Abstract
The Communication describes a textile-based wearable multi-ion potentiometric sensor array. The printed flexible sensors operate favorably under extreme mechanical strains (that reflect daily activity) while offering attractive real-time non-invasive monitoring of electrolytes such as sodium and potassium.
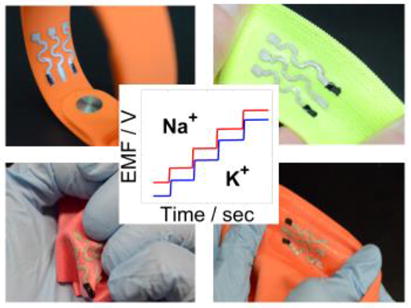
Keywords: screen printing, wearable textile, stretchable potentiometric sensor, electrolytes
Recent trends in personalized healthcare have led to growing demands for real-time monitoring of the physiological status of human subjects. Detecting disorders in electrolyte levels, such as hyperkalemia, hypernatremia, hypokalemia or hyponatremia is very important and challenging.[1,2] Electrolyte imbalance represents a potential risk of fatal abnormal heart rhythms.[3,4] Electrolyte loss is a major concern in disorders such as Cystic Fibrosis[5] and hyperhidrosis.[6] Monitoring key electrolytes may also alert to latent cardiac problems[7] and diagnose apparent life-threatening events.[8,9] Overall, real-time measurements of electrolyte concentrations could indicate the patient, doctor, coach or athlete to potential electrolyte loss,[10] dehydration status[11] and the associated need for electrolytes replenishment.[12] Cumbersome methods to measure the sodium and potassium imbalance, such as electrocardiogram and sweat patch, have been reported.[13,14] Recent activity have led to wearable electrochemical (potentiometric) devices for non-invasive electrolyte monitoring.[15,16] However, the success of wearable sensing devices for health monitoring requires proper attention to key challenges concerning their mechanical resilience and large-scale manufacturing. Recent efforts to address these issues have relied on stretchable printable electrochemical devices.[17]
This article reports for the first time on a highly stretchable and printable textile-based potentiometric sensor array for simultaneous multi-ion sweat analysis using variety of fabric materials towards diverse healthcare and fitness applications. Screen-printing has been applied recently for fabricating amperometric sensors[18] and biofuel cells[19] on common textiles, but not in connection to potentiometric sensors or stretchable textile devices.[20] Textiles are attractive components of wearable sensing devices and offer rich elastomeric properties towards achieving conformal contact between the sensor and the body. Integrating chemical sensors directly into fabrics offer major advantages for future healthcare monitoring systems. Yet, a key issue involving textile-based sensing devices is the ability to operate under extreme mechanical tensions (that reflect daily activity) without compromising their analytical performance. In this work, we realized highly stretchable textile-based potentiometric sensors by combining polyurethane (PU)-based ion-selective membranes and inks with a serpentine sensor pattern and recently developed stretch-enduring printed electrodes.[21] The compositions of the selective potentiometric membrane and of the printed inks have thus been tailored for ensuring selectivity, electrical conductivity, reproducible printing, and strong adherence to conventional textiles. To provide the necessary biocompatibility and further resistance to mechanical stress we relied on polyurethane as replacement to the common PVC matrix of ion-selective sensing membranes as well as the binder of the printed CNT trace. Polyurethanes have attractive mechanical and biocompatibility properties that make them suitable for many wearable devices. These materials are known to minimize unwanted inflammation, fouling and other adverse physiological effects,[22] while providing exceptional analytical performance using potentiometric technique.[23] As illustrated in Figures 1A and 1B, such PU-based membrane 2 and CNT ink have been coupled to an Ecoflex-containing Ag/AgCl ink and combined with a solid-contact reference electrode, leading to highly stretchable textile-based potentiometric sensors that transduce their potential response under extreme mechanical stress. As shown in Video S1 (Supporting Information) the resulting textile-based potentiometric sensors sustain extreme conditions, including large stretching and bending deformations. Such resiliency is crucial for realizing high-performance wearable potentiometric sensing devices.
Figure 1.
