Abstract
Vascular response is an essential aspect of an effective immune response to periodontal disease pathogens, as new blood vessel formation contributes to wound healing and inflammation. Gaining a greater understanding of the factors that affect vascular response may then contribute to future breakthroughs in dental medicine. In this study, we have characterized the endothelial cell response to the common bacterium Fusobacterium nucleatum, an important bridging species that facilitates the activity of late colonizers of the dental biofilm. Endothelial cells were infected with Fusobacterium nucleatum (strain 25586) for periods of 4, 12, 24, or 48 h. Cell proliferation and tube formation were analyzed, and expression of adhesion molecules (CD31 and CD34) and vascular endothelial growth factor (VEGF) receptors 1 and 2 was measured by fluorescence-activated cell sorter (FACS) analysis. Data indicate that F. nucleatum impaired endothelial cell proliferation and tube formation. The findings suggest that the modified endothelial cell response acts as a mechanism promoting the pathogenic progression of periodontal diseases and may potentially suggest the involvement of periodontopathogens in systemic diseases associated with periodontal inflammation.
INTRODUCTION
The process of new blood vessel formation, known as angiogenesis, contributes to inflammation both by facilitating the transportation of immune cells to the site of injury and through the supply of oxygen and nutrients to the inflamed tissues (1). This vascular response is an essential aspect of an effective inflammatory reaction (2); however, many infectious diseases directly impact vascular structures and cells, reducing the efficacy of the immune response. Several systemic conditions and inflammatory diseases associated with bacteria affect vascular responses, through either the direct or indirect modulation of endothelial function and vascular tissues. This can be observed under a wide range of conditions: Crohn's disease has been linked to Mycobacterium species and Escherichia coli in genetically susceptible patients (3, 4); cirrhosis has been connected with Enterobacteriaceae, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Acinetobacter baumannii, Staphylococcus aureus, and Enterococcus (5); and Aspergillus has been related to meningitis and associated with vascular complications (6). Thus, the pathogenesis of complex infectious and inflammatory diseases can, in part, be seen as representing an active interaction between bacteria and the vasculature, where endothelial cells may be directly involved with the inflammation process.
Periodontitis results from the inflammatory response to bacterial colonization and biofilm formation. The most diverse and highly populated biofilm in the human body is found in the periodontal tissues, which contain highly complex microbial interactions both among bacterial species and with the host (7). During the initiation of periodontal infection, biofilm levels increase, and formerly commensal microbial species become pathogenic. This process involves pathological changes in gingival tissues and their attachment to tooth surfaces (8). Bridging species, such as Fusobacterium nucleatum, create a favorable environment for late colonizers and for other potentially more pathogenic bacteria (e.g., Porphyromonas gingivalis). This process occurs as bridging species adhere to early colonizers (e.g., Gram-positive cocci), which enhances the adherence of red complex species (9). F. nucleatum plays an essential role in coaggregation (9, 10), the process enabling interspecies communication. In this way, F. nucleatum may directly impact the host tissues and cells. Increased production of inflammatory mediators, stimulated by host cells in response to the presence of F. nucleatum, may further impact vascular changes (1). However, it is not yet fully clear how the endothelium responds to the progression of the inflammation initiated by F. nucleatum.
In the early stages of periodontal inflammation, perivascular tissues are disrupted (11). This is followed by the destruction of collagenous fibers and the proliferation of capillaries into the disrupted connective tissue (12). A prolonged exposure to bacteria and inflammation may predispose host tissues to greater damage, which in turn may modulate vessel formation and further amplify the inflammation. Previous studies have shown that bacterial challenge with Pseudomonas aeruginosa (13), Porphyromonas gingivalis (14), and Enterococcus faecalis (15) modulates endothelial cell function. Data also suggest that endothelial cells respond to P. gingivalis by increasing the levels of leukocyte adhesion molecules, elevating levels of inflammatory cytokines and chemokines, and inducing prothrombotic properties (16, 17). However, P. gingivalis emerges later, after inflammation is established. As a bridging species, F. nucleatum may modulate endothelial cell function and vasculature, creating a favorable environment for late colonizers such as P. gingivalis. In order to elucidate the endothelial cell response to F. nucleatum, we have analyzed surface markers involved in cell adhesion, development, and function and expression of receptors and targeted genes.
MATERIALS AND METHODS
Growth and culture of bacteria.
In order to test our hypothesis, F. nucleatum strain 25586 was cultured on blood agar plates in an anaerobic system using 10% H2, 80% N2, and 10% CO2 for 2 to 6 days. The cultures were then inoculated with brain heart infusion broth, supplemented with hemin, and incubated at 37°C for 2 days until they reached an optical density at 540 nm (OD540) of 0.8, corresponding to 109 CFU ml−1. The bacteria were then diluted at 107 CFU ml−1, corresponding to a multiplicity of infection (MOI) of 100. In preparation for this study, we ran experiments with MOIs of 10, 100, and 1,000 (data not shown). Results of studies using various MOIs, including the MOIs of 10 (18), 100 (19, 20), and 1,000 (18), have been previously reported in the literature. The optimum concentration achieved in our experiments—in line with most publications in the literature—was the MOI of 100.
