Abstract
Purpose
To report the training/certification process of nonphysician imagers, image quality, and factors that affected image quality in the National Eye Institute sponsored multicentered e-ROP study.
Methods
Nonphysician imagers underwent rigorous training and certification in obtaining retinal images, with attention to clarity, focus, and optic disk placement. Image readers measured pupil size in pupil image and graded posterior pole, temporal, nasal, superior, and inferior retinal images and classified them as good, adequate, poor, or missing. Good and adequate images were deemed acceptable.
Results
In 4,003 image sessions of 1,257 infants, 3,453 (86.8%) were complete. Of 39,550 retinal images, 91.7% had acceptable quality, 5.6% poor, and 2.7% were missing. Inadequate pupil dilation negatively affected acceptable image quality: 54% acceptable images for pupil <5 mm versus 93% for >6 mm (P < 0.0001). When ventilatory equipment obstructed access to imaged infant, the percent of acceptable image quality decreased: 94% for no support versus 66.6% for oscillatory ventilation (P < 0.0001). Acceptable image quality rates improved from 87% to 90% (P = 0.03) from first 6 months to last 6 months at low patient volume centers, while high patient volume centers remained stable at 95%.
Conclusions
Nonphysicians successfully obtained acceptable quality images for ROP evaluation. Skills improved with experience. Image quality was negatively affected by inadequate pupil dilation and the presence of obstructive ventilatory equipment.
Retinopathy of prematurity (ROP), which remains a significant comorbidity of very-low-birth-weight (VLBW) infants, can lead to blindness. Worldwide, an estimated 20,000 to 30,000 premature infants go blind or are severely visually handicapped from ROP each year.1 Although blindness from ROP can largely be prevented by timely treatment, ROP of any stage is associated with a poorer prognosis for child development.2 In the US, approximately 14,000–16,000 preterm infants undergo ROP screening annually, with 1,100–1,500 who develop severe acute-phase ROP considered for treatment.3
Screening for ROP, based on the American Academy of Pediatrics (AAP)/American Academy of Ophthalmology (AAO)/American Association of Pediatric Ophthalmology and Strabismus (AAPOS) guidelines,4 has traditionally been the responsibility of an ROP-trained ophthalmologist. With the mismatch between the limited number of ophthalmologists and the large number of at-risk infants, other methods, such as using retinal images for remote evaluation, are gaining currency for efficiently, effectively, and safely evaluating infants at risk for ROP. Telemedicine-based remote evaluation of digital fundus imaging is now recognized by the AAP5 as a potential means of ROP screening, helping to fill a void left by lack of ROP-trained ophthalmologists. The use of digital imaging enables nonophthalmologists to obtain retinal images that can be reviewed by ophthalmologists or trained readers to identify infants with potentially severe ROP. Such projects are already underway on a large scale in India6 and California,7 using different models.8 The training of nonophthalmologists to obtain quality images is a cornerstone to the widespread use of retinal imaging in ROP screening.
The term referral-warranted ROP (RW-ROP)9 describes morphology on retinal images that should activate an ophthalmic consultation. RW-ROP is defined as ROP in zone I, any stage 3 or worse ROP, or plus disease noted by the evaluation of retinal images. To evaluate the presence of RW-ROP reliably, image readers need to have diagnostic images of acceptable quality; therefore, a robust and reliable method for imager training and certification and maintenance of skills is required.
The protocol used to train nonphysician imagers to acquire and submit retinal images in the Telemedicine Approaches to Evaluating Acute Phase ROP (e-ROP) Study was rigorous and systematic and can be implemented in a nonresearch setting. The e-ROP study was the first large-scale, National Eye Institute-sponsored, multicenter study in the US to train and assess the ability of nonphysicians to successfully obtain retinal images using a wide-angle 130° retinal camera (RetCam Shuttle, Clarity Inc, Pleasanton, CA) in obtaining digital images of preterm infants with birth weight of <1251 g. These images were evaluated by nonphysician trained readers to identify eyes with RW-ROP.9 From May 2011 to October 2013, 1,257 infants were enrolled and underwent imaging in each eye. Trained nonphysician readers (vs trained nonphysician imagers) were able to detect the presence of RW-ROP in one or both eyes of an infant with a sensitivity of 90% and specificity of 87%.10 The purpose of this study was to describe the retinal imagers’ training and certification process and examine the factors that affected image acquisition and image quality in the e-ROP study.
