Abstract
A huge wetland (the ‘Pebas system’) covered western Amazonia during the Miocene, hosting a highly diverse and endemic aquatic fauna. One of the most contentious issues concerns the existence, potential pathways and effects of marine incursions on this ecosystem. Palaeontological evidences (body fossils) are rare. The finding of a new, presumably marine ostracod species (Pellucistoma curupira sp. nov.) in the upper middle Miocene Solimões Formation initiated a taxonomic, ecological and biogeographical review of the genus Pellucistoma. We demonstrate that this marine (sublittoral, euhaline), subtropical–tropical taxon is biogeographically confined to the Americas. The biogeographical distribution of Pellucistoma largely depends on geographical, thermal and osmotic barriers (e.g. land bridges, deep and/or cold waters, sea currents, salinity). We assume an Oligocene/early Miocene, Caribbean origin for Pellucistoma and outline the dispersal of hitherto known species up to the Holocene. Pellucistoma curupira sp. nov. is dwarfed in comparison to all other species of this genus and extremely thin-shelled. This is probably related to poorly oxygenated waters and, in particular, to strongly reduced salinity. The associated ostracod fauna (dominated by the eurypotent Cyprideis and a few, also stunted ostracods of possibly marine ancestry) supports this claim. Geochemical analyses (δ18O, δ13C) on co-occurring ostracod valves (Cyprideis spp.) yielded very light values, indicative of a freshwater setting. These observations point to a successful adaptation of P. curupira sp. nov. to freshwater conditions and therefore do not signify the presence of marine water. Pellucistoma curupira sp. nov. shows closest affinities to Caribbean species. We hypothesize that Pellucistoma reached northern South America (Llanos Basin) during marine incursions in the early Miocene. While larger animals of marine origin (e.g. fishes, dolphins, manatees) migrated actively into the Pebas wetland via fluvial connections, small biota (e.g. P. curupira sp. nov.) were phoretically freighted and developed freshwater tolerance over long timescales.
http://zoobank.org/urn:lsid:zoobank.org:pub:886C6476-393D-4323-8C0E-06BB8BD02FD9
Keywords: Pellucistoma, biogeography, palaeogeography, palaeoecology, dispersal mechanisms, freshwater adaptation
Introduction
During the Miocene epoch, an enormous wetland shaped western Amazonia's landscapes and biota (the ‘Pebas system’; for comprehensive synopses see Hoorn & Wesselingh 2010; Hoorn et al. 2010a). The general understanding of this unique ecosystem has significantly improved in the last two decades. In detail, however, its nature remains controversial and disputed (e.g. ‘mega-lake’, Wesselingh et al. 2002; ‘mega-wetland’, Hoorn et al. 2010b; ‘mega-fan’, Latrubesse et al. 2010; Wilkinson et al. 2010).
In particular, the existence, chronology, spatial extent and potential sources of marine interferences continue to be a heavily (and sometimes paradigmatically) discussed subject of western Amazonia's past. A plethora of sedimentological, palaeontological and geochemical indications were depicted to infer marine influences (e.g. Sheppard & Bate 1980; Hoorn 1993, 1994; Räsänen et al. 1995; Gingras et al. 2002; Wesselingh et al. 2002; Vonhof et al. 2003; Hovikoski et al. 2005, 2007, 2010; Rebata et al. 2006; Linhares et al. 2011; for recent compilation of arguments in favour see Boonstra et al. 2015). Nonetheless, the evidence is equivocal and permits differing interpretations (Cozzuol 2006; Westaway 2006; Latrubesse et al. 2007, 2010; Lundberg et al. 2010; Riff et al. 2010; Silva-Caminha et al. 2010; Gross et al. 2011, 2013; for comparable discussions see, e.g. Nicolaidis & Coimbra 2008; Ruskin et al. 2011).
Aside from mangrove-related pollen, dinoflagellate cysts, foraminiferal linings and remains of several marine vertebrate clades, body fossils that are specific for marine environments are scarce and restricted to thin intervals (e.g. Linhares et al. 2011; Boonstra et al. 2015). Remarkably, the highly endemic aquatic invertebrate fauna of the ‘Pebas system’ is strongly dominated by the abundance of pachydontine bivalves and the cytheroid ostracod Cyprideis. Otherwise common freshwater and marine taxa are rare and typical marginal marine molluscs (e.g. arcids, oysters, mangrove cerithioidean snails) are absent (e.g. Whatley et al. 1998a; Wesselingh 2006, 2007; Gross et al. 2013, 2014). Based on the mollusc and ostracod faunas, brackish waters (e.g. Purper 1979; Nuttall 1990; Whatley et al. 1998a) or extensive marine transgressions (Sheppard & Bate 1980) were proposed. However, geochemical investigations performed on the shells of these biota consistently indicate freshwater conditions. Elevated salinity (∼5 PSU) is only evident for a few localities (e.g. Vonhof et al. 2003; Kaandorp et al. 2006; Wesselingh et al. 2006; Gross et al. 2013).
Wesselingh (2007) considered the ‘Pebas system’ to be a predominantly freshwater environment, in which adaptations to predation pressure and a muddy, poorly oxygenated substrate triggered speciation, as well as habitat dominance by pachydontine bivalves. Gross et al. (2013) suggested that it was a locally unstable but on a regional scale long-lived wetland, where euryoecious biology, passive dispersal predispositions and reproduction modes (brood care) favoured the success of the genus Cyprideis. Thus, a conclusive, ‘simple’ explanation about the nature of this ‘mega-wetland’ is still pending.
The current study was initiated by the finding of a very small-sized, marine ostracod (Pellucistoma) in late middle Miocene sediments of western Brazil (Fig. 1). This posed three central questions: (1) does this record prove the existence of marine incursions, thousands of kilometres away from the next (palaeo-)coastline; (2) which provenance could Pellucistoma be from; and (3) what are the potential migration pathways? It is certainly tempting to approve the first question by applying uniformitarian principles, to suppose a Caribbean origin (as suggested for e.g. molluscs; Wesselingh & Ramos 2010) and to relate our record directly to far-reaching marine incursions (e.g. Boonstra et al. 2015). However, in view of the conflicting discussions about this topic, we performed an extensive taxonomic, ecological and biogeographical appraisal of Pellucistoma. Based on this, we explore possible answers to the above-mentioned questions.
Figure 1.
Location of the studied well 1AS-10-AM in western Amazonia. A, overview map; B, position of exploration wells (after Maia et al. 1977); star = herein investigated core; compare Gross et al. (2014).
Geological setting and age
The investigated material originates from a well (1AS-10-AM) drilled ∼62 km south-west of Benjamin Constant in western Brazil (site Sucuriju, close to Rio Ituí; 04 ° 50′ S, 70 ° 22′ W; state of Amazonia; Fig. 1). Based on the available subsurface information (Maia et al. 1977; Del' Arco et al. 1977), it is located in the Solimões Basin (e.g. Wanderley-Filho et al. 2010) and penetrates (except Holocene soils) sediments of the Solimões Formation (for substantial discussions about this formation see Purper 1979; Hoorn 1994; Latrubesse et al. 2010; Hoorn et al. 2010b).
Well 1AS-10-AM was continuously cored down to 400.25 m. Its lithology consists of alternations of semi-indurated clay and silt. Up to metre-thick, sandy as well as decimetre-thick, lignite intercalations occur subordinately. Recently, Gross et al. (2014) studied the microfauna (in particular the ostracod genus Cyprideis). For more detailed core descriptions and an illustration of the section, we refer to this work. The herein discussed findings stem from sample AM10/30 (depth = 141.2 m), which is a clayey silt, rich in mollusc remains. According to Gross et al. (2014) this sample is biostratigraphically dated to the Cyprideis obliquosulcata ostracod zone sensu Muñoz-Torres et al. (2006), corresponding to a late middle Miocene age (Wesselingh & Ramos 2010).
Material and methods
Samples (250 g of dried sediment; 40 °C, 24 h) from core 1AS-10-AM were washed through standard sieves (63/125/250/500 µm) using diluted hydrogen peroxide for disintegration (H2O2:H2O = 1:5). Wet sieve residuals were washed with ethanol (70%) before drying (40 °C, 24 h; Gross et al. 2014). Residuals ≥125 µm of sample AM10/30, from which the species under discussion originates, were picked out completely for their micropalaeontological content.
Prior to scanning electron microscope imaging (SEM: JEOL JSM-6610LV), the shells were photographed in transmitted light (Leitz Orthoplan microscope, camera: Leica DFC290) and measured (Leica Application Suite V3.6.0). Focus stacked images (Fig. 2A, B) were obtained by combining ∼35 transmitted light photographs per specimen (covered with distilled water) with the software CombineZP.
Figure 2.
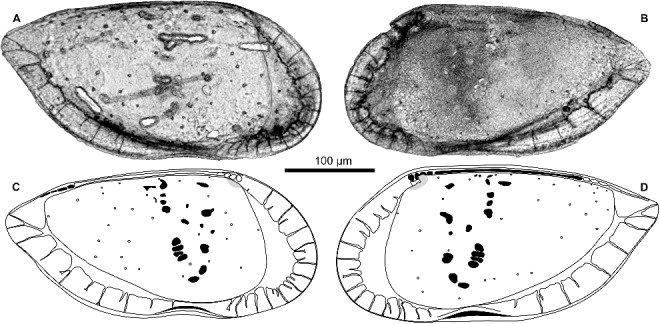
Transmitted light photographs (A, B, focus stacked) and schematic drawings (C, D) of Pellucistoma curupira sp. nov. A, MPEG-513-M, left valve, internal view (length = 0.37 mm, height = 0.18 mm); B, MPEG-509-M, right valve, internal view of Figure 3F; C, left valve, internal view, based on A and Figure 3E; D, right valve, internal view, based on B and Figure 3H (compare also Fig. 3F).
For stable isotope analyses (δ18O, δ13C), ostracod valves were additionally washed with distilled water and rinsed in ethanol. From core 1AS-10-AM (sample AM10/30) adults of three species were measured: Cyprideis machadoi (Purper, 1979), Cyprideis multiradiata (Purper, 1979) and Cyprideis sulcosigmoidalis (Purper, 1979) (for taxonomy see Gross et al. 2014). The number of specimens required for analyses (∼50 µg) varied between 1 and 3 per measurement. A Thermo-Finnigan Kiel II automated reaction system and a Thermo-Finnigan Delta Plus isotope-ratio mass spectrometer were used to conduct the analyses (University of Graz; standard deviation = 0.1‰ relative to NBS-19; results in per mille relative to the Vienna Pee Dee Belemnite (VPDB) standard).
All specimens are housed in the micropalaeontological collection of the Museu Paraense Emílio Goeldi, Belém (Inv. No. MPEG-503-M to MPEG-514-M).
Systematic palaeontology
Suprageneric classification follows Horne et al. (2002).
Class Ostracoda Latreille, 1802
Order Podocopida Sars, 1866
Superfamily Cytheroidea Baird, 1850
Family Cytheromatidae Elofson, 1939
Genus Pellucistoma Coryell & Fields, 1937
Type species
Pellucistoma howei Coryell & Fields, 1937.
Pellucistoma curupira sp. nov.
Figure 3.
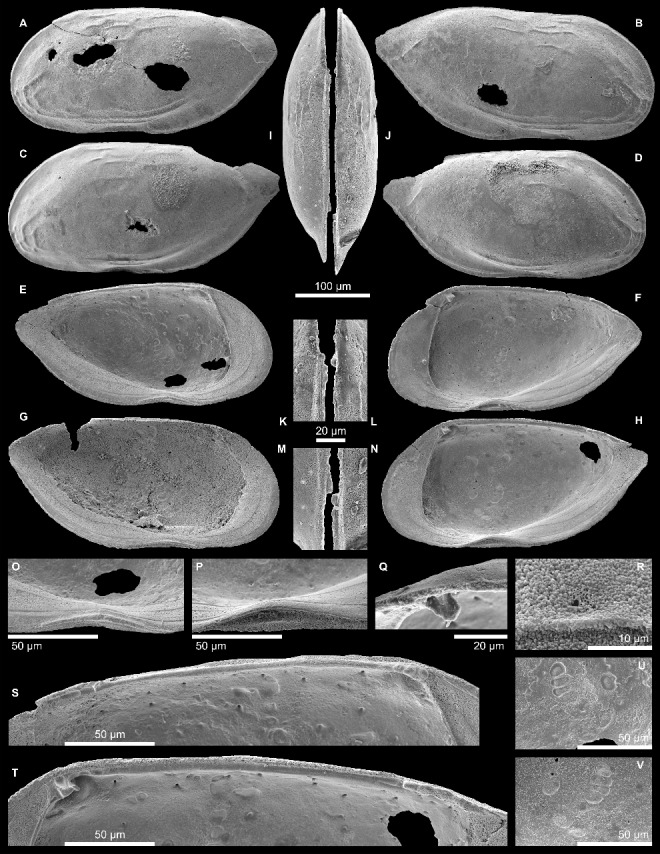
Pellucistoma curupira sp. nov. A, MPEG-504-M, left valve, external view (length = 0.36 mm, height = 0.17 mm); B, MPEG-505-M, right valve, external view (length = 0.38 mm, height = 0.18 mm); C, MPEG-506-M, left valve, external view (length = 0.38 mm, height = 0.18 mm); D, MPEG-507-M, right valve, external view (length = 0.36 mm, height = 0.18 mm); E, MPEG-508-M, left valve, internal view (length = 0.35 mm, height = 0.17 mm); F, MPEG-509-M, right valve, internal view (length = 0.34 mm, height = 0.17 mm); G, MPEG-510-M, left valve, internal view (length = 0.37 mm, height = 0.18); H, holotype MPEG-503-M, right valve, internal view (length = 0.36 mm, height = 0.18 mm); I, MPEG-511-M, left valve, dorsal view (length = 0.34 mm, height = 0.17 mm); J, MPEG-512-M, right valve, dorsal view (length = 0.36 mm, height = 0.17 mm); K, anterior hinge element of I; L, anterior hinge element of J; M, posterior hinge element of I; N, posterior hinge element of J; O, ventral concavity of E; P, ventral concavity of H; Q, anti-slip tooth of J (oblique dorsal view); R, normal pore, sieve-type of B; S, hinge of E; T, hinge of H; U, central muscle scars of E; V, central muscle scars of H.
