Abstract
We previously reported that dietary genistein inhibits mammary tumor growth and metastasis of the highly metastatic MDA-MB-435 cancer cells in immunocompromised mice. The purpose herein was to characterize the role of the novel oncogenic microRNA (miRNA) miR-155 in the anticancer effects of genistein in metastatic breast cancer. The effect of genistein was determined on breast cancer cell viability, apoptosis, and expression of miR-155 and its targets. At low physiologically relevant concentrations, genistein inhibits cell viability and induces apoptosis in metastatic MDA-MB-435 and Hs578t breast cancer cells, without affecting the viability of nonmetastatic MCF-7 breast cancer cells. In parallel with reduced cell viability, miR-155 is downregulated, whereas proapoptotic and anticell proliferative miR-155 targets FOXO3, PTEN, casein kinase, and p27 are upregulated in MDA-MB-435 and Hs578t cells in response to genistein treatment. However, miR-155 levels remain unchanged in response to genistein in the MCF-7 cells. Ectopic expression of miR-155 in MDA-MB-435 and Hs578t cells decreases the effects of genistein on cell viability and abrogates the effects of genistein on apoptosis and expression of proapoptotic genes. Therefore, genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein in metastatic breast cancer.
Introduction
Isoflavones are found in nutritionally relevant amounts in soybeans and comprise ~3.5 mg/g soy protein in traditional soy foods. Soy is one of the major cash crops in the United States, and consumption of soy products is increasing due to the heightened awareness of the health benefits of plant-based diets. Moreover, ~50% of Americans use dietary supplements that contain various plant products, including soy isoflavones, without adequate knowledge of their mechanism of action. Thus, it is critical to understand the risks and benefits of consuming soy for cancer patients, survivors, and those at risk. However, most studies on soy and cancer have focused on cancer prevention (1–4), whereas the effects of soy foods in established cancers, or as substitutes for hormone replacement therapies, remain controversial (5). A more comprehensive understanding of the effects of individual soy isoflavones, their effective concentrations, and effects and molecular mechanisms on different stages of breast cancer is important for rational recommendations on soy isoflavone supplementation.
Of the soy isoflavones, genistein has been specifically associated with reduced breast cancer risk (2,6). Genistein is the major isoflavone in soy foods comprising ~50% of the isoflavone content. The commonly found glycosidic forms of soy isoflavones are rapidly absorbed and converted to the biologically active aglycone forms (7). Following consumption of soy foods, ~1–10 μM concentrations of genistein may accumulate in the circulation (8–10), thus regulating a number of physiological activities in target tissues. Because of its estrogenic and antiestrogenic activities, genistein has been shown to be both cancer preventive and promoting, especially in estrogen receptor (ER) positive (+) breast cancers (11). For example, a number of studies with ER (+) human breast cancer cell lines demonstrated that genistein induced cell and mammary tumor growth, increased breast cancer-associated aromatase expression, and elevated estrogen levels in mouse models and consequently abrogated the effect of anti-estrogen therapy (12,13). Even though genistein may have estrogenic properties in ER (+) breast cancers, our studies with ER negative (−), human epidermal growth factor receptor 2 (HER2) (++) mammary tumors in nude mice demonstrated decreased tumor growth and metastasis in response to genistein, whereas daidzein (the other abundant isoflavone in soy) or combined soy isoflavones increased tumor growth and metastasis (14). Others have also reported that genistein inhibits tumor growth, cell proliferation, and invasion and induces apoptosis in ER (+) and ER (−) breast cancer cells (15–18). Some of these discrepancies in the responses of breast cancers to genistein may be due to estrogen-independent mechanisms of genistein, as well as the high concentrations (>50 μM) of genistein used in these reports.
The molecular basis of the anticancer effects of genistein have been attributed to a number of signaling pathways (19). Genistein has been implicated in cell cycle arrest and apoptosis in different cancers, including aggressive breast cancer cells, by the following: inhibition of phosphoinositide 3-kinase (PI3-K)/Akt pathway; regulation of tumor suppressors and cell cycle check point proteins such as phosphatase and tensin homolog deleted in chromosome 10 (PTEN), ataxia telangiectasia mutated (ATM), p53, p21, p16, p27; upregulation of pro-death transcription factors such as forkhead box O3 (FOXO3), as well as through the inhibition of β-catenin-mediated Wnt and nuclear factor κB signaling to reduce cyclins and antiapoptotic proteins (Bcl-2, Bcl-XL) (20–28). Moreover, genistein has been implicated in epigenetic control, including regulation of DNA methylation, histone acetylation, and miRNA expression (28–30).
Noncoding RNAs (ncRNAs), such as microRNAs (miRNAs), are major regulators of cancer initiation and progression that act as oncogenes and tumor suppressors in many types of cancer, including breast cancer (31, 32). The anticancer properties of genistein have been recently implicated in the regulation of miRNA expression (33). Genistein has been shown to regulate differential expression of miRNAs in prostate cancer (34–36), ovarian cancer (33, 37), renal cancer (38), melanoma (39), and pancreatic cancer (40). In prostate cancer cells, genistein was shown to downregulate a number of mRNAs that include miR-221/222, miR-1260b, miR-151, miR-574-3p, miR-223, mir-34a, and the long ncRNA HOTAIR (35,36,41–44). In addition, studies have implicated genistein’s anticancer activities through regulation of miR-27 in pancreatic and ovarian cancer (37, 45).
However, scarce information exists on the role of genistein in breast cancer miRNA regulation. Therefore, we performed quantitative reverse transcription polymerase chain reaction (RT-qPCR) on known breast cancer oncomiRs, in breast cancer cells following genistein treatment. Similar to the reports with prostate cancer (35), genistein also decreased miR-221/222 expression in breast cancer cells; however, this decrease was not statistically significant (Supplemental Fig. S1). The present report investigates the effect of genistein on miR-155 regulation in ER (−) breast cancer cells.