Methods
A standardized protocol for image submission and certification was developed for the e-ROP Study. The protocol and informed consent processes were approved by the Institutional Review Boards of the participating study centers, and informed consent was obtained. Monitoring, reporting of patient volume, image acquisition, and quality by clinical center was performed throughout the study in order to maintain proficiency of the certified retinal imagers (CRIs). Monthly conference calls were held among imagers to share technical tips for successful imaging.
Image acquisition requires a team of at least two persons: a CRI proficient in imaging and another person to monitor and support the infant. The imaging team selection was an essential component in e-ROP. The CRIs were registered nurses, nurse practitioners, ophthalmic technicians, or photographers. The support person was either another CRI or an experienced neonatal intensive care unit (NICU) nurse. The study visits were planned and timed around clinically indicated ROP examinations.
Imager Training and Certification
Imagers underwent an extensive training process. At the initial meeting of the entire e-ROP Cooperative Group, imagers learned about ROP, VLBW infants, and image acquisition, selection, and grading criteria. In addition to addressing the challenges of imaging VLBW infants, optimal positioning and comfort measures were emphasized. Additional training included further onsite instruction by representatives from Clarity Medical Systems. Also, hands-on technical training with the RetCam and use of a model eye allowed imagers familiarity with the camera and imaging techniques before imaging an infant in the NICU. Further education requirements included review of the e-ROP manual of procedures, the RetCam and the e-ROP imaging manuals, data entry, export, image selection, as well as import and transfer of images through a secure server to the Image Data Center. After completion of these tasks, imagers embarked on the certification process described below.
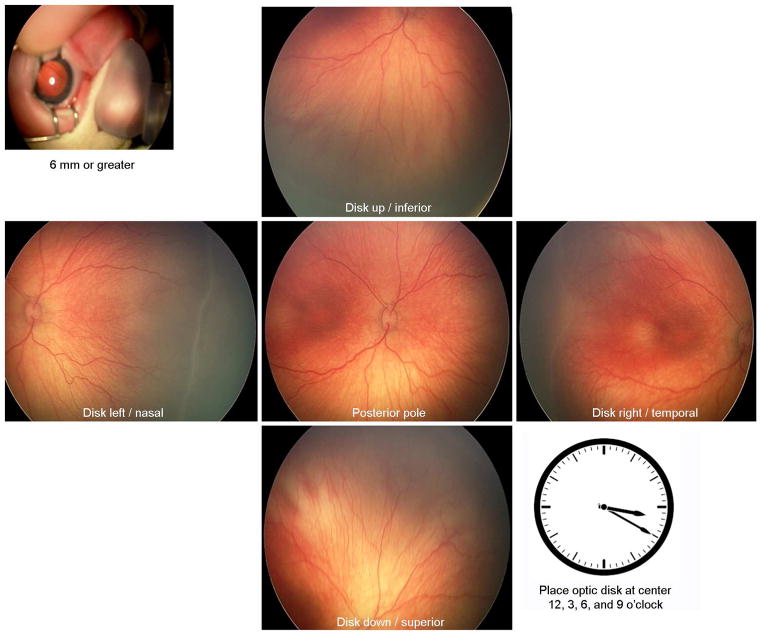
As per the e-ROP protocol, an imaging session included 2 sets of 6 images, one set from each eye for a total of 12 still images at each session selected from a video stream and uploaded to the server for grading at the e-ROP Reading Center. An image set included an external image to assess pupillary dilation, and 5 retinal views: disk center and 4 disk off-centered, giving views of the inferior, superior, temporal, and nasal retina. Off-center disk placement was emphasized at 12, 3, 6, and 9 o’clock positions, with the disk visible but as close to the edge of the image as possible (Figure 1).
FIG 1.
Required retinal views. An image set included an external image to assess pupillary dilation, and 5 retinal views that provided views of the posterior, inferior, superior, temporal, and nasal retina.