Endothelial cell culture.
Primary human umbilical vein endothelial cells (HUVEC) (ATCC PCS-100-010) were purchased from American Type Culture Collection (ATCC). Cells were cultured in vascular basal cell medium (ATCC PCS-100-030) supplemented with endothelial cell growth kit-VEGF (ATCC PCS-100-041), penicillin, and streptomycin. Cells were cultured in 75-cm2 flasks (Corning) and maintained in an incubator with 5% CO2 at 37°C. Cells from passages 4 to 8 were used. The medium was changed every 3 days, in accordance with the manufacturer's recommendations. Cell characterization was accomplished through morphological analysis after confluence was reached. HUVEC (2 × 105) were placed in 12-well plates and were preincubated at 37°C for 2 h. Cells were then incubated with F. nucleatum; cells and supernatants were collected and analyzed at baseline level and after different time points at up to 48 h after infection.
Endothelial cell proliferation in response to F. nucleatum.
In order to determine the impact of F. nucleatum on endothelial cell proliferation, we used an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (21–23). Endothelial cells were seeded at 1 × 104 cells/well in 96-well plates and incubated for 24 h at 37°C. The cells were then infected with F. nucleatum (MOI, 1:100) for 4, 12, 24, or 48 h. After each time point, medium was removed and cells were washed with phosphate-buffered saline (PBS). PBS (90 μl) and MTT (10 μl) (10% MTT solution; concentration, 0.5 mg/ml) were added. The plates were shaken gently and incubated at 37°C. After 4 h, 75 μl of the MTT solution was removed from the well and 50 μl of dimethyl sulfoxide (DMSO) was added to each well and mixed using the pipette. Plates were incubated at 37°C. After 10 min, the A570 was read.
Tube formation assay.
In order to study how F. nucleatum affects endothelial cell tube formation as a measure of in vitro vessel formation, 45 μl of Matrigel (BD Biosciences) was added to each well in 96-well plates. Plates were incubated at 37°C for 1 h to allow gelling, and endothelial cells were added at a concentration of 5 × 103 in each well. The plates were then incubated at 37°C for one more hour. Following this process, F. nucleatum was added at an MOI of 1:100. Plates were again incubated at 37°C, and images were captured at the 4-, 12-, 24-, and 48-h marks. No bacteria were added to the control plates. Cells were observed directly under the optical microscope. Images were evaluated by the use of Image-Pro Plus Version 4.5.0.29 (Media Cybernetics, Silver Spring, MD, USA). The numbers of tubes were counted, and their areas were measured.
Expression of endothelial cell surface markers in response to F. nucleatum.
In order to study the impact of F. nucleatum on endothelial cell surface markers, endothelial cells were plated in 12-well plates (1 ml of media containing 2 × 105 cells) and bacteria were added at an MOI of 100. After 4, 12, and 24 h of infection with F. nucleatum, cells were collected and washed twice. The cells were then incubated and labeled with allophycocyanin (APC) (Alexa Fluor 647) anti-human CD31 antibody (Biolegend), APC (Alexa Fluor 647) anti-human CD34 antibody (Biolegend), phycoerythrin (PE) anti-human VEGF receptor 1 (VEGFR1) antibody (Biolegend), or APC (Alexa Fluor 647) anti-human VEGFR2 antibody (Biolegend) for 45 min. The cells were then washed twice with PBS-bovine serum albumin (BSA) and analyzed using a flow cytometer (FACScan, using CellQuest software; BD Bioscience). Isotype controls for APC and PE (Biolegend) were used in all experiments. Data were expressed as percentages of positive cells compared to target molecules.
Quantitative PCR for gene expression in endothelial cells.
Endothelial cells were cultured and infected with F. nucleatum in order to elucidate the role of F. nucleatum in modulating the expression of target genes. Total RNA was extracted using TRIzol reagent and was quantified in a spectrophotometer at A260. RNA samples with a 260:280 ratio (around 2.0) were employed to ensure high purity. Total RNA (1 μg) from each sample was used for reverse transcription. Each assay was carried out in triplicate using a 20-μl reaction mixture. Experiments were performed at least three times and in triplicate. The primers used for real-time PCR analysis (purchased from Life Technologies) were as follows: Hs02585826_cn (VEGF growth factor A [VEGFA]), Hs04047136_cn (PLCG1), Hs00164893_CE (inducible nitric oxide synthase [iNOS]), and Hs00725652_CE (endothelial NOS [eNOS]). β-actin (Hs01060665_g1) was used as the housekeeping gene control for normalization. Data analysis was performed using the threshold cycle (ΔΔCT) method, and β-actin was used as the reference gene.
PGE2 and proinflammatory cytokine release by endothelial cells in response to F. nucleatum.