MiR-155 is a designated oncomiR and a potential therapeutic target in breast cancer (46–49). MiR-155 has also been correlated with poor prognosis via ER/PR expression, breast cancer stage, triple negative breast cancer (47,50–52). Moreover, genomic and proteomic studies have identified miR-155 as a prioritized cancer target, due to the predicted and confirmed regulation of over 100 cell cycle regulatory and cancer pathway molecules (49,53,54). Therefore, there is an urgent need to design therapeutic interventions against miR-155 in cancer. However, the use of anti-miRs as therapeutic agents for miR-155 overexpressing cancers has been hindered by the instability of anti-miRs and issues with targeting nano-delivery systems (47,50–52). Naturopathic remedies offer an attractive nontoxic and stable alternative, as has been demonstrated for the grape polyphenol resveratrol, which was shown to reduce miR-155 in colon cancer cells via modulation of miR-663 (55). In addition, pomegranate polyphenolics were also shown to target miR-155 in breast cancer.
Herein, we add to this body of increasing evidence that common dietary polyphenols may be an alternative for anti-miR-155 therapy in cancer by investigating a role for the soy isoflavone genistein in reducing miR-155 expression and thus breast cancer malignancy. Results are presented to show that in metastatic breast cancer cells, in parallel with decreased cell viability and increased apoptosis, genistein decreases miR-155 and increases its proapoptotic targets, in a statistically significant manner. Therefore, the anticancer properties of genistein in breast cancer, can at least partially, be attributed to the downregulation of miR-155.
Materials and methods
Cell stimulation
Quiescent (24 h serum-starved) MDA-MB-435, Hs578t, and MCF-7 breast cancer cells were treated with vehicle (0.1% DMSO) or 99% pure genistein at various concentrations (LKT Laboratories, Inc., St. Paul, MN) in 5% serum plus media for 12, 24, or 48 h.
The origin of the MDA-MB-435 cell line has been questioned. However, recent karyotype and comparative genomic hybridization studies have supported the idea that the current stocks of MDA-MB-435 cells and M14 melanoma cells are identical but of breast cancer origin (56). Since the MDA-MB-435 variant that was used in this study consistently produces mammary fatpad tumors and metastases and responds to gensitein treatment by reduced tumor growth and metastases (14), we have selected to use this cell line as a model system for metastatic breast cancer.
Cell viability assays
As described in Ref. 57, cell viability was determined using the CellTiter 96 Non-Radioactive Cell Proliferation Kit according to manufacturer’s instructions (Promega, Madison, WI). Briefly, quiescent MCF-7, MDA-MB-435, and Hs578t cells were treated with genistein (0–50 μM) for 48 h. Next, 150 μL/well of MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] reagent was added and the cells were incubated at 37°C for 4 h, prior to measuring absorbance at 570 nm. Results are the means ± SEM for 3 biological replicates with 4 technical replicates each.
Apoptosis assays
Apoptosis was measured by relative caspase 3/7 activity, using a Caspase-Glo3/7 Luminescence Assay Kit as per manufacturer’s instructions (Promega, Madison, WI). Following treatment with genistein (0–25 μM), 100 μL of Caspase-3/7 Glo reagent was added and incubated at room temperature for 60 min. The caspase-3/7 activities were determined by quantifying luminescence. Results are the means ± SEM for 3 biological replicates with 4 technical replicates each.
RT-qPCR
MicroRNA extraction was performed using the miR-Neasy Mini Kit (Qiagen, Valencia, CA). MiRNA expression was measured with the TaqMan miRNA RT-qPCR method (Applied Biosystems, Houston, TX), using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA). Ten ng of total RNA was used per reverse transcription reaction. Appropriate negative controls were used. Primers for RT and probes for quantitative PCR (Hsa-miR-155, U6, and U48) were from Applied Biosystems by Life Technologies and used as directed. U6 and U48 snRNA were used as internal controls, and the fold change in response to genistein was calculated by the 2ΔΔCT method. Results are the means ± SEM for 3 biological replicates with 4 technical replicates each.
Western blotting
Cell lysates were Western blotted using routine laboratory procedures, as previously described (58). Primary antibodies to FOXO3a, CK1α, PTEN, p27, β-catenin, and actin (Cell Signaling, Danvers, MA, and Sigma-Aldrich Comp., St Louis, MO) were used at a 1:1000 concentration. The integrated density of positive bands was quantified using Image J software. Results are the means ± SEM for 3 biological replicates.
Ectopic expression of miR-155
MDA-MB-435 and Hs578t cells were infected with control or miR-155 lentiviral vectors, as per manufacturer’s instructions (Biosettia, CA). Stable cell lines were selected by red fluorescence and puromycin resistance.
Statistical analysis
Data are expressed as the mean ± SEM for a minimum of 3 biological replicates. Statistical analyses were done using Microsoft Excel and GraphPad Prism®. Differences between groups were considered to be statistically significant at P≤0.05. Students t-test and 1-way analysis of variance using Turkey’s multiple comparison tests were performed for data comparisons from cells expressing miR-155 vector, with those expressing control vector for all concentrations of genistein tested.
Results
The following effects of genistein on breast cancer cells were determined at physiological 1–5 μM range (10) and pharmacological concentrations (10, 25, and 50 μM). These concentrations were selected to fall within the 1–10 μM concentrations of soy isoflavones in the circulation following consumption of soy products (18). Moreover, these concentrations have been used to demonstrate epigenetic effects and inhibition of cancer cell growth by genistein (59,60).
Genistein reduces er (−) breast cancer cell viability
We previously reported that dietary genistein reduced MDA-MB-435 mammary tumor growth (by 30%) and metastasis to lung and bone (by 100%) in a nude mouse model of experimental metastasis (14). To investigate the molecular mechanisms by which genistein reduces mammary tumor growth and metastasis, we determined the effect of genistein on the viability of MCF-7 (non-metastatic luminal A type), MDA-MB-435 (HER2 type), and Hs578t (basal-like) breast cancer cell lines. Genistein, starting at 1 μM, significantly decreased the viability of the MDA-MB-435 and Hs578t cells (P < 0.05), with a ~50–60% decrease in viability at 10–25 μM. The effect of genistein on MDA-MB-435 cell viability plateaued at 10 μM, whereas the decrease in Hs578t cell viability in response to genistein remained linear up to 50 μM (Fig. 1). This higher sensitivity of Hs578t cells to genistein may be attributed to the higher expression levels of miR-155 in this cell line compared to MDA-MB-435 (61) (Table 1). Alternatively, this difference could be due to the less metastatic but triple negative features of the Hs578t cell line compared to the highly metastatic HER2++ MDA-MB-435 cells, which may express alternate pathways of malignancy.