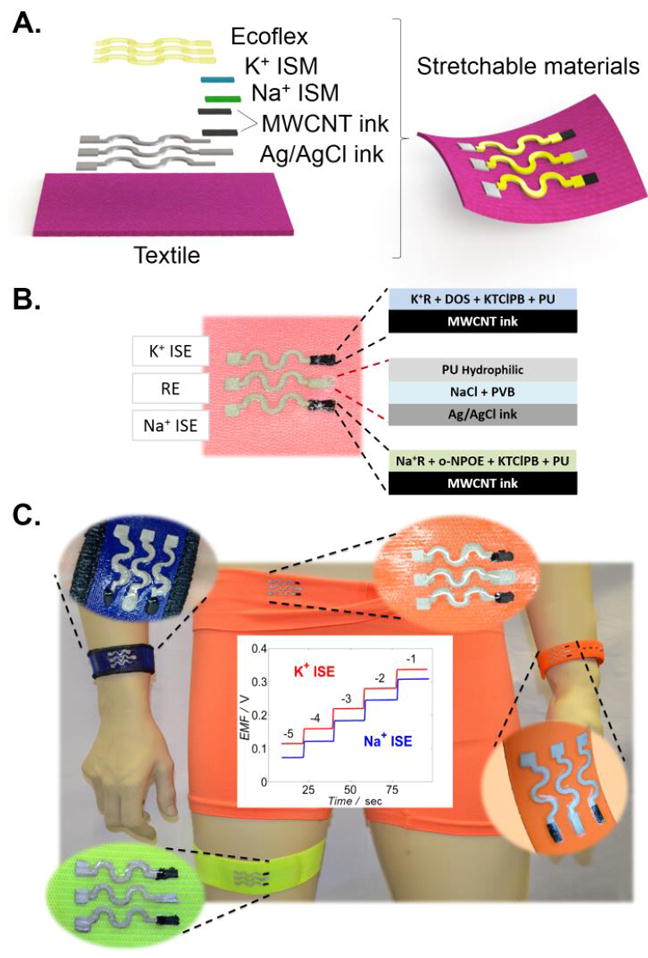
(A) Schematic representation of the tailor-made stretchable materials and manufacturing process. (B) Image depicting the wearable sensor based on textile and ion-selective membranes (ISM) composition. (C) Image of the stretchable printed sensors on different common textiles and typical time trace plots for potassium and sodium.
The resilience and analytical performance of the novel dual-electrolyte textile-based printable wearable potentiometric sensor was examined using open-circuit potential measurements (Figure 1B). A calibration curve was executed by recording the electromotive force (EMF) versus the time and changing the concentration of NaCl and KCl. Sensor array in Figure 1C exhibited a Nernstian response of 59.4 mV/log [Na+], linear range from 10-4 m up to 10-1 m and limit of detection (LOD) of 10-4.9 m for its sodium selective electrode (Na+ISE) and a Nernstian response of 56.5 mV/log [K+] from 10-4 m up to 10-1 m and a LOD of 10-4.9m for the potassium selective electrode (K+ISE). Both results are comparable to the values reported previously using similar ion-selective membranes.[24] These dynamic ranges cover the physiological sodium and potassium levels in sweat before, during and after prolonged exercise.[25] Electrolyte concentrations progressively increase during such exercise activity to provide a useful indicator of the dehydration status.[26] Moreover, understanding the amount of ionic species loss during exercise could facilitate recovery of the ionic balance during and after exercise.[27]
Stretchability tests were performed initially employing different strain conditions (Figure 2A). The textile was anchored and moved away at a speed of 1 mm·s-1. This process was repeated 10 times at 0%, 25%, 50%, 75% and 100% strains. Subsequently, the stretchability was tested by using a fixed 75% linear strain during 60 minutes, emulating a prolonged strain force during exercise. A calibration curve of each electrode was recorded every 15 minutes (Figure 2B). Both tests indicated a robust analytical performance under the different strains. Larger (<100%) strains of the textile led to some damage in the reference membrane and hence to an inferior performance. Bending represents another common mechanical stress expected during daily wear. Bending of the textile sensor was tested by twisting it back and forth for 0°, 45°, 90° and 180° 10 times in each angle. The subsequently recorded calibration curve indicates no apparent change in the sensitivity (Figure 2C). A crumpling test was also performed, consisting of wrinkling the textile with the hand for multiple times (Figure 2D). A calibration curve was recorded after 0, 5, 15 and 30 such wrinklings. No apparent variation in the performance was observed under these repeated textile manipulations. Finally, as shown in in Figure 2E and Supporting Video S2, a simulation of a conventional washing procedure was performed. This test consisted on immersing the sensor in a beaker full of water under a vigorous agitation for periods of 10 and 40 minutes. A calibration plot of each electrode was recorded after complete drying of the sensor, leading to favourable analytical results. Such attractive behaviour indicates strong surface adherence of the organic polymeric membranes with no apparent leaching of the membrane components. In addition, placing the hydrophilic polyurethane membrane on top of the reference membrane prevents leaching of NaCl from the porous PVB membrane and maintains the baseline potential even after a prolonged mechanical agitation. Table 1 summarizes the analytical performance of the sensor under different mechanical deformations shown in Figure 2.