After training on a model eye, the imager underwent general and role-specific e-ROP knowledge assessments along with a practical examination including submission of image sets with required fields from infants. To be certified, imagers were required to submit to the e-ROP Reading Center11 image sets of good quality for 3 right and 3 left eyes; images were judged according to placement, clarity, and focus. Feedback was provided to the imager and additional sets submitted if necessary until sufficient image quality was achieved. During the study, an image set was scored for both quality and the presence of RW-ROP (see Table 2 and Table 1 in Daniel and colleauges11).
Table 2.
Image quality overall and by pupil size
| Image | Pupil sizea | Acceptable | Poor | Missing | P value |
|---|---|---|---|---|---|
| Posterior pole | <5 mm | 34 (60.7%) | 8 (14.3%) | 14 (25.0%) | |
| 5–6 mm | 1323 (94.1%) | 58 (4.1%) | 25 (1.8%) | <0.001 | |
| >6 mm | 6172 (97.6%) | 103 (1.6%) | 51 (0.8%) | ||
| All | 7630 (96%) | 176 (2%) | 104 (1%) | ||
| Nasal retina | <5 mm | 27 (48.2%) | 13 (23.2%) | 16 (28.6%) | |
| 5–6 mm | 1096 (78.0%) | 248 (17.6%) | 62 (4.4%) | <0.001 | |
| >6 mm | 5383 (85.1%) | 755 (11.9%) | 188 (3.0%) | ||
| All | 6577 (83%) | 1039 (13%) | 294 (4%) | ||
| Temporal retina | <5 mm | 35 (62.5%) | 8 (14.3%) | 13 (23.2%) | |
| 5–6 mm | 1297 (92.2%) | 81 (5.8%) | 28 (2.0%) | <0.001 | |
| >6 mm | 6134 (97.0%) | 131 (2.1%) | 61 (1.0%) | ||
| All | 7555 (96%) | 235 (3%) | 120 (2%) | ||
| Inferior retina | <5 mm | 30 (53.6%) | 9 (16.1%) | 17 (30.4%) | |
| 5–6 mm | 1227 (87.3%) | 94 (6.7%) | 85 (6.0%) | <0.001 | |
| >6 mm | 5837 (92.3%) | 245 (3.9%) | 244 (3.9%) | ||
| All | 7171 (91%) | 362 (5%) | 377 (5%) | ||
| Superior retina | <5 mm | 26 (46.4%) | 13 (23.2%) | 17 (30.4%) | |
| 5–6 mm | 1267 (90.1%) | 106 (7.5%) | 33 (2.3%) | <0.001 | |
| >6 mm | 5967 (94.3%) | 251 (4.0%) | 108 (1.7%) | ||
| All | 7344 (93%) | 390 (5%) | 176 (2%) | ||
| All retinal images | <5 mm | 152 (54.3%) | 51 (18.2%) | 77 (27.5%) | |
| 5–6 mm | 6210 (88.3%) | 587 (8.3%) | 233 (3.3%) | <0.001 | |
| >6 mm | 29493 (93.2%) | 1485 (4.7%) | 652 (2.1%) | ||
| All | 36277 (92%) | 2202 (6%) | 1071 (3%) |
Pupil size measured by e-ROP grading center.
Once imagers were certified, the clinical centers could initiate the acquisition and submission of images for the e-ROP study. During this early period, site visits were undertaken by the teams from Office of the Study Chair and the Data Coordinating Center to evaluate imaging onsite and establish readiness for enrolling patients. Image acquisition and quality for each retinal view was assessed and general feedback provided throughout the study and reported at monthly CRI calls and yearly technical group meetings.
Imaging Procedure
When approaching an infant for imaging, CRIs were instructed to concentrate on safety while obtaining highest image quality, with clarity and focus (especially of the periphery) and disk placement that optimized the view of the peripheral retina. Imagers acquired the techniques to overcome the physical barriers around the eye, such as the obstructive modes of ventilatory support, poor dilation, and low-contrast fundi, all of which may affect image acquisition and quality. CRIs were instructed to record findings if images were difficult to obtain, such as hazy vitreous or tunica vasculosa lentis, if present.
The numerous modes of ventilatory support that premature infants require often obstruct access to the infant’s eye. In such difficult circumstances, imagers may devise new techniques to acquire quality images. The imaging team was trained in optimum positioning of the infant and the ventilator apparatus so that the equipment was away from the infant’s eye and positioning the imager and support person in a stable, comfortable position to manipulate the camera head with minimal disturbance to the infant. Copious amounts of coupling gel were essential in aiding the movement of the camera head safely within that tight space. To complete the imaging sessions, CRIs used gentle manipulation and comfort measures, including sucrose, pacifier, swaddling, and sedation (if ordered by the NICU staff).