Supernatants were collected after 4 to 24 h of infection with F. nucleatum and stored in aliquots at −80°C in order to analyze the impact that F. nucleatum has on cytokine release by endothelial cells. In accordance with the manufacturer's instructions, samples were thawed on ice before measurements of prostaglandin E2 (PGE2) levels were performed using a specific enzyme-linked immunosorbent assay (ELISA) obtained from R&D Systems. Levels of VEGF, interleukin-1α (IL-1α), and tumor necrosis factor alpha (TNF-α) were measured using specific Milliplex multiplex assays, according to the instructions of the manufacturer (Millipore). Results were expressed after correction to total protein contents of the supernatant media in picograms of protein.
Nitric oxide release by endothelial cells in response to F. nucleatum.
Levels of nitric oxide metabolites (nitrite and nitrate) and NO synthase (NOS) were measured in order to evaluate if F. nucleatum changes the nitric oxide pathway of endothelial cells. Supernatants of infected endothelial cells were collected after 4, 12, and 24 h of infection and were studied using a specific assay kit from R&D Systems (catalog number KGE001), in accordance with the manufacturer's instructions. This assay determines NO concentrations based on the enzymatic conversion of nitrate to nitrite by nitrate reductase. The reaction is followed by colorimetric detection of nitrite as an azo dye product of the Griess reaction. The Griess reaction is based on the two-step diazotization reaction, in which acidified NO- produces a nitrosation agent which reacts with sulfanilic acid to produce the diazonium ion. This ion is then coupled to N-(1-naphthyl) ethylenediamine to form the chromophoric azo derivative which absorbs light at 540 to 570 nm. Results were expressed in micromoles per liter.
Statistical analysis.
All experiments were performed a minimum of three times and in triplicate samples. Measurements were expressed as means ± standard deviations (SD). Data were analyzed using one-way analysis of variance (ANOVA) with Bonferroni's post hoc correction or Student's t test (GraphPad Prism, 5.01; Graphpad Software, Inc., San Diego, CA, USA). P values of <0.05 were considered significant.
RESULTS
F. nucleatum modulates proliferation and tube formation of endothelial cells.
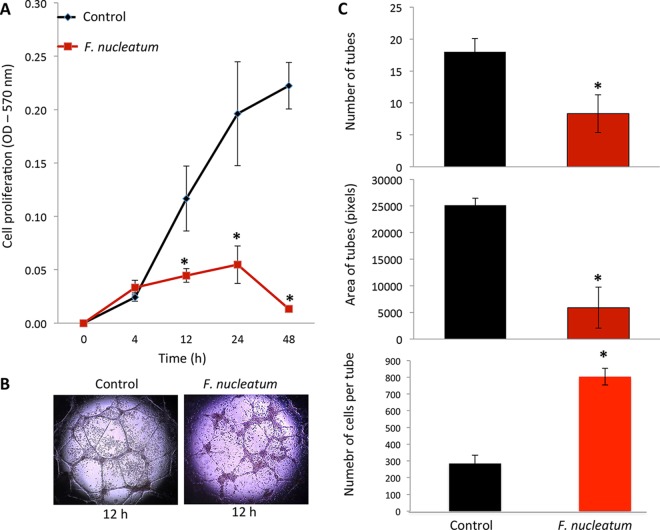
F. nucleatum was shown to cause significant and substantial suppression of endothelial cell proliferation after 12 h of incubation (Fig. 1A). Tube formation as a correlate for endothelial cell function in angiogenesis in vitro was decreased when cells were cultured with F. nucleatum (Fig. 1B and C). These changes were found to be statistically significant after adjusting for the decrease in cell viability, as measured by Trypan blue exclusion.
FIG 1.
HUVEC impairment of cell proliferation and tube formation. (A) F. nucleatum had no observable effect on endothelial cell proliferation at up to 4 h postinfection. After the 4-h mark, cell proliferation by infected endothelial cells occurred at a markedly lower rate than that observed in the control group. (B) Tube formation is impaired in cells infected with F. nucleatum in terms of both numbers of tubes and tube area; endothelial cells form tube-like structures after 12 h (control); endothelial cells infected with F. nucleatum for 24 h are unable to form tube-like structures (F. nucleatum). Magnification for images, ×400. (C) Average number of tubes (top), average area of tubes (middle), and number of cells per tube (bottom). *, P < 0.05.
Impact of F. nucleatum on adhesion molecule expression on endothelial cells.
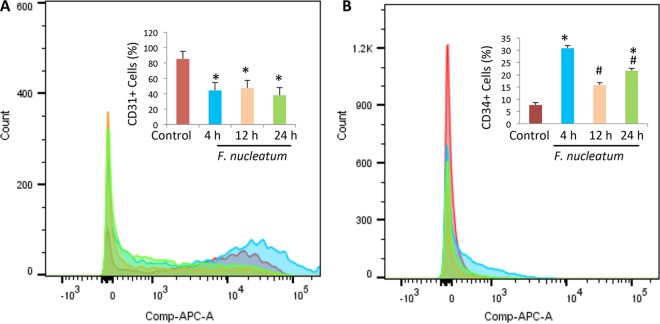
CD31 and CD34 expression levels were analyzed by flow cytometry. A significant decrease in CD31 expression was observed (Fig. 2A), a phenomenon which occurs when endothelial cells are exposed to inflammatory cytokines as early as 4 h after infection. CD31 expression remained low (P < 0.05), suggesting reduced cell-cell interaction and increased vascular permeability. Figure 2B shows that there was a significant increase in CD34 expression after 4 h of infection that remained higher than the level seen with the control. This suggests an increase in the numbers of undifferentiated endothelial cells.