Figure 2.
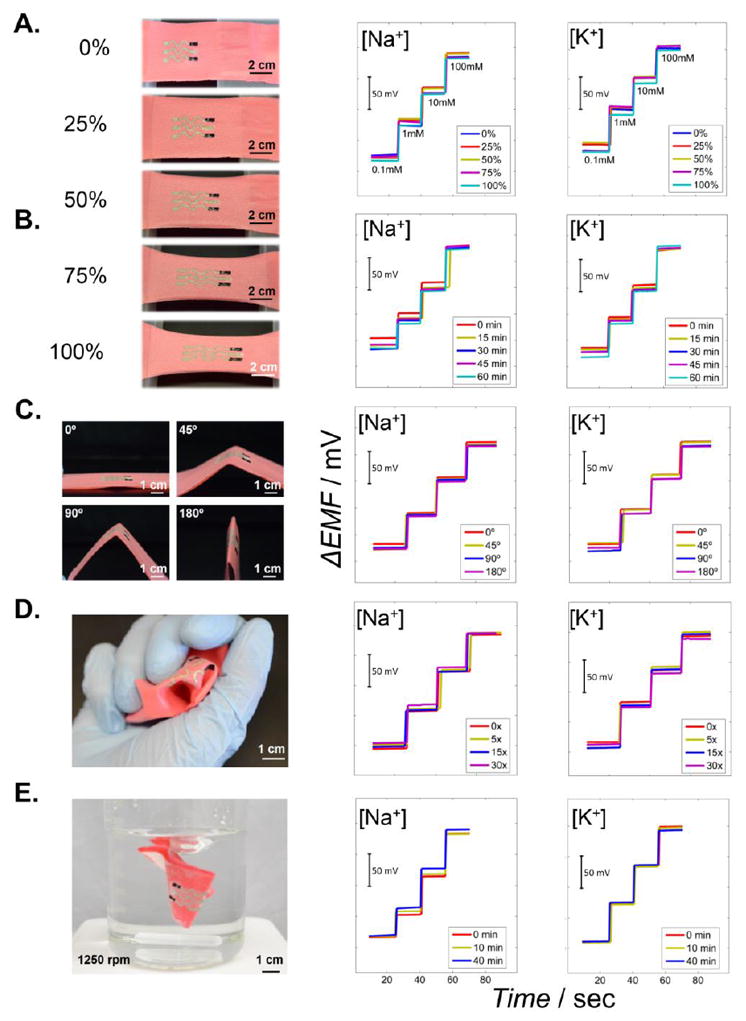
Images illustrating resilience studies involving exposing the printable textile potentiometric sensor to increasing levels of strain (left) along with the corresponding time trace calibration plots (right). (A) Linear stretchability test up to a total of 100% strain, 10 repetitions in each strain step. (B) Stretchability test, using a 75% linear strain for a total of 60 min (time trace recorded after every 15 min). (C) Bending assessment, up to a total of 180°, 10 repetitions in each angle. (D) Crumpling evaluation, up to 30 times wrinklings. (E) Washing step simulations (without soap) using short and long (10 and 40 minutes) periods.
