relevance or it may be coincidental or occurring in response to the symptom of enuresis[9]. Children with enuresis had 2.88 times increased odds (95%CI: 1.26-6.57) of having attention deficit hyperactivity disorder (ADHD) as compared with those without enuresis[21]. It has been suggested that both enuresis and ADHD might be related to delays in central nervous system maturation[22]. Enuresis has sometimes been described as a masturbatory equivalent, an expression of bisexuality, or the somatic expression of a defect in body image.
Adverse event to medications
Enuresis may rarely results as a side effect of a medication such as lithium, valproic acid, clozapine and theophylline (secondary enuresis).
MECHANISM
The pathophysiology of enuresis is complex, involving the central nervous system (several neurotransmitters and receptors), circadian rhythm (sleep and diuresis), and bladder function derangements. Urinary continence is obtained in three sequential steps: Enlargement of the bladder capacity, voluntary control of the sphincter muscles, and voluntary control of the micturition reflex.
There are three commonly proposed mechanisms to bedwetting (Figure 1)[23,24].
Figure 1.
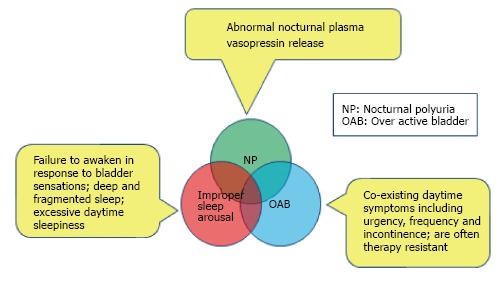
Mechanisms to bedwetting: Three commonly proposed mechanisms often overlap each other.
The locus coeruleus (LC), a noradrenergic neuron group in the upper pons is crucial for arousal from sleep and overlaps both functionally and anatomically with the pontine micturition centre, which coordinates the micturition reflex. The LC also has axonal connections with the hypothalamic cells that produce vasopressin[25-27]. Hence disturbances in this region of brainstem might be the missing link to a unifying pathogenic mechanism.
EVALUATION
Evaluation of a child with enuresis consists of detailed history, focused examination and appropriate investigations.
History
Detailed history is the key to the treatment success of a child suffering from enuresis. In every instance, both the parents and the child should be interviewed, and sensitivity to the emotional consequences of the symptoms should be high. Special focus in history should include: (1) Daytime wetting/urgency/holding maneuvers/weak or interrupted urinary stream including dribbling or straining; (2) Primary or secondary enuresis; (3) Frequency and pattern of nocturnal enuresis (including number of wet nights per week or month, number of episodes per night, time of episodes, approximate volume of each episode); (4) Daily fluid intake and urine output diary. (This will identify whether the child drinks adequately as well as whether the majority of fluid intake happens in late afternoon/evening, daytime urinary frequency, presence of polyuria - which might indicate other underlying cause such as diabetes, kidney disease or psychogenic polydipsia); (5) Stool history (including history of constipation/fecal soiling/encoperesis); (6) Any relevant medical history (e.g., review of history of sleep apnea, sickle cell disease or trait, diabetes, recurrent urinary tract infection, gait/neurological abnormalities); (7) Details of any previous interventions for enuresis; (8) Family history of nocturnal enuresis; (9) Social history (may be important in secondary enuresis); (10) Importantly effort should be made to understand how the problem has affected the child and family and the degree of motivation in both the child and family; (11) Behavioral history including behavior screening questionnaire; and (12) The sleeping arrangements for the child at home should be explored.
A voiding diary is helpful in not only identifying any underlying bladder dysfunction such as day time frequency but also in establishing a baseline record of the enuresis pattern. This may serve as standard against which the success of subsequent interventions can be gauged. Not infrequently, this baseline monitoring itself is associated with a dramatic improvement.
Physical examination
Although in most of the cases (particularly in children with MNE) physical examination is usually normal, detailed physical examination is still important to ensure any other underlying etiology is not being missed. A quick but focused physical examination in children with enuresis should include: (1) Growth: Poor growth may indicate an underlying renal problem and should prompt further examination attempting to identify any other renal disorder related signs such as hypertension; (2) Adenotonsillar hypertrophy or other signs of sleep apnea: Rarely they may be the underlying cause for enuresis; (3) Abdominal palpation: Will help in identifying fecal mass (severe constipation/encoparesis) or distended bladder (bladder outlet obstruction); (4) Perianal excoriation or vulvovaginitis which may indicate pinworm infection; (5) Detailed examination of lumbosacral spine as well as neurologic examination of perineum and lower limbs will aid in identifying any occult spinal cord abnormalities; and (6) Detection of wetness in the undergarments may be a sign of daytime incontinence.
Appropriate investigations
Investigations are usually minimally required in children with MNE. Cayan reported that the findings of ultrasonography and uroflowmetry were no different in children with nocturnal enuresis than in children without the condition[28]. So performing more than a urinalysis and culture for children with nocturnal enuresis would neither be cost effective nor helpful to the child. More invasive tests are indicated only in NMNE.
Urinalysis: This can aid in ruling out ketoacidosis, diabetes insipidus, water intoxication, and/or occult urinary tract infection[29]. First-morning specific gravity may be helpful in predicting who will respond to desmopressin treatment.