Holotype
MPEG-503-M, right valve (Fig. 3H, P, T, V).
Paratypes
Additional material
MPEG-514-M, 26 adult specimens from sample AM10/30.
Diagnosis
A very small-sized, extremely thin-shelled species of Pellucistoma with subrhomboidal shape, ornamented with wrinkle-like ridges forming anteroventrally a weak reticulum and a unique combination of hinge structures.
Derivation of name
‘Curupira’, the name of a mythic dwarf of Brazilian legends with backward turned feet, which should confuse pursuers; used as a noun in apposition; in reference to the small size of the species and its baffling discovery.
Type locality
Borehole 1AS-10-AM at Sucuriju close to Rio Ituí (04 ° 50′ S, 70 ° 22′ W, ∼62 km south-west of Benjamin Constant; municipality Atalaia do Norte, state of Amazonia, Brazil; Fig. 1).
Type horizon
Sample AM10/30 ( = depth: 141.2 m, altitude: –56.2 m; Gross et al. 2014).
Description
Shape
Subrhomboidal in lateral view; anterior margin moderately infracurvate, dorsal margin almost straight and subhorizontal, ventral margin with slight concavity below the mandibular scars, posterior margin with a blunted subdorsal caudal process; valves anterodorsally, posteroventrally and posteriorly laterally flattened; lens-shaped in dorsal view with beaked posterior end.
Ornamentation
The very thin-shelled valves are basically smooth except for shallow, wrinkle-like ridges along the free valve margin as well as in the centro- and ventrodorsal area, forming a weak reticulum anteroventrally; at the caudal process an additional, oblique ridge is always present.
Inner lamella
Anterior and posterior wide; anterior vestibulum large, posterior one narrow-elongated, extending up to the caudal process; several inner lists developed; selvage subperipheral, inconspicuous anteriorly, forming at the ventral concavity a bulge, which fits into a groove of the other valve (groove more prominent in right valves).
Marginal pore canals
Widened at their base, leading to an irregular line of concrescence; occasionally bifurcated, some branches developed as false pore canals.
Hinge
Right valve – anterior element with four roundish sockets (the most anterior being the largest, the most posterior one is barely developed) and a spatulate anti-slip tooth below; median element consists of a smooth groove, which is deepened at its posterior end; posterior hinge element with three elongated teeth, succeeded by a fourth, indistinct tooth, which merges backwards into a thin expansion of the posterior margin; left valve – anterior element with a trilobate tooth and a further weak tooth postjacent; median element consisting of a smooth bar, which forms an elongated tooth-like structure at its posterior end; posterior element with three, elongated, shallow sockets followed by a fourth, indefinite, elongated socket fading out towards the posterior end.
Normal pores
Widely scattered, very small (∼3–5 µm in diameter); sieve-type.
Central muscle scars
A row of four, slightly posteriorly inclined adductor scars; two oval mandibular scars, one irregularly ovate frontal scar; numerous dorsal muscle scars; well above the row of adductor scars a row of four dorsal scars is developed, probably corresponding to the ‘lucid spot’ (Morkhoven 1963; Sandberg 1969), which, however, is not a discrete, single spot here.
Eye-spot
Slight eye-spot developed.
Sexual dimorphism
Unclear; a few specimens (e.g. Fig. 3B) are slightly larger and display a somewhat higher posterior valve proportion, which could be related to sexual dimorphism.
Dimensions
Right valve (number of measured specimens = 9): length = 0.34–0.38 (mean = 0.36) mm, height = 0.17–0.18 (mean = 0.18) mm; left valve (number of measured specimens = 7): length = 0.34–0.38 (mean = 0.36) mm, height = 0.17–0.18 (mean = 0.17) mm.
Remarks
Generic classification
The current species imitates several genera of different families by its subrhomboidal outline and almost smooth shells.
Amongst the Bythocytheridae Sars, 1866, some species of Bythocythere Sars, 1866 and Pseudocythere Sars, 1866 superficially resemble Pellucistoma curupira sp. nov. However, both genera differ explicitly due to the development of five adductor scars, and, less clearly, in their hinges (Pseudocythere: adont; Bythocythere: adont, lophodont or merodont; e.g. Morkhoven 1963; Athersuch et al. 1989; Stepanova 2006; Sciuto 2009).
The loxoconchid genera Palmoconcha Swain & Gilby, 1974 ( = syn. Lindisfarnia Horne & Kilenyi, 1981; Horne & Whatley 1985; Athersuch et al. 1989), Elofsonia Wagner, 1957, Pseudoconcha Witte, 1993 and, especially, Phlyctocythere Keij, 1958, are similar to some degree.
However, Palmoconcha is distinguished by its gongylodont hinge (right valve: anterior socket–tooth–socket sequence; median smooth furrow; posterior tooth–socket–tooth sequence; Swain & Gilby 1974; Horne & Kilenyi 1981; Athersuch & Horne 1984; Horne & Whatley 1985; Athersuch et al. 1989) and to a minor degree by its less prominent caudal process, strong fulcral point and Y-shaped frontal scar.
Pseudoconcha has a bipartite hinge (right valve: anterior element formed by a strong bar, with a groove below; posterior half with groove and bar below), a less developed caudal process, a well-punctate surface and a narrower inner lamella (Witte 1993; Sarr et al. 2008).
Although some variability in details of the hinge, muscle scar patterns and pore canals seem to be present in Elofsonia (Aiello & Szczechura 2002), this genus differs by its less prominent, less pointed caudal process (except Elofsonia sp. in Keyser & Schöning 2000) and its more simple hinge (right valve: dorsally crenulated anterior socket; smooth median groove; weak posterior tooth; Whittaker 1973; Athersuch & Horne 1984; Athersuch et al. 1989).
Originally, Phlyctocythere was characterized by its inflated, almost spherical carapaces with a peripherally compressed zone and an obtuse, subdorsal caudal process. Its surface is smooth, lacks eye-spots and the valves are very thin-shelled. The hinge is adont (right valve: curved, smooth bar), marginal pore canals are simple, and one frontal muscle scar is developed (Keij 1958; compare also Morkhoven 1963). Subsequently, several species were included in Phlyctocythere, which blur the prime generic diagnosis. For example: (1) outline: Phlyctocythere hamanensis Ikeya & Hanai, 1982 (more elongated, less arched dorsal margin), Phlyctocythere japonica Ishizaki, 1981 (subovate), Phlyctocythere recta Bold, 1988 (straight dorsal margin), Phlyctocythere sicula Sciuto & Pugliese, 2013 (more elongated) and Phlyctocythere stricta Bold, 1988 (straight dorsal margin); (2) ornament: Phlyctocythere curva Bold, 1988, Phlyctocythere retifera Bonaduce, Masoli & Pugliese, 1978, P. sicula and P. stricta display a faint reticulation and/or longitudinal ridges/wrinkles; (3) Phlyctocythere curva, P. recta, P. stricta and probably P. fennerae Mostafawi, 1992 have a slight eye-spot; (4) for P. fennerae, P. japonica and P. retifera few (anteroventrally) branched marginal pore canals are described and these structures are often observed to be bifurcated in P. curva; (5) for P. hamanensis normal pores are ‘presumably’ of sieve-type; (6) in P. hamanensis two frontal scars and one elongated mandibular scar are mentioned; in the illustration of P. retifera a double frontal scar is indicated; in P. sicula the adductor scars are very elongated; and (7) hinge: in Phlyctocythere caudata Hartmann, 1979, P. curva, Phlyctocythere pellucida (Müller, 1894), P. retifera and P. sicula right valves display a smooth median groove (crenulated in P. curva) and (two) terminal sockets (in P. curva: teeth); P. hamanensis has a reduced gongylodont hinge. (Note: Phlyctocythere hartmanni Omatsola, 1970 is attributed to Elofsonia (Athersuch & Horne 1984; Schornikov 2011) or to Pseudoconcha (Witte 1993). Phlyctocythere pellucida is discussed as belonging to Loxocauda Schornikov, 1969 (Athersuch & Horne 1984; Schornikov 2011)). Consequently, Phlyctocythere is either quite variable or has turned into a collective genus due to inclusion of profuse species. An in-depth revision is obviously needed but is beyond the scope of the present work. In particular, P. retifera from the Red Sea is similar but it is more ovate (but note sexual dimorphism displayed in Mostafawi (1992) for P. fennerae), it diverges in details of the hinge (as far as reproducible) and has two frontal scars. However, by following the original diagnosis of Phlyctocythere (Keij 1958; Schornikov 2011) especially its outline (much more arched dorsal margin), its adont, right-bar hinge and simple marginal pore canals are considered herein to exclude an assignment of the current specimens to that genus.
Some authors (Bold 1950, 1958; Benson et al. 1961; Morkhoven 1963) have discussed a possible synonymy of Javanella Kingma, 1948 with Pellucistoma Coryell & Fields, 1937 (see also Gou & Chen 1988; Howe & McKenzie 1989; Ayress 1996). Lately, Bergue & Coimbra (2007) revised Javanella, revalidated it and reassigned it into the family Cytheridae Baird, 1850. According to this work only two species are left in Javanella, which clearly differ in outline (more elongated; caudal process below the middle of valves height) and in details of the terminal hinge elements from the present material.
Amongst the Cytheromatidae Elofson, 1939, the genus Paracytheroma Juday, 1907 is closely related to Pellucistoma. Nevertheless, Paracytheroma can be differentiated from the latter by lacking strong terminal anti-slip hinge elements, the absence of a caudal process and – to a minor degree – by missing a complex marginal zone with branched marginal canals (Hartmann 1978; Ayress 1990; compare also Sandberg 1969; Keyser 1976; Garbett & Maddocks 1979). Based on those features, our specimens do not belong to Paracytheroma but fit best with Pellucistoma as originally defined by Coryell & Fields (1937; for genus definition compare also Edwards 1944; Morkhoven 1963; Sanguinetti 1979). A few, minor differences concern the valves’ hinge and surface ornament.
For the hinge of the left valve an “anterior long blade-like triangular tooth” is indicated (Coryell & Fields 1937, p. 17), being trilobate in the present specimens. The median element is formed by a “serrated bar”, which “terminates at the posterior cardinal angle” (Coryell & Fields 1937, p. 17). Here, that bar is smooth – at least as preserved. As far as described or perceptible on the provided figures, a smooth median element occurs in Pellucistoma scrippsi Benson, 1959 and Pellucistoma bensoni McKenzie & Swain, 1967 (Benson & Kaesler 1963; McKenzie & Swain 1967; Swain & Gilby 1967, 1974). For the type species, P. howei, the drawings of Bold (1967) and Teeter (1975) do not show such a crenulation. The crenulation of the median hinge element is in some species of Pellucistoma probably very delicate or indeed not developed.
A posterior hinge element, consisting of four elongated sockets/teeth as in our examples, has not been mentioned for Pellucistoma so far. However, based on the dorsal view of a left valve in Coryell & Fields (1937), behind the thickened, tooth-like terminal end of the bar, a shallow groove may be present which might analogously receive tiny teeth of the right valves. Moreover, the illustration of P. scrippsi in Swain & Gilby (1967) implies the presence of small posterior teeth in the right valve (note that this feature is not indicated in e.g. Benson 1959; Benson & Kaesler 1963; McKenzie & Swain 1967; Swain 1967). The description and illustration of the hinge structure of Pellucistoma spurium Bold, 1963 (p. 406: “In the left valve the selvage curves around the sockets and forms the upper border of the groove”) also hints at the presence of a posterior element. Thus, the subtle posterior sockets/teeth, clearly visible in our species (under the SEM), seem to be present in other Pellucistoma species equally. Garbett & Maddocks (1979, p. 871) carefully described a similar posterior hinge structure for Paracytheroma stephensoni (Puri, 1954) of which Pellucistoma atkinsi Hall, 1965 is a synonym (Keyser 1976): “[a] posterior tooth formed by the expanded end of the posterior margin.” That resemblance mirrors the close relation between Pellucistoma and Paracytheroma again (see above).
The surface of Pellucistoma is described as “finely perforated” (Coryell & Fields 1937, p. 17) and “smooth or finely punctate” (Morkhoven 1963, p. 436). Here, the valves are basically smooth, but display a weak reticulate pattern anteroventrally, some wrinkle-like ridges dorsocentrally and ventrocentrally, as well as a characteristic, oblique, light ridge on the caudal process. Shallow, posterocentral and posteroventral ridges, which converge towards the caudal process, can be seen on P. magniventra in Garbett & Maddocks (1979). True eye-spots have not been included in the genus definition so far. However, a slight eye-spot – like in P. curupira sp. nov. – is recognized in P. scrippsi (Swain 1967; Swain & Gilby 1974).
Comparison with other Pellucistoma species
To our knowledge, 15 Pellucistoma species have been formally described so far (e.g. Kempf 1986, 1995, 2008; Brandão 2015).
The type species, Pellucistoma howei Coryell & Fields, 1937 (first record: Panama, latest middle–early late Miocene), is quite similar but differs by: its more ovate outline; its more projecting posteroventral margin; a more acuminate caudal process; a narrower anterior vestibulum (which seems to be almost restricted to the lower half of valve height); the ventral snap-mechanism is less developed; and its larger size (holotype: length/height = 0.48/0.27 mm; Coryell & Fields 1937; Bold 1967; see also e.g. Teeter 1975; Bold 1988). For differences in hinge and ornamentation see above.