Table 1.
Average sensitivity values and intercept values obtained during several mechanical deformations of the textile ISE system.
| Na+ selective electrode | ||||
|---|---|---|---|---|
| Test | Sensitivity [mV/log [Na+]] | %RSD | Intercept [mV] | %RSD |
| Stretching | 54.1±1.5 | 2,7 | 239.5±6.3 | 2,6 |
| Hold stretched | 61.3±1.9 | 3,1 | 179.8±2.3 | 1,3 |
| Bending | 64.4±0.7 | 1,0 | 282.6±3.5 | 1,2 |
| Crumpling | 62.6±0.9 | 1,5 | 250.3±1 | 0,4 |
| Washing | 56.2±0.9 | 1,7 | 186±6.4 | 3,5 |
|
| ||||
| K+ selective electrode | ||||
| Test | Sensitivity [mV/log [K+]] | %RSD | Intercept [mV] | %RSD |
|
| ||||
| Stretching | 56.9±1.6 | 2,9 | 313.3±4.2 | 1,3 |
| Hold stretched | 64.9±2.8 | 4,3 | 235.4±4.1 | 1,7 |
| Bending | 64.1±1.1 | 1,7 | 335±4.4 | 1,3 |
| Crumpling | 60.6±2.6 | 4,2 | 307.5±8 | 2,6 |
| Washing | 61.8±1.1 | 1,8 | 258.3±2.8 | 1,1 |
Overall, the wearable textile potentiometric array displays an attractive analytical performance before and after exposures to severe mechanical stress. Such resilience can be explained by considering the behavior of the printed materials at the microscopic level. When an external force is applied to the stretchable sensor, the stress is absorbed mostly by the stretchable Ecoflex component in the Ag/AgCl ink and by the PU as a stretchable binder in the CNT ink. When the printed Ag/AgCl trace undergo high strain, its Ecoflex polymeric component stretches while maintaining the physical contact between randomly oriented multilayers of its conductive particles. Similarly, when the printed CNT trace is subject to extreme stress, its PU binder stretches while maintaining the electrical contact of the randomly distributed CNT. This ability to operate under high strain conditions meets the demands of using these printed textile potentiometric sensors in real-life scenarios involving extreme and repeated movements of the wearer.
Repeatability represents another important feature of wearable biomedical sensors. It is crucial to obtain highly reproducible results for prolonged monitoring of fluctuating ions concentrations. Hence, the repeatability was evaluated through a carry-over test before, during and after the exposure to middle-high strain (50% stretched) (Figure 3A). The results from 10-4 m to 10-1 m showed a slope of 61.2±0.7 mV/log [Na+] and an intercept of 243.7±4 mV before mechanical stress, a slope of 59.2±1.4 mV/log [Na+] and an intercept of 247.6±4.4 mV during 50% strain and a slope of 61.5±0.9 mV/log [Na+] and an intercept of 247.1±2 mV after the resilience test. These carry-over tests demonstrate the ability to follow rapidly fluctuating ions levels. Finally, a carry-over test was performed in artificial sweat over the narrow physiological range of the target electrolytes (Figure 3B), using 10 - 110 mm NaCl (with 5 mm KCl) and the potassium sensor from 1 - 8 mm KCl (with 80 mm NaCl). The response of the array yielded a slope of 64.3±1.2 mV/log [Na+] with an intercept of 343.3±1.5 mV from 10-2 m to 10-0.96 m and a slope of 57.4±1.6 mV/log [K+] with an intercept of 383.4±3.8 mV from 10-3 m to 10-2.09 m. These data showed favorable reproducibility, stability and repeatability while varying the sodium and potassium concentrations within the physiological range, demonstrating the promise of the new potentiometric textile sensors in different real-life scenarios.
Figure 3.