The CRI recorded the reasons for incomplete image sets, such as unstable infant, determined by preset ranges, and other parameters. Reporting adverse events and severe adverse events followed standardized procedures.
Statistical Analysis
Image quality was analyzed as the percentage of retinal images with acceptable quality, poor quality and missing image for each retinal view and for all retinal images combined. Acceptable images were a combined category of images graded as good and adequate by the trained readers. We analyzed factors associated with image acquisition and quality, including pupil size (as determined by the trained readers), clinical center patient volume, and infants’ ventilatory support status (because it can affect success of imaging and/or change the stability of the infant). Image quality in each study center’s first 6 months and last 6 months was compared to evaluate improvement over time in higher patient volume centers (HPVCs) and lower patient volume centers (LPVCs), based on a cut-off point of 17 visits per month on average. The χ2 test was used to compare the proportions between groups. To account for the correlation among images from multiple image sessions of an eye, and among images from both eyes of an infant, the generalized estimating equation (GEE)12 was used for P value calculation.
Results
A total of 28 imagers from 13 participating clinical centers were trained and certified for the e-ROP study. The number of certification image sets submitted for certification ranged from 1 to 5, with an average of 1.8. The certification submission process took from 1 to 93 days because of availability of infants for imaging, frequency of ROP rounds, and the individual imager’s learning curve.
During the e-ROP study, 1,257 infants were enrolled and imaged from May 2011 to October 2013, with an average of 3.4 image sessions per infant. There were 4,205 study visits that included the diagnostic examination, in which the ophthalmologist examination preceded imaging 65% of the time. Imaging sessions occurred at 4,003 visits (95.%), with 202 sessions (5%) not attempted because of parent refusal or the infant’s medical status. In 26 sessions no images were submitted because of technical issues (eg, the images for an infant were recorded with the wrong ID). Of 3,977 image sessions with image submission, 3,453 (86.8%) were complete (6 required images in both eyes) and 550 (14%) had incomplete sets in one or both eyes.
The reasons for incomplete sets (not mutually exclusive) as recorded by CRIs on the case report form are reported in Table 1. Table 2 presents the image quality of 5 retinal images and the pupil dilation of each eye. Of the 39,550 images from 7,910 image sets that were evaluated by trained readers, 91.7% had acceptable image quality, 5.6% were poor, and 2.7% were missing.
Table 1.
Completeness of imaging in image session and reasons for incomplete image sets
| Label | Baby | Right eye | Left eye |
|---|---|---|---|
| No. infant approaches for imaging | 4003/4205 (95.2%) | 4003/4205 (95.2%) | 4003/4205 (95.2%) |
| No. imaging sessions with any images sent | 3977/4003 (99.4%) | 3971/4003 (99.2%) | 3939/4003 (98.4%) |
| No. complete images sets sent | 3453/3977 (86.8%) | 3679/3971 (92.6%) | 3617/3939 (91.8%) |
| Reasons for incomplete image sets (n = 550) | |||
| Agitated baby | 12/550 (2.2%) | 7/324 (2.2%) | 9/386 (2.3%) |
| Baby became unstable | 60/550 (10.9%) | 38/324 (11.7%) | 51/386 (13.2%) |
| Bell’s phenomenon | 78/550 (14.2%) | 46/324 (14.2%) | 46/386 (11.9%) |
| Poor access to eye | 228/550 (41.5%) | 152/324 (46.9%) | 148/386 (38.3%) |
| Poor dilation | 104/550 (18.9%) | 63/324 (19.4%) | 79/386 (20.5%) |
| Technical reasons | 64/550 (11.6%) | 36/324 (11.1%) | 47/386 (12.2%) |
| Other | 26/550 (4.7%) | 16/324 (4.9%) | 12/386 (3.1%) |
| Unknown | 63/550 (11.5%) | 25/324 (7.7%) | 42/386 (10.9%) |
The effect of pupil size on the image acquisition and quality is also reported in Table 2. Of the 280 attempted retinal images taken with pupils <5 mm in diameter, only 54% of images were graded as having acceptable quality, compared to 88% for pupils 5–6 mm and 93% for pupils >6 mm in diameter. The percentage of missing retinal images was also higher when pupil size was smaller; 28% missing for pupil size <5 mm compared to 3.3% for pupil size 5–6 mm and 2.1% in pupil size of >6 mm (P < 0.0001, Table 2).