Figure 1.
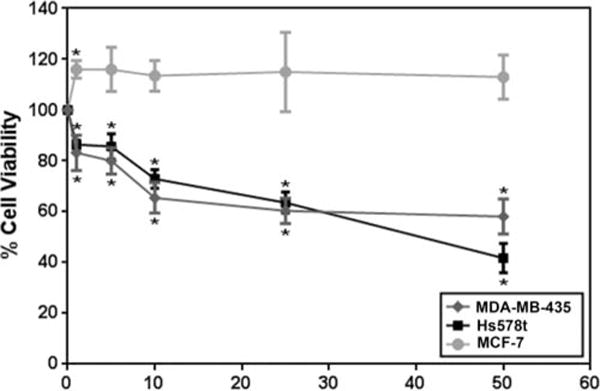
Effect of genistein on cancer cell viability. MCF-7, MDA-MB-435, or Hs578t cells were treated with genistein at 0–50 μM for 48 h and tested for cell viability using a MTT assay. Percentage (%) cell number is shown for N = 3 ± SEM. *P < 0.05.
Table 1.
miR-155 expression in human breast cancer cell lines.
| Cell line | Ct miR-155 |
|---|---|
| MDA-MB-435.Parental | 27.32 |
| MDA-MB-435.Control | 27.58 |
| MDA-MB-435.miR-155 | 24.33 |
| Hs578t. Parental | 25.47 |
| Hs578t.Control | 25.01 |
| Hs578t.miR-155 | 22.20 |
| MCF-7 | 34.75 |
RT-qPCR was conducted for miR-155 in parental MDA-MB-435, Hs578t, and MCF-7 cells, and MDA-MB-435 and Hs578t cells stably expressing control or miR-155 expression vectors. Mean cycle threshold (Ct), N = 3.
When the effect of genistein was tested on the viability of MCF-7 cell line, which expresses negligible levels of miR-155 (Table 1) (61), we found that genistein had no significant effects on the growth of this cell line (Fig. 1). Therefore the null effect of genistein on growth may also be attributed to the relatively low miR-155 expression in this cell line, which may not be dependent on miR-155 for increased growth but on alternative pathways.
Genistein downregulates mir-155 and upregulates miR-155 targets in breast cancer cells
As shown in Fig. 2, we determined the potential of the oncomir miR-155 as a regulator of the effects of genistein on breast cancer cells. MiR-155 was selected due to its novelty and importance in breast cancer, as well as the reported regulation of pro-apoptotic tumor suppressors such as FOXO3, a target of genistein (27). RT-qPCR assays for miR-155 demonstrate that, similar to the inhibitory effects on cell viability, 1–5 μM genistein significantly reduced miR-155 expression in MDA-MB-435 cells by ~1.8-fold. A similar statistically significant downregulation of miR-155 by genistein in Hs578t cells remained constant at 1, 5, and 25 μM concentrations. The 25 μM concentration of genistein failed to reduce miR-155 levels in the MDA-MB-435 cell line, which may be due to additional effects of genistein at high concentrations. In contrast to the ER (−) cells, the MCF-7 ER (+) breast cancer cell line did not demonstrate significant changes in miR-155 levels in response to genistein (Fig. 2A). Moreover, the inhibitory effect of genistein on miR-155 expression was observed at 12 h following 5 μM genistein and remained constant up to 48 h suggesting a stable response to genistein treatment (Fig. 2B).
Figure 2.
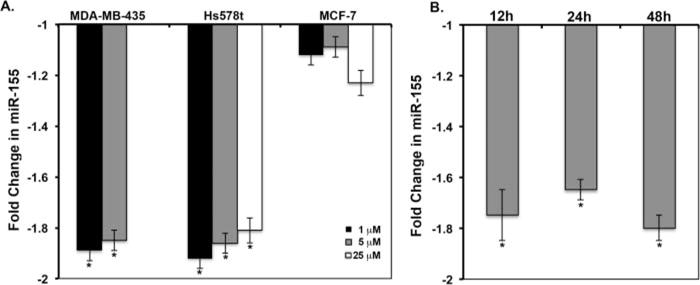
Expression of miR-155 in response to genistein. Quiescent MDA-MB-435, Hs578t, or MCF-7 cells were treated with vehicle or genistein, followed by RT-qPCR for miR-155. The comparative Ct method was used to calculate the relative abundance of miR-155 with respect to U6 RNA expression. Fold changes from vehicle are shown (N = 3 ± SD, *P < 0.05). A: Average fold change in miR-155 levels at 48 h following 0–25 μM genistein in MDA-MB-435, Hs578t, and MCF-7 cells. B: miR-155 expression in the MDA-MB-435 cell line in response to 5 μM genistein, as a function of time.
Next, we determined the effect of genistein on the expression of known miR-155 targets. Of the ~149 potential miR-155 targets, the expression of selected pro-apoptotic molecules that are also known to be regulated by genistein (27, 39) was determined by Western blot. The proapoptotic forkhead family transcription factor FOXO3 has been shown to be downregulated in breast cancer via PI3-K/Akt signaling and miR-155 regulation (61). The FOXO downstream target p27/kip cyclin dependent kinase inhibitor is also downregulated by miR-155 (49,61,62). Our results show that, in a statistically significant manner, in response to 1–5 μM genistein, FOXO3 is upregulated by ~1.3-fold in MDA-MB-435 cells and by 1.4-fold in HS578t cells. Similarly, the FOXO3 target p27 is upregulated by ~2.0-fold at 1 and 5 μM and by 1.3-fold at 25 μM genistein in MDA-MB-435 cells. Genistein effects could not be ascertained for p27 in the Hs578t cell line, which did not express sufficient levels of p27 for detection by Western blot. However, unlike the PTEN null cell line MDA-MB-435, the Hs578t cells expressed PTEN, an anticell survival tumor suppressor, which is also a miR-155 target (63). PTEN was upregulated by 1.2-fold in response to 1 μM genistein in the Hs578t cell line (Fig. 3). Consistent with the results where 25 μM genistein did not affect miR-155 levels in the MDA-MB-435 cell line, miR-155 targets did not demonstrate statistically significant changes in response to 25 μM genistein treatment. These results suggest that miR-155 independent pleiotrophic effects of gensitein, such as direct inhibition of survival signaling, may be occurring at these high genistein concentrations (64).