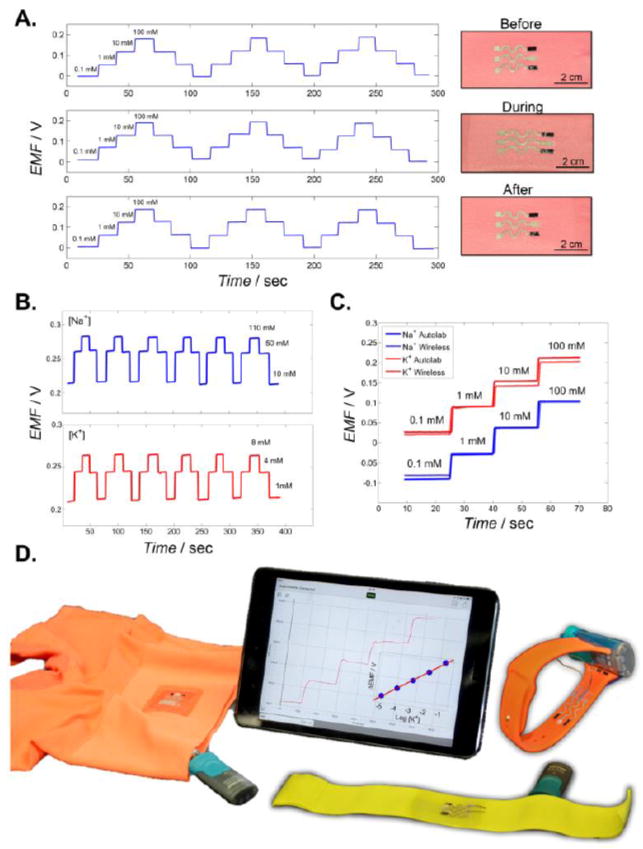
(A) Carry-over test of a textile-based sensor before, during (50% strain) and after such mechanical deformation. (B) Carry-over testing using artificial sweat test within the physiological range. (C) Correlation of calibration experiments carried out using a compact wireless high-input voltmeter and a conventional potentiostat. (D) Images depicting the versatility of the printable and stretchable sensor array on different common wearable substrates. The tablet displays a real-time trace of increasing potassium levels obtained wirelessly by the underwear printed sensor.
Following the detailed characterization of the textile sensors, it was necessary to meet further the practical demands of their real-life wearable operation. Particular important is the integration of the textile sensors with wearable supporting electronics and wireless transmission devices. Hence, we addressed the miniaturization of the reader device and a real-time wireless data transmission to another portable device (e.g., cell phone or a tablet). Complete integration and miniaturization of the textile sensor system was accomplished using a wireless high-input impedance voltmeter, with the potentiometric data being recorded with an iOS® application on an Ipad® tablet. Figure 3C compares calibration curves using the textile sensor array obtained with the compact wireless high-input voltmeter and with a benchtop Autolab potentiostat. The excellent agreement between the plots obtained using the two instruments support the integration of the wireless device with the textile-based potentiometric sensor. The response of the array from 10-4 m to 10-1 m yielded a slope of 63.1±2.7 mV/log [Na+] with an intercept of 164.8±5.3 mV and a slope of 61.1±1.8 mV/log [K+] with an intercept of 270.1±9.5 mV.
Wearable devices have large versatility in terms of the diverse types of substrate materials used. It is thus important to take advantage of existing commercial platforms to print the wearable electrolyte sensors. Accordingly, we printed the new potentiometric sensors onto conventional textiles such as underwear, watch straps and elastic band (Figure 3D). Coupling screen printing technology with commodity textiles could offer large-scale low-cost production of variety of electrochemical sensors and flexible electronics systems.[28] Nevertheless, in some cases, such as elastic band or underwear, the rough material surface might become a problem when external tensile load is applied, requiring pretreatment by printing of the stretchable ink. Firstly, an Ecoflex layer was deposited on top of the sensor zone, creating a smooth thin layer. Subsequently, a PU layer was deposited on top of the Ecoflex layer prior to printing the CNT and Ag/AgCl inks. This step was essential to maintain strong adherence of the potentiometric sensor onto the substrate, and hence a remarkable sensor stretchability and durability. The performance of the potentiometric sensor array printed in this way on different substrates was tested. The watch-straps based sensors yielded a sensitivity of 51.6 mV/log [K+] and 51.8 mV/log [Na+], compared to 50 mV/log [K+] and 52.6 mV/log [Na+] for the elastic-band sensors and 56.6 mV/log [K+] and 52.5 mV/log [Na+] using the underwear sensors. All the sensors performed favorably within a 10-3 m to 10-1 m linear range, yielding a near-Nernstian sensor response.