The mode of the infant’s ventilatory support during the imaging session affected image quality (Table 3). The percentage of acceptable images decreased with the increasing difficulty of access to the infant’s eye created by mode of ventilation; 94% for room air to 66.6% for JET/HFOV ventilation, both having very stiff, short tubing. NCPAP and NIMV had the next lowest percentage of acceptable quality images (89%), with extensive equipment centered on the nose and eyes; conventional mechanical ventilation had 92% acceptable quality images.
Table 3.
Image quality by respiratory status before imaging
| Image | Quality | HFOV/JET | CPAP/NIPPV/HFNC | CMV | NC | Room air | P value |
|---|---|---|---|---|---|---|---|
| Posterior pole | Acceptable | 49 (73.1%) | 1710 (94.7%) | 799 (96.0%) | 2576 (96.8%) | 2485 (98.1%) | <0.001 |
| Poor/missing | 18 (26.9%) | 95 (5.3%) | 33 (4.0%) | 86 (3.2%) | 47 (1.9%) | ||
| Nasal retina | Acceptable | 45 (70.3%) | 1429 (79.2%) | 716 (86.1%) | 2191 (82.3%) | 2185 (86.3%) | <0.001 |
| Poor/missing | 22 (34.4%) | 376 (20.8%) | 116 (13.9%) | 471 (17.7%) | 347 (13.7%) | ||
| Temporal retina | Acceptable | 45 (67.2%) | 1694 (93.9%) | 794 (95.4%) | 2554 (95.9%) | 2457 (97.0%) | <0.001 |
| Poor/missing | 22 (32.8%) | 111 (6.1%) | 38 (4.6%) | 108 (4.1%) | 75 (3.0%) | ||
| Inferior retina | Acceptable | 37 (55.2%) | 1569 (86.9%) | 745 (89.5%) | 2434 (91.4%) | 2374 (93.8%) | <0.001 |
| Poor/missing | 30 (44.8%) | 236 (13.1%) | 87 (10.5%) | 228 (8.6%) | 158 (6.2%) | ||
| Superior retina | Acceptable | 47 (70.1%) | 1607 (89.0%) | 768 (92.3%) | 2491 (93.6%) | 2421 (95.6%) | <0.001 |
| Poor/missing | 20 (29.8%) | 198 (11.0%) | 64 (7.7%) | 171 (6.4%) | 111 (4.4%) | ||
| All retinal images | Acceptable | 223 (66.6%) | 8009 (88.7%) | 3822 (91.9%) | 12246 (92.0%) | 11922 (94.2%) | <0.001 |
| Poor/missing | 112 (33.4%) | 1016 (11.3%) | 338 (8.1%) | 1064 (8.0%) | 738 (5.8%) |
CMV, conventional mechanical vventilation; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula; HFOV, high-frequency oscillator ventilation; JET, JET ventilation; NC, nasal cannula; NIPPV, nasal intermittent positive pressure ventilation.
Among all clinical centers, incomplete image sets decreased from 10.4% in the first 6 months of imaging to 5.9% in the last 6 months of the study (P < 0.001, Table 4). Image quality also improved from the first 6 months to the last 6 months, particularly for the images of the inferior retina (89% vs 94% acceptable quality, respectively; P < 0.001) and nasal retina (82% vs 85%, respectively; P < 0.001; Table 4).
Table 4.