Imaging: Ultrasonography may be useful in NMNE for estimating bladder capacity, post-void residual volume, and bladder wall thickness. Voiding cystourethrogram can be useful in children with significant daytime complaints or history of recurrent urinary tract infection. Abdominal radiograph although rarely used for determining the presence and/or extent of stool retention is also helpful in convincing the parents about the severity of the constipation[30]. Neuroimaging such as magnetic resonance imaging of the spine will be needed if the lumbosacral/perianal/lower limb neurological examination demonstrates any abnormality[31].
Urodynamic studies: Urodynamic studies are limited to children with suspected bladder dysfunction as per history/examination and or ultrasound or voiding cystourethrogram results.
Frequently, the psychological and developmental damage may actually be more significant and devastating to the child than the symptom of enuresis itself so many a times psychological evaluation may be needed.
DIFFERENTIAL DIAGNOSIS
Although with detailed history and examination the diagnosis is not very difficult but following underlying conditions should not be overlooked: (1) Underlying medical conditions resulting in polyuria such as sickle cell disease, diabetes mellitus, diabetes insipidus, etc.; (2) Severe constipation/encoperesis; (3) Bladder bowel dysfunction; (4) Spinal dysraphism; (5) Chronic kidney disease such as nephronopthisis; (6) Pinworms; (7) Psychogenic polydipsia; and (8) Upper airway tract obstruction.
TREATMENT
Bed wetting while asleep is considered normal at least till 5 years. Even subsequently need for intervention is often not a medical decision being influenced primarily by the family and the child’s perception towards enuresis.
Evaluating the impact on the child and family
In children aged ≥ 5 years, enuresis is considered abnormal. Reasons for proactive management can be manifold including the distress caused to child and family, difficulty of “sleeping over” on holiday or at friends’ houses, social withdrawal, reduced self-esteem, and potential disturbance of the child’s and the parents’ sleep architecture that may have an impact on daytime functioning and health[32]. Additional reasons include the risk that some parents may be intolerant of their child’s wetting and the significant inconvenience and costs associated with frequent laundering of bed-sheets and clothing[33]. In primary care, “trial and error” treatment for enuresis is often the rule rather than the exception; this approach is a waste of time and money and increases frustration among families and doctors. It may also have an adverse psychological effect on the child.
Prior to initiation of any management it is important to understand the prime concern of the family as well as their expectation. The age at which enuresis is considered to be a “problem” varies depending upon the family. If both parents wet the bed until late childhood, they may not be concerned that their seven-year-old wets the bed. In contrast, parents may be concerned about a four-year-old who wets if he has a three-year-old sibling who is already dry. Often the family may not want any active intervention once they understand the self resolving nature of the problem as well as the usual absence of any identifiable underlying physical anomaly. Sometimes the family wants a quick solution (maybe for a planned travel or sleep over) or often is aiming for a long term cure. Therapy should be goal-oriented, and follow-up should be consistent.
Goals of treatment
The goals of interventions for nocturnal enuresis include[34]: (1) To stay dry on particular occasions (e.g., sleep over); (2) To reduce the number of wet nights; (3) To reduce the impact of enuresis on the child and family; and (4) To avoid recurrence.
Historically nocturnal eneuresis management as per Glicklich’s review explored fascinating “treatments” as cautery of sacral nerves, penile ligation, inflated vaginal balloons to compress the bladder neck, and electric shocks to the genitalia. Structured approach has been show to be beneficial and is professed to be the approach of choice. History, physical examination and or laboratory tests give clues as to the management plans. Daytime wetting, abnormal voiding (unusual posturing, discomfort, straining, or a poor urine stream), a history of urinary tract infections or evidence of infection on urinalysis or culture, and genital abnormalities are indications for nephro-urologic referral and subsequent treatment plan is influenced by any identified underlying aetiology. In case of coexisting constipation- disimpaction and establishment of a healthy bowel regimen leads to better control of enuresis. Snoring and enlarged tonsils or adenoids may signal sleep apnea and surgical correction of upper airway obstruction may result in improvement or cure of enuresis.
Step 1
Simple interventions: Initial interventions are usually restricted to educational and simple behavioral interventions. A number of common sense approaches (Table 2) to enuresis have evolved over time and despite lack of evidence they can be considered supportive for uncomplicated MNE.
Table 2.
Common sense approaches for the management of uncomplicated monosymptomatic nocturnal enuresis[39,40]
| What to do | How to do | How it works |
| Educate parents about | High prevalence of enuresis | Reduce their guilt |
| Relatively high spontaneous cure rate | Encourage hope | |
| Non-volitional nature of the symptom | Avoid a punitive response or the development of a control struggle | |
| Encourage child | Keeping of a journal | Raises awareness in the child |
| Keeping a dry bed chart | ||
| Changing the wet bed | ||
| Maintain voiding diary record | Daytime diary used to: Measurement of maximum voiding volume (excluding the first morning void); over a minimum of 3-4 d for accuracy; measurement on weekends or school holidays are ideal | Assess the child’s bladder capacity |
| Bedwetting diary completed for seven consecutive days/nights | Assess for the presence of nocturnal polyuria | |
| Fluid intake regulation | Decrease fluids especially caffeinated beverages, before bedtime. Ensuring adequate fluid consumption in the morning and afternoon and avoiding excessive fluid during evening | Decrease nocturnal urine production |
Behavioral interventions: Behavioral interventions for treating bedwetting are defined as interventions that require a behavior or action by the child that promotes night dryness and includes strategies which reward that behavior. These include: (1) Simple behavioral interventions - behaviors or actions that can be achieved by the child without great effort; (2) Complex behavioural interventions - multiple behavioural interventions which require greater effort by the child and parents to achieve, including enuresis alarm therapy.
