Abstract:
Eicosapentaenoic acid (EPA) is a triglyceride-lowering agent that reduces circulating levels of the apolipoprotein B (apoB)-containing lipoprotein particles small dense low-density lipoprotein (sdLDL), very–low-density lipoprotein (VLDL), and oxidized low-density lipoprotein (LDL). These benefits may result from the direct antioxidant effects of EPA. To investigate this potential mechanism, these particles were isolated from human plasma, preincubated with EPA in the absence or presence of atorvastatin (active) metabolite, and subjected to copper-initiated oxidation. Lipid oxidation was measured as a function of thiobarbituric acid reactive substances formation. EPA inhibited sdLDL (IC50 ∼2.0 μM) and LDL oxidation (IC50 ∼2.5 μM) in a dose-dependent manner. Greater antioxidant potency was observed for EPA in VLDL. EPA inhibition was enhanced when combined with atorvastatin metabolite at low equimolar concentrations. Other triglyceride-lowering agents (fenofibrate, niacin, and gemfibrozil) and vitamin E did not significantly affect sdLDL, LDL, or VLDL oxidation compared with vehicle-treated controls. Docosahexaenoic acid was also found to inhibit oxidation in these particles but over a shorter time period than EPA. These data support recent clinical findings and suggest that EPA has direct antioxidant benefits in various apoB-containing subfractions that are more pronounced than those of other triglyceride-lowering agents and docosahexaenoic acid.
Key Words: omega-3 fatty acid, eicosapentaenoic acid, small, dense LDL, docosahexaenoic acid, atorvastatin, triglycerides
INTRODUCTION
Consumption of fish or fish oils rich in omega-3 fatty acids has been shown to be associated with reduced risk of cardiovascular disease.1,2 Omega-3 fatty acids incorporate directly into the membranes of cells associated with the atherosclerotic plaque, where they interfere with signal transduction pathways involved in inflammation and endothelial dysfunction.2,3 Eicosapentaenoic acid (EPA) is an omega-3 fatty acid that has been evaluated in various clinical studies, including those involving statin-treated patients. In studies that included investigation of proinflammatory and oxidative stress markers, EPA was shown to decrease high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, arachidonic acid/EPA ratio, and oxidized low-density lipoprotein (oxLDL) levels compared with placebo.4–10 In the Japan EPA Lipid Intervention Study, purified EPA (1.8 g/d) combined with statin treatment resulted in a 19% relative reduction in major coronary events compared with statin treatment alone (P = 0.011).11 In patients with type 2 diabetes mellitus or coronary artery disease (CAD), treatment with purified EPA (1.8 g/d) slowed atherosclerotic disease progression; effects were also observed in addition to statin therapy.12–14 However, other triglyceride-lowering agents such as niacin (in the Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events [HPS2-THRIVE] and Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes [AIM-HIGH] trials)15,16 and fibrates (in the Action to Control Cardiovascular Risk in Diabetes [ACCORD]–Lipid and Evaluation of Choline Fenofibrate on Carotid Intima-Media Thickness in Subjects with Type IIb Dyslipidemia with Residual Risk in Addition to Atorvastatin Therapy [FIRST] trials)17,18 failed to reduce cardiovascular events or decrease intima-media thickness compared with statin treatment alone. These differences between EPA and other triglyceride-lowering agents may be due, in part, to the unique inhibitory effects of EPA on oxidation of apolipoprotein B (apoB)-containing lipoproteins, which result from its distinct lipophilic and electron stabilization properties.19
Oxidative modification of LDL has long been understood to play an important role in the development of atherosclerosis by contributing to endothelial dysfunction, vascular inflammation, and other processes.20–22 High plasma levels of oxLDL are associated with an increased risk for myocardial infarction and metabolic syndrome as well as the severity of acute coronary syndromes.23,24 In addition, we previously demonstrated that levels of oxidized lipids are predictive of clinical events in patients with CAD.25
Several studies suggest that small, dense low-density lipoprotein (sdLDL) is highly atherogenic compared with larger LDL particles.26–30 The risk associated with sdLDL has been attributed to some of its unique properties, including reduced affinity for the LDL receptor, enhanced interaction with arterial wall proteoglycans, and increased permeability through the endothelial barrier.31–34 In addition, human sdLDL has been shown to be significantly more susceptible to oxidative modification than larger LDL subfractions.35,36 These differences may influence the clearance of sdLDL from the body relative to other LDL particles. By inhibiting lipoprotein oxidation, EPA may enhance the clearance of sdLDL from the circulation. Very–low-density lipoprotein (VLDL) represents the main triglyceride-carrying particles in the circulation and the cholesterol within these particles contributes to atherosclerotic plaque development; elevated VLDL levels are thus also associated with increased cardiovascular risk.37
In this study, we examined the direct effects of EPA on rates of lipid oxidation in different-sized apoB-containing subfractions (sdLDL, LDL, VLDL) isolated from human plasma. We hypothesized that EPA, based on its unique physicochemical properties, would inhibit lipoprotein oxidation. We tested EPA in a dose-dependent manner and compared its effects with those of other triglyceride-lowering agents, including fenofibrate, niacin, and gemfibrozil. We also combined EPA with atorvastatin active metabolite, as it has also been shown to have potent antioxidant properties and because EPA and atorvastatin are often used together in patients with dyslipidemia.38,39 Finally, we compared EPA with another long-chain omega-3 fatty acid, docosahexaenoic acid (DHA), regarding their antioxidant effects in these various lipoprotein particles.