Image quality in the first 6 months and last 6 months of the imaging period
| First 6 monthsa | Last 6 monthsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Image view | N | Acceptable | Poor | Missing | N | Acceptable | Poor | Missing | P value |
| Pupil size | 1860 | 1763 (95%) | 66 (4%) | 31 (2%) | 2007 | 1874 (93%) | 109 (5%) | 24 (1%) | 0.01 |
| Posterior pole | 1789 (96%) | 37 (2%) | 34 (2%) | 1932 (96%) | 59 (3%) | 16 (1%) | 0.003 | ||
| Nasal retina | 1519 (82%) | 256 (14%) | 85 (5%) | 1713 (85%) | 248 (12%) | 46 (2%) | <0.001 | ||
| Temporal retina | 1774 (95%) | 49 (3%) | 37 (2%) | 1935 (96%) | 55 (3%) | 17 (1%) | 0.01 | ||
| Inferior retina | 1650 (89%) | 100 (5%) | 110 (6%) | 1881 (94%) | 76 (4%) | 50 (2%) | <0.001 | ||
| Superior retina | 1736 (93%) | 87 (5%) | 37 (2%) | 1858 (93%) | 103 (5%) | 46 (2%) | 0.64 | ||
| All retinal images | 8468 (91%) | 529 (6%) | 303 (3%) | 9319 (93%) | 541 (5%) | 175 (2%) | 0.02 | ||
The first 6 months and the last 6 months is center specific, depending on when they start imaging and stop imaging.
Patient volume varied across clinical centers, with HPVCs having a higher percentage of acceptable image quality early on compared to LPVCs (Table 5). In the first 6 months the HPVCs had a mean (with standard deviation) of 25.4 ± 6.1 image sessions per month compared to the LPVCs, with 12.8 ± 5.6 image sessions per month; in the last 6 months, the HPVCs had 23.3 ± 5.0 versus LPVCs 11.9 ± 4.0 image sessions per month. Table 5 shows image quality and number of incomplete image sets in HPVCs and LPVCs. The image quality of the inferior retina showed improvement in both low- and high-volume centers. Image quality at HPVCs remained relatively stable throughout the study (95% acceptable quality), but the LPVC continued to show improvement over time, with 87% acceptable image quality in the first 6 months and 90% in the last 6 months (P = 0.03). In LPVCs, nasal and temporal missing images dropped from 8% to 4%, and 3% to 1%, respectively, from the first 6 months to the last 6 months, whereas in the HPVCs quality remained stable.
Table 5.
Image quality in high versus low patient volume centers in first and last 6 months of the imaging period
| First 6 months | Last 6 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Clinic | Image | N | Acceptable | Poor | Missing | N | Acceptable | Poor | Missing | P value |
| LPVCs | Pupil size | 891 | 834 (94%) | 28 (3%) | 29 (3%) | 929 | 844 (91%) | 63 (7%) | 22 (2%) | 0.001 |
| Posterior pole | 837 (94%) | 29 (3%) | 25 (3%) | 876 (94%) | 45 (5%) | 8 (1%) | 0.002 | |||
| Nasal retina | 661 (74%) | 162 (18%) | 68 (8%) | 773 (83%) | 123 | 33 (4%) | 0.001 | |||
| Temporal retina | 831 (93%) | 32 (4%) | 28 (3%) | 880 (95%) | 40 (4%) | 9 (1%) | 0.004 | |||
| Inferior retina | 747 (84%) | 60 (7%) | 84 (9%) | 848 (91%) | 47 (5%) | 34 (4%) | 0.001 | |||
| Superior retina | 802 (90%) | 58 (7%) | 31 (3%) | 819 (88%) | 69 (7%) | 41 (4%) | 0.42 | |||
| All retinal images | 4455 | 3878 (87%) | 341 (8%) | 236 (5%) | 4645 | 4196 (90%) | 324 (7%) | 125 | 0.03 | |
| HPVCs | Pupil size | 969 | 929 (96%) | 38 (4%) | 2 (0%) | 1078 | 1030 (96%) | 46 (4%) | 2 (0%) | 0.92 |
| Posterior pole | 952 (98%) | 8 (1%) | 9 (1%) | 1056 (98%) | 14 (1%) | 8 (1%) | 0.53 | |||
| Nasal retina | 858 (89%) | 94 (10%) | 17 (2%) | 940 (87%) | 125 | 13 (1%) | 0.24 | |||
| Temporal retina | 943 (97%) | 17 (2%) | 9 (1%) | 1055 (98%) | 15 (1%) | 8 (1%) | 0.72 | |||
| Inferior retina | 903 (93%) | 40 (4%) | 26 (3%) | 1033 (96%) | 29 (3%) | 16 (1%) | 0.03 | |||
| Superior retina | 934 (96%) | 29 (3%) | 6 (1%) | 1039 (96%) | 34 (3%) | 5 (0%) | 0.87 | |||
| All retinal images | 4845 | 4590 (95%) | 188 (4%) | 67 (1%) | 5390 | 5123 (95%) | 217 (4%) | 50 (1%) | 0.57 | |
HPVC, higher patient volume centers; LPVC, lower patient volume center.
