Abstract
AIM: To determine the incidence, clinical characteristics and outcomes of patients with metformin associated lactic acidosis (MALA).
METHODS: Auckland City Hospital drains a population of just over 400000 people. All cases presenting with metabolic acidosis between July 2005 and July 2009 were identified using clinical coding. A retrospective case notes review identified patients with MALA. Prescribing data for metformin was obtained from the national pharmaceutical prescribing scheme.
RESULTS: There were 42 cases of metabolic lactic acidosis over 1718000 patient years. There were 51000 patient years of metformin prescribed to patients over the study period. There were thirty two cases of lactic acidosis due to sepsis, seven in patients treated with metformin. Ten cases of MALA were identified. The incidence of MALA was estimated at 19.46 per 100000 patient year exposure to metformin. The relative risk of lactic acidosis in patients on metformin was 13.53 (95%CI: 7.88-21.66) compared to the general population. The mean age of patients with MALA was 63 years, range 40-83 years. A baseline estimated glomerular filtration rate was obtained in all patients and ranged from 23-130 mL/min per 1.73 m2. Only two patients had chronic kidney disease G4. Three patients required treatment with haemodialysis. Two patients died.
CONCLUSION: Lactic acidosis is an uncommon but significant complication of use of metformin which carries a high risk of morbidity.
Keywords: Acute kidney injury, Lactic acidosis, Metformin
Core tip: Metformin is an effective therapy for type 2 diabetes mellitus. Although few side effects are described in clinical trials, here, we describe observational evidence that suggests that use of metformin is associated with an increased risk of lactic acidosis. We recommend dose reduction in the elderly, withholding the drug if an intercurrent illness occurs and that metformin be halted in patients with chronic kidney disease G4.
INTRODUCTION
The oral hypoglycaemic agent metformin has been used for close to 50 years[1]. It has been found to reduce mortality compared to other agents and is recommended as first line therapy for patients with type 2 diabetes mellitus[2-4]. It is also used for patients with the metabolic syndrome[5] and overweight women with polycystic ovarian syndrome[6].
The biguanide, phenformin, clearly caused lactic acidosis[7]. It has been hypothesized that this severe and significant side-effect is also associated with metformin. Several mechanisms of action have been proposed for the hypoglycaemic effect of the metformin: A reduction in hepatic glucose production, an increase in peripheral glucose uptake, a reduction in gastrointestinal glucose production and a reduction in lipolysis by adipocytes[8]. The major mechanism is through reduction in hepatic production, mediated by phosphorylation of the transcriptional co-activator cAMP response element-binding protein thus reducing the expression of genes inducing gluconeogenesis[9].
It is thought that metformin associated lactic acidosis (MALA) may occur through anaerobic stimulation of lactate production by intestinal cells, with impaired elimination of lactate from the liver and contributed to by accumulation of metformin if there is renal failure, overdose or liver failure[8].
New Zealand has a pharmaceuticals scheme with metformin freely available and fully subsidised. The purpose of this report was to review all cases of lactic acidosis in patients on metformin at Auckland City Hospital.
MATERIALS AND METHODS
Auckland City Hospital is an adult tertiary referral centre which serves a population of just over 400000 people. Using a health information technology system all cases of metabolic acidosis between July 2005 and July 2009 were identified. Acidosis was defined as a pH ≤ 7.35. Lactic acidosis was defined as a lactate of ≥ 5 mmol/L, in association with a low bicarbonate and a low PCO2. Patients with a mixed respiratory and metabolic acidosis were excluded.
The clinical records were available and reviewed for all potential cases. The dose and duration of metformin, other medications, co-morbidities and baseline laboratory data were obtained from the clinical records and primary practice.
Population estimates were obtained from Statistics New Zealand[10]. Data about metformin use in the Auckland region was obtained from the Pharmaceutical Management Agency of New Zealand (PHARMAC). The incidence of diabetes mellitus was estimated from data from the New Zealand Health Survey[11].
Poisson regression statistics were used to determine the risk of lactic acidosis, using the general population as the reference.
RESULTS
Eight hundred cases of lactic acidosis were identified by the health information technology system. Two hundred and eighty-eight cases of metabolic acidosis were identified by review of laboratory data. Forty-three cases of metabolic lactic acidosis were identified. One was in a nineteen-year-old female, who had an intentional overdose with ten grams of metformin, and was not included in the analysis, thus leaving forty-two cases. Thirty-two patients had metabolic lactic acidosis which was clearly associated with sepsis (Figure 1). Seven of these cases were in patients also taking metformin (Table 1). Four were in patients with diabetes mellitus, not on metformin. Ten patients were taking metformin and did not have a strong alternate cause for lactic acidosis (Table 2).