Finally, Video S3 (Supporting information) demonstrates the proof of concept of creating a compact wearable sensor system by integrating the textile ISEs with a portable wireless data transmission device. The corresponding Figure 3D illustrates the time-trace plot obtained with the underwear textile sensor for increasing potassium levels, as recorded wirelessly with the Bluetooth application. This resulted in a favorable Nernstian response, with a slope of 59.9 mV/log [K+] over the 10-5 m to 10-1 m range.
In conclusion, we have demonstrated for the first time a stretchable textile-based potentiometric sensor that exhibits a Nernstian behavior under extreme conditions. Combining stretchable components as the PU, Ecoflex and stretch-enduring inks, along with a serpentine design, this printed textile sensor array can withstands high tensile stress without provoking 10 major cracking common to previous electrochemical devices. Mechanical deformation studies revealed that stretching up to 100%, along with repeated bending, crumpling or prolonged washing have negligible effects on the potentiometric response. Moreover, the screen printing process offers large-scale mass production of low-cost reproducible textile sensors. Further functionalization of the stretchable electrodes with other materials could enable a large arsenal of wearable textile sensors. Such integration of flexible chemical sensors with common textiles will lead to next-generation platform for personalized medicine, and will bring new opportunities for using wearable chemical sensing in the diagnostics, healthcare and sport fields.
Supplementary Material
Acknowledgments
M.P. and R.C. contributed equally to this work. Support from the National Institute of Biomedical Imaging and Bioengineering of NIH (R21EB019698) is acknowledged.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Marc Parrilla, Department of NanoEngineering, University of California, San Diego, La Jolla, CA 92093, USA.
Rocío Cánovas, Department of NanoEngineering, University of California, San Diego, La Jolla, CA 92093, USA.
Itthipon Jeerapan, Department of NanoEngineering, University of California, San Diego, La Jolla, CA 92093, USA.
Francisco J. Andrade, Email: franciscojavier.andrade@urv.cat, Departament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/Marcel·lí Domingo 1, Tarragona 43007, Spain.
Joseph Wang, Email: josephwang@eng.ucsd.edu, Department of NanoEngineering, University of California, San Diego, La Jolla, CA 92093, USA.
References
- 1.Rosner MH, Kirven J. Clin J Am Soc Nephrol. 2006;2:151. doi: 10.2215/CJN.02730806. [DOI] [PubMed] [Google Scholar]
- 2.Lehnhardt A, Kemper MJ. Pediatr Nephrol. 2011;26:377. doi: 10.1007/s00467-010-1699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leier CV, Dei Cas L, Metra M. Am Heart J. 1994;128:564. doi: 10.1016/0002-8703(94)90633-5. [DOI] [PubMed] [Google Scholar]
- 4.von Duvillard SP, Braun WA, Markofski M, Beneke R, Leithäuser R. Nutrition. 2004;20:651. doi: 10.1016/j.nut.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Scurati-Manzoni E, Fossali EF, Agostoni C, Riva E, Simonetti GD, Zanolari-Calderari M, Bianchetti MG, Lava SaG. Pediatr Nephrol. 2014;29:1015. doi: 10.1007/s00467-013-2712-4. [DOI] [PubMed] [Google Scholar]