FIG 2.
F. nucleatum modulates expression of HUVEC surface markers. The percentage of endothelial cells expressing CD31 was seen to decrease after infection with F. nucleatum (A), while the percentage of endothelial cells expressing CD34 was seen to increase following infection (B). Measurements were taken at baseline levels and after 4, 12, and 24 h of infection with F. nucleatum, as analyzed by flow cytometry. *, P < 0.05 (compared to control results at 12 h); #, P < 0.05 (compared to control results at 4 h).
F. nucleatum modulates VEGF receptor expression and VEGF release.
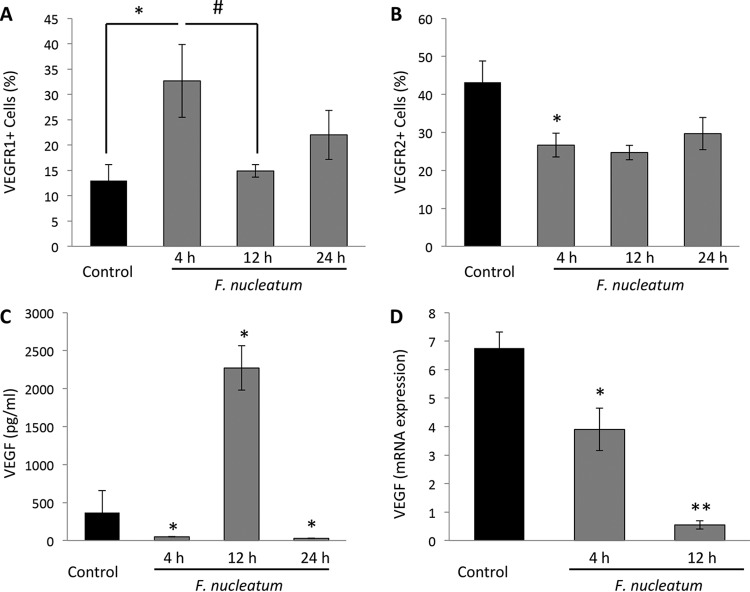
F. nucleatum was found to modulate receptors for VEGF on endothelial cells. The levels of VEGFR1 increased 2-fold after the 4-h mark of infection (Fig. 3A) and subsequently returned to normal. VEGFR2 expression was decreased after 4 h (P < 0.05) and remained low up to the 24-h mark. The switch in the VEGFR2/VEGFR1 ratio suggests a reduction in proangiogenic signaling and an increase in the inflammatory role played by endothelial cells. VEGF release, a major regulator of endothelial proliferation and cell migration, was significantly increased after 12 h (Fig. 3C), while its expression was seen to decrease over time (Fig. 3D) (P < 0.05).
FIG 3.
F. nucleatum modulates VEGF. (A and B) F. nucleatum was observed to modulate VEGFR1 (A) and VEGFR2 (B). Results are shown as percentages of expression of endothelial cells at baseline and following 4, 12, or 24 h of infection. (C and D) VEGF release in supernatants showed some fluctuation (C), while VEGF mRNA was downregulated (D) (data represent fold decreases after the 4- and 12-h marks expressed in a time-dependent manner). *, P < 0.05 (compared to control results at 4 h); #, P < 0.05 (compared to control results at 4 h and 12 h); **, P < 0.05 (compared 12 h results to control and 12 h results to 4 h).
F. nucleatum promotes PGE2 release and inflammatory cytokine production by endothelial cells.
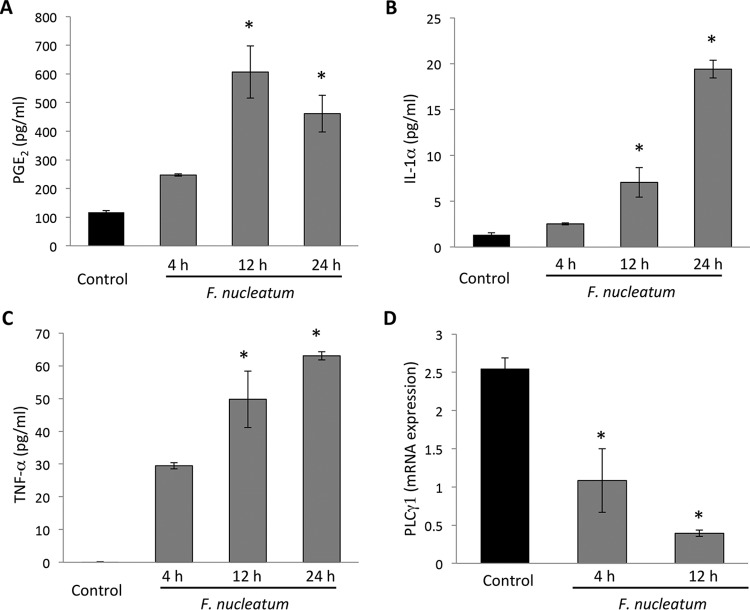
Twelve hours after infection, PGE2 release was elevated, and it remained at an increased level up to 24 h after infection (Fig. 4A; P < 0.05). IL-1α (Fig. 4B) and TNF-α (Fig. 4C) levels were seen to increase, while PLCY1 expression (Fig. 4D) was observed to decrease (*, P < 0.05). This finding further suggests that endothelial cells play a direct role in progressive inflammation and possibly affect autocrine inflammatory impact.