Figure 3.
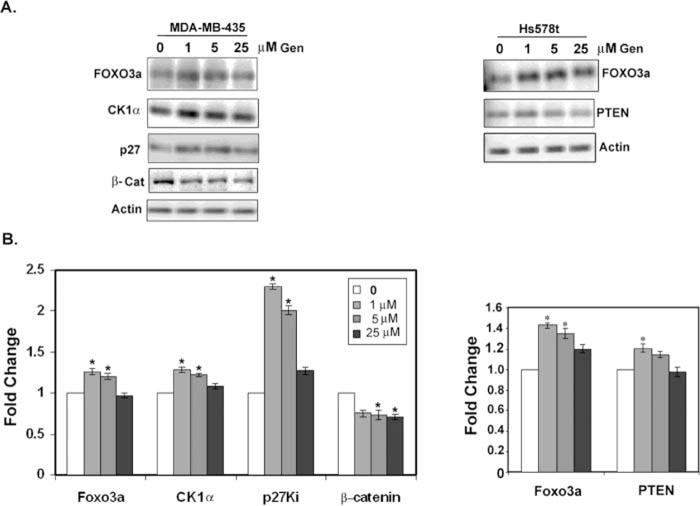
Expression of miR-155 targets in response to genistein. MDA-MB-435 or Hs578t cells were treated with vehicle or genistein for 48 h. Lysates were western blotted for the indicated proteins. A: Representative Western blots. B: Fold changes in protein expression compared to vehicle as calculated from the integrated density of positive bands normalized for actin expression. Values shown are the mean ± SEM for N = 3. *P < 0.05.
Another direct target of miR-155, casein kinase 1α (CK1α) (65) was not expressed in the Hs578t cell line but was expressed in sufficient levels for detection by Western blot in the MDA-MB-435 cell line. CK1α was upregulated ~1.3-fold in the MDA-MB-435 cells in a statistically significant manner by 1 and 5 μM genistein. Because CK1α can phosphorylate and target β-catenin for degradation (65), we also tested for β-catenin stability in response to genistein, and found a −1.4-fold reduction in β-catenin protein levels at all concentrations of genistein tested (Fig. 3).
To demonstrate a direct role for the observed effects of genistein on cell viability and expression of pro-apoptotic molecules, we created stable MDA-MB-435 and HS578t cell lines expressing miR-155 or a control miRNA via lentiviral vectors. These stable cell lines, ectopically expressing miR-155 vector, demonstrated an eightfold increase in miR-155 expression compared to the cells expressing the scrambled control in the MDA-MB-435 cell line and a sixfold increase in expression in the Hs578t cells (Supplemental Fig. S2). As expected the cells expressing a control vector responded to gensitein treatment by a −1.62-fold reduction in miR-155 levels, whereas the cells expressing the miR-155 vector were insensitive to gensitein treatment. Interestingly, the cells expressing miR-155 via a constitutive promoter were less sensitive to the effects of genistein on cell viability and insensitive to genistein-mediated induction of apoptosis. Similar to the results in Fig. 2, both MDA-MB-435 and Hs578t cells expressing the scrambled control miRNA demonstrated decreased cell viability in response to genistein (Fig. 4A). Control cells demonstrated a statistically significant ~45% decrease in cell viability at 25 μM genistein, whereas the cells ectopically expressing miR-155 demonstrated only a 20% decrease in cell viability. The effect of ectopic expression of miR-155 was more pronounced in the Hs578t cell line where genistein at 50 μM caused a ~60% decrease in cell viability in the control cells, whereas the decrease in cell viability in cells expressing miR-155 plateaued at 20%. Therefore, even though genistein exerts miR-155 independent effects on cell viability, the effects of genistein on cell viability may be exerted mainly via decreased expression of miR-155.
Figure 4.
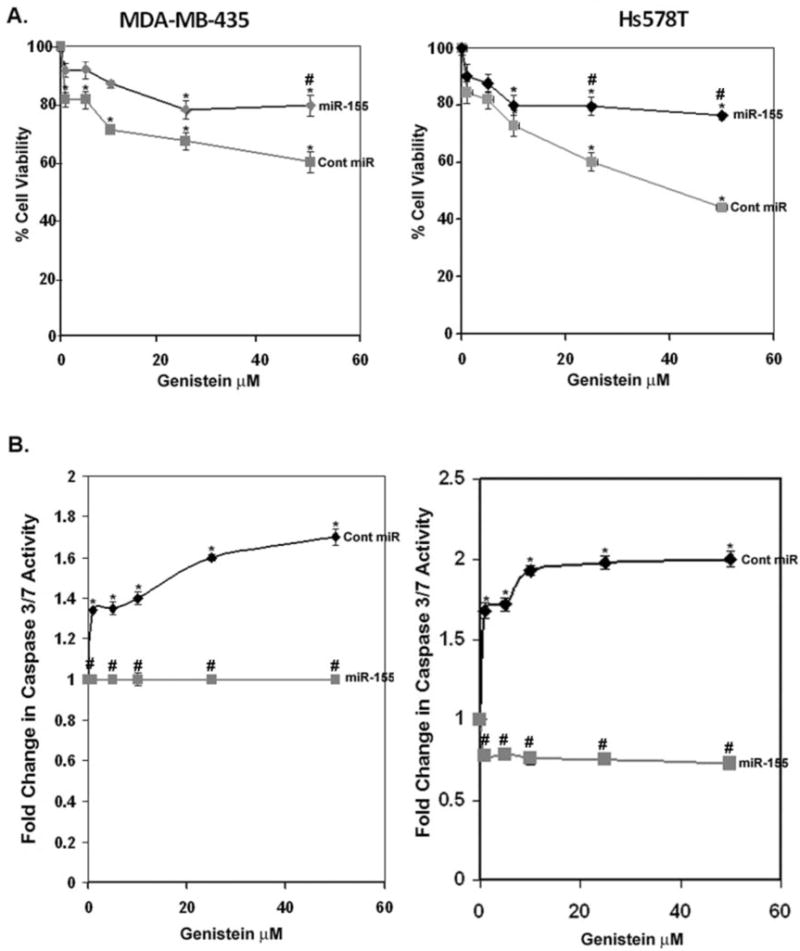
Effect of genistein on cell viability and apoptosis in breast cancer cells expressing control or miR-155 vector. MDA-MB-435 or Hs578t cells stably expressing control or miR-155 vector were treated with vehicle or genistein for 48 h. Left, MDA-MB-435; right, Hs578t. A: Cell viability as a percentage of vehicle (100%). B: Apoptosis. Fold changes in caspase 3/7 activity from vehicle are shown (N = 3 ± SEM, *P < 0.05). Genistein treatments compared to vehicle. #P < 0.05, cells ectopically expressing miR-155 compared with cells expressing control vector.