Simple behavioral interventions are often used as a first attempt to improve nocturnal enuresis and include reward systems such as star charts given for dry nights, lifting or waking the children at night to urinate, retention control training to enlarge bladder capacity (bladder training) and fluid restriction.
Awakening the child to void during the night (to pre-empt the symptom). Generally, given the enuretic child’s sound sleeping ability, this does not lead to significant sleep disruption.
Lifting: Involves taking the child to the toilet during the night usually before the time that bedwetting is expected, without necessarily waking the child.
Waking: Involves waking the child to allow him/her to get up and urinate.
Neither waking nor lifting children and young people will promote long-term dryness but can be used in the short-term management of nocturnal enuresis.
Reward systems (e.g., star charts): The child might receive a star for every dry night, and a reward after a preset number of stars have been earned[35].
Bladder-stretching exercises to increase functional bladder capacity have been used without consistent evidence of effectiveness. The effort not to void despite considerable urgency is unpleasant for both the child and the family.
Retention control training: Attempting to increase the functional bladder capacity by delaying urination for extended periods of time during the day.
Stop-start training: Teaching children to interrupt their stream of urine in order to strengthen their pelvic floor muscles.
The impact of bedwetting can be reduced by using bed protection and washable/disposable products; using room deodorizers; thoroughly washing the child before dressing; and using emollients to prevent chafing.
Urotherapy is a commonly used terminology and usually includes education on normal bladder function, regular voiding habits and voiding posture, life-style advice regarding fluid intake and prevention of constipation and instruction on the use of bladder diaries or frequency-volume charts[36]. It encompasses various methods of pelvic floor muscle training, behavioral modification, neuromodulation and catheterization. The first-line treatment of daytime incontinence in childhood is basic urotherapy, i.e., advice regarding fluid intake and regular voiding habits. The same advice is routinely given to enuretic children as well. This is not illogical, given the role of detrusor over activity in enuresis, but to date evidence for the efficacy of this approach is weak. However, urotherapy is certainly not harmful and alleviates concomitant daytime symptoms.
Simple behavioural methods in twelve randomised controlled trials including a Cochrane review found it to be superior to no active treatment but appear to be inferior to enuresis alarm therapy and some drug therapy (such as imipramine and amitriptyline).
Despite anecdotal reports, there is no empirical evidence to suggest efficacy of hypnotherapy, dietary manipulation, acupuncture, chiropractic treatment and psychotherapy and desensitization to allergens[37].
Step 2
Active interventions (enuresis alarms/pharmacotherapy): Active interventions are usually planned if simple strategies as discussed above fail to yield positive results even after 3 to 6 mo. These interventions are usually based on recommendations of ICCS standardization document on MNE which have been reviewed and endorsed by committees representing the American Academy of Pediatrics, European Society for Paediatric Urology, European Society for Paediatric Nephrology, and the ICCS. Two first-line treatment options are suggested - desmopressin and enuresis alarm[38]. The choice of initial treatment may be based on the parents’ and child’s preference, their motivations, the physician’s experience and local resources.
Information from diaries may identify one of four subtypes of MNE and allow further fine-tuning of treatment (Figure 2).
Figure 2.
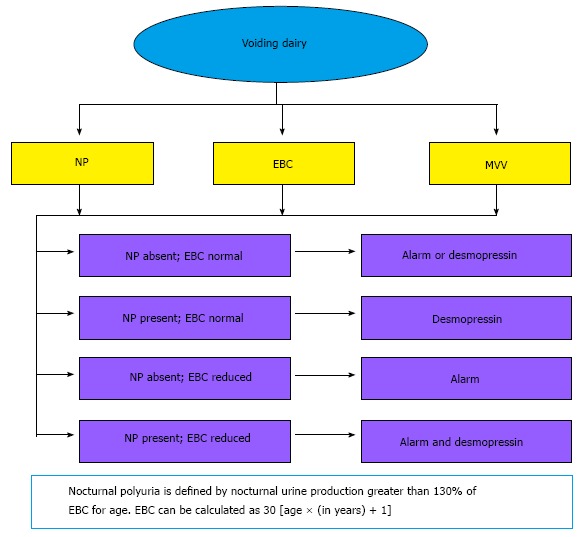
First line management of monosymptomatic nocturnal enuresis as per voiding dairy[45]. NP: Nocturnal polyuria; EBC: Expected bladder capacity; MVV: Maximum voiding volume.