FIG 4.
F. nucleatum infection increases prostaglandin and cytokine release in supernatants and decreases mRNA PLCY1 expression in HUVEC. (A) The inflammatory component of supernatants is characterized by high-level release of PGE2 after 12 and 24 h. Endothelial cells infected with F. nucleatum were observed to release a high level of PGE2, suggesting a vasodilatory state. (B and C) Levels of IL-1α (B) and TNF-α (C) were increased after the 12- and 24-h marks following infection. (D) mRNA PLCY1 expression was reduced after the 4- and 12-h marks following infection (*, P < 0.05).
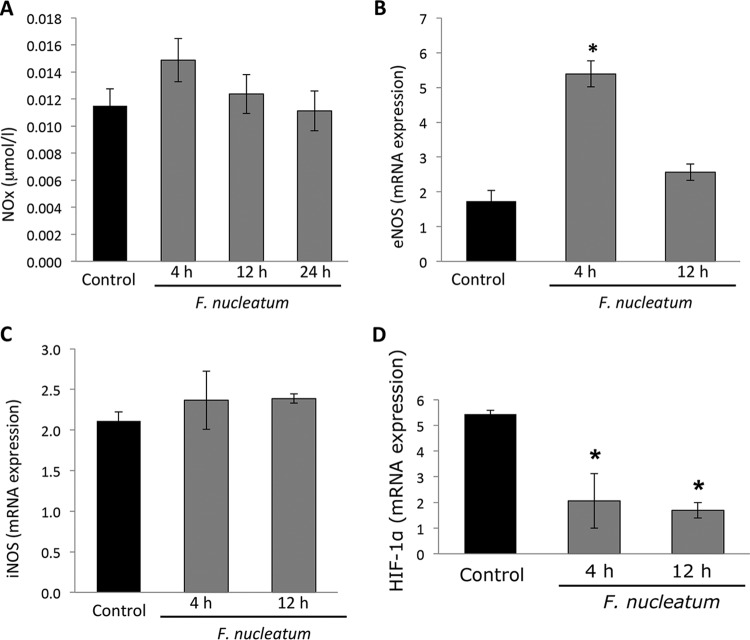
F. nucleatum upregulates endothelial nitric oxide synthase and reduces HIF-1α mRNA expression.
F. nucleatum was not seen to cause a substantial change in nitric oxide release by the endothelial cells (Fig. 5A), while endothelial nitric oxide synthase (eNOS) levels were seen to increase after the 4-h mark (P < 0.05) (Fig. 5B). There was no impact on the levels of inducible NOS (iNOS) (Fig. 5C), which suggests increased expression of the endothelial form of nitric oxide synthase at an early phase without affecting the inducible form of NO or its overall generation. HIF-1α expression was reduced after 4 and 12 h (P < 0.05) (Fig. 5D).
FIG 5.
F. nucleatum modulates mRNA eNOS without changing mRNA iNOS or NOx release. (A) F. nucleatum infection was not observed to affect NOx release (measured as micromoles per liter) by endothelial cells at any time point. (B and C) After the 4-h mark following infection, levels of eNOS mRNA were observed to increase (B), while those of iNOS were not observed to be affected (C). (D) HIF-1α mRNA expression was analyzed by quantitative PCR (qPCR). Data show a reduction in HIF-1α expression after 4 and 12 h. *, P < 0.05 (compared to control results).
DISCUSSION
Endothelial cells are among the first in contact with bacteria when bacterial products are disseminated into the bloodstream, as they form the vascular tree of all organs. Therefore, endothelial cells are directly impacted by infectious/inflammatory processes (2). Vascular changes occur during the onset of chronic periodontitis (24–26) and are also affected by other forms of inflammation, cancer metastasis, healing, blood flow, atherosclerosis, tumor growth, and angiogenesis (27). The data from this study demonstrate that endothelial cell function is modulated toward a proinflammatory cell response upon exposure to F. nucleatum. These findings suggest that vascular changes are critical in the establishment of an environment which is favorable for late colonizers and pathogens such as P. gingivalis.