The role of genistein-mediated downregulation of miR-155 appears to be more relevant for apoptosis induction by genistein. In Fig. 4B, 1 and 5 μM genistein significantly induced apoptosis in the MDA-MB-435 cell line expressing control miRNA by ~1.4-fold with increased effects (1.6- and 1.8-fold increases) at 25 and 50 μM. However, cells ectopically expressing miR-155 were unresponsive to apoptosis induction by genistein at all concentrations tested. Therefore, this result indicates that genistein may regulate cell viability and apoptosis of ER (−) breast cancer cells via transcriptional regulation of miR-155.
Figure 5.
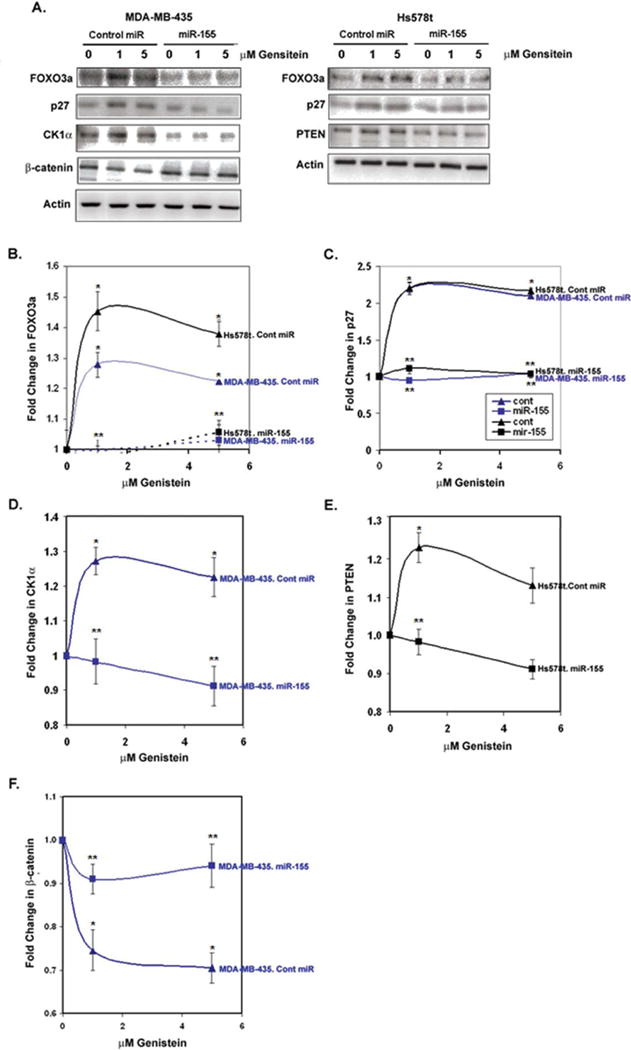
Expression of miR-155 targets in response to genistein in breast cancer cells expressing control or miR-155 vector. MDA-MB-435 or Hs578t cells expressing control or miR-155 vector were treated with vehicle or genistein (1 or 5μM) for 48 h. Lysates were western blotted for miR-155 targets. A: Representative western blots are shown for MDA-MB-435 (left) or Hs578t (right) cell lysates. B–F: The fold changes in protein expression from N = 3 ± SEM are indicated for (B) FOXO3a, (C) p27, (D) casein kinase 1α, (E) PTEN, or (F) β-catenin. *P < 0.05, genistein treatments compared to vehicle; #P < 0.05, cells ectopically expressing miR-155 compared with cells expressing control vector.
To determine whether the observed changes in miR-155 targets in response to genistein (Fig. 3) were dependent on downregulation of miR-155 by genistein, the expression of these targets were monitored from lysates of MDA-MB-435 and Hs578t cells expressing control miRNA or miR-155. As shown in Fig. 5A–5C, FOXO3a and its target p27 were both significantly upregulated (P < 0.05) in response to physiological genistein concentrations in both MDA-MB-435 and Hs578t cells expressing control miRNA but not in miR-155 expressing cells. Similarly, CK1α, which was only upregulated in the MDA-MB-435 cells in response to genistein (Fig. 3), was significantly upregulated by ~1.3-fold by genistein in the control miRNA cells but not in the miR-155 expressing cells. In parallel, the CK1α target β-catenin, which is expected to be phosphorylated and degraded by the excess CK1α, was decreased by 30% in genistein-treated MDA-MB-435 cells, while the β-catenin levels remained constant in the cells expressing miR-155. In the PTEN positive Hs578t cells, PTEN levels were increased in control miRNA expressing cells in response to genistein but not in the miR-155 expressing cells, in a statistically significant manner. As expected, expression of miR-155 driven by a viral promoter demonstrated reduced levels of the miR-155 targets in both MDA-MB-435 and Hs578t cell lines. These low levels of expression may indicate that both endogenous and ectopically expressed miR-155 levels are not sufficient to saturate all of the transcripts of the target genes examined. Taken together, these results indicate that the observed upregulation of the proapoptotic proteins FOXO3a, p27, PTEN, and CK1α, a negative regulator of β-catenin, in response to gensitein, may at least partially be under miR-155 control.