Figure 1.
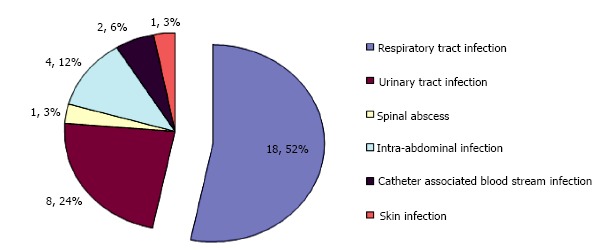
Cause of sepsis in patients with lactic acidosis.
Table 1.
Patients on presenting with lactic acidosis and sepsis
| All patients | Patients on metformin | |
| No. of patients | 32 | 7 |
| Age (yr)1 | 22-85 | 46-81 |
| Sex | 19 females | 4 female |
| Ethnicity | 19 Europeans, | 3 Europeans, |
| 9 Pacific People, | 3 Pacific People | |
| 1 NZ Maori, | ||
| 1 Indian | ||
| 2 Chinese | ||
| Baseline Creatinine (μmol/L) | 41-200 | 58-140 |
| 2eGFR mL/min per 1.73 m2 | 25-90 | 31-87 |
| Creatinine at presentation (μmol/L) | 50-600 | 103-463 |
| Number who died | 15 | 3 |
| Number receiving acute haemodialysis | Three | Nil |
| Creatinine at discharge (μmol/L) | 53-245 | 56-60 |
| eGFR mL Tab/min per 1.73 m2 | 22-90 | 77-90 |
The age of the patient is rounded down to the nearest year;
eGFR: The estimated glomerular filtration rate based on the four variable MDRD formula[12]. eGFR: Estimated glomerular filtration rate; MDRD: Modification of diet in renal disease.
Table 2.
Demographic and clinical details of patients with metformin associated lactic acidosis
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Age (yr)1 | 68 | 53 | 68 | 63 | 80 | 40 | 83 | 55 | 67 | 72 |
| Sex | Male | Male | Male | Female | Male | Female | Female | Female | Female | Female |
| Ethnicity | Pacific Islander | Indian | European | Pacific Islander | Pacific Islander | Pacific Islander | Pacific Islander | Pacific Islander | European | European |
| Metformin dose (g/d) | 2.5 | 1.7 | 2 | 3 | 2 | 2 | 1 | 2 | 1.7 | 1 |
| Duration of Metformin2 | 5 yr | 4 yr | 5 yr | 4 yr | 2 yr | 1 yr | 4 yr | 4 yr | 4 yr | 2 mo |
| Baseline Creatinine (μmol/L) | 114 | 57 | 138 | 123 | 106 | 123 | 180 | 105 | 154 | 139 |
| 3eGFR mL/min per 1.73 m2 | 55 | 130 | 44 | 38 | 58 | 42 | 23 | 47 | 29 | 32 |
| Creatinine at Presentation (μmol/L) | 449 | 90 | 333 | 612 | 381 | 612 | 376 | 895 | 973 | 304 |
| pH | 7.3 | 7.23 | 7.14 | 7.32 | 6.99 | 7.21 | 7.34 | 6.9 | 7.35 | 7.09 |
| Bicarbonate (mmol/L) | 15 | 13 | 13 | 16 | 6 | 12 | 12 | 3 | 15 | 15 |
| Lactate (mmol/L) | 9.2 | 6.7 | 15 | 6 | 16 | 8.4 | 6 | 22 | 6.2 | 7 |
| Received acute haemodialysis | Yes | No | Yes | No | No | No | No | No | Yes | No |
| Outcome | Dead | Alive | Alive | Alive | Dead | Alive | Alive | Alive | Alive | Alive |
| Creatinine at discharge (μmol/L) | - | 73 | 121 | 95 | - | 90 | 167 | 123 | 129 | 96 |
| 3eGFR mL/min per 1.73 m2 | - | 97 | 52 | 52 | - | 60 | 25 | 39 | 36 | 50 |
The age of the patient is rounded down to the nearest year;
The duration of metformin is rounded down to completed years of therapy, except for patient 10;
eGFR: The estimated glomerular filtration rate based on the four variable MDRD formula[12]. eGFR: Estimated glomerular filtration rate; MDRD: Modification of diet in renal disease.