It has been previously demonstrated that P. gingivalis impairs endothelial cell proliferation, function, and migration (26), but P. gingivalis is a dominant species during the later stages of periodontal infection. Vascular response to biofilm, however, can be induced as soon as the supragingival plaque extends into the subgingival region. This conversion is associated with an environmental change toward hypoxia and anaerobiosis (28). In light of this, bridging species such as F. nucleatum can be seen as critical to the development of periodontal infection during this transition, as they potentially create a favorable ecosystem for late colonizers such as P. gingivalis.
We have focused on the endothelial cells, as vascular changes precede the immune cell response. The data show impairment in cell proliferation and cell death after 12 h of infection. F. nucleatum infection was also observed to affect the expression of endothelial cell surface markers. CD31 levels were reduced as early as 4 h after infection and remained low at all measured time points. This finding suggests an increase in vascular permeability. CD31, an adhesion molecule of 130 kDa identified in endothelial cells, platelets (29), and leukocytes (30), had previously been considered simply a cellular adhesion molecule (31). However, later studies suggested that CD31 triggers downstream inhibitory signaling upon transhomophilic CD31 engagement during cell-cell interaction (32). CD31 signaling participates in the regulation of leukocytes, T cell and platelet activation, and angiogenesis (33). Meanwhile, another surface marker on endothelial cells, CD34, was found to be overexpressed 4 h postinfection (P < 0.05). This finding corroborates the presence of undifferentiated cells at this time point, and levels returned to normal after the 12-h mark. CD34 is a marker of vascular endothelial progenitor cells (34, 35), and results of in vitro studies show that endothelial cells lose CD34 expression after several passages, with only a small population of CD34+ cells remaining (34). Endothelial function is sustained by the net balance between injury and repair. The quantification of the detachment of mature endothelial cells and derived microparticles may represent the degree of damage, while it may also determine the number and functional characteristics of circulating endothelial progenitor cells to reflect the endogenous repair potential. Beyond injury, endothelial cells may detach and become identifiable by flow cytometry analysis of the expression of surface markers (36). Therefore, the presented findings suggest that the increase in CD34 expression is due to the bacterial challenge against HUVEC. F. nucleatum initially stimulated undifferentiated cells. Taking these data together, a clear picture emerges demonstrating that the maturation of endothelial cells was impaired when they were exposed to F. nucleatum.
Endothelial proliferation and migration are regulated by vascular endothelial growth factor (VEGF) (37), which is the most potent inducer of angiogenesis and capillary permeability (38) and is an essential regulator of vascularization (39). F. nucleatum infection was found to increase the level of VEGF release after 12 h. VEGF production is regulated by two receptors during angiogenesis and endothelial cell function: R1 and R2 (40, 41). VEGFR1 modulates VEGF activity, creating heterodimers with VEGFR2 (42). A VEGFA “trap” sequesters ligand to reduce VEGFR2-mediated endothelial signaling (43), which promotes the angiogenic function of the endothelial cells (42). Our results show that the levels of VEGFR1 were increased 4 h following infection (P < 0.05) and returned to normal after the 12-h mark. The literature shows that endothelial cells have been observed to express at least 10 times less VEGFR1 than VEGFR2 (44, 45). In our study, after 4 h of infection, endothelial cells showed a VEGFR1/VEGFR2 ratio of 1.2. VEGFR2 levels were reduced 4 h postinfection (P < 0.05) and remained lower than the control levels at all time points. Additionally, levels of expression of mRNA VEGF and mRNA PLCγ1 were reduced after the 4- and 12-h marks (P < 0.05). The modulation of the phenotypic properties of endothelial cells and the increases in proinflammatory properties of the endothelium can be viewed as parallel developments in response to the changes in VEGF receptor signaling. As a result, PGE2 release was increased, representing a stimulus causing increased vasodilation (46). Proinflammatory cytokine release was found to increase along with structural changes. This suggests that F. nucleatum actively promotes the proinflammatory changes of endothelial cells.
Increases and alterations in the metabolic regulation of VEGF during angiogenesis that occurred when endothelial cells were exposed to stressful conditions have been previously reported. Previous studies performed with endothelial cells and P. gingivalis have not resulted in observed differences in the levels of expression of VEGF or its receptor (VEGFR2), while lipopolysaccharide (LPS) from P. gingivalis is known to stimulate angiogenesis (47). There was higher release of VEGF in supernatants without an increase in VEGF mRNA expression, which suggests that F. nucleatum may activate the autocrine function of endothelial cells, leading to higher release of VEGF that is not, however, followed by higher VEGF production. VEGF mRNA expression was reduced after 4 and 12 h (P < 0.05) (Fig. 3D). Since VEGF is associated with vessel growth and angiogenesis (48), these data are in accordance with the proliferation and tube formation data shown in Fig. 1. Suppression of VEGF expression accompanied by a reduction in tube formation due to bacterial challenge has also been shown in endothelial cells of chick embryos (49). Additionally, expression of HIF-1α—which is a precursor of VEGF—was also suppressed (Fig. 5D); VEGF reduction, accompanied by HIF-1α reduction, has been previously shown in the literature (50).