Discussion
The anticancer effects of genistein on tumor growth and metastasis have been attributed to cancer cell cycle arrest and apoptosis via upregulation of a number of pro-apoptotic and cell cycle checkpoint proteins (16,22,64,66). Confirming the published data for MDA-MB-435 cells that show reduced cell viability and induction of apoptosis by genistein (67), we found that genistein inhibits cell growth and induces apoptosis in HER2-type MDA-MB-435, as well as the basal-type Hs578t cancer cells but not in the luminal A MCF-7 cells. Even though gensitein has been shown to decrease viability and induce apoptosis in MCF-7 cells by some reports, most of these studies were conducted at pharmacological concentrations of >50 μM, which are too high to be achieved via dietary consumption of gensitein (68–70). Moreover, others have shown that the anticancer effect of gensitein is dependent on the ERα/ERβ ratio, where gensitein is estrogenic at high ERα concentrations, as may be the case with the MCF-7 cell line (71,72). Genistein has also been shown to inhibit the growth of cancer cells using a 3-D gel culture system, which is more physiologically relevant than the 2-D culture approach of the present study (73,74).
To identify novel mechanism for the anticancer effects of genistein, we investigated the role of miR-155, a well-established oncomiR in breast cancer. Our results reveal a functional role for genistein as a potential antibreast cancer agent via downregulation of miR-155, one of the most significantly altered miRNAs in breast cancer (46–49,75). The regulation of a single miRNA, such as miR-155, is predicted to exert a considerable impact on cancer progression by altering the plethora of cancer regulatory molecules under miR-155 control. Accordingly, we found genistein to upregulate a number of miR-155 targets with proapoptotic and tumor suppressor functions. Consumption of foods rich in soy products has been shown to yield ~1–10 μM concentrations of genistein in the circulation (8–10). Therefore, the observed consistent response of both metastatic (ER/PR negative) breast cancer cell lines, but not the non-metastatic ER/PR (+) MCF-7 cells to genistein by decreased miR-155 expression and increased upregulation of miR-155 targets may demonstrate an ER-independent response at physiologically relevant concentrations of gensitein.
Because genistein has been shown to regulate cell growth via a plethora of effectors (6,76), downregulation of miR-155 is not expected to be the only mechanism by which genistein regulates cancer cell and tumor growth. However, our results, where stable ectopic expression of miR155 in MDA-MB-435 and Hs578t cells reduced the effects of genistein on cell viability and abrogated the effects of genistein on apoptosis and expression of proapoptotic proteins, implicates miR-155 as a central regulator of the anticancer effects of genistein.
Our data show that genistein-mediated downregulation of miR-155 resulted in increased FOXO3a levels; thus, providing a potential mechanism for the reported upregulation of FOXO3 in response to genistein (27). We also report that genistein downregulates β-catenin, an established cancer promoting transcriptional enhancer of the Wnt signaling pathway, in a miR-155 dependent manner. Direct miR-155 knockdown has been shown to reduce cancer cell and tumor growth and induce cell-cycle arrest via upregulation of CK1α, which consequently degraded β-catenin and reduced expression of the β-catenin target, cyclin D1 (65). Therefore, our data that demonstrate enhanced CK1α in response to genistein may, at least partially, be responsible for the reported anticancer inhibitory effects of genistein on Wnt/β-catenin signaling (77).
In conclusion, we report a novel anticancer mechanism for genistein: downregulation of miR-155, a potent oncogene in breast cancer. This study suggests that decreased miR-155 in response to genistein may in parallel upregulate a large number of anticancer molecules, thus reducing breast cancer cell survival and proliferation, and inducing apoptosis. This mechanism is presumably the molecular basis for our previously reported reduced tumor growth and metastasis in response to dietary genistein, using the same MDA-MB-435 model (14). Therefore, genistein holds promise as a natural nontoxic miR-155 targeted anticancer therapeutic. Moreover, this study augments the body of knowledge on the role of dietary soy isoflavones in established breast cancer and has the potential to impact dietary and therapeutic decisions for breast cancer patients, survivors, and those at risk for breast cancer.
Supplementary Material
Acknowledgments
Funding
This work was sponsored by the United States Army/Breast Cancer Research Program W81XWH-11-1-0199 to CDP; National Institutes of Health (NIH)/National Institute of General Medical Sciences SC3GM084824 to SD; NIH/National Institute on Minority Health and Health Disparities (NIMHD) G12RR035051 to the University of Puerto Rico Medical Sciences Campus; and NIH/NIMHD G12RR003035, NIH/NIMHHD Research Centers in Minority Institutions (RCMI) 8G12MD007583, Title V PPOHA P031S130068 from United States Department of Education, and Title V Promoting Postbaccalaureate Opportunities for Hispanic Americans Program United States Department of Education P031M105050 to Universidad Central del Caribe.