Pellucistoma magniventra Edwards, 1944 (first record: North Carolina, Pliocene) is distinguished by (largely based on the redescription of Garbett & Maddocks 1979): its more arched, upwards rising dorsal margin and its strongly projecting posteroventral margin, respectively (if not aligned to the base line = reversal points in front and backwards of the ventral concavity); its much more infracurvate anterior margin; its more pointed and acuminate caudal process; its ornament (see above); its anterior vestibulum, which is largely restricted to the anteroventral area (for variability see Garbett & Maddocks 1979); details of the hinge (crenulated median element; simple anterior and posterior sockets/teeth; lack of posterior hinge elements); and its larger size (holotype: length/height = 0.62/0.33 mm; Edwards 1944; Garbett & Maddocks 1979; see also e.g. Puri 1960; Bold 1963; Benson & Coleman 1963; Hall 1965; Morales 1966; Grossman 1967; Sandberg 1969; Cronin 1979; Krutak 1982; King Lyon 1990).
Pellucistoma scrippsi Benson, 1959 (first record: Baja California, Recent) has more convex dorsal and ventral margins and a less oblique anterior margin as well as a smooth surface. The posterior vestibulum is almost absent (but see Swain & Gilby 1967); the hinge is slightly different (simple anterior socket (right valve) and tooth (left valve); posterior teeth are lacking (except illustration of Swain & Gilby 1967)); marginal pore canals are missing on the apex of the caudal process; and it is larger (holotype: length/height = 0.69/0.33 mm; Benson 1959; Benson & Kaesler 1963; McKenzie & Swain 1967; Swain 1967; Swain & Gilby 1967, 1974).
Pellucistoma bensoni McKenzie & Swain, 1967 (first record: Baja California, Recent) differs in its strongly arched dorsal margin resulting in a subtriangular outline and a posterocentrally located caudal process, its smooth surface, its almost absent posterior vestibulum; and it is larger (holotype: length/height = 0.44/0.25 mm).
Pellucistoma spurium Bold, 1963 (first record: Trinidad, late Miocene) has a more convex dorsal margin; a more accentuated caudal process; and a hinge with a simple knob-like anterior tooth (left valve) and minutely crenulated median elements. The anterior vestibulum is restricted to the anterocentral area, the posterior vestibulum is only developed at the caudal process; and it is larger (holotype: length/height = 0.49/0.25 mm; compare also Pellucistoma? spurium of Bold (1988)).
Pellucistoma santafesinensis Zabert, 1978 (first record: Argentina, middle–late Miocene; correct spelling according to Kempf (2008): P. santafesinense; note: in the following we refer to correct spellings of species names but retain the original spellings in this work) is similar to Pellucistoma gibosa Sanguinetti, 1979 (see below) and possibly both are synonyms. However, it differs significantly in outline (subtriangular-elongate in lateral view; ventromedian long, pointed caudal process) and hinge structures from the current material. Size of holotype: length/height = 0.53/0.26 mm.
Pellucistoma gibosa Sanguinetti, 1979 (first record: southern Brazil, late Miocene; correct spelling according to Kempf (1986): P. gibosum) has an extremely humped (right valve) dorsal margin and an acuminate, long caudal process well below the half valves’ height. It is smooth; it has a hinge with a simple, strong anterior tooth (left valve) and a slightly serrated bar; the anterior vestibulum is restricted to lower half of valves height; and it is larger (holotype: length/height = 0.51/0.23 mm).
Pellucistoma elongata Whatley et al., 1997a (first record: Argentina, Recent; correct spelling according to Kempf (2008): P. elongatum) differs by: its more convex dorsal and posteroventral margin; more acuminate and more ventrally located caudal process; the inner lamella curves inwards posteroventrally; it is smooth (lacks eye-spots); and it is larger (holotype: length/height = 0.52/0.24 mm; Whatley et al. 1997a).
Further species of questionable generic classification
Bold (1950) considered his Miocene Venezuelan species Pellucistoma kendengensis (Kingma) to be synonymous with Javanella kendengensis Kingma, 1948 (Pliocene, Java). Later, Bold (1972a) included P. kendengensis of Bold (1950) in his new species Pellucistoma? kingmai Bold, 1972a and assumed J. kendengensis of Kingma (1948) not to be a synonym of P.? kingmai. Bergue & Coimbra (2007) re-examined the type material of Bold (1950), excluded it from Javanella but left the generic status of P. kendengensis (according to Bold 1972a: P.? kingmai) open. Although there are some features perceptible that are unlike Pellucistoma (anterior margin almost equicurvate (cf. Bold 1950, p. 86: “obliquely rounded”); subventral caudal process; quite heavily punctate surface (as shown in Bergue & Coimbra 2007), as well as short and straight marginal pore canals, it cannot be definitively excluded from Pellucistoma.
Due to their simple marginal pore canals, the species Pellucistoma? sp. (Bold 1958, 1972a), Pellucistoma? compactum Bold, 1972a and Pellucistoma? kingmai Bold, 1972a, that are questionably attributed to Pellucistoma, differ in outline, hinge structure and development of the inner lamella. Only Pellucistoma sp. in Bold (1970, 1972b, 1988) is rather similar in its shape to the current species. Nevertheless, it has a smooth surface and is larger (length/height = 0.48/0.25 mm). Unfortunately, neither the hinge nor inner characters are accessible, which does not enable further comparisons.
Pellucistoma tumida Puri, 1954 (correct spelling according to Kempf (1986): P. tumidum) from the Pliocene of Florida is poorly described and a re-examination of the type material already failed (Bold 1988). Although its outline is comparable with the present individuals (except the equicurvate anterior margin), without additional traits (see also Bold 1963; Hulings 1967) a generic assignment or a species-specific identification is unfeasible. Bold (1988) discussed possible congruence with his Phlyctocythere sp. 2, which again demonstrates the superficial similarity of that loxoconchid genus with Pellucistoma (see above).
Pellucistoma atkinsi Hall, 1965 (see above) is a junior synonym of Paracytheroma stephensoni (Puri, 1954) (Keyser 1976; Garbett & Maddocks 1979).
Pellucistoma ovaliphylla Hu, 1981 from the Plio-/Pleistocene of southern Taiwan has been recognized by Hu (1984) to belong to Paradoxostoma Fischer, 1855. Two further Taiwanese species, Pellucistoma magnolioidea Hu & Tao, 2008 and Pellucistoma chushunshui Hu & Tao, 2008, differ notably in outline, especially due to the almost lacking caudal process, from both the current as well as from other Pellucistoma species (important internal characters are not accessible because of missing illustrations and descriptions). Most likely, these species belong to another genus (Paracytheroma?) but this claim needs additional investigations.
Ayress (1990, 1996) described Pellucistoma coombsi Ayress, 1990, Pellucistoma fordycei Ayress, 1990 and Pellucistoma punctata Ayress, 1996 (correct spelling according to Kempf (2008): P. punctatum) from New Zealand and the Tasman and Coral Seas. These species diverge significantly from the present species and the genus Pellucistoma in general (outline: much more elongated-rectangular; hinge structures: e.g. P. coombsi and P. punctata have a right-bar hinge; inner lamella: much wider; ornament: P. punctata), which shed doubt on their generic allocation. However, those species are not comparable with the material described herein.
The illustration and description of Pellucistoma sp. from Henderson Island (Pitcairn Islands, S. Pacific; Recent; Whatley & Roberts 1995; Whatley et al. 2004) do not offer enough details (e.g. hinge) for an assured generic attribution. Its laterally inflated valves are rather unlike those of Pellucistoma.
Faugères et al. (1984) mentioned Pellucistoma from the Ghubbet el Kharab (Djibouti; Holocene) but provided no figure. Presumably, this material belongs to another genus (Phlyctocythere?).
Whatley et al. (1997b) recorded – without figure or description – Pellucistoma sp. 1 from the southern Strait of Magellan (Chile; Recent). Due to extremely low water temperatures at the sampling sites, this record is ecologically very unlikely for Pellucistoma (see below). Thus, we do not consider it subsequently.
The Late Cretaceous Pennyella foveolata Majoran & Widmark, 1998 from the Maud Rise (Southern Ocean, off Antarctica), listed under Pellucistoma in the ‘World Ostracoda Database’ (Brandão et al. 2015), actually belongs to the former genus (Yasuhara et al. 2013).
To conclude, all the above compared species can be clearly differentiated from Pellucistoma curupira sp. nov. and are noticeably larger. Most similar are P. howei from the Miocene of Panama and Pellucistoma sp. of Bold (1970, 1972b, 1988; Miocene: Antilles and Panama), however, the latter is little known and a closer examination is not possible. Most likely, the Australasian and Taiwanese species (Ayress 1990, 1996; Hu & Tao 2008) do not belong to Pellucistoma. The records from the Pitcairn Islands and southern Chile (Whatley et al. 1997b, 2004) need additional affirmation. Currently, we assume that the genus Pellucistoma is confined to the Americas.
Results
Stable isotope (δ18O and δ13C) analyses
Due to the minute size of Pellucistoma curupira sp. nov., geochemical analyses were performed on three Cyprideis species, co-occurring in the same sample (AM10/30). All measurements provided very light values with a range for δ18O from –7.25 to –10.41‰ and for δ13C from –8.74 to –13.54‰ (Fig. 4).
Figure 4.
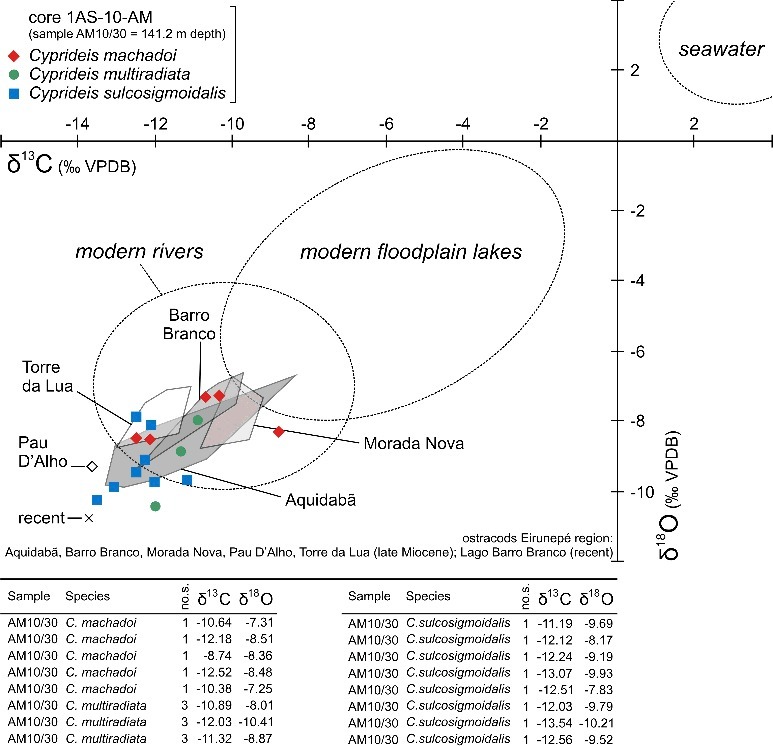
δ18O and δ13C isotopic ratios of Cyprideis species associated with Pellucistoma curupira sp. nov. Abbreviation: no.s., number of shells used for analysis. Grey shaded polygons display the range of results obtained from fossil and Recent ostracods from the Eirunepé region (Gross et al. 2013). (Note: the indicated range for modern rivers and floodplain lakes is based on aragonitic mollusc shells (Wesselingh et al. 2006), which give somewhat heavier values for the same environmental parameters compared to ostracod calcite (Grossman & Ku 1986)).
Spatiotemporal distribution and autecology of hitherto known Pellucistoma species
We evaluated all published Pellucistoma records (fossil and Recent) known to us (Fig. 5; for references and details see Supplemental Material 1 and 2). This review might be incomplete due to overlooked literature and, probably more importantly, because of sampling biases. Partially poor stratigraphical assessment of sampling sites and the inclusion of reports without illustrations may additionally blur our results. Nevertheless, the dataset reveals some significant biogeographical and ecological patterns, which further enable a discussion of the erratic occurrence of P. curupira sp. nov. in the Miocene of western Amazonia.
Figure 5.
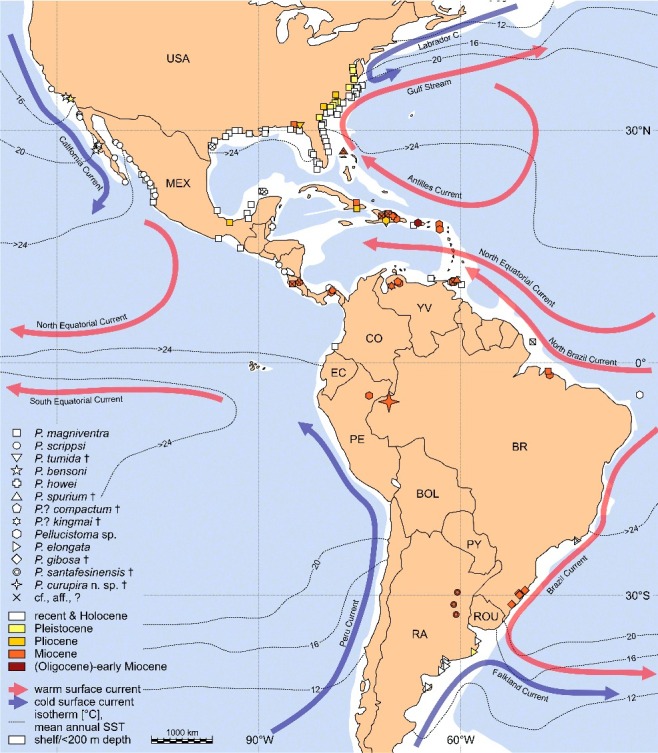
Fossil and Recent records of Pellucistoma species (mean annual sea surface temperature (SST) based on NASA data (http://svs.gsfc.nasa.gov/index.html; accessed 18 September 2014); for details see Supplemental Material 1 and 2; species only known from the fossil record marked with †.