The population in Auckland City over this period was estimated at 419000 people and increased to 444000 people over the study period. Eighteen point eight percent of the Auckland population are children and cared for by the regional paediatric institution. Thus, we estimate a total of 1395000 patient years over the study period.
The number of patients receiving metformin between July 2005 and July 2009, in Auckland City, was estimated at 51400 patient years. It was estimated that there were 15600 adult patients with diabetes in Auckland each year.
The incidence of metabolic lactic acidosis was estimated to be 3.01 per 100000 patient years for the general population. The incidence of metabolic lactic acidosis due to sepsis was estimated as 2.29 per 100000 patient years.
The incidence of metabolic lactic acidosis was 33.07 per 100000 patient years’ exposure to metformin. There was a significant increase in the relative risk of lactic acidosis in patients on metformin compared to the general population RR = 13.53 (95%CI: 7.88-21.66).
The incidence of MALA was 19.46 per 100000 patient years exposure to metformin. There were four male and six female patients whose mean age was 63 years, range 40-83 years (Table 2). All patients were prescribed metformin for type 2 diabetes mellitus and were also on either an angiotensin converting enzyme inhibitors or an angiotensin two receptor antagonist. Four patients presented with congestive heart failure, two patients had ischaemic events, three patients had gastroenteritis and one patient had a bradyarrhythmia as the primary diagnosis. All patients had their renal function tested in the community prior to their presenting illness; the baseline eGFR, as determined using the modified MDRD formula[12], ranged from 23-90 mL/min per 1.73 m2. Only two patients had chronic kidney disease (CKD) 4, five patients had CKD3. In addition to other therapy three patients were treated with emergent haemodialysis (patients 1, 3 and 9, Table 2). Two patients died, one of cardiac ischaemia and one of multi-organ failure (patient 1 and 5 respectively, Table 2).
DISCUSSION
Metformin remains an attractive option in the treatment of type 2 diabetes: It promotes weight loss and has been shown to reduce the complications of and the mortality associated with diabetes[13]. Monotherapy with metformin appears to carry greater benefits than monotherapy with other hypoglycaemic agents[14].
The main concern with the use of metformin is the risk of developing lactic acidosis. Although a number of case series exist in the literature it is controversial as to whether MALA actually occurs. In a Cochrane review, with 70490 patient years of exposure to metformin, no cases of MALA were identified[14]. It was estimated that the hypothetical incidence of lactic acidosis in patients treated with metformin was 4.3 per 100000 patient years and 5.4 per 100000 patient years in non-metformin users[15]. In our series we report a low rate of lactic acidosis in the general population but that the rate of lactic acidosis in patients on metformin is significantly greater. Other population based studies have also reported a much greater rate of lactic acidosis in patients on metformin: In a recent series Scale and Harvey reported a rate of 120 per 100000 patient years[16]. There are several potential reasons for the discrepancy between the observational studies and the clinical trial cohorts. Lactic acidosis is uncommon and may occur some time after the initiation of therapy, and thus may be missed in studies with short term follow-up. Lactic acidosis is not commonly listed as a primary discharge diagnosis, and thus may be underdiagnosed. There may be reporting bias in clinical trials. In addition, clinical trials may exclude patients such as the elderly or other with co-morbidities that may also contribute to the risk of developing lactic acidosis.
We used data from the New Zealand health survey to estimate the incidence of diabetes in Auckland. This survey estimated the prevalence of diabetes in children as 0.1%-0.4% and the number of diagnosed adult patients at between 3.4% to 6.3%. This is in line with national estimates but does not account potential patients with undiagnosed diabetes. Thus, we are likely to be underestimating the overall incidence of diabetes in the region. We used data from Pharmac to estimate the use of metformin in the region. Pharmac records the subsided use of metformin. Currently, all New Zealanders enrolled with a general practice are eligible for subsidised metformin and a free health check if they have diabetes. However, this does not extend to non-New Zealand residents in the region. Further, if a number of patients with diabetes are not enrolled with a primary practice, then they are also ineligible for subsidised metformin. Finally, a phased rollout of subsidized medications occurred between 2003 and 2007. All of these factors may also lead to underestimation of the use of metformin in the region.
We describe a series of ten patients with MALA. The mortality in this group of patients is high but not as great as that seen in lactic acidosis associated with sepsis, and all cases were associated with acute kidney injury. Renal replacement therapy is an attractive therapeutic option as it aids in the correction of acidosis and also the removal of lactate and metformin. However, it is not clear that haemodialysis confers any survival benefit[17]. In our series only three patients received dialysis. Interestingly, the patient who presented with the worst laboratory parameters, case 8, was managed with supportive therapy, did not receive dialysis, and survived with recovery of her renal function.