References
- 1.Lampe JW, Nishino Y, Ray RM, Wu C, Li W, et al. Plasma isoflavones and fibrocystic breast conditions and breast cancer among women in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2007;16:2579–2586. doi: 10.1158/1055-9965.EPI-07-0368. [DOI] [PubMed] [Google Scholar]
- 2.Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le ML, et al. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev Res (Phila) 2009;2:887–894. doi: 10.1158/1940-6207.CAPR-09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constantinou AI, Lantvit D, Hawthorne M, Xu X, van Breemen RB, et al. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer. 2001;41:75–81. doi: 10.1080/01635581.2001.9680615. [DOI] [PubMed] [Google Scholar]
- 4.Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140:2326S–2334S. doi: 10.3945/jn.110.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, et al. Soy Isoflavone supplementation for breast cancer risk reduction: a randomized Phase II trial. Cancer Prev Res (Phila) 2012;5:309–319. doi: 10.1158/1940-6207.CAPR-11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67:398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 7.Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol. 2009;304:30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal wome. J Sci Food Agric. 2011;91:658–663. doi: 10.1002/jsfa.4228. [DOI] [PubMed] [Google Scholar]
- 9.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 10.Chau MN, El Touny LH, Jagadeesh S, Banerjee PP. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis. 2007;28:2282–2290. doi: 10.1093/carcin/bgm148. [DOI] [PubMed] [Google Scholar]
- 11.Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr. 2010;140:1350S–1354S. doi: 10.3945/jn.109.118315. [DOI] [PubMed] [Google Scholar]
- 12.van Duursen MB, Nijmeijer SM, de Morree ES, de Jong PC, van den BM. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology. 2011;289:67–73. doi: 10.1016/j.tox.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Du M, Yang X, Hartman JA, Cooke PS, Doerge DR, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis. 2012;33:895–901. doi: 10.1093/carcin/bgs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De LM-P, Cubano LA, et al. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis. 2010;27:465–480. doi: 10.1007/s10585-010-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitsett TG, Jr, Lamartinier CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev Anticancer Ther. 2006;6:1699–1706. doi: 10.1586/14737140.6.12.1699. [DOI] [PubMed] [Google Scholar]
- 16.Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29:465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farina HG, Pomies M, Alonso DF, Gomez DE. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep. 2006;16:885–891. [PubMed] [Google Scholar]
- 18.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27:31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]
- 19.Messina M, Watanabe S, Setchell KD. Report on the 8th International Symposium on the Role of Soy in Health Promotion and Chronic Disease Prevention and Treatment. J Nutr. 2009;139:796S–802S. doi: 10.3945/jn.108.104182. [DOI] [PubMed] [Google Scholar]
- 20.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–560. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 21.Rahal OM, Simmen RC. PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis. 2010;31:1491–1500. doi: 10.1093/carcin/bgq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, et al. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013;43:289–296. doi: 10.3892/ijo.2013.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Privat M, Aubel C, Arnould S, Communal Y, Ferrara M, et al. AKT and p21 WAF1/CIP1 as potential genistein targets in BRCA1-mutant human breast cancer cell lines. Anticancer Res. 2010;30:2049–2054. [PubMed] [Google Scholar]
- 24.Pan H, Zhou W, He W, Liu X, Ding Q, et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-kappaB activity via the Notch-1 pathway. Int J Mol Med. 2012;30:337–343. doi: 10.3892/ijmm.2012.990. [DOI] [PubMed] [Google Scholar]
- 25.Rajah TT, Peine KJ, Du N, Serret CA, Drews NR. Physiological concentrations of genistein and 17beta-estradiol inhibit MDA-MB-231 breast cancer cell growth by increasing BAX/BCL-2 and reducing pERK1/2. Anticancer Res. 2012;32:1181–1191. [PubMed] [Google Scholar]
- 26.Li Y, Chen H, Hardy TM, Tollefsbol TO. Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PLoS One. 2013;8:e54369. doi: 10.1371/journal.pone.0054369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Chen H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp Biol Med (Maywood) 2011;236:714–722. doi: 10.1258/ebm.2011.010347. [DOI] [PubMed] [Google Scholar]
- 29.Majid S, Dar AA, Ahmad AE, Hirata H, Kawakami K, et al. BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis. 2009;30:662–670. doi: 10.1093/carcin/bgp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, et al. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer Res. 2008;68:2736–2744. doi: 10.1158/0008-5472.CAN-07-2290. [DOI] [PubMed] [Google Scholar]
- 31.Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs—the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 32.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 33.Parker LP, Taylor DD, Kesterson J, Metzinger DS, Gercel-Taylor C. Modulation of microRNA associated with ovarian cancer cells by genistein. Eur J Gynaecol Oncol. 2009;30:616–621. [PubMed] [Google Scholar]
- 34.Rabiau N, Trraf HK, Adjakly M, Bosviel R, Guy L, et al. miRNAs differentially expressed in prostate cancer cell lines after soy treatment. In Vivo. 2011;25:917–921. [PubMed] [Google Scholar]
- 35.Chen Y, Zaman MS, Deng G, Majid S, Saini S, et al. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev Res (Phila) 2011;4:76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiyomaru T, Yamamura S, Zaman MS, Majid S, Deng G, et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS One. 2012;7:e43812. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, Xiang J, Shen J, Zou X, Zhai S, et al. Oncogenic microRNA-27a is a target for genistein in ovarian cancer cells. Anticancer Agents Med Chem. 2013;13:1126–1132. doi: 10.2174/18715206113139990006. [DOI] [PubMed] [Google Scholar]
- 38.Hirata H, Ueno K, Nakajima K, Tabatabai ZL, Hinoda Y, et al. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br J Cancer. 2013;108:2070–2078. doi: 10.1038/bjc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Q, Cong R, Yan H, Gu H, Zeng Y, et al. Genistein inhibits growth of human uveal melanoma cells and affects microRNA-27a and target gene expression. Oncol Rep. 2009;22:563–567. doi: 10.3892/or_00000472. [DOI] [PubMed] [Google Scholar]
- 40.Xia J, Duan Q, Ahmad A, Bao B, Banerjee S, et al. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr Drug Targets. 2012;13:1750–1756. doi: 10.2174/138945012804545597. [DOI] [PubMed] [Google Scholar]