Amongst extant Pellucistoma species, P. magniventra has a continuous Holocene record along the east coast of the USA (Maryland (∼38 ° N) to southern Florida) and in the northern and southern Gulf of Mexico (Fig. 5). This is the ‘core area’ of P. magniventra today. Further scattered indications come from the Pacific coast of Mexico (Sinaloa, Nayarit, Gulf of Tehuantepec) and Colombia (Bahía de Tumaco), from Puerto Rico, Venezuela and Trinidad and the Brazilian equatorial shelf (∼2 ° S; the latter as P. ex gr. magniventra). To summarize the ecological data (Supplemental Material 1), P. magniventra prefers shallow marine (inner sublittoral, ∼10–20 m water depth), subtropical–tropical (minimal water temperature of coldest month (Tmin) ∼10 °C; average annual water temperature (Tav) >20 °C), oxygenated, euhaline waters (∼30–40 PSU; e.g. Valentine 1971; King Lyon 1990; Fig. 5). Fossil occurrences (late Miocene–Pleistocene) of P. magniventra match with the ‘core area’ as outlined above. Additionally, it is reported from Cuba (middle Miocene–Pliocene), Trinidad (early Miocene–Pliocene) and northern Brazil (early Miocene). Pellucistoma aff. magniventra is noted from Costa Rica (late Miocene) and Hispaniola (middle Miocene–early Pliocene). Palaeoecological data for these fossil occurrences coincide with the ecology of Recent P. magniventra.
Pellucistoma scrippsi, up to now only with Recent and sub-Recent records, is restricted to the Pacific coast of North and Meso America (∼Santa Barbara/USA (∼34 ° N) to San Juan del Sur/Nicaragua (∼11 ° N)). The rare Recent and Pleistocene findings of P. bensoni plot within the distribution area of P. scrippsi. For P. scrippsi and P. bensoni the following autecological data can be summarized: shallow marine (inner sublittoral, ∼10 m water depth), warm temperate–tropical (Tmin ∼12 °C, Tav >15 °C), euhaline.
Pellucistoma howei, although principally a fossil species (Panama and Dominican Republic; middle–late Miocene), is recorded from the Holocene of Belize as well as with uncertainty (P. cf. howei or P. aff. howei) from the Gulf of Mexico (Recent) and the Miocene of Costa Rica. Ecological data characterize it as a shallow marine (sublittoral, ∼5–50 m water depth), tropical–subtropical, euhaline species.
Except for a few further Recent Pellucistoma sp. records (Panama, Trinidad, Rocas Atoll/Brazil), P. elongata remains the last extant species to be discussed. It is confined to the Argentinean coast from about the Isla de Los Pájaros (∼42 ° S) towards the north (∼36 ° S) to the Río de la Plata estuary (with one Pleistocene report: Mar Chiquita). Further in the north, P. cf. elongata is noted off Cabo Frio (Rio de Janeiro, ∼23 ° S; subrecent). Based on the available information, P. elongata is a shallow marine (littoral–inner sublittoral; littoral rock pools to ∼14 m water depth), euhaline species. It is reported from shallower settings than the species treated above, however, at least Tmin can be assumed to be comparable to the occurrences of other species (e.g. Mar del Plata: Tmin ∼10 °C, Tav ∼15 °C; www.seatemperature.org, accessed 12 January 2015).
Pellucistoma tumida (Florida), P. spurium (Trinidad, ?Bahamas), P.? compactum (Venezuela), P.? kingmai (Venezuela), P. gibosa (Brazil) and P. santafesinensis (Argentina) are exclusively fossil taxa. As far as it is known, these species, as well as further fossil Pellucistoma records left in open nomenclature, lived in shallow marine, subtropical–tropical, euhaline habitats.
In summary, Pellucistoma is unquestionably a marine (sublittoral, euhaline) taxon of subtropical–tropical, oxygenated waters with a seasonally lower temperature limit of about 10 °C. All fossil records point in the same direction. So far, there is no evidence for a different autecology in the geological past.
Discussion
Constraints of dispersal and potential dispersal modes of Pellucistoma
Dispersal capacity of organisms depends on various biotic and abiotic factors (e.g. autecology, reproduction mode, predation, competition, active/passive dispersal capacity, medium of transport). In the case of Pellucistoma, water temperature, depth and salinity appear to be the most important physico-chemical parameters (e.g. Valentine 1971, 1976; Bold 1974; Cronin 1979; Cronin & Dowsett 1990). Little is known about its reproduction; however, due to the proof of female and male individuals in some species, sexual reproduction can be assumed. As female carapaces lack an apparent brood pouch (for storage of eggs and/or early instars), brood care seems unlikely. Planktonic larval stages – like in all marine podocopid ostracods – are missing (Titterton & Whatley 1988). Presumably, Pellucistoma belongs to the marine meiobenthos. Synecological information (e.g. predation, competition) is not available for this genus.
By considering the small size of Pellucistoma and the lack of planktonic stages, active dispersal can be expected to be very slow (Sandberg 1964). Land bridges (e.g. Panamian isthmus), deep-water areas (e.g. Cayman Trench), cold waters/ocean currents (e.g. Peru Current) as well as massive river discharge (e.g. Amazon River; lowering of salinity, instability of the seabed) will be effective dispersal barriers for Pellucistoma (compare Cronin 1987; Coimbra et al. 1999; Iturralde-Vinent & MacPhee 1999).
However, passive dispersal by animals (e.g. birds, fishes), wind, water currents (fluvial and marine) or, in modern times, by man, are frequently quoted to affect ostracod migrations (e.g. Mesquita-Joanes et al. 2012).
Birds can transport ostracods (adults, juveniles, eggs) on e.g. their feet or feathers, preferably encased in sediment. In addition, intestinal transport (and survival) has been successfully demonstrated (e.g. Löffler 1964; Frisch et al. 2007; Brochet et al. 2010). Although bird-mediated transport is conceivable for Pellucistoma, three counter-arguments should be mentioned (Teeter 1973): (1) species of this genus (except P. elongata) prefer sublittoral settings, which makes their adhesion to shorebirds/waterfowls difficult; (2) Pellucistoma is rather small and thin-shelled (P. curupira sp. nov. is very small and very thin-shelled) and thus prone to digestion (also by fishes); and (3) torpid stages, desiccation-resistant eggs (as in freshwater Cypridoidea; e.g. Horne 1993; Rossi et al. 2011) and brood care (as e.g. in the cytheroid Cyprideis; Sandberg 1964; Bold 1976) facilitate aerial dispersal via birds, but the presence of such features is implausible for Pellucistoma. Comparable arguments seem to rule out transport by wind.
Aquatic displacement by rivers (downstream) and by tidal currents (up- and downstream) appears unlikely for the euhaline, sublittoral Pellucistoma (Barker 1963). Conversely, ocean currents are regarded as a prominent means of passive ostracod transport (e.g. Titterton & Whatley 1988). Especially, drifting aquatic plants/algae – inclusively adhering sediments – significantly contribute to ostracod dispersal (e.g. Teeter 1973; Cronin 1987).
Human induced dispersal by ships (e.g. in ballast water or on fouling of the hull) has been inferred to influence modern ostracod distribution (Teeter 1973; Witte & Harten 1991). Although the evidence is quite poor, we would refer to the possibility of phoretic dispersal via larger aquatic animals. For instance, manatees (Sirenia: Trichechidae) carry diverse epibionts on their skin (e.g. barnacles, copepods, algae and ostracods; Hartman 1979; Suárez-Morales et al. 2010; Marsh et al. 2011). Possibly, such animals act – like vessels – as means of dispersal for shallow marine ostracods. In this case, the sublittoral lifestyle of Pellucistoma, its susceptibility to intestinal digestion and decease due to desiccation, will be no constraint, as in bird transport. In addition, stream, tidal and ocean currents can be surmounted.
In conclusion, due to the lack of specific, dispersal-favouring traits and its autecology, the colonization of new habitats is subject to more restrictions compared to, for example, the euryoecious, brood care practicing Cyprideis.
Origin and dispersal of Pellucistoma
Based on his extensive works on Caribbean ostracods, Bold (1974) previously outlined the dispersal of Pellucistoma. Bold suggested an early Miocene origin in northern South America and its subsequent spread towards the Lesser Antilles and Panama before the onset of the middle Miocene. Following our evaluations, the potentially earliest records stem from the Lares Formation of Puerto Rico (upper Oligocene–lower Miocene; Fig. 6).
Figure 6.
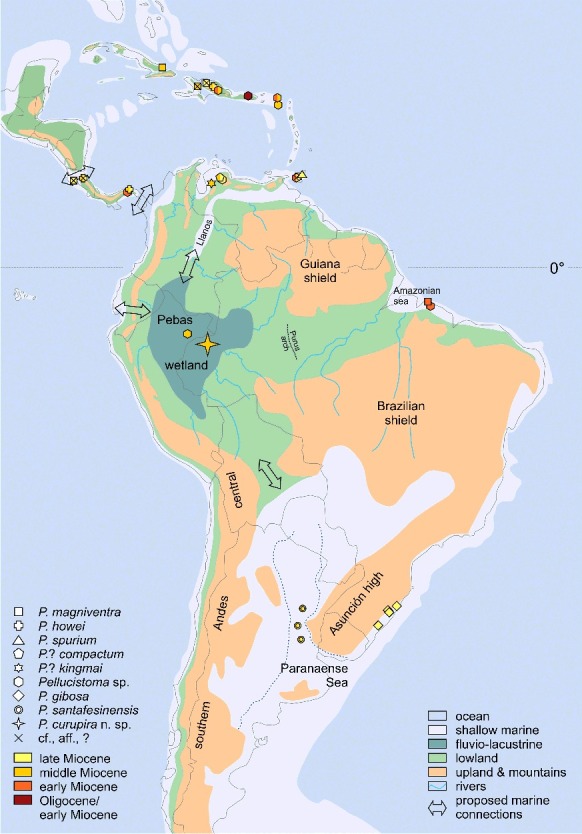
Tentative middle Miocene palaeogeography of the Caribbean realm and South America (based on Iturralde-Vinent & MacPhee 1999; Del Río 2000; Hernández et al. 2005; Hoorn et al. 2010b; Candela et al. 2012; extent of the Paranaense Sea probably too large (dashed blue line); compare Aceñolaza 2000; Cione et al. 2011; Ruskin et al. 2011) and Miocene records of Pellucistoma (the late Miocene P. magniventra (Florida) and P. aff. spurium (Bahamas) records are not displayed; compare Fig. 5).
Either way, early Miocene occurrences are noted from Trinidad (Brasso Formation), Panama (Culebra Formation) and Brazil (Pirabas Formation), which support a substantial dispersal event in the early Miocene (Bold 1974). Afterwards (middle Miocene), Pellucistoma colonized the Greater Antilles (Hispaniola, Cuba) and arrived during the late Miocene at the south-eastern coasts of North America and the Pacific coast of Costa Rica. Amazingly, it also has been spread far to the south, to southern Brazil (Pelotas basin) and north-eastern Argentina (Paraná basin) at that time. During the Pliocene, Pellucistoma expanded along the Atlantic coast of the USA (up to North Carolina) and settled the Gulf of Mexico (P. magniventra).
Its Miocene and Pliocene dispersal in the Caribbean, Gulfian and Carolinian provinces (sensu Cronin 1987) can be explained by repeated, short-distance transport via floating water plants between the islands and along the shoals of the American continent, following the generally west and north-west directed sea surface currents (Bold 1974; Cronin 1987; Iturralde-Vinent & MacPhee 1999). More difficult to assert is the ‘rapid’ early Miocene spread of Pellucistoma towards the south-east (Pirabas Formation; ∼2000 km south-east of Trinidad), directed against the modern North Brazil Current. However, during the Miocene the Panama isthmus was open, causing ocean current patterns different from today. Due to the inflow of Pacific waters through the Central American Seaway, a reversed North Brazil Current, flowing along the north-east South American coast towards the south-east, has been proposed (Prange & Schulz 2004; Heinrich & Zonneveld 2013 and references therein). As neither the Orinoco River (Díaz de Gamero 1996) nor the Amazon River (Hoorn et al. 2010b) were fully developed, a significant, riverine dispersal barrier (like the modern Amazon; Coimbra et al. 1999) was not installed during the early/middle Miocene. Tentatively, a reversed Miocene North Brazil Current facilitated the south-eastward dispersal of Pellucistoma along the Brazilian shelf. Comparably, the colonization of areas far in the south (Pelotas and Paraná basin) in the late Miocene could have been triggered by the south-west directed Brazil Current (Wood et al. 1999). At this time, the cold Falkland Current is assumed to have been less effective (Coimbra et al. 2009; Le Roux 2012), which enabled the proliferation of Pellucistoma in the Paranaense Sea. Although highly speculative, larger animals (e.g. sirenians) might have accelerated the expansion along the east coast of South America by acting as ectophoretic vectors. This hypothesis receives some support by the proposed north–south dispersal of sirenians along the eastern South American coast and their invasion of the Paranaense Sea from the south (Vélez-Juarbe et al. 2012).
In warmer periods of the Pleistocene, Pellucistoma extended further northwards on the west Atlantic coast (P. magniventra; e.g. Valentine 1971; Cronin 1979; Cronin & Dowsett 1990) and started to settle the Pacific coast of the USA (P. bensoni; Valentine 1976). In southern South America, a new species appeared (P. elongata; Ferrero 2009). Based on comparative morphology, P. elongata is much more closely related to P. magniventra than to the late Miocene species P. gibosa and P. santafesinensis. Thus, P. elongata is probably a descendant of P. magniventra and derives from a pre-Holocene dispersal event of the latter.
During the Holocene, Pellucistoma achieved its present distribution. Obviously, its latitudinal extension is limited by water temperature. While on the US Atlantic coast (∼38 ° N) the cold Labrador Current forms a thermal barrier, on the east Pacific coast the California Current confines its northward dispersal (∼34 ° N). On the south-west Atlantic coast (Argentina), the cold Falkland Current restricts its migration (P. elongata) further to the south. P. elongata has the highest latitudinal occurrence (∼42 ° S) of all Pellucistoma species but it is also the species with the shallowest records (at 42 ° S: littoral rock pool). As it is found in restricted bays, locally significantly warmer waters can be assumed, which permit its survival. A substantial northward migration could be hindered by the freshwater discharge of the Río de la Plata (Whatley et al. 1998b).