Indications for referral: MNE usually can be managed effectively by the primary care provider. However, children with refractory nocturnal enuresis may benefit from referral to a healthcare professional who specializes in the management of recurrent or refractory enuresis (e.g., developmental-behavioral pediatrician or urologist if structural or anatomic abnormalities are suspected). Additional indications for referral include non-monosymptomatic enuresis; developmental, attention or learning difficulties; behavioral or emotional problems; and known or suspected physical or neurologic problems.
Conditioning regime: Conditioning awakening to the sensation of a full bladder is the most benign and successful of the generic treatments of enuresis since its description in 1938. A careful meta-analysis of decades of conditioning studies has shown an initial success rate (defined as a reduction to less than one wet night per month) of approximately 66%, with more than half the subjects experiencing long-term success[39]. The few existing studies that compare conditioning with pharmacologic treatments have generally shown conditioning to be significantly more effective than imipramine[40] and desmopressin (DDAVP)[41].
Enuresis alarm
Enuresis alarms is the most commonly prescribed conditioning regime which has a level 1, grade A International Consultation on Incontinence (ICI) recommendation. Portable transistorized alarms that the child wears on the body have replaced the old bell-and-pad type, but the principle is the same. The first drops of urine moisten the fabric separating two electrodes, thereby completing the circuit and setting off the alarm that the child is wearing. Initially if children do not wake with the noise or vibration, it is important for their parents to wake them. Gradually the child awakens earlier and earlier in the course of the enuretic episode and the wet spot diminishes in size until the sensation of bladder fullness causes the child to awaken before wetting. Response is not immediate and treatment should be continued for 2-3 mo or until the child is dry for 14 consecutive nights (whichever comes first). Success is followed by over-learning (e.g., extra drinks are given at bedtime to cause additional stress to the detrusor muscles in the bladder. Alarm treatment is then continued until 14 consecutive dry nights are once again achieved) and intermittent reinforcement in which the child uses the alarm every other day before discontinuing it. Lack of parental help to awaken the child to finish voiding in the toilet is a major reason for failure of the conditioning treatment.
Enuresis alarm should not be tried if: (1) The child wets the bed only once or twice per week; (2) The child or parents do not seem to be enthusiastic about the enuresis alarm; (3) Rapid or short-term improvement seems to be the goal for the parents; and (4) The parents seem to express negative feelings/blame their child for wetting the bed.
Lack of success with the approach in the past or a relapse after previous success does not preclude successful subsequent treatment with a conditioning device. Throughout the behavioral treatment, rewarding the success with a sticker chart and reinforcing positive change is critical to maintaining the child’s investment in the process. Enuresis alarms are by far the most effective means of long term control as well as preventing relapses. In a meta-analysis of 56 randomized trials (3257 children), sixty-six percent of children became dry for 14 consecutive nights during alarm use vs only 4% in the no-treatment control group [relative risk (RR) for treatment failure 0.38, 95%CI: 0.33-0.45]. Additionally nearly a half of children remained dry even after stopping the treatment, compared with almost none in the no-treatment group (45% vs 1%, RR for relapse 0.56, 95%CI: 0.46-0.680)[42].
Pharmacotherapy: Two medications, DDAVP and imipramine have proven efficacy in the treatment of enuresis.
Desmopressin is a synthetic analogue of the ADH vasopressin which has been used to treat central diabetes insipidus, bleeding disorders such as von Willebrand disease and primary nocturnal enuresis. It decreases urine production at night when taken at bedtime. Desmopressin has a level 1, grade A recommendation from the ICI in 2009[43]. It is administered orally in 0.2 mg tablets in doses of 0.2 to 0.6 mg nightly or, less commonly, intra-nasally as a spray in doses of 10 to 40 μg (one to four sprays) nightly; the lowest effective dose is determined empirically with each child. Due to variable absorption and risk of over dosage nasal sprays are usually not advocated. Desmopressin is also available as a fast-melting oral lyophilisate (melt; dosage, 120-360 μg). As this form does not require extra water to take this medication, it has become popular particularly for children under 12 years medication should be taken 1 h before the last void before bedtime to allow timely enhanced concentration of urine to occur. Fluid intake should be reduced from 1 h before desmopressin administration and for 8 h subsequently to encourage optimal concentrating capacity and treatment response, as well as to reduce the risk of hyponatremia/water intoxication. Desmopressin is primarily utilized as an alternative to enuresis alarms for children and families who seek rapid or short-term improvement of enuresis; where enuresis alarms have failed or have been refused by family or are unlikely to succeed because of family dynamics. The initial duration of treatment should be for 2-6 wk, to ascertain its anti-enuretic effect. If a sufficient degree of improvement is experienced, then treatment can be continued for an additional 3 mo - where appropriate. Structured withdrawal of medication may reduce relapse rates[44]. If a second voiding diary indicates that nocturnal urinary production is not reduced, consider a dose increase. As a rule of thumb, one third of unselected enuretic children are reliably dry as long as they take the drug, one third has a partial response and one third is not helped at all. Fluid overload (water intoxication) is potentially the most serious complication with desmopressin. It is associated with overdrinking at bedtime and its symptoms include headache, nausea, hyponatraemia, cerebral oedema, and convulsions. Overall desmopressin has an excellent safety profile with very few significant adverse events reported[45]. The reported success rates of DDAVP treatment for enuresis have ranged from 10% to 65%, but as many as 80% of patients relapse after treatment[46]. Depression of endogenous ADH secretion is not a concern as children who have used DDAVP for as long as 1 year have demonstrated the ability to concentrate their urine appropriately in response to a water deprivation challenge. It seems reasonable at least to consider a trial of withdrawal of DDAVP at 3- to 6-mo intervals.