Taken together, the data show that F. nucleatum orchestrates changes in endothelial cells which modulate the responses of the cells and promote their active involvement in the inflammatory process, leading to impaired tissue vascularization during inflammation. This research is particularly relevant to the understanding of complex infectious and inflammatory diseases such as periodontitis.
ACKNOWLEDGMENTS
This work was supported by NIH/NIDCR grants DE020906 and DE15566 and by CAPES/BRAZIL—Science Without Borders.
REFERENCES
- 1.Johnson RB, Serio FG, Dai X. 1999. Vascular endothelial growth factor and progression of periodontal disease. J Periodontol 70:848–852. doi: 10.1902/jop.1999.70.8.848. [DOI] [PubMed] [Google Scholar]
- 2.Air WC. 2007. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100:158–173. [DOI] [PubMed] [Google Scholar]
- 3.Roifman I, Sun YC, Fedwick JP, Panaccione R, Buret AG, Liu H, Rostom A, Anderson TJ, Beck PL. 2009. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 7:175–182. doi: 10.1016/j.cgh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Principi M, Mastrolonardo M, Scicchitano P, Gesualdo M, Sassara M, Guida P, Bucci A, Zito A, Caputo P, Albano F, Ierardi E, Di Leo A, Ciccone MM. 2013. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis 7:e427–e433. doi: 10.1016/j.crohns.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Acevedo J, Fernández J. 2014. New determinants of prognosis in bacterial infections in cirrhosis. World J Gastroenterol 20:7252–7259. doi: 10.3748/wjg.v20.i23.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow FC, Marra CM, Cho TA. 2011. Cerebrovascular disease in central nervous system infections. Semin Neurol 31:286–306. doi: 10.1055/s-0031-1287658. [DOI] [PubMed] [Google Scholar]
- 7.Feng Z, Weinberg A. 2006. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000 40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 8.Slots J. 1979. Subgingival microflora and periodontal disease. J Clin Periodontol 6:351–382. doi: 10.1111/j.1600-051X.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 9.Kolenbrander PE. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 10.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ Jr. 2002. Communication among oral bacteria. Microbiol Mol Biol Rev 66:486–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warwick JW, Counsell A. 1927. A histological investigation into so-called pyorrhea alveolaris. Br Dent J 48:1237–1252. [Google Scholar]
- 12.Orban B, Weinmann JP. 1942. Diffuse atrophy of the alveolar bone (periodontitis). J Periodontol 13:31–45. [Google Scholar]
- 13.Valente E, Assis MC, Alvim IM, Pereira GM, Plotkowski MC. 2000. Pseudomonas aeruginosa induces apoptosis in human endothelial cells. Microb Pathog 29:345–356. doi: 10.1006/mpat.2000.0400. [DOI] [PubMed] [Google Scholar]
- 14.Roth GA, Ankersmit HJ, Brown VB, Papapanou PN, Schmidt AM, Lalla E. 2007. Porphyromonas gingivalis infection and cell death in human aortic endothelial cells. FEMS Microbiol Lett 272:106–113. doi: 10.1111/j.1574-6968.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.Millán D, Chiriboga C, Patarroyo MA, Fontanilla MR. 2013. Enterococcus faecalis internalization in human umbilical vein endothelial cells (HUVEC). Microb Pathog 57:62–69. doi: 10.1016/j.micpath.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Kang IC, Kuramitsu HK. 2002. Induction of monocyte chemoattractant protein-1 by Porphyromonas gingivalis in human endothelial cells. FEMS Immunol Med Microbiol 34:311–317. doi: 10.1111/j.1574-695X.2002.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 17.Roth GA, Moser B, Roth-Walter F, Giacona MB, Harja E, Papapanou PN, Schmidt AM, Lalla E. 2007. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis 190:271–281. doi: 10.1016/j.atherosclerosis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Hashizume T, Kurita-Ochiai T, Yamamoto M. 2011. Porphyromonas gingivalis stimulates monocyte adhesion to human umbilical vein endothelial cells. FEMS Immunol Med Microbiol 62:57–65. doi: 10.1111/j.1574-695X.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuramitsu HK, Kang IC, Qi M. 2003. Interactions of Porphyromonas gingivalis with host cells: implications for cardiovascular diseases. J Periodontol 74:85–89. doi: 10.1902/jop.2003.74.1.85. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, Zheng H, Zhao J, Lin L, Li C, Liu J, Pan Y. 2011. Porphorymonas gingivalis induces intracellular adhesion molecule-1 expression in endothelial cells through the nuclear factor-kappaB pathway, but not through the p38 MAPK pathway. J Periodont Res 46:31–38. doi: 10.1111/j.1600-0765.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Garn H, Krause H, Enzmann V, Drössler K. 1994. An improved MTT assay using the electron-coupling agent menadione. J Immunol Methods 168:253–256. doi: 10.1016/0022-1759(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Peterson DA, Kimura H, Schubert D. 1997. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69:581–593. [DOI] [PubMed] [Google Scholar]