Clearly metformin is an effective therapy. Here we describe observational evidence that suggests that use of metformin is associated with an increased risk of lactic acidosis. The standard recommendations are to use metformin cautiously in patients with hepatic impairment and reduce the dose in the elderly. We recommend reducing the dose of metformin in CKD G4 and advise stopping when the eGFR is less than 20 mL/min. We suspect that this later message is well heeded, and may be a reason that only two patients in our series with CKD G4 were found to develop MALA. In addition, we routinely recommend to patients that if they develop an intercurrent illness that metformin be withheld and medical review is sought.
Further investigation of this issue is suggested to confirm the findings in this study, using more robust design and controlling more potential confounding factors, e.g., indication for metformin, co-morbidities and age.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Li-Feng Zhou, Epidemiologist, Waitemata District Health Board for biostatistical advice and performing data analysis, and Dr. Janeen Milner, General Pracitioner, Auckland, for thoughtful comments and review of the manuscript.
COMMENTS
Background
The incidence of metformin associated lactic acidosis (MALA) is small when assessed by systematic review, however, randomised controlled trials may underestimate the true incidence by using strict inclusion and exclusion criteria. This retrospective review describes the incidence of MALA and highlights the significant morbidity and mortality that is associated with this condition.
Research frontiers
No randomised clinical trial has been undertaken to assess the safety of metformin in patients with mild to moderate renal impairment. This would be challenging due to the low incidence of MALA. Use of observational cohort data or national patient registries may better quantify risk and acceptable clinical practice.
Applications
The authors recognise the efficacy of metformin as a therapeutic agent for type 2 diabetes and recommend reducing the dose of metformin in mild to moderate renal impairment, and advise halting metformin when the estimated glomerular filtration rate is less than 20 mL/min. In addition, they recommend to patients that if they develop an intercurrent illness that metformin be withheld and medical review sought.
Peer-review
The paper gives interesting information about the incidence of metformin associated lactic acidosis which is important for clinical practice. It is well written and the analysis has been performed adequately.
Footnotes
Institutional review board statement: This retrospective review was approved by the Northern X Regional Ethics Committee (NTX/EXP).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous, de-identified clinical data.
Conflict-of-interest statement: There are no conflicts of interest in the publication of this paper.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 27, 2016
First decision: February 29, 2016
Article in press: April 22, 2016
P- Reviewer: Nechifor G, Pedersen EB, Saleem M, Zuo L S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
References
- 1.Sterne J. [Report on 5-years’ experience with dimethylbiguanide (metformin, glucophage) in diabetic therapy] Wien Med Wochenschr. 1963;113:599–602. [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 4.Colagiuri S, Dickenson S, Girgis S, Colagiuri R. Canberra: Diabetes Australia and the NHMRC; 2009. National evidence based guideline for blood glucose control in type 2 diabetes. [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover A, Yialamas MA. Metformin or thiazolidinedione therapy in PCOS? Nat Rev Endocrinol. 2011;7:128–129. doi: 10.1038/nrendo.2011.16. [DOI] [PubMed] [Google Scholar]
- 7.Simpson IJ, Henley JW, Sharpe DN. Lactic acidosis associated with phenformin therapy in diabetes mellitus. N Z Med J. 1974;79:645–648. [PubMed] [Google Scholar]
- 8.Lalau JD. Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf. 2010;33:727–740. doi: 10.2165/11536790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics New Zealand. [accessed 2011 May] Available from: http://www.stats.govt.nz.
- 11.New Zealand Ministry of Health. [accessed 2011 Jun] Available from: http://www.moh.govt.nz/moh.nsf/indexmh/dataandstatistics-survey-nzhealth.
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 14.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(4):CD002967. doi: 10.1002/14651858.CD002967.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Scale T, Harvey JN. Diabetes, metformin and lactic acidosis. Clin Endocrinol (Oxf) 2011;74:191–196. doi: 10.1111/j.1365-2265.2010.03891.x. [DOI] [PubMed] [Google Scholar]
- 17.Peters N, Jay N, Barraud D, Cravoisy A, Nace L, Bollaert PE, Gibot S. Metformin-associated lactic acidosis in an intensive care unit. Crit Care. 2008;12:R149. doi: 10.1186/cc7137. [DOI] [PMC free article] [PubMed] [Google Scholar]