Based on the fossil and Recent records, a huge distributional gap is obvious (Fig. 5). Pellucistoma is missing along practically the entire Pacific coast of South America (there is only one P. magniventra record (one valve); Bahía de Tumaco, Colombia). Potentially this is an enormous sampling bias; however, we expect the Peru Current impeded a successful settlement of Pellucistoma. The Peru Current has been in existence at least since middle Miocene times (Le Roux 2012 and references therein). Its cold waters and northward-directed drift probably hampered the dispersal of Pellucistoma on the western coast of South America from the Miocene up to present times.
Recent P. scrippsi is restricted to the west coast of North and Meso America. Off Sinaloa and Nayarit (Mexico), it co-occurs with P. magniventra. This sympatric occurrence could be a taxonomic artefact or – speculatively – the result of a quite recent, passive, man-made dispersal event, tracing major ocean lanes from the Panama Canal towards California (Teeter 1973). Comparably, the single P. magniventra record on the western Colombian coast might reflect such a scattered displacement.
The enigmatic occurrence of Pellucistoma curupira sp. nov. in western Amazonia: proof of marine incursions?
The spatiotemporal distribution pattern of thus far known Pellucistoma species can be explained by vicariant barriers (land bridges, water temperature, depth, salinity), as well as by passive transport (sea currents (drifting matter) and, possibly, phoresy). However, the crucial questions of the palaeoenvironmental implication and the provenance of our new species remain to be discussed.
Our appraisal of records of Pellucistoma demonstrates that it is a shallow marine taxon. No evidence of substantial deviations in habitat preferences or adaptations to non-marine environments has been reported so far. Consequently, the finding of P. curupira sp. nov. appears to be amongst the most solid (body fossil) biotic evidence for proposed marine incursions, affecting the centre of Amazonia in Miocene times.
Autecology of Pellucistoma curupira sp. nov
The new species, Pellucistoma curupira sp. nov., is evidently dwarfed in comparison to all other Pellucistoma species (in length ∼20–50% smaller) and very thin-shelled. There are multiple causes of size reduction or dwarfism in ostracods (temperature, oxygenation, salinity, food resources, etc.; e.g. Neale 1988; Majoran et al. 2000; Yin et al. 2001; Hunt & Roy 2006; Finston 2007; Hunt et al. 2010; Scheihing et al. 2011; Yamaguchi et al. 2012). Here, we cannot identify a single or several factors to be the reason for the small size of P. curupira sp. nov. Nevertheless, it obviously had to cope with some kind of environmental stress and lived close to its tolerance limits. Its extremely thin-shelled valves also support this theory (e.g. Frenzel & Boomer 2005 for references; compare also Vermeij & Wesselingh 2002 for marine-derived gastropods). During the preparation of this paper, Boonstra et al. (2015) reported Pellucistoma (conspecific with P. curupira sp. nov.; MIFR pers. obs.) from western Amazonia (middle Miocene; Nuevo Horizonte), accompanied by euryhaline foraminifers. Based on the low diversity foraminiferal assemblage and the high proportion of abnormal tests, these authors proposed a highly stressful habitat with poorly oxygenated bottom waters, close to freshwater conditions (Vonhof et al. 2003: <1 PSU). Hence, it seems plausible that low oxygenation (Wesselingh et al. 2006) and, in particular, low salinity are the main abiotic stress factors leading to the dwarfism and the poorly calcified valves of P. curupira sp. nov.
Pellucistoma curupira sp. nov. is associated in sample AM10/30 with a typical Pebasian ostracod fauna (Whatley et al. 1998a; Gross et al. 2013, 2014), totally dominated by Cyprideis (12 sympatric species; ∼99% of the total ostracod fauna). This ostracod genus is holoeuryhaline, able to survive hypoxic periods and especially successful in stressful settings (e.g. Gross et al. 2008 and references therein). The only other ostracods found in this sample are Perissocytheridea ornellasae (Purper, 1979), Rhadinocytherura amazonensis Sheppard & Bate, 1980 and Skopaeocythere tetrakanthos Whatley et al., 2000 (MG in prep.). These species are – like P. curupira sp. nov. – endemic for western Amazonia and supposedly of marine origin. Interestingly, they are also of minute size (∼0.30–0.35 mm in length). An adaptation to freshwater settings during the late Miocene has been demonstrated for R. amazonensis and Perissocytheridea, as well as for Cyprideis spp. (Gross et al. 2013).
Stable isotope analyses (δ18O, δ13C) performed on three Cyprideis species associated with P. curupira sp. nov. yielded very light values (Fig. 4). Such depleted δ18O and δ13C ratios are indicative of a freshwater system (Leng & Marshall 2004). Our results are consistent with previous isotopic data obtained from outcrop material (ostracods: Gross et al. 2013). Earlier O/C-isotopic investigations (mainly molluscs) yielded closely comparable results (Vonhof et al. 2003; Kaandorp et al. 2006; Ramos 2006; Wesselingh et al. 2006).
The morphology of P. curupira sp. nov. (very small and extremely thin-shelled), the associated ostracod fauna (co-occurrence of further stunted ostracods of potentially marine ancestry) and our geochemical evidence, suggest that this Pellucistoma species – exceptionally – managed to adapt to freshwater conditions in the late middle Miocene.
Provenance of Pellucistoma curupira sp. nov
We presume: (1) the genus Pellucistoma originated in the Caribbean realm around the Oligo-/Miocene boundary; (2) Pellucistoma curupira sp. nov. is most closely related to Caribbean species; and (3) Pellucistoma is a shallow marine clade but adapted (P. curupira sp. nov.) over geological timescales (about 10 million years) to freshwater conditions in western Amazonia. Based on this, we explore possible migration scenarios:
(A) Migration of Pellucistoma curupira sp. nov. via aerial (bird) transport
Bird-mediated transport plays a certain role in ostracod dispersal. In such a case, Pellucistoma (and other small aquatic invertebrates, e.g. foraminifers) could have entered western Amazonia without aquatic connections (neither fluvial nor marine). By considering the palaeobiogeographical distribution of Pellucistoma, there are two potential sources (Fig. 6): the Caribbean and the Amazonian Sea. Due to the close relationship of P. curupira sp. nov. to Caribbean species and half the distance to travel, the first source seems more likely. Although less well developed than today, north–south bird migration was already in existence during the Miocene (Tambussi & Degrange 2013).
Nevertheless, Pellucistoma is not predisposed for aerial dispersal, and successful transfer as well as ‘ad hoc’ colonization of new (freshwater) habitats appears to be demanding. (Note: such a mode of migration is much more conceivable for the eurypotent Cyprideis. This ostracod achieved evidently a ‘habitat monopoly’ in the Miocene of western Amazonia, possibly causing additional biotic stress for other, rather stenopotent arrivals like Pellucistoma). However, a multitude (over millions of years) of short-distance transport ‘accidents’ through a patchy structured wetland is possible, enabling a stepwise, long-distance spread and gradual adaptation to freshwater. Even if this scenario applies for small invertebrates, it is hardly appropriate for larger, marine-derived vertebrates occurring in Amazonia (e.g. Lovejoy et al. 2006; Boonstra et al. 2015 and references therein).
(B) Migration of Pellucistoma curupira sp. nov. through marine incursions
Several marine pathways have been proposed to have linked western Amazonia with the sea during the Miocene (for comprehensive discussions see e.g. Nuttall 1990; Rebata et al. 2006; Wesselingh & Salo 2006; Hovikoski et al. 2010). Such connections have been envisaged towards the north (Caribbean Sea), the east (Amazonian Sea), the south (Paranaense Sea) and the west (Pacific, southern Ecuador).
Concerning Pellucistoma, we have no evidence for an eastern Pacific source, which corroborates low faunistic affinities in the mollusc record (Wesselingh & Salo 2006). Pellucistoma occurs in the Pirabas Formation (early Miocene) and the Amazonian Sea could be a potential source. Nevertheless, marine incursions, originating from the eastern Brazilian Atlantic, are unlikely since the ‘Purus arch’ formed a significant watershed at least until the late Miocene (e.g. Figueiredo et al. 2009; Hoorn et al. 2010b; Latrubesse et al. 2010; Dino et al. 2012).
Relations between aquatic biota of the Paranaense Sea and the Pebas system are poor (e.g. Marengo 2000; Hulka et al. 2006; Wesselingh & Salo 2006; Nicolaidis & Coimbra 2008). Similarly, P. curupira sp. nov. is not closer related to the species of the Paraná and Pelotas basins, for which we already suggested migration along the eastern coast of South America. Potential marine connections with the Paranaense Sea are dated to the late Miocene (Hovikoski et al. 2007; Uba et al. 2009). Thus, P. curupira sp. nov. (late middle Miocene) pre-dates the southern South American species and immigration from the south is improbable.
Most authors agree with a linkage between the early–middle Miocene Pebas wetland and the Caribbean Sea through corridors in the Llanos basin (e.g. Nuttall 1990; Hoorn et al. 1995; Anderson et al. 2006; Lovejoy et al. 2006; Wesselingh & Macsotay 2006). Morphological affinities of P. curupira sp. nov. to the Caribbean also support this claim. Hence, a Caribbean–Llanos–Pebas-dispersal pathway is most plausible; however, fossil evidence is pending for Pellucistoma in the Llanos region.
Marine incursions, deriving from the Caribbean realm, have been assumed to affect western Amazonia's environments and biota (e.g. Boonstra et al. 2015). At first glance, our Pellucistoma record is a profound confirmation of such far-reaching marine influences, as it could have followed the ingressions. But, as argued above, this record does not confirm the influx of marine waters. To clarify, we do not reject the possibility of marine incursion throughout the entire Miocene history of western Amazonia. For instance, the probably slightly older middle Miocene Pellucistoma records from oligo-/mesohaline layers of Nuevo Horizonte (Boonstra et al. 2015) could be interpreted to mirror an incursion-related immigration as well as the stepwise freshwater adaptation of P. curupira sp. nov. Nevertheless, in our case direct marine connections are not mandatory.
(C) Migration of Pellucistoma curupira sp. nov. through aquatic pathways.
The Caribbean-derived Pellucistoma probably reached the Llanos Basin (like other marine biota) during sporadic marine incursions in the early/middle Miocene (e.g. Wesselingh & Macsotay 2006; Jiménez & Hammen 2007; Bayona et al. 2007; Gomez et al. 2009; Boonstra et al. 2015 and references therein). Larger animals of marine ancestry (e.g. fishes, dolphins, manatees; Lovejoy et al. 2006; Lundberg et al. 2010; Bianucci et al. 2013) were able to migrate actively into the Pebas system via fluvial pathways. Smaller biota (e.g. ostracods, foraminifers, mollusc larvae) could have entered this ‘mega-wetland’ actively too but, more likely, could have been freighted ectophoretically and gradually developed freshwater tolerance over long timescales (e.g. P. curupira sp. nov.). Thus, “it would not seem to be necessary for the connection between the sea and the heart of the basin to be direct at any one time. A series of lakes continually splitting and merging with each other, or perhaps becoming reconnected by streams, would enable taxa to progress gradually from one area to another.” (Nuttall 1990, p. 351; compare Lundberg et al. 2010).
Based on our investigations and the data available, we favour hypothesis (C). However, we cannot explicitly reject hypotheses (A) and (B), which might also contribute to the enigmatic occurrence of Pellucistoma curupira sp. nov.
Conclusions
Based on our in-depth evaluation of a new species of the ostracod genus Pellucistoma from the late middle Miocene of western Amazonia, we conclude that this genus is: (1) biogeographically restricted to the Americas; (2) in general a typical shallow marine clade; and (3) of Oligocene/early Miocene Caribbean origin. We assume that Pellucistoma entered the Llanos Basin during the early Miocene, migrated into the fluvio-lacustrine Pebas mega-wetland by phoresy through aquatic (fluvial) connections and adapted to freshwater conditions. Our finding emphasizes again that palaeoenvironmental interpretations based on a straightforward application of uniformitarian principles are problematical for the endemic biota of western Amazonia (Wesselingh 2006; Gross et al. 2013). Thus we conclude that this record of Pellucistoma is not evidence for marine incursions.
Supplementary Material
Acknowledgements
This study was financed by the Austrian Science Fund project P21748-N21 and supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico/Ministério da Ciência e Tecnologia (CNPq/MCT; process number EXC 010389/2009-1). For access to core materials as well as to unpublished reports we acknowledge the Departamento Nacional de Produção Mineral (DNPM/Manaus, especially Gert Woeltje) and the Companhia de Pesquisa de Recursos Minerais (CPRM/Manaus, especially Marco Antônio de Oliveira). The authors are grateful to Dan L. Danielopol (University of Graz), Frank Gitter (UM Joanneum, Graz), Eugen Kempf (University of Cologne) and Sylvain Richoz (University of Graz) for discussions. We thank Hsi-Jen Tao (University of Taipei), Moriaki Yasuhara (University of Hong Kong), Claudia Wrozyna (University of Graz) and Yanlong Chen (University of Graz) for providing literature and translations as well as Hans-Peter Bojar (UM Joanneum, Graz) for access to the SEM of the Joanneum. Sincere thanks are given to Claudia Wrozyna and Marco Caporaletti (University of Graz) for δ18O- and δ13C-analyses. We express our gratitude to Elsa Gliozzi (Roma Tre University) and an anonymous reviewer for constructive comments on the manuscript.