- 24.Zoellner H, Hunter N. 1991. Vascular expansion in chronic periodontitis. J Oral Pathol Med 20:433–437. doi: 10.1111/j.1600-0714.1991.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 25.Wirthlin MR, Hussain MZ. 1992. Clinical and light microscopic observations of gingivitis and early ligature-induced periodontitis in the Cynomolgus monkey. J Periodontol 63:533–539. doi: 10.1902/jop.1992.63.6.533. [DOI] [PubMed] [Google Scholar]
- 26.Bartruff JB, Yukna RA, Layman DL. 2005. Outer membrane vesicles from Porphyromonas gingivalis affect the growth and function of cultured human gingival fibroblasts and umbilical vein endothelial cells. J Periodontol 76:972–979. doi: 10.1902/jop.2005.76.6.972. [DOI] [PubMed] [Google Scholar]
- 27.Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ. 2002. In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res 64:384–397. doi: 10.1006/mvre.2002.2434. [DOI] [PubMed] [Google Scholar]
- 28.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. 2009. Regulation of angiogenesis by oxygen and metabolism. Dev Cell 16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.van Mourik JA, Leeksma OC, Reinders JH, de Groot PG, Zandbergen-Spaargaren J. 1985. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J Biol Chem 260:11300–11306. [PubMed] [Google Scholar]
- 30.Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, Strass B, Schnabl E, Knapp W. 1990. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol 145:3889–3897. [PubMed] [Google Scholar]
- 31.Newman PJ, Berndt MC, Gorski J, White GC II, Lyman S, Paddock C, Muller WA. 1990. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 32.Newton JP, Buckley CD, Jones EY, Simmons DL. 1997. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J Biol Chem 272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Shi GP. 2012. CD31: beyond a marker of endothelial cells. Cardiovasc Res 94:3–5. doi: 10.1093/cvr/cvs108. [DOI] [PubMed] [Google Scholar]
- 34.Fina L, Molgaard HV, Robertson D, Bradley Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. 1990. Expression of the CD34 gene in vascular endothelial cells. Blood 75:2417–2426. [PubMed] [Google Scholar]
- 35.Hristov M, Weber C. 2008. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res 58:148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Deanfield JE, Halcox JP, Rabelink TJ. 2007. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13:9–22. [PubMed] [Google Scholar]
- 38.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Gerber HP. 2001. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol 106:148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya M. 2001. Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1). Int J Biochem Cell Biol 33:409–420. doi: 10.1016/S1357-2725(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 41.Ferrara N, Gerber HP, LeCouter J. 2003. The biology of VEGF and its receptors. Nat Med 9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 42.Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, Miller MR, Newby DE, Gu Y, Barleon B, Weich H, Ahmed A. 2012. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun 3:972. doi: 10.1038/ncomms1977. [DOI] [PubMed] [Google Scholar]
- 43.Park JE, Chen HH, Winter J, Houck KA, Ferrara N. 1994. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269:25646–25654. [PubMed] [Google Scholar]
- 44.Lin MI, Sessa WC. 2006. Vascular endothelial growth factor signaling to endothelial nitric oxide synthase: more than a FLeeTing moment. Circ Res 99:666–668. doi: 10.1161/01.RES.0000245430.24075.a4. [DOI] [PubMed] [Google Scholar]
- 45.Imoukhuede PI, Popel AS. 2011. Quantification and cell-to-cell variation of vascular endothelial growth factor receptors. Exp Cell Res 317:955–965. doi: 10.1016/j.yexcr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams TJ, Peck MJ. 1977. Role of prostaglandin-mediated vasodilatation in inflammation. Nature 270:530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- 47.Koo TH, Jun HO, Bae SK, Kim SR, Moon CP, Jeong SK, Kim WS, Kim GC, Jang HO, Yun Il Kim KW, Bae MK. 2007. Porphyromonas gingivalis, periodontal pathogen, lipopolysaccharide induces angiogenesis via extracellular signal-regulated kinase 1/2 activation in human vascular endothelial cells. Arch Pharm Res 30:34–42. doi: 10.1007/BF02977776. [DOI] [PubMed] [Google Scholar]
- 48.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. 2006. VEGF receptor signaling—in control of vascular function. Nat Rev Mol Cell Biol 7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 49.Gheorghescu AK, Tywonuik B, Duess J, Buchete NV, Thompson J. 12 September 2015. Exposure of chick embryos to cadmium changes the extra-embryonic vascular branching pattern and alters expression of VEGF-A and VEGF-R2. Toxicol Appl Pharmacol doi: 10.1016/j.taap.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Yang X, Ding Y, Liu J, Lu J, Zhan Qin L Q, Zhang H, Chen X, Yang Y, Liu Z, Yang M, Zhou X, Cheng H, Sun X. 2015. Recombinant human endostatin enhances the redio response in esophageal squamous cell carcinoma by normalizing tumor vasculature and reducing hypoxia. Sci Rep 5:14503. doi: 10.1038/srep14503. [DOI] [PMC free article] [PubMed] [Google Scholar]