In comparison to arousal alarms, treatment effects were not sustained after discontinuation of therapy (the rate of failure or relapse was 65% and 46% with desmopressin and alarms, respectively; relative risk of failure 1.42, 95%CI: 1.05-1.91). Comparison with some tricyclic drugs (e.g., amitriptyline) suggests that they might be as effective as desmopressin although in two trials, children were less likely to achieve 14 dry nights with imipramine than desmopressin (RR 0.44, 95%CI: 0.27-0.73) but there was not enough information about subsequent relapse[47]. There were more side effects with the tricyclics.
The British National Formulary currently suggests that drug therapy is not usually appropriate for children under 7 years of age and should be reserved for children in whom alternative measures have failed.
Failure to therapy
Inability to achieve > 50% improvement in symptoms is defined as resistant to therapy. If this happens despite an adequate trial of treatment with an enuresis alarm (i.e., three months) and/or desmopressin (at a dose of 0.4 mg) and in absence of any concern regarding the family/child’s motivation then referral to a healthcare professional who specializes in the management of bedwetting (e.g., developmental behavioral pediatrician, pediatric urologist) may be warranted.
Possible reasons for lack of response include: (1) Overactive bladder; (2) Underlying disease (e.g., diabetes mellitus/diabetes insipidus); (3) Occult constipation; (4) Sleep apnea; (4) Incorrect use of alarm; and (5) Social and emotional factors.
If on additional evaluation (which may include repeat bladder diary, ultrasound scan (if not done before), rectal examination/abdominal X ray for constipation) no underlying aetiology is found then combination therapy or switch to imipramime, may be considered. For children with suspected day and night detrusor over activity/small functional bladder capacity, a combination of oxybutynin and desmopressin may be indicated (level 2, grade B).
Imipramine (a tri-cyclic anti-depressant) in a single bedtime dose of 1.0-2.5 mg/kg had been used for many years as a third line agent for enuresis. Tricyclic antidepressants (TCAs, e.g., imipramine, amitriptyline and desipramine) decrease the amount of time spent in REM sleep, stimulate vasopressin secretion, and relax the detrusor muscle although the exact mechanism of action in treating enuresis is unknown. Major rare significant adverse effects include cardiotoxicity and hepatotoxicity. Minor side-effects are related to their anti-cholinergic actions and include postural hypotension, dry mouth, constipation, perspiration, tachycardia, nausea, lethargy and insomnia. A pretreatment electrocardiogram may be obtained to detect any underlying rhythm disorder. Treatment can be continued for 4-6 mo. In a systematic review, compared with placebo, treatment with TCA was associated with reduction of approximately one wet night per week[48].
Other drugs
Anti-cholinergic drug tolterodine, oxybutynin and propiverine is in fact useful as an add-on therapy in enuretic children who have not responded to desmopressin alone[49]. They carry a risk for constipation and for UTI due to the accumulation of residual urine. Other drugs, including indomethacin, phenmetrazine, amphetamine sulfate, ephedrine, atropine, furosemide, diclofenac, and chlorprothixene have been tried in the treatment of nocturnal enuresis. A 2012 systematic review of randomized trials of drugs other than tricyclic antidepressants and desmopressin found that although indomethacin, diclofenac, and diazepam were better than placebo in reducing the number of wet nights, none of the drugs was better than desmopressin[50]. Atomoxetine used in ADHD has been found to decrease frequency of bedwetting among children with enuresis with or without[51].
In summary: Differentiating between MNE and NMNE forms the cornerstone in the management of these children. Once MNE is identified and initial steps like counseling, bladder diarys, etc., have failed the first treatment for the family who is well-motivated and well informed is the enuresis alarm. Desmopressin is the first line treatment for families who are not sufficiently motivated to use the alarm, who have recently used the alarm (correctly) without success or who are considered unlikely to comply with alarm treatment. Anti-cholinergic therapy has the greatest chance of success in the child with signs of detrusor over activity, i.e., low daytime voided volumes. If desmopressin, the alarm and the anti-cholinergic treatment have all been tried without success, or have been judged unsuitable, the cautious use of imipramine may be considered (Figure 3).
Figure 3.