METHODS
Materials
EPA, DHA, vitamin E (α-tocopherol), fenofibrate, nicotinic acid (niacin), and gemfibrozil were purchased from Sigma-Aldrich (St Louis, MO). EPA and DHA were solubilized in ethanol to 1 mM under nitrogen atmosphere. Immediately before experimental use, vitamin E was prepared in ethanol at 1.0 mM (ε = 3.06 × 104 M−1·cm−1 at 294 nm). Atorvastatin ortho- (o-) hydroxy (active) metabolite (atorvastatin metabolite) was purchased from Toronto Research Chemicals (North York, Ontario, Canada) and solubilized in methanol to 1.0 mM. Fenofibrate, niacin, and gemfibrozil were solubilized in ethanol to 1.0 mM. All test compounds were further diluted in ethanol or aqueous buffer as needed.
Isolation of sdLDL, LDL, VLDL and Oxidation
ApoB-containing lipoprotein fractions were isolated from the plasma of healthy volunteers by iodixanol density gradient centrifugation and adjusted to a final apolipoprotein B-100 concentration of 2 mg/mL as previously described.40 We adapted previous methods to isolate and characterize sdLDL, LDL, and VLDL fractions using agarose gel electrophoresis.41
Sample aliquots (100 μg apolipoprotein B-100) were incubated with EPA, fenofibrate, gemfibrozil, niacin, DHA, or vehicle (ethanol) control (at concentrations indicated in figure legends) for 30 minutes at 37 °C in a shaking water bath. In the case of VLDL, we used 50 μg apolipoprotein B-100 due to its higher lipid content. We also tested vitamin E as a positive control and atorvastatin metabolite (alone or in combination with EPA) and fenofibrate, niacin, or gemfibrozil (at concentrations indicated in figure legends).
Lipid oxidation was initiated with 10 μM CuSO4 and assayed at various time points for up to 4 hours. For VLDL samples, we used 20 μM CuSO4 due to its larger particle size. Oxidation was monitored by spectrophotometric detection of thiobarbituric acid reactive substances as previously described.19,40
Statistical Analyses
Mean ± SD was calculated for (n) separate samples or experiments. The 2-tailed Student's t test was used for comparisons between only 2 groups; analysis of variance, followed by Dunnett or Student–Newman–Keuls multiple comparisons post hoc analysis, was used for comparisons between 3 or more groups. Differences were considered to be significant for probability values less than 0.05.
RESULTS
Dose-dependent Effects of EPA on Human sdLDL, LDL, and VLDL Oxidation
We tested the pretreatment effects of EPA on sdLDL, LDL, and VLDL oxidation by treating the samples with EPA at 1.0, 2.5, 5.0, and 10.0 μM followed by initiation of oxidative stress. Significant and reproducible inhibition was observed with all doses examined (Fig. 1). EPA inhibited sdLDL oxidation by 19 ± 8% (P < 0.001) at the lowest dose (1.0 μM) and by 93 ± 2% (P < 0.001) at the highest dose (10.0 μM). The IC50 calculated for EPA in this assay was approximately 2 μM, which was similar to that determined for LDL samples. In VLDL, EPA had much more potent antioxidant activity with >90% inhibition at 2.5 μM (P < 0.001) and an IC50 of <1.0 μM.
FIGURE 1.