Supplemental material
Supplemental material for this article can be accessed here: http://dx.doi.org/10.1080/14772019.2015.1078850
References
- Aceñolaza F. G. La Formación Paraná (Miocene medio: estratigrafia, distribucion regional y unidades equivalentes) Instituto Superior de Correlación Geológica (INSUGEO), Serie Correlación Geológica. 2000:9–28. [Google Scholar]
- Aiello G. Szczechura J. Appearance of the genus Elofsonia Wagner, 1957 (Loxoconchidae: Ostracoda) in the Middle Miocene of Central Paratethys. Revue de Micropaléontologie. 2002:3–7. [Google Scholar]
- Anderson L. C. Hartman J. H. Wesselingh F. Close evolutionary affinities between freshwater corbulid bivalves from the Neogene of western Amazonia and Paleogene of the northern Great Plains, USA. Journal of South American Earth Sciences. 2006:28–48. [Google Scholar]
- Athersuch J. Horne D. J. A Review of some European genera of the Family Loxoconchidae (Crustacea: Ostracoda) Zoological Journal of the Linnean Society. 1984:1–22. [Google Scholar]
- Athersuch J. Horne D. J. Whittaker J. E. Marine and brackish water ostracods (Superfamilies Cypridacea and Cytheracea) Synopses of the British Fauna (New Series) 1989:1–343. [Google Scholar]
- Ayress M. A. New Cytheromatid Ostracoda from the Cenozoic of New Zealand. New Zealand Natural Sciences. 1990:67–72. [Google Scholar]
- Ayress M. A. On Pellucistoma punctata Ayress sp. nov. Stereo-Atlas of Ostracod Shells. 1996:5–8. [Google Scholar]
- Barker D. Size in relation to salinity in fossil and Recent euryhaline ostracods. Journal of the Marine Biological Association of the UK. 1963:785–795. [Google Scholar]
- Bayona G. Jaramillo C. Rueda M. Reyes-Harker A. Torres V. Paleocene-Middle Miocene flexural-margin migration of the nonmarine Llanos foreland basin of Colombia. Ciencia, Tecnología y Futuro. 2007:51–70. [Google Scholar]
- Benson R. H. Ecology of recent ostracodes of the Todos Santos Bay, Baja California, Mexico. The University of Kansas Paleontological Contributions. 1959:1–80. [Google Scholar]
- Benson R. H. Coleman G. L. Recent marine ostracodes from the eastern Gulf of Mexico. The University of Kansas Paleontological Contributions. 1963:1–52. [Google Scholar]
- Benson R. H. Kaesler R. L. Recent marine and lagoonal ostracodes from the Estero de Tastiota region, Sonora, Mexico (Northeastern Gulf of California) The University of Kansas Paleontological Contributions. 1963:1–34. [Google Scholar]
- Benson R. H. Berdan J. M. Bold W. A. van den. Hanai T. Hessland I. Howe H. V. Kesling R. V. Levinson S. A. Reyment R. A. Moore R. C. Scott H. W. Shaver R. H. Sohn I. G. Stover L. E. Swain F. M. Sylvester-Bradley P. C. Wainwright J. Treatise on invertebrate paleontology. Part Q, Arthropoda 3, Crustacea, Ostracoda. Geological Society of America, Boulder and University of Kansas Press; Lawrence: 1961. p. 442. [Google Scholar]
- Bergue C. T. Coimbra J. C. Javanella sanfordae, a new Cytheridae (Crustacea: Ostracoda) species with a discussion on the validity of the genus Javanella Kingma. Revista Brasileira de Paleontologia. 2007:151–156. [Google Scholar]
- Bianucci G. Lambert O. Salas-Gismondi R. Tejada J. Pujos F. Urbina M. Antoine P.-O. A Miocene relative of the Ganges River Dolphin /Odontoceti, Platanistidae) from the Amazonian basin. Journal of Vertebrate Paleontology. 2013:741–745. [Google Scholar]
- Bold W. A. van den. Miocene Ostracoda from Venezuela. Journal of Paleontology. 1950:76–88. [Google Scholar]
- Bold W. A. van den. Ostracoda of the Brasso Formation of Trinidad. Micropaleontology. 1958:391–418. [Google Scholar]
- Bold W. A. van den. Upper Miocene and Pliocene Ostracoda of Trinidad. Micropaleontology. 1963:361–424. [Google Scholar]
- Bold W. A. van den. Ostracoda of the Gatún Formation, Panama. Micropaleontology. 1967:306–318. [Google Scholar]
- Bold W. A. van den. Ostracoda of the Lower and Middle Miocene of St. Croix, St. Martin and Anguilla. Caribbean Journal of Science. 1970:35–61. [Google Scholar]
- Bold W. A. van den. Ostracodos del post-Eoceno de Venezuela y regions vecinas. Boletín de Geología, Ministerio de Minas e Hidrocarburos, Publicación Especial. 1972a:999–1071. [Google Scholar]
- Bold W. A. van den. Ostracoda of the La Boca Formation, Panama Canal Zone. Micropaleontology. 1972b:410–442. [Google Scholar]
- Bold W. A. van den. Ostracode Associations in the Caribbean Neogene. Verhandlungen der Naturforschenden Gesellschaft in Basel. 1974:214–221. [Google Scholar]
- Bold W. A. van den. Ostracode correlation of brackish-water beds in the Caribbean Neogene. Transactions of the 7th Caribbean Geological Conference. 1976:169–175. [Google Scholar]
- Bold W. A. van den. Neogene Paleontology in the northern Dominican Republic. 7. The Subclass Ostracoda (Arthropoda: Crustacea) Bulletins of American Paleontology. 1988:1–105. [Google Scholar]
- Bonaduce G. Masoli M. Pugliese N. Ostracoda from the Gulf of Aqaba (Red Sea) Pubblicazioni della Stazione Zoologica di Napoli. 1978:372–428. [Google Scholar]
- Boonstra M. Ramos M. I. F. Lammertsma E. I. Antoine P.-O. Hoorn C. Marine connections of Amazonia: Evidence from foraminifera and dinoflagellate cysts (early to middle Miocene, Colombia/Peru) Palaeogeography, Palaeoclimatology, Palaeoecology. 2015:176–194. [Google Scholar]
- Brandão S. World Ostracoda Database. World Register of Marine Species; 2015. Pellucistoma Coryell & Fields, 1937.www.marinespecies.org Accessed 3 April 2015. [Google Scholar]
- Brochet A. L. Gauthier-Clerc M. Guillemain M. Fritz H. Waterkeyn A. Baltanás Á. Green A. J. Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camargue (southern France) Hydrobiologia. 2010:255–261. [Google Scholar]
- Candela A. M. Bonini R. A. Noriega J. I. First continental vertebrates from the marine Paraná Formation (late Miocene, Mesopotamia, Argentina): Chronology, biogeography, and palaeoenvironments. Geobios. 2012:515–526. [Google Scholar]
- Cione A. L. Cozzuol M. A. Dozo M. T. Hospitaleche C. A. Marine vertebrate assemblages in the southwest Atlantic during the Miocene. Biological Journal of the Linnean Society. 2011:423–440. [Google Scholar]
- Coimbra J. C. Pinto I. D. Würdig N. L. Carmo D. A. Zoogeography of Holocene Podocopina (Ostracoda) from the Brazilian Equatorial shelf. Marine Micropaleontology. 1999:365–379. [Google Scholar]
- Coimbra J. C. Carreño A. L. Anjos-Zerfass G. S. Biostratigraphy and paleoceanographical significance of the Neogene planktonic foraminifera from the Pelotas Basin, southernmost Brazil. Revue de Micropaléontologie. 2009:1–14. [Google Scholar]
- Coryell H. N. Fields S. A Gatun ostracode fauna from Cativa, Panama. American Museum Novitates. 1937:1–18. [Google Scholar]
- Cozzuol M. A. The Acre vertebrate fauna: Age, diversity, and geography. Journal of South American Earth Sciences. 2006:185–203. [Google Scholar]
- Cronin T. M. Late Pleistocene marginal marine ostracodes from the southeastern Atlantic coastal plain and their palaeoenvironmental implications. Geographie Physique et Quaternaire. 1979:121–173. [Google Scholar]
- Cronin T. M. Evolution, biogeography, and systematic of Puriana: Evolution and Speciation in Ostracoda, III. The Paleontological Society, Memoir. 1987:1–71. [Google Scholar]
- Cronin T. M. Dowsett H. J. A Quantitative Micropaleontologic Method for Shallow Marine Paleoclimatology: Application to Pliocene Deposits of the Western North Atlantic Ocean. Marine Micropaleontology. 1990:117–147. [Google Scholar]
- Del' Arco J. O. Santos R. O. B. Rivetti M. Olivera Alves E. D. Fernandes C. A. C. Silva L. L. Folha SB. 19 Juruá. I–Geologia. Projeto Radambrasil. Levantamento de Recursos Naturais. 1977:19–88. [Google Scholar]
- Del Río C. J. Malacofauna de las Formaciones Paraná y Puerto Madryn (Mioceno marino, Argentina): su origen, composición y significado bioestratigráfico. Instituto Superior de Correlación Geológica (INSUGEO), Serie Correlación Geológica. 2000:77–101. [Google Scholar]
- Díaz de Gamero M. L. The changing course of the Orinoco River during the Neogene: a review. Palaeogeography, Palaeoclimatology, Palaeoecology. 1996:385–402. [Google Scholar]
- Dino R. Soares E. A. A. Antonioli L. Riccomini C. Nogueira A. C. R. Palynostratigraphy and sedimentary facies of Middle Miocene fluvial deposits of the Amazonas Basin, Brazil. Journal of South American Earth Sciences. 2012:61–80. [Google Scholar]
- Edwards R. A. Ostracoda from the Duplin Marl (Upper Miocene) of North Carolina. Journal of Paleontology. 1944:505–528. [Google Scholar]
- Faugères J. C. Duprat J. Gonthier E. Peypouquet J. P. La sédimentation marine holocène dans le Ghubbet el Kharab (territoire des Afars et Issas): importance du context regional. Oceanologica Acta. 1984:5–12. [Google Scholar]
- Ferrero L. Foraminíferos y ostrácodos del Pleistoceno tardío (Mar Chiquita, provincial de Buenos Aires, Argentina. Ameghiniana. 2009:637–656. [Google Scholar]
- Figueiredo J. Hoorn C. Ven P. van der. Soares E. late Miocene onset of the Amazon River and the Amazon deep-sea fan: Evidence from the Foz do Amazonas Basin. Geology. 2009:619–622. [Google Scholar]
- Finston T. Size, shape and development time are plastic traits in salt lake ostracods of the Mytilocypris mytiloides (Ostracoda : Cyprididae) species complex. Marine and Freshwater Research. 2007:511–518. [Google Scholar]
- Fischer S. Beitrag zur Kenntniss der Ostracoden. Abhandlungen der mathematisch-physikalischen Classe der königlich-bayerischen Akademie der Wissenschaften. 1855:635–666. [Google Scholar]
- Frenzel P. Boomer I. The use of ostracods from marginal marine, brackish waters as bioindicators of modern and Quaternary environmental change. Palaeogeography, Palaeoclimatology, Palaeoecology. 2005:68–92. [Google Scholar]
- Frisch D. Green A. J. Figuerola J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Sciences. 2007:568–574. [Google Scholar]
- Garbett E. C. Maddocks R. F. Zoogeography of Holocene cytheracean ostracodes in the bays of Texas. Journal of Paleontology. 1979:841–919. [Google Scholar]
- Gingras M. J. Räsänen M. E. Pemberton S. G. Romero L. P. Ichnology and sedimentology reveal depositional characteristics of bay-margin parasequences in the Miocene Amazonian foreland basin. Journal of Sedimentary Research. 2002:871–883. [Google Scholar]
- Gomez A. A. Jaramillo C. A. Parra M. Mora A. Huesser Horizon: A lake and a marine incursion in northwestern South America during the Early Miocene. Palaios. 2009:199–210. [Google Scholar]
- Gou Y.-S. Chen D.-Q. On the occurrence of Javanella and Saida in the Pliocene of Leizhou peninsula, Guangdong, China. In: Hanai T., editor; Ikeya N., editor; Ishizaki K., editor. Evolutionary Biology of Ostracoda: its fundamentals and applications. Elsevier; Tokyo: 1988. pp. 797–803. [Google Scholar]
- Gross M. Minati K. Danielopol D. L. Piller W. E. Environmental changes and diversification of Cyprideis in the late Miocene of the Styrian Basin (Lake Pannon, Austria) Senckenbergiana lethaea. 2008:161–181. [Google Scholar]
- Gross M. Piller W. E. Ramos M. I. Paz J. D. S. late Miocene sedimentary environments in south-western Amazonia (Solimões Formation; Brazil) Journal of South American Earth Sciences. 2011:169–181. doi: 10.1016/j.jsames.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. Ramos M. I. Caporaletti M. Piller W. E. Ostracods (Crustacea) and their palaeoenvironmental implication for the Solimões Formation (late Miocene; Western Amazonia/Brazil) Journal of South American Earth Sciences. 2013:216–241. doi: 10.1016/j.jsames.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. Ramos M. I. F. Piller W. E. On the Miocene Cyprideis species flock (Ostracoda; Crustacea) of Western Amazonia (Solimões Formation): Refining taxonomy on species level. Zootaxa. 2014:1–69. doi: 10.11646/zootaxa.3899.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S. Part 1. Living and subfossil rhizopod and ostracode populations. The University of Kansas Paleontological Contributions. 1967:7–82. [Google Scholar]
- Grossman E. L. Ku T.-L. Oxygen and carbon isotope fractionation in biogenic aragonite: Temperature effects. Chemical Geology. 1986:59–74. [Google Scholar]
- Hall D. D. Paleoecology and taxonomy of fossil Ostracoda in the vicinity of Sapelo Island, Georgia. In: Kesling R. V., editor; Darby D. G., editor; Smith R. N., editor; Hall D. D., editor. Four Reports of Ostracod Investigations. University of Michigan; 1965. pp. 1–79. National Science Foundation Project GB-26, Report 4. [Google Scholar]
- Hartman D. S. Ecology and behavior of the manatee (Trichechus manatus) in Florida. The American Society of Mammalogists, Special Publication. 1979:1–150. [Google Scholar]