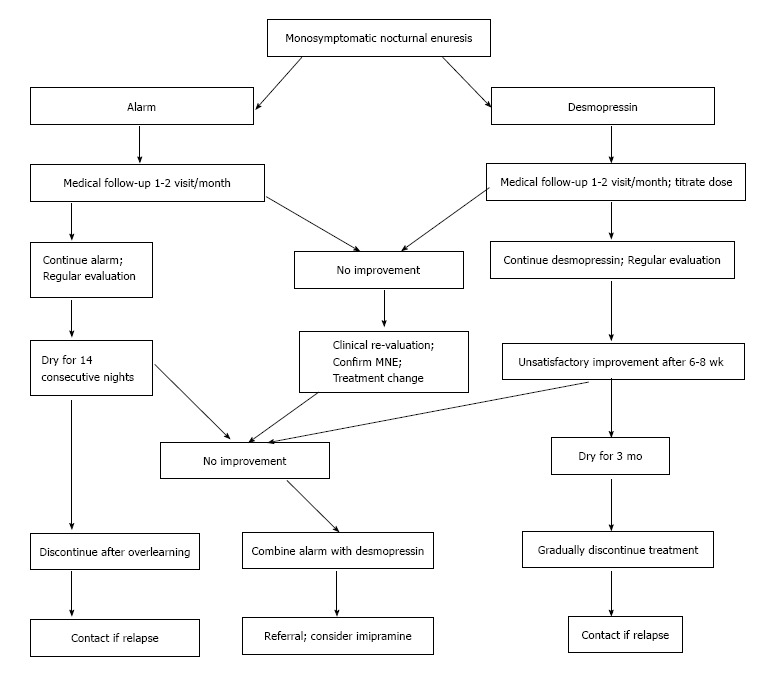
Treatment flowchart for monosymptomatic nocturnal enuresis. MNE: Monosymptomatic enuresis.
Follow-up: Following successful treatment with either the alarm or desmopressin, patients should be advised to contact the clinic if relapse is experienced after discontinuation of therapy. If relapse occurs, desmopressin, alarm, or combined therapy should be re-considered. The most likely fundamental reason for not responding to alarm or desmopressin therapy is that the actual diagnosis is NMNE and not MNE. When a detailed history is obtained, the majority of these children have at least subtle daytime symptoms. If a patient is treatment-resistant and a bladder diary has not been completed, it is imperative this is undertaken or to refer the child to a specialty center as OAB and dysfunctional voiding may be present.
Footnotes
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 1, 2016
First decision: February 29, 2016
Article in press: May 11, 2016
P- Reviewer: Chang CC, Friedman EA, Trkulja V S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
References
- 1.Fritz G, Rockney R, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, et al. Practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:1540–1550. doi: 10.1097/01.chi.0000142196.41215.cc. [DOI] [PubMed] [Google Scholar]
- 2.Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, Jørgensen TM, Rittig S, Walle JV, Yeung CK, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176:314–324. doi: 10.1016/S0022-5347(06)00305-3. [DOI] [PubMed] [Google Scholar]
- 3.von Gontard A, Mauer-Mucke K, Plück J, Berner W, Lehmkuhl G. Clinical behavioral problems in day- and night-wetting children. Pediatr Nephrol. 1999;13:662–667. doi: 10.1007/s004670050677. [DOI] [PubMed] [Google Scholar]
- 4.Spee-van der Wekke J, Hirasing RA, Meulmeester JF, Radder JJ. Childhood nocturnal enuresis in The Netherlands. Urology. 1998;51:1022–1026. doi: 10.1016/s0090-4295(98)00106-x. [DOI] [PubMed] [Google Scholar]
- 5.Feehan M, McGee R, Stanton W, Silva PA. A 6 year follow-up of childhood enuresis: prevalence in adolescence and consequences for mental health. J Paediatr Child Health. 1990;26:75–79. doi: 10.1111/j.1440-1754.1990.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 6.Yeung CK, Sihoe JD, Sit FK, Bower W, Sreedhar B, Lau J. Characteristics of primary nocturnal enuresis in adults: an epidemiological study. BJU Int. 2004;93:341–345. doi: 10.1111/j.1464-410x.2003.04612.x. [DOI] [PubMed] [Google Scholar]
- 7.Yeung CK, Sreedhar B, Sihoe JD, Sit FK, Lau J. Differences in characteristics of nocturnal enuresis between children and adolescents: a critical appraisal from a large epidemiological study. BJU Int. 2006;97:1069–1073. doi: 10.1111/j.1464-410X.2006.06074.x. [DOI] [PubMed] [Google Scholar]
- 8.Shreeram S, He JP, Kalaydjian A, Brothers S, Merikangas KR. Prevalence of enuresis and its association with attention-deficit/hyperactivity disorder among U.S. children: results from a nationally representative study. J Am Acad Child Adolesc Psychiatry. 2009;48:35–41. doi: 10.1097/CHI.0b013e318190045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sureshkumar P, Jones M, Caldwell PH, Craig JC. Risk factors for nocturnal enuresis in school-age children. J Urol. 2009;182:2893–2899. doi: 10.1016/j.juro.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 10.Forsythe WI, Redmond A. Enuresis and spontaneous cure rate. Study of 1129 enuretis. Arch Dis Child. 1974;49:259–263. doi: 10.1136/adc.49.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eiberg H, Berendt I, Mohr J. Assignment of dominant inherited nocturnal enuresis (ENUR1) to chromosome 13q. Nat Genet. 1995;10:354–356. doi: 10.1038/ng0795-354. [DOI] [PubMed] [Google Scholar]
- 12.Arnell H, Hjälmås K, Jägervall M, Läckgren G, Stenberg A, Bengtsson B, Wassén C, Emahazion T, Annerén G, Pettersson U, et al. The genetics of primary nocturnal enuresis: inheritance and suggestion of a second major gene on chromosome 12q. J Med Genet. 1997;34:360–365. doi: 10.1136/jmg.34.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfish NM, Pivik RT, Busby KA. Elevated sleep arousal thresholds in enuretic boys: clinical implications. Acta Paediatr. 1997;86:381–384. doi: 10.1111/j.1651-2227.1997.tb09027.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeung CK, Diao M, Sreedhar B. Cortical arousal in children with severe enuresis. N Engl J Med. 2008;358:2414–2415. doi: 10.1056/NEJMc0706528. [DOI] [PubMed] [Google Scholar]