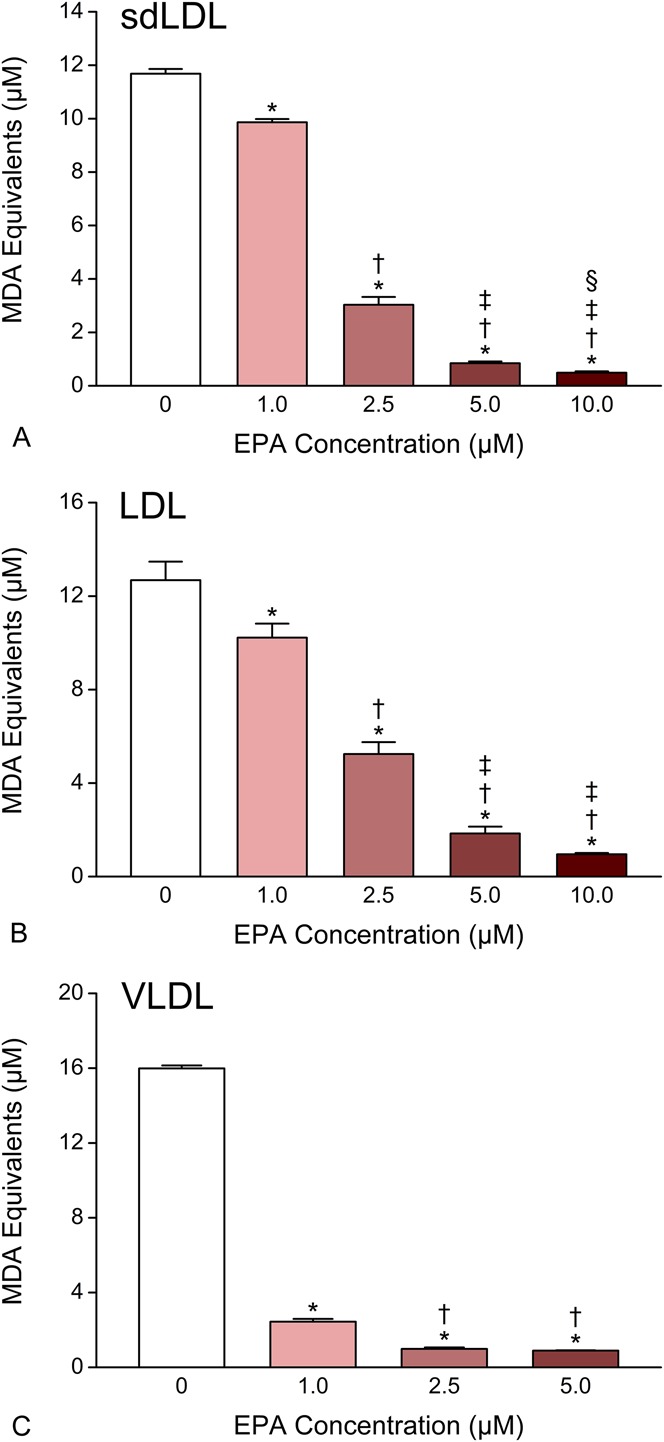
Dose-dependent effects of EPA on human sdLDL (A), LDL (B), and VLDL (C) oxidation. Data were collected after 1–2 hours of exposure to oxidative conditions. Values are mean ± SD (N = 3) and are expressed as molar equivalents of MDA. *P < 0.001 versus vehicle-treated control; †P < 0.001 versus 1.0 μM EPA; ‡P < 0.001 versus 2.5 μM EPA; §P < 0.05 versus 5.0 μM EPA (Student–Newman–Keuls multiple comparisons test; overall ANOVA—sdLDL data: P < 0.0001, F = 2960.1; LDL data: P < 0.0001, F = 298.14; VLDL data: P < 0.0001, F = 12,055). ANOVA, analysis of variance; MDA, malondialdehyde.
Effect of EPA and Atorvastatin Metabolite Combination Treatment on Human sdLDL, LDL, and VLDL Oxidation
We also tested the separate and combined pretreatment effects of EPA and atorvastatin metabolite (each at 1.0 μM) on copper-initiated oxidation in the different-sized LDL particles (Fig. 2). When combined, EPA and atorvastatin metabolite inhibited sdLDL oxidation to an extent that was not observed with equimolar concentrations of either agent alone. EPA and atorvastatin metabolite, tested separately at 1.0 μM, inhibited sdLDL oxidation by 19 ± 8% (P < 0.01) and 18 ± 5% (P < 0.01), respectively, whereas the combination treatment inhibited sdLDL oxidation by 75 ± 8% (P < 0.001) compared with vehicle-treated controls. We also tested the effect of these agents at a lower dose of atorvastatin metabolite (0.5 μM), which inhibited oxidation by only 5 ± 5% when tested separately but by 57 ± 9% (P < 0.001) when combined with equimolar EPA. The inhibitory effects of EPA and atorvastatin metabolite combination treatment were much greater than the sum of their separate effects at all concentrations examined in this study. Similar effects with EPA and atorvastatin metabolite were seen in LDL particles. In VLDL, atorvastatin metabolite treatment alone had no significant antioxidant effect, but EPA inhibited oxidation by 25% (P < 0.001). Of note, the combination of EPA plus atorvastatin metabolite inhibited oxidation by 34% (P < 0.001), which was significantly greater than EPA alone (P < 0.05).