- Hartmann G. Die Ostracoden der Ordnung Podocopida G. W. Müller, 1894 der tropisch-subtropischen Westküste Australiens (zwischen Derby im Norden und Perth im Süden) Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut. 1978:64–219. [Google Scholar]
- Hartmann G. Die Ostracoden der Ordnung Podocopida G. W. Müller, 1894 der warm-temperierten (antiborealen) West- und Südwestküste Australiens (zwischen Perth im Norden und Eucla im Süden) Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut. 1979:219–301. [Google Scholar]
- Heinrich S. Zonneveld K. A. F. Influence of the Amazon River development and constriction of the Central American Seaway on Middle/late Miocene oceanic conditions at the Ceara Rise. Palaeogeography, Palaeoclimatology, Palaeoecology. 2013:599–606. [Google Scholar]
- Hernández R. M. Jordan T. E. Farjat A. Echavarría L. Idleman B. D. Reynolds J. H. Age, distribution, tectonics and eustatic controls of the Paranense and Caribbean marine transgressions in southern Bolivia and Argentina. Journal of South American Earth Sciences. 2005:495–512. [Google Scholar]
- Hoorn C. Marine incursions and the influence of Andean tectonics on the Miocene depositional history of northwestern Amazonia: results of a palynostratigraphic study. Palaeogeography, Palaeoclimatology, Palaeoecology. 1993:267–309. [Google Scholar]
- Hoorn C. An environmental reconstruction of the palaeo-Amazon River system (Middle–late Miocene, NW Amazonia) Palaeogeography, Palaeoclimatology, Palaeoecology. 1994:187–238. [Google Scholar]
- Hoorn C. Wesselingh F. P. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. p. 464. [Google Scholar]
- Hoorn C. Guerrero J. Sarmiento G. A. Lorente M. A. Andean tectonics as a cause for changing drainage patterns in Miocene northern South America. Geology. 1995:237–240. [Google Scholar]
- Hoorn C. Wesselingh F. P. Steege H. ter. Bermudez M. A. Mora A. Sevink J. Sanmartín I. Sanchez-Meseguer A. Anderson C. L. Figueiredo J. P. Jaramillo C. Riff D. Negri F. R. Hooghiemstra H. Lundberg J. Stadler T. Särkinen T. Antonelli A. Amazonia Through Time: Andean Uplift, Climate Change, Landscape Evolution, and Biodiversity. Science. 2010a:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- Hoorn C. Wesselingh F. P. Hovikoski J. Guerrero J. The development of the Amazonian mega-wetland (Miocene; Brazil, Colombia, Peru, Bolivia) In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010b. pp. 123–142. [Google Scholar]
- Horne F. R. Survival strategy to escape desiccation in a freshwater ostracod. Crustaceana. 1993:53–61. [Google Scholar]
- Horne D. J. Kilenyi T. I. Lindisfarnia laevata (Norman) Stereo-Atlas of Ostracod Shells. 1981:107–116. [Google Scholar]
- Horne D. J. Whatley R. C. On Palmoconcha laevata (Norman) Stereo-Atlas of Ostracod Shells. 1985:158. [Google Scholar]
- Horne D. J. Cohen A. Martens K. Taxonomy, Morphology and Biology of Quaternary and Living Ostracoda. American Geophysical Union Monograph. 2002:5–36. [Google Scholar]
- Hovikoski J. Räsänen M. Gingras M. Roddaz M. Brusset S. Hermoza W. Pittman L. R. Lertola K. Miocene semidiurnal tidal rhythmites in Madre de Dios, Peru. Geology. 2005:177–180. [Google Scholar]
- Hovikoski J. Räsänen M. Gingras M. Lopez S. Romero L. Ranzi A. Melo J. Palaeogeographical implications of the Miocene Quendeque Formation (Bolivia) and tidally-influenced strata in southwestern Amazonia. Palaeogeography, Palaeoclimatology, Palaeoecology. 2007:23–41. [Google Scholar]
- Hovikoski J. Wesselingh F. P. Räsänen M. Gingras M. Vonhof H. B. Marine influence in Amazonia: evidence from the geological record. In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 143–161. [Google Scholar]
- Howe H. V. McKenzie K. G. Recent marine Ostracoda (Crustacea) from Darwin and north-western Australia. Northern Territory Museum of Arts and Sciences, Monograph Series. 1989:1–49. [Google Scholar]
- Hu C.-H. New Ostracod Faunas from the Maanshan Mudstone of Hengchun Peninsula, Southern Taiwan. Petroleum Geology of Taiwan. 1981:81–109. [Google Scholar]
- Hu C.-H. New Fossil Ostracod Faunas from Hengchun Peninsula, Southern Taiwan. Journal of Taiwan Museum. 1984:65–129. [Google Scholar]
- Hu C.-H. Tao H.-J. Studies on the ostracod fauna of Taiwan and its adjacent seas. National Taiwan Museum, Special Publication Series. 2008:1–910. In Chinese. [Google Scholar]
- Hulings N. C. Marine Ostracoda from the western North Atlantic Ocean between Cape Hatteras, North Carolina, and Jupiter Inlet, Florida. Bulletin of Marine Science. 1967:629–659. [Google Scholar]
- Hulka C. Gräfe K.-U. Sames B. Uba C. E. Heubeck C. Depositional setting of the Middle to late Miocene Yecua Formation of the Chaco Foreland Basin, southern Bolivia. Journal of South American Earth Sciences. 2006:135–150. [Google Scholar]
- Hunt G. Roy K. Climate change, body size evolution, and Cope's Rule in deep-sea ostracods. Proceedings of the National Academy of Sciences of the United States of America. 2006:1347–1352. doi: 10.1073/pnas.0510550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G. Wicaksono S. A. Brown J. E. Macleod K. G. Climate-driven body-size trends in the ostracod fauna of the deep Indian Ocean. Palaeontology. 2010:1255–1268. [Google Scholar]
- Ikeya N. Hanai T. Ecology of recent ostracods in the Hamana-Ko region, the Pacific coast of Japan. The University Museum, The University of Tokyo, Bulletin. 1982:15–59. [Google Scholar]
- Ishizaki K. Ostracoda from the East China Sea. Science Report of Tohoku University, Second Series, Geology. 1981:37–65. [Google Scholar]
- Iturralde-Vinent M. A. MacPhee R. D. E. Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bulletin of the American Museum of Natural History. 1999:1–95. [Google Scholar]
- Jiménez H. D. Hammen T. van der. Significado geológico y asociaciones palinológicas de las formaciones Diablo Inferior (Mioceno Tardío) y San Fernando Superior (Mioceno Medio), Piedemonte cuenca de los Llanos Orientales, Colombia. Revista de la Academia Colombiana de Ciencias. 2007:481–498. [Google Scholar]
- Juday C. Ostracoda of the San Diego region. II: Littoral forms. University of California Publications in Zoology. 1907:135–156. [Google Scholar]
- Kaandorp R. J. G. Wesselingh F. P. Vonhof H. B. Ecological implications from geochemical records of Miocene Western Amazonian bivalves. Journal of South American Earth Sciences. 2006:54–74. [Google Scholar]
- Keij A. J. Note on the Lutetian Ostracoda of Damery (Marne), France. Proceedings Koninklijke Nederlandse Akademie van Wetenschappen, Series B. 1958:63–73. [Google Scholar]
- Kempf E. K. Index and bibliography of marine Ostracoda 1: Index A. Sonderveröffentlichungen des Geologischen Instituts der Universität zu Köln. 1986:1–762. [Google Scholar]
- Kempf E. K. Index and bibliography of marine Ostracoda 6: Index A: Supplement 1. Sonderveröffentlichungen des Geologischen Instituts der Universität zu Köln. 1995:1–244. [Google Scholar]
- Kempf E. K. Index and bibliography of marine Ostracoda 11: Index A: Supplement 2. CD-ROM edition only 2008 [Google Scholar]
- Keyser D. Zur Kenntnis der brackigen mangrovebewachsenen Weichböden Südwest-Floridas unter besonderer Berücksichtigung ihrer Ostracodenfauna. University of Hamburg; 1976. p. 142. Unpublished PhD thesis. [Google Scholar]
- Keyser D. Schöning C. Holocene Ostracoda (Crustacea) from Bermuda. Senckenbergiana lethaea. 2000:567–591. [Google Scholar]
- King Lyon S. Biostratigraphic and palaeoenvironmental interpretation from late Pleistocene Ostracoda, Charleston, South Carolina. U.S. Geological Survey Professional Paper. 1990:D1–48. [Google Scholar]
- Kingma J. T. Contribution to the knowledge of the young Caenozoic Ostracoda from the Malayan Region. University of Utrecht; 1948. p. 118. Unpublished PhD thesis. [Google Scholar]
- Krutak P. R. Modern ostracodes of the Veracruz-Anton Lizardo reefs, Mexico. Micropaleontology. 1982:258–288. [Google Scholar]
- Latrubesse E. M. Silva S. A. F. Cozzuol M. Absy M. L. late Miocene continental sedimentation in the southwestern Amazonia and its regional significance: Biotic and geological evidence. Journal of South American Earth Sciences. 2007:61–80. [Google Scholar]
- Latrubesse E. M. Cozzuol M. Silva-Caminha S. A. F. Rigsby C. A. Absy M. L. Jaramillo C. The late Miocene paleogeography of the Amazon Basin and the evolution of the Amazon River system. Earth-Science Reviews. 2010:99–124. [Google Scholar]
- Leng M. J. Marshall J. D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quaternary Science Reviews. 2004:811–831. [Google Scholar]
- Le Roux J. P. A review of Tertiary climate changes in southern South America and the Antarctic Peninsula. Part 1: Oceanic conditions. Sedimentary Geology. 2012:247–248. 1–20. [Google Scholar]
- Linhares A. P. Ramos M. I. F. Gross M. Piller W. E. Evidence for marine influx during the Miocene in southwestern Amazonia, Brazil. Geología Colombiana. 2011:91–103. [Google Scholar]
- Löffler H. Vogelzug und Crustaceenverbreitung. Zoologischer Anzeiger, Supplement. 1964:311–316. [Google Scholar]
- Lovejoy N. R. Albert J. S. Crampton W. G. R. Miocene marine incursions and marine/freshwater transitions: Evidence from Neotropical fishes. Journal of South American Earth Sciences. 2006:5–13. [Google Scholar]
- Lundberg J. G. Sabaj Pérez M. H. Dahdul W. M. Aguilera O. A. The Amazonian Neogene fish fauna. In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 281–301. [Google Scholar]
- Maia R. G. N. Godoy H. K. Yamaguti H. S. Moura P. A. Costa F. S. F. Holanda M. A. Costa J. A. Projeto Carvão no Alto Solimões. Relatório Final. Companhia de Pesquisa de Recursos Minerais-Departamento Nacional da Produçáo Mineral; Manaus: 1977. p. 142. [Google Scholar]
- Majoran S. Widmark J. G. V. Response of deep-sea ostracod assemblages to Late Cretaceous palaeoceanographical changes: ODP Site 689 in the Southern Ocean. Cretaceous Research. 1998:843–872. [Google Scholar]
- Majoran S. Agrenius S. Kucera M. The effect of temperature on shell size and growth rate in Krithe praetexta praetexta (Sars) Hydrobiologia. 2000:141–148. [Google Scholar]
- Marengo H. G. Rasgos micropaleotológicos de los depósitos de la transgression Entrerriense-Paranense en la Cuenca Chaco-Paranense y Noroeste Argentino. Instituto Superior de Correlación Geológica (INSUGEO), Serie Correlación Geológica. 2000:29–45. [Google Scholar]
- Marsh H. O'Shea T. J. Reynolds J. E., III . Ecology and Conservation of the Sirenia. Dugongs and Manatees. Cambridge University Press; Cambridge: 2011. p. 536. [Google Scholar]
- McKenzie K. G. Swain F. M. Recent Ostracoda from Scammon Lagoon, Baja California. Journal of Paleontology. 1967:281–305. [Google Scholar]
- Mesquita-Joanes F. Smith A. J. Viehberg F. A. The Ecology of Ostracoda Across Levels of Biological Organisation from Individual to Ecosystem: A Review of Recent Developments and Future Potential. Developments in Quaternary Science. 2012:15–35. [Google Scholar]
- Morales G. A. Ecology, distribution, and taxonomy of recent Ostracoda of the Laguna de Terminos, Campeche, Mexico. Universidad Nacional Autonoma de Mexico, Boletin. 1966:1–103. [Google Scholar]
- Morkhoven F. P. C. M. van. Post-Palaeozoic Ostracoda, their Morphology, Taxonomy, and Economic Use. Volume 2, Generic descriptions. Elsevier Publishing Company; Amsterdam: 1963. p. 478. [Google Scholar]
- Mostafawi N. Rezente Ostracoden aus dem mittleren Sunda-Schelf, zwischen der Malaiischen Halbinsel und Borneo. Senckenbergiana lethaea. 1992:129–168. [Google Scholar]
- Müller G. W. Die Ostracoden des Golfes von Neapel und der angrenzenden Meeresabschnitte. Zoologische Station zu Neapel, Monograph 21; Berlin: 1894. p. 404. [Google Scholar]
- Muñoz-Torres F. A. Whatley R. C. Harten D. van. Miocene ostracod (Crustacea) biostratigraphy of the upper Amazon Basin and evolution of the genus Cyprideis . Journal of South American Earth Sciences. 2006:75–86. [Google Scholar]
- Neale J. W. Ostracods and palaeosalinity reconstructions. In: De Deckker P., editor; Colin J. P., editor; Peypouquet J. P., editor. Ostracoda in the Earth Sciences. Elsevier; Amsterdam: 1988. pp. 125–157. [Google Scholar]
- Nicolaidis D. D. Coimbra J. C. Perissocytheridea carrenoae sp. nov. (Crustacea, Ostracoda) and associated calcareous microfauna from Yecua Formation (Miocene), Bolivia. Revista Brasileira de Paleontologia. 2008:179–186. [Google Scholar]
- Nuttall C. P. A review of the Tertiary non-marine molluscan faunas of the Pebasian and other inland basins of north-western South America. Bulletin of the British Museum (Natural History), Geology Series. 1990:165–371. [Google Scholar]