Discussion
The e-ROP study identified several key factors to improve imaging as a clinical tool. Maximizing pupil dilation is crucial to imaging success. Image quality often suffered as a result of the infant’s medical condition, and the need for certain modes of ventilation that obstructed the eye. A thorough training program provides instruction on how to handle a fragile, premature infant and the surrounding equipment. Training also emphasized the need for the imager to image frequently with a varied patient population to maintain optimal imaging skills. A successful imaging program will also provide frequent feedback to the imager from the reading center with respect to clarity, field and focus, and optic disk placement. Training must also stress accurate data input; of course, proper safeguards be in place to ensure errors are corrected prior to evaluation of image sets. Overall success will rely on a consistent volume of patients so that proper systems can be developed and maintained.
In conclusion, the e-ROP Study demonstrated that nonphysicians can consistently acquire and submit quality images, with a 92% success rate in providing acceptable quality images to the e-ROP readers for evaluation. Such a system presents a way to offer preterm infants worldwide a safe13 means for image acquisition and an effective system for ROP evaluation.10,15 Imaging has also provided an important teaching aid for families and medical staff to illustrate the infant’s ROP status, reinforcing the seriousness of the disorder and need for careful follow-up.
Supplementary Material
Acknowledgments
Supported by National Eye Institute of the National Institutes of Health, Department of Health and Human Services. U10 EY017014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Lawn JE, Vasquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74:35–39. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt B, Roberts RS, Davis PG, et al. Prediction of late death or disability at age 5 year using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167:982–6. doi: 10.1016/j.jpeds.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 3.National Eye Institute. Retinopathy of prematurity. 2014 Jun; Available at https://nei.nih.gov/health/ROP.
- 4.Fierson WM American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 5.Fierson WM, Capone A, Jr American Association of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association of Certified Orthoptists. Telemedicine for Evaluation of Retinopathy of Prematurity. Pediatrics. 2015;135:e238–54. doi: 10.1542/peds.2014-0978. [DOI] [PubMed] [Google Scholar]
- 6.Vinekar A, Gilbert C, Dogra M, et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014;62:41–9. doi: 10.4103/0301-4738.126178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fijalkowski N, Zheng LL, Henderson MT, Wallenstein MB, Leng T, Moshfeghi DM. Stanford University Network for Diganosis of Retinopathy of Prematurity (SUNDROP): four-years of Screening with telemedicine. Curr Eye Res. 2013;38:283–91. doi: 10.3109/02713683.2012.754902. [DOI] [PubMed] [Google Scholar]
- 8.Fijalkowski N, Zheng LL, Henderson MT, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic Surg Lasers Imaging Retina. 2014;45:106–13. doi: 10.3928/23258160-20140122-01. [DOI] [PubMed] [Google Scholar]
- 9.Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity: a pilot study. Ophthalmology. 2003;110:2113–17. doi: 10.1016/S0161-6420(03)00831-5. [DOI] [PubMed] [Google Scholar]
- 10.Quinn GE, Ying GS, Daniel E, et al. e-ROP Cooperative Group. Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmology. 2014;132:1178–84. doi: 10.1001/jamaophthalmol.2014.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel E, Quinn GE, Hildebrand PL, et al. e-ROP Cooperative Group. Validated system for centralized grading of retinopathy of prematurity: telemedicine approaches to evaluating acute phase ROP (e-ROP) study. JAMA Ophthalmology. 2015;133:675–82. doi: 10.1001/jamaophthalmol.2015.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 13.Wade K, Pistilli M, Baumritter A, et al. e-ROP Cooperative Group. Safety of retinopathy of prematurity examination and imaging in premature infants. J Pediatr. 2015;167:994–1000. doi: 10.1016/j.jpeds.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn GE e-ROP Cooperative Group. Telemedicine approaches to evaluating acute-phase retinopathy of prematurity: study design. Ophthalmic Epidemiol. 2014;21:256–67. doi: 10.3109/09286586.2014.926940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.