- 15.von Gontard A, Freitag CM, Seifen S, Pukrop R, Röhling D. Neuromotor development in nocturnal enuresis. Dev Med Child Neurol. 2006;48:744–750. doi: 10.1017/S0012162206001599. [DOI] [PubMed] [Google Scholar]
- 16.Koff SA. Estimating bladder capacity in children. Urology. 1983;21:248. doi: 10.1016/0090-4295(83)90079-1. [DOI] [PubMed] [Google Scholar]
- 17.Norgaard JP. Urodynamics in enuretics. I: Reservoir function. Neurourol Urodyn. 1989;8:199–211. [Google Scholar]
- 18.Rasmussen PV, Kirk J, Borup K, Nørgaard JP, Djurhuus JC. Enuresis nocturna can be provoked in normal healthy children by increasing the nocturnal urine output. Scand J Urol Nephrol. 1996;30:57–61. doi: 10.3109/00365599609182350. [DOI] [PubMed] [Google Scholar]
- 19.Rittig S, Schaumburg HL, Siggaard C, Schmidt F, Djurhuus JC. The circadian defect in plasma vasopressin and urine output is related to desmopressin response and enuresis status in children with nocturnal enuresis. J Urol. 2008;179:2389–2395. doi: 10.1016/j.juro.2008.01.171. [DOI] [PubMed] [Google Scholar]
- 20.Kawauchi A, Watanabe H, Nakagawa S, Miyoshi K. [Development of bladder capacity, nocturnal urinary volume and urinary behavior in nonenuretic and enuretic children] Nihon Hinyokika Gakkai Zasshi. 1993;84:1811–1820. doi: 10.5980/jpnjurol1989.84.1811. [DOI] [PubMed] [Google Scholar]
- 21.Fehlow P. [EEG findings in 130 enuretics with special reference to spike potentials] Psychiatr Neurol Med Psychol (Leipz) 1985;37:221–227. [PubMed] [Google Scholar]
- 22.Oppel WC, Harper PA, Rider RV. The age of attaining bladder control. Pediatrics. 1968;42:614–626. [PubMed] [Google Scholar]
- 23.Kamperis K, Rittig S, Jørgensen KA, Djurhuus JC. Nocturnal polyuria in monosymptomatic nocturnal enuresis refractory to desmopressin treatment. Am J Physiol Renal Physiol. 2006;291:F1232–F1240. doi: 10.1152/ajprenal.00134.2006. [DOI] [PubMed] [Google Scholar]
- 24.Yeung CK, Chiu HN, Sit FK. Bladder dysfunction in children with refractory monosymptomatic primary nocturnal enuresis. J Urol. 1999;162:1049–1054; discussion 1054-1055. doi: 10.1016/S0022-5347(01)68062-5. [DOI] [PubMed] [Google Scholar]
- 25.Cohen-Zrubavel V, Kushnir B, Kushnir J, Sadeh A. Sleep and sleepiness in children with nocturnal enuresis. Sleep. 2011;34:191–194. doi: 10.1093/sleep/34.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayama Y, Koyama Y. Brainstem neural mechanisms of sleep and wakefulness. Eur Urol. 1998;33 Suppl 3:12–15. doi: 10.1159/000052235. [DOI] [PubMed] [Google Scholar]
- 27.Lightman SL, Todd K, Everitt BJ. Ascending noradrenergic projections from the brainstem: evidence for a major role in the regulation of blood pressure and vasopressin secretion. Exp Brain Res. 1984;55:145–151. doi: 10.1007/BF00240508. [DOI] [PubMed] [Google Scholar]
- 28.Cayan S, Doruk E, Bozlu M, Akbay E, Apaydin D, Ulusoy E, Canpolat B. Is routine urinary tract investigation necessary for children with monosymptomatic primary nocturnal enuresis? Urology. 2001;58:598–602. doi: 10.1016/s0090-4295(01)01338-3. [DOI] [PubMed] [Google Scholar]
- 29.Robson WL. Clinical practice. Evaluation and management of enuresis. N Engl J Med. 2009;360:1429–1436. doi: 10.1056/NEJMcp0808009. [DOI] [PubMed] [Google Scholar]
- 30.Hodges SJ, Anthony EY. Occult megarectum--a commonly unrecognized cause of enuresis. Urology. 2012;79:421–424. doi: 10.1016/j.urology.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Pippi Salle JL, Capolicchio G, Houle AM, Vernet O, Jednak R, O’Gorman AM, Montes JL, Farmer JP. Magnetic resonance imaging in children with voiding dysfunction: is it indicated? J Urol. 1998;160:1080–1083. doi: 10.1097/00005392-199809020-00030. [DOI] [PubMed] [Google Scholar]