- Omatsola M. E. Podocopid Ostracoda from the Lagos Lagoon, Nigeria. Micropaleontology. 1970:407–445. [Google Scholar]
- Prange M. Schulz M. A coastal upwelling seesaw in the Atlantic Ocean as a result of the closure of the Central American Seaway. Geophysical Research Letters. 2004;17207:1–4. [Google Scholar]
- Puri H. S. Contribution to the study of the Miocene of the Florida Panhandle, Part 3: Ostracoda. Geological Bulletin, Florida Geological Survey. 1954:215–345. [Google Scholar]
- Puri H. S. Recent Ostracoda from the west coast of Florida. Transactions of the Gulf Coast Association of Geological Societies. 1960:107–149. [Google Scholar]
- Purper I. Cenozoic Ostracodes of the Upper Amazon Basin, Brazil. Pesquisas. 1979:209–281. [Google Scholar]
- Ramos M. I. F. Ostracods from the Neogene Solimões Formation (Amazonas, Brazil) Journal of South American Earth Sciences. 2006:87–95. [Google Scholar]
- Räsänen M. E. Linna A. M. Santos J. C. R. Negri F. R. late Miocene tidal deposits in the Amazonian Foreland Basin. Science. 1995:386–390. doi: 10.1126/science.269.5222.386. [DOI] [PubMed] [Google Scholar]
- Rebata L. A. Gingras M. K. Räsänen M. E. Barberi M. Tidal-channel deposits on a delta plain from the Upper Miocene Nauta Formation, Marañón Foreland Sub-basin, Peru. Sedimentology. 2006:971–1013. [Google Scholar]
- Riff D. Romano P. S. R. Oliveira G. R. Aguilera O. A. Neogene crocodile and turtle fauna in northern South America. In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 259–280. [Google Scholar]
- Rossi V. Benassi G. Belletti F. Menozzi P. Colonization, population dynamics, predatory behaviour and cannibalism in Heterocypris incongruens (Crustacea: Ostracoda) Journal of Limnology. 2011:102–108. [Google Scholar]
- Ruskin B. G. Dávila F. M. Hoke G. D. Jordan T. E. Astini R. A. Alonso R. Stable isotope composition of middle Miocene carbonates of the Frontal Cordillera and Sierras Pampeanas: Did the Paranaense seaway flood western and central Argentina? Palaeogeography, Palaeoclimatology, Palaeoecology. 2011:293–303. [Google Scholar]
- Sandberg P. A. The ostracod genus Cyprideis in the Americas. Acta Universitatis Stockholmiensis. 1964:1–178. [Google Scholar]
- Sandberg P. A. Appendages and family placement of the ostracod genus Pellucistoma . Journal of Paleontology. 1969:1174–1178. [Google Scholar]
- Sanguinetti Y. T. Miocene ostracodes of the Pelotas Basin, state of Rio Grande do Sul, Brasil. Pesquisas. 1979:119–187. [Google Scholar]
- Sarr R. Sow E. H. Sarr B. Holocene marine intrusions in Retba and Mbawane lakes (Senegal) evidenced by ostracod faunas. Revue de Micropaléontologie. 2008:327–338. [Google Scholar]
- Sars G. O. Oversigt af Norges marine Ostracoder. Forhandlinger i Videnskabs-Selskabet i Christiania. 1866:1–130. [Google Scholar]
- Scheihing R. Labarca P. Cardenas L. Nespolo R. F. Viability selection on body size in a non-marine ostracod. Hydrobiologia. 2011:193–203. [Google Scholar]
- Schornikov E. I. Podklass ostracoda, ili rakushkovye rachki, Ostracoda Latreille, 1816. Opredelitel Fauny Chernogo i Azovskogo Morey. 1969:163–259. [Google Scholar]
- Schornikov E. I. Loxocaudinae: A New Subfamily in the Ostracod Family Loxoconchidae. Russian Journal of Marine Biology. 2011:185–192. [Google Scholar]
- Sciuto F. Bythocythere mylaensis n. sp. (Crustacea, Ostracoda) from the Early Pleistocene of Capo Milazzo (NE Sicily) Bollettino della Società Paleontologica Italiana. 2009:183–188. [Google Scholar]
- Sciuto F. Pugliese N. Description of two new species of ostracods from the Strait of Messina (central Mediterranean) Zoosystema. 2013:35–43. [Google Scholar]
- Sheppard L. M. Bate R. H. Plio-Pleistocene ostracods from the Upper Amazon of Colombia and Peru. Palaeontology. 1980:97–124. [Google Scholar]
- Silva-Caminha S. A. F. Jaramillo C. A. Absy M. L. Neogene palynology of the Solimões Basin, Brazilian Amazonia. Palaeontographica, Abteilung B. 2010:13–79. [Google Scholar]
- Stepanova A. Y. Late Pleistocene-Holocene and Recent Ostracoda of the Laptev Sea and their importance for paleoenvironmental reconstructions. Paleontological Journal. 2006:91–204. [Google Scholar]
- Suárez-Morales E. Morales-Vela B. Padilla-Saldívar J. Silva-Briano M. The copepod Balaenophilus manatorum (Ortíz, Lalana and Torres, 1992) (Harpacticoida), an epibiont of the Caribbean manatee. Journal of Natural History. 2010:847–859. [Google Scholar]
- Swain F. M. Ostracoda from the Gulf of California. Geological Society of America Memoir. 1967:1–134. [Google Scholar]
- Swain F. M. Gilby J. M. Recent Ostracoda from Corinto Bay, western Nicaragua, and their relationship to some other assemblages of the Pacific coast. Journal of Paleontology. 1967:306–334. [Google Scholar]
- Swain F. M. Gilby J. M. Marine Holocene Ostracoda from the Pacific coast of North and Central America. Micropaleontology. 1974:257–352. [Google Scholar]
- Tambussi C. P. Degrange F. J. South American and Antarctic Continental Cenozoic Birds. SpringerBriefs in Earth System Sciences. 2013:1–113. [Google Scholar]
- Teeter J. W. Geographic distribution and dispersal of some recent shallow-water marine Ostracoda. The Ohio Journal of Science. 1973:46–54. [Google Scholar]
- Teeter J. W. Distribution of Holocene Marine Ostracoda from Belize. American Association of Petroleum Geologists, Studies in Geology. 1975:400–499. [Google Scholar]
- Titterton R. Whatley R. C. The Provincial Distribution of Shallow Water Indo-Pacific Marine Ostracoda: Origins, Antiquity, Dispersal Routes and Mechanisms. In: Hanai T., editor; Ikeya N., editor; Ishizaki K., editor. Evolutionary Biology of Ostracoda: its fundamentals and applications. Elsevier; Tokyo: 1988. pp. 759–786. [Google Scholar]
- Uba C. E. Hasler C.-A. Buatois L. A. Schmitt A. K. Plessen B. Isotopic, paleontologic, and ichnologic evidence for late Miocene pulses of marine incursions in the central Andes. Geology. 2009:827–830. [Google Scholar]
- Valentine P. C. Climatic Implication of a Late Pleistocene Ostracode Assemblage from Southeastern Virginia. U.S. Geological Survey Professional Paper. 1971:D1–28. [Google Scholar]
- Valentine P. C. Zoogeography of Holocene Ostracoda off Western North America and Paleoclimatic Implications. U.S. Geological Survey Professional Paper. 1976:1–47. [Google Scholar]
- Vélez-Juarbe J. Noriega J. I. Ferrero B. S. Fossil Dugongidae (Mammalia, Sirenia) from the Paraná Formation (late Miocene) of Entre Ríos province, Argentina. Ameghiniana. 2012:585–593. [Google Scholar]
- Vermeij G. J. Wesselingh F. P. Neogastropod Molluscs from the Miocene of Western Amazonia, with Comments on Marine to Freshwater Transitions in Molluscs. Journal of Paleontology. 2002:265–270. [Google Scholar]
- Vonhof H. B. Wesselingh F. P. Kaandorp R. J. G. Davies G. R. Hinte J. E. van. Guerrero J. Räsänen M. Romero-Pittman L. Ranzi A. Paleogeography of Miocene Western Amazonia: Isotopic composition of molluscan shells constrains the influence of marine incursions. Geological Society of America Bulletin. 2003:983–993. [Google Scholar]
- Wagner C. W. Sur les Ostracodes du Quaternaire récent des Pays-Bas et leur utilisation dans l'étude géologique des dépots holocenes. Université de Paris; 1957. p. 259. Unpublished PhD thesis. [Google Scholar]
- Wanderley-Filho J. R. Eiras J. F., Cunha P. R. C. Ven P. H. van der. The Paleozoic Solimões and Amazonas basins and the Acre foreland basin of Brazil. In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. pp. 29–37. [Google Scholar]
- Wesselingh F. P. Molluscs from the Miocene Pebas Formation of Peruvian and Colombian Amazonia. Scripta Geologica. 2006:19–290. [Google Scholar]
- Wesselingh F. P. Long-Lived Lake Molluscs as Island Faunas: A Bivalve Perspective. In: Renema W., editor. Biogeography, Time, and Place: Distributions, Barriers, and Islands. Springer; Dordrecht: 2007. p. Pp. 275–314. [Google Scholar]
- Wesselingh F. P. Macsotay O. Pachydon hettneri (Anderson, 1928) as indicator for Caribbean–Amazonian lowland connections during the Early–Middle Miocene. Journal of South American Earth Sciences. 2006:49–53. [Google Scholar]
- Wesselingh F. P. Ramos M. I. F. Amazonian aquatic invertebrate faunas (Mollusca, Ostracoda) and their development over the past 30 million years. In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. p. Pp. 302–316. [Google Scholar]
- Wesselingh F. P. Salo J. A. A Miocene perspective on the evolution of the Amazonian biota. Scripta Geologica. 2006:439–458. [Google Scholar]
- Wesselingh F. P. Räsänen M. E. Irion G. Vonhof H. B. Kaandorp R. Renema W. Romero Pittman L. Gingras M. Lake Pebas: a palaeoecological reconstruction of a Miocene, long-lived lake complex in western Amazonia. Cainozoic Research. 2002:35–81. [Google Scholar]
- Wesselingh F. P. Kaandorp R. J. G. Vonhof H. B. Räsänen M. E. Renema W. Gingras M. The nature of aquatic landscapes in the Miocene of western Amazonia: an integrated palaeontological and geochemical approach. Scripta Geologica. 2006:363–393. [Google Scholar]
- Westaway R. Late Cenozoic sedimentary sequences in Acre state, southwestern Amazonia: Fluvial or tidal? Deductions from the IGCP 449 fieldtrip. Journal of South American Earth Sciences. 2006:120–134. [Google Scholar]
- Whatley R. Roberts R. Marine Ostracoda from Pitcairn, Oeno and Henderson Islands. Biological Journal of the Linnean Society. 1995:359–364. [Google Scholar]
- Whatley R. Moguilevsky A. Toy N. Chadwick J. Ramos M. I. F. Ostracoda from the south west Atlantic. Part II. The littoral fauna from between Tierra del Fuego and the Río de la Plata. Revista Española de Micropaleontología. 1997a:5–83. [Google Scholar]
- Whatley R. C. Staunton M. Kaesler R. L. The depth distribution of recent marine Ostracoda from the southern Strait of Magellan. Journal of Micropalaeontology. 1997b:121–130. [Google Scholar]
- Whatley R. C. Muñoz-Torres F. Harten D. van. The Ostracoda of an isolated Neogene saline lake in the western Amazon Basin, Peru. Bulletin du Centres de Recherches Elf Exploration-Production, Memoires. 1998a:231–245. [Google Scholar]
- Whatley R. C. Ramos M. I. F. Moguilevsky A. Chadwick J. The provincial distribution of Recent littoral and shelf Ostracoda in the SW Atlantic. Bulletin du Centres de Recherches Elf Exploration-Production, Memoires. 1998b:317–327. [Google Scholar]
- Whatley R. C. Muñoz-Torres F. Harten D. van. Skopaeocythere: a minute new limnocytherid (Crustacea, Ostracoda) from the Neogene of the Amazon Basin. Ameghiniana. 2000:163–167. [Google Scholar]
- Whatley R. Jones R. Roberts R. The marine Ostracoda of Pitcairn, Oeno and Henderson Islands, southern Pacific. Revista Española de Micropaleontología. 2004:493–528. [Google Scholar]
- Whittaker J. E. On Elofsonia baltica (Hirschmann) Stereo-Atlas of Ostracod Shells. 1973:193–200. [Google Scholar]
- Wilkinson M. J. Marshall L. G. Lundberg J. G. Kreslavsky M. H. Megafan environments in northern South America and their impact on Amazon Neogene aquatic ecosystems. In: Hoorn C., editor; Wesselingh F. P., editor. Amazonia, Landscape and Species Evolution: A Look into the Past. Wiley-Blackwell; Oxford: 2010. p. Pp. 162–184. [Google Scholar]
- Witte L. Taxonomy and Biogeography of West African Beach Ostracodes. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, Afdeling Natuurkunde, 1. Reeks. 1993:1–84. [Google Scholar]
- Witte L. Harten D. van. Polymorphism, Biogeography and Systematics of Kotoracythere inconspicua (Brady, 1880) (Ostracoda: Pectocytheridae) Journal of Biogeography. 1991:427–436. [Google Scholar]
- Wood A. M. Ramos M. I. F. Whatley R. C. The palaeozoogeography of Oligocene to Recent marine Ostracoda from the Neotropics (mid- and South America) and Antarctica. Marine Micropaleontology. 1999:345–364. [Google Scholar]
- Yamaguchi T. Norris R. D. Bornemann A. Dwarfing of ostracodes during the Paleocene–Eocene Thermal Maximum at DSDP Site 401 (Bay of Biscay, North Atlantic) and its implication for changes in organic carbon cycle in deep-sea benthic ecosystem. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012:130–144. [Google Scholar]
- Yasuhara M. Hunt G. Okahashi H. Brandão S. N. The ‘Oxycythereis’ Problem: Taxonomy and palaeobiogeography of deep-sea ostracod genera Pennyella and Rugocythereis . Palaeontology. 2013:1045–1080. [Google Scholar]
- Yin Y. Li W. Yang X. Wang S. Li S. Xia W. Morphological responses of Limnocythere inopinata (Ostracoda) to hydrochemical environment factors. Science in China. 2001:316–323. [Google Scholar]
- Zabert L. L. Micropaleontologia de la formacion Parana (Mioceno superior) en el subsuelo de la provincial de Santa Fe, Republica Argentina. Facena. 1978:101–165. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