- 41.Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, et al. Genistein inhibits prostate cancer cell growth by targeting mir-34a and oncogenic HOTAIR. PLoS One. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirata H, Hinoda Y, Shahryari V, Deng G, Tanaka Y, et al. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br J Cancer. 2014;110:1645–1654. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, Majid S, et al. Genistein up-regulates tumor suppressor micro-RNA-574-3p in prostate cancer. PLoS One. 2013;8:e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Cheng L, Liu H, Zhang J, Shi Y, et al. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr Drug Targets. 2013;14:1150–1156. doi: 10.2174/13894501113149990187. [DOI] [PubMed] [Google Scholar]
- 45.Xia J, Cheng L, Mei C, Ma J, Shi Y, et al. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr PharmDes. 2014;20:5348–5353. doi: 10.2174/1381612820666140128215756. [DOI] [PubMed] [Google Scholar]
- 46.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Zhao J, Deng H. 17beta-Estradiol up-regulates miR-155 expression and reduces TP53INP1 expression in MCF-7 breast cancer cells. Mol Cell Biochem. 2013;379:201–211. doi: 10.1007/s11010-013-1642-6. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Wang M, Lin G, Sun S, Li X, et al. Serum micro-RNA-155 as a potential biomarker to track disease in breast cancer. PLoS One. 2012;7:e47003. doi: 10.1371/journal.pone.0047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 50.Kong W, He L, Richards EJ, Challa S, Xu CX, et al. Upregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancer. Oncogene. 2013;33:679–689. doi: 10.1038/onc.2012.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasparini P, Cascione L, Fassan M, Lovat F, Guler G, et al. microRNA expression profiling identifies a four micro-RNA signature as a novel diagnostic and prognostic biomarker in triple negative breast cancers. Oncotarget. 2014;15:1174–1184. doi: 10.18632/oncotarget.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higgs G, Slack F. The multiple roles of microRNA-155 in oncogenesis. J Clin Bioinforma. 2013;3:17. doi: 10.1186/2043-9113-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng N, Puetter A, Zhang K, Johnson K, Zhao Z, et al. Isoform-level microRNA-155 target prediction using RNA-seq. Nucleic Acids Res. 2011;39:e61. doi: 10.1093/nar/gkr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lossner C, Meier J, Warnken U, Rogers MA, Lichter P, et al. Quantitative proteomics identify novel miR-155 target proteins. PLoS One. 2011;6:e22146. doi: 10.1371/journal.pone.0022146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tili E, Michaille JJ, Alder H, Volinia S, Delmas D, et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem Pharmacol. 2010;80:2057–2065. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 57.Castillo-Pichardo L, Martinez-Montemayor MM, Martinez JE, Wall KM, Cubano LA, et al. Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin Exp Metastasis. 2009;26:505–516. doi: 10.1007/s10585-009-9250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de la PC, Otero-Franqui E, Martinez-Montemayor M, Dharmawardhane S. The soy isoflavone equol may increase cancer malignancy via upregulation of eukaryotic protein synthesis initiation factor eIF4G. J Biol Chem. 2012;287:41640–41650. doi: 10.1074/jbc.M112.393470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, et al. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 60.Mai Z, Blackburn GL, Zhou JR. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol Carcinog. 2007;46:534–542. doi: 10.1002/mc.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong W, He L, Coppola M, Guo J, Esposito NN, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869–17879. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Lu C, Huang X, Zhang X, Roensch K, Cao Q, et al. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–4303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo YM, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114:3265–3275. doi: 10.1182/blood-2009-06-222794. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang P, Bill K, Liu J, Young E, Peng T, et al. MiR-155 is a liposarcoma oncogene that targets casein kinase-1alpha and enhances beta-catenin signaling. Cancer Res. 2012;72:1751–1762. doi: 10.1158/0008-5472.CAN-11-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan N, Adhami VM, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010;17:R39–R52. doi: 10.1677/ERC-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Bhuiyan M, Sarkar FH. Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein. Int J Oncol. 1999;15:525–533. doi: 10.3892/ijo.15.3.525. [DOI] [PubMed] [Google Scholar]
- 68.Choi EJ, Jung JY, Kim GH. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERalpha expression and induction of apoptosis. Exp Ther Med. 2014;8:454–458. doi: 10.3892/etm.2014.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prietsch RF, Monte LG, da Silva FA, Beira FT, Del Pino FA, et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol Cell Biochem. 2014;390:235–242. doi: 10.1007/s11010-014-1974-x. [DOI] [PubMed] [Google Scholar]
- 70.Tsuboy MS, Marcarini JC, de Souza AO, de Paula NA, Dorta DJ, et al. Genistein at maximal physiologic serum levels induces G0/G1 arrest in MCF-7 and HB4a cells, but not apoptosis. J Med Food. 2014;17:218–225. doi: 10.1089/jmf.2013.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pons DG, Nadal-Serrano M, Del MB-R, Sastre-Serra J, Oliver J, et al. Genistein modulates proliferation and mitochondrial functionality in breast cancer cells depending on ERalpha/ERbeta ratio. J Cell Biochem. 2014;115:949–958. doi: 10.1002/jcb.24737. [DOI] [PubMed] [Google Scholar]
- 72.Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, et al. Mechanisms enforcing the estrogen receptor beta selectivity of botanical estrogens. FASEB J. 2013;27:4406–4418. doi: 10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tome Y, Uehara F, Mii S, Yano S, Zhang L, et al. 3-dimensional tissue is formed from cancer cells in vitro on Gelfoam(R), but not on Matrigel. J Cell Biochem. 2014;115:1362–1367. doi: 10.1002/jcb.24780. [DOI] [PubMed] [Google Scholar]
- 74.Geller J, Sionit L, Partido C, Li L, Tan X, et al. Genistein inhibits the growth of human–patient BPH and prostate cancer in histoculture. Prostate. 1998;34:75–79. doi: 10.1002/(sici)1097-0045(19980201)34:2<75::aid-pros1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 75.Zheng SR, Guo GL, Zhang W. Clinical significance of miR-155 expression in breast cancer and effects of miR-155 ASO on cell viability and apoptosis. Oncol Rep. 2012;27:1149–1155. doi: 10.3892/or.2012.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagaraju GP, Zafar SF, El-Rayes BF. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr Rev. 2013;71:562–572. doi: 10.1111/nure.12044. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Li Q, Zhou D, Chen H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/beta-catenin signalling and reduces colon pre-neoplasia in rats. Br J Nutr. 2013;109:33–42. doi: 10.1017/S0007114512000876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