- 32.Ertan P, Yilmaz O, Caglayan M, Sogut A, Aslan S, Yuksel H. Relationship of sleep quality and quality of life in children with monosymptomatic enuresis. Child Care Health Dev. 2009;35:469–474. doi: 10.1111/j.1365-2214.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 33.Carskadon MA. Sleep deprivation: health consequences and societal impact. Med Clin North Am. 2004;88:767–776. doi: 10.1016/j.mcna.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt BD. Nocturnal enuresis. Pediatr Rev. 1997;18:183–190; quiz 91. doi: 10.1542/pir.18-6-183. [DOI] [PubMed] [Google Scholar]
- 35.Glazener CM, Peto RE, Evans JH. Effects of interventions for the treatment of nocturnal enuresis in children. Qual Saf Health Care. 2003;12:390–394. doi: 10.1136/qhc.12.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maternik M, Krzeminska K, Zurowska A. The management of childhood urinary incontinence. Pediatr Nephrol. 2015;30:41–50. doi: 10.1007/s00467-014-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seabrook JA, Gorodzinsky F, Freedman S. Treatment of primary nocturnal enuresis: A randomized clinical trial comparing hypnotherapy and alarm therapy. Paediatr Child Health. 2005;10:609–610. doi: 10.1093/pch/10.10.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamperis K, Hagstroem S, Rittig S, Djurhuus JC. Combination of the enuresis alarm and desmopressin: second line treatment for nocturnal enuresis. J Urol. 2008;179:1128–1131. doi: 10.1016/j.juro.2007.10.088. [DOI] [PubMed] [Google Scholar]
- 39.Houts AC, Berman JS, Abramson H. Effectiveness of psychological and pharmacological treatments for nocturnal enuresis. J Consult Clin Psychol. 1994;62:737–745. doi: 10.1037//0022-006x.62.4.737. [DOI] [PubMed] [Google Scholar]
- 40.Wagner W, Johnson SB, Walker D, Carter R, Wittner J. A controlled comparison of two treatments for nocturnal enuresis. J Pediatr. 1982;101:302–307. doi: 10.1016/s0022-3476(82)80146-7. [DOI] [PubMed] [Google Scholar]
- 41.Wille S. Comparison of desmopressin and enuresis alarm for nocturnal enuresis. Arch Dis Child. 1986;61:30–33. doi: 10.1136/adc.61.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glazener CM, Evans JH, Peto RE. Alarm interventions for nocturnal enuresis in children. Cochrane Database Syst Rev. 2003;(2):CD002911. doi: 10.1002/14651858.CD002911. [DOI] [PubMed] [Google Scholar]
- 43.Lottmann H, Froeling F, Alloussi S, El-Radhi AS, Rittig S, Riis A, Persson BE. A randomised comparison of oral desmopressin lyophilisate (MELT) and tablet formulations in children and adolescents with primary nocturnal enuresis. Int J Clin Pract. 2007;61:1454–1460. doi: 10.1111/j.1742-1241.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 44.Marschall-Kehrel D, Harms TW. Structured desmopressin withdrawal improves response and treatment outcome for monosymptomatic enuretic children. J Urol. 2009;182:2022–2026. doi: 10.1016/j.juro.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 45.Donoghue MB, Latimer ME, Pillsbury HL, Hertzog JH. Hyponatremic seizure in a child using desmopressin for nocturnal enuresis. Arch Pediatr Adolesc Med. 1998;152:290–292. doi: 10.1001/archpedi.152.3.290. [DOI] [PubMed] [Google Scholar]
- 46.Thompson S, Rey JM. Functional enuresis: is desmopressin the answer? J Am Acad Child Adolesc Psychiatry. 1995;34:266–271. doi: 10.1097/00004583-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Glazener CM, Evans JH. Desmopressin for nocturnal enuresis in children. Cochrane Database Syst Rev. 2002;(3):CD002112. doi: 10.1002/14651858.CD002112. [DOI] [PubMed] [Google Scholar]
- 48.Glazener CM, Evans JH, Peto RE. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2003;(3):CD002117. doi: 10.1002/14651858.CD002117. [DOI] [PubMed] [Google Scholar]
- 49.Austin PF, Ferguson G, Yan Y, Campigotto MJ, Royer ME, Coplen DE. Combination therapy with desmopressin and an anticholinergic medication for nonresponders to desmopressin for monosymptomatic nocturnal enuresis: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2008;122:1027–1032. doi: 10.1542/peds.2007-3691. [DOI] [PubMed] [Google Scholar]
- 50.Deshpande AV, Caldwell PH, Sureshkumar P. Drugs for nocturnal enuresis in children (other than desmopressin and tricyclics) Cochrane Database Syst Rev. 2012;12:CD002238. doi: 10.1002/14651858.CD002238.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shatkin JP. Atomoxetine for the treatment of pediatric nocturnal enuresis. J Child Adolesc Psychopharmacol. 2004;14:443–447. doi: 10.1089/cap.2004.14.443. [DOI] [PubMed] [Google Scholar]