- 6.Moraites E, Vaughn OA, Hill S. Dermatol Clin. 2014;32:457. doi: 10.1016/j.det.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Bielecka-Dabrowa A, Mikhailidis DP, Jones L, Rysz J, Aronow WS, Banach M. Int J Cardiol. 2012;158:12. doi: 10.1016/j.ijcard.2011.06.121. [DOI] [PubMed] [Google Scholar]
- 8.Tirosh E, Haddad F, Lanir A, Tal Y, Cohen A. Acta Paediatr. 1994;83:1268. doi: 10.1111/j.1651-2227.1994.tb13012.x. [DOI] [PubMed] [Google Scholar]
- 9.Almond CSD, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, Duncan CN, Olson DP, Salerno AE, Newburger JW, Greenes DS. N Engl J Med. 2005;352:1550. doi: 10.1056/NEJMoa043901. [DOI] [PubMed] [Google Scholar]
- 10.Maughan RJ, Shirreffs SM. J Sports Sci. 1997;15:297. doi: 10.1080/026404197367308. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong LE. J Am Coll Nutr. 2007;26:575S. doi: 10.1080/07315724.2007.10719661. [DOI] [PubMed] [Google Scholar]
- 12.Maughan RJ, Shirreffs SM. Int J Sport Nutr Exerc Metab. 2008;18:457. doi: 10.1123/ijsnem.18.5.457. [DOI] [PubMed] [Google Scholar]
- 13.Dziedzic CE, Ross ML, Slater GJ, Burke LM. Int J Sport Physiol Perform. 2014;9:832. doi: 10.1123/ijspp.2013-0480. [DOI] [PubMed] [Google Scholar]
- 14.Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. J Emerg Med. 2004;27:153. doi: 10.1016/j.jemermed.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Florea L, Diamond D. Sensors Actuators B Chem. 2015;211:403. [Google Scholar]
- 16.Bandodkar AJ, Molinnus D, Mirza O, Guinovart T, Windmiller JR, Valdés-Ramírez G, Andrade FJ, Schöning MJ, Wang J. Biosens Bioelectron. 2014;54:603. doi: 10.1016/j.bios.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Bandodkar AJ, Nuñez-Flores R, Jia W, Wang J. Adv Mater. 2015;27:3060. doi: 10.1002/adma.201500768. [DOI] [PubMed] [Google Scholar]
- 18.Chuang MC, Windmiller JR, Santhosh P, Ramírez GV, Galik M, Chou TY, Wang J. Electroanalysis. 2010;22:2511. [Google Scholar]
- 19.Jia W, Wang X, Imani S, Bandodkar AJ, Ramírez J, Mercier PP, Wang J. J Mater Chem A. 2014;2:18184. [Google Scholar]
- 20.Windmiller JR, Wang J. Electroanalysis. 2013;25:29. [Google Scholar]
- 21.Bandodkar AJ, Jeerapan I, You J-M, Nuñez-Flores R, Wang J. Nano Lett. 2015;16:721. doi: 10.1021/acs.nanolett.5b04549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun SY, Hong YK, Oh BK, Cha GS, Nam H, Lee SB, Jin JI. Anal Chem. 1997;69:868. doi: 10.1021/ac9605455. [DOI] [PubMed] [Google Scholar]
- 23.Cosofret VV, Erdosy M, Raleigh JS, Johnson Ta, Neuman MR, Buck RP. Talanta. 1996;43:143. doi: 10.1016/0039-9140(95)01724-0. [DOI] [PubMed] [Google Scholar]
- 24.Guinovart T, Parrilla M, Crespo Ga, Rius FX, Andrade FJ. Analyst. 2013;138:5208. doi: 10.1039/c3an00710c. [DOI] [PubMed] [Google Scholar]
- 25.Baker LB, Stofan JR, Hamilton Aa, Horswill Ca. J Appl Physiol. 2009;107:887. doi: 10.1152/japplphysiol.00197.2009. [DOI] [PubMed] [Google Scholar]
- 26.Buono MJ, Ball KD, Kolkhorst FW. J Appl Physiol. 2007;103:990. doi: 10.1152/japplphysiol.00015.2007. [DOI] [PubMed] [Google Scholar]
- 27.Coyle EF. J Sports Sci. 2004;22:39. doi: 10.1080/0264041031000140545. [DOI] [PubMed] [Google Scholar]
- 28.Metters JP, Kadara RO, Banks CE. Analyst. 2011;136:1067. doi: 10.1039/c0an00894j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