FIGURE 2.
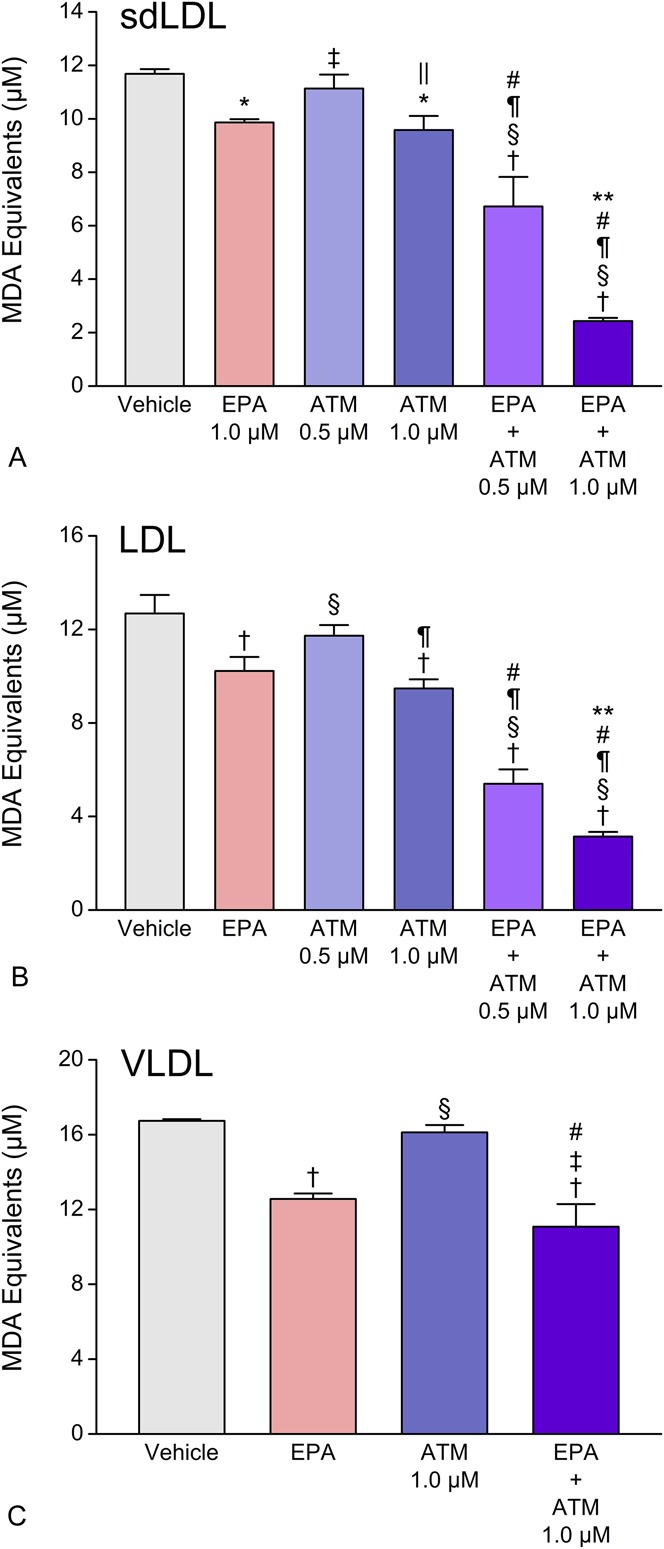
Separate and combined effects of EPA and atorvastatin (active) metabolite (ATM) on human sdLDL (A), LDL (B), and VLDL (C) oxidation. EPA was tested at 1.0 μM. Data were collected after 1 hour (sdLDL and LDL) and 4 hours (VLDL) exposure to oxidative conditions. Values are mean ± SD (N = 3) and are expressed as molar equivalents of MDA. *P < 0.01 and †P < 0.001 versus vehicle-treated control; ‡P < 0.05 and §P < 0.001 versus EPA; ‖P < 0.05 and ¶P < 0.001 versus 0.5 μM atorvastatin metabolite; #P < 0.001 versus 1.0 μM atorvastatin metabolite; **P < 0.001 versus EPA + 0.5 μM atorvastatin metabolite (Student–Newman–Keuls multiple comparisons test; overall ANOVA—sdLDL data: P < 0.0001, F = 118.22; LDL data: P < 0.0001, F = 142.74; VLDL data: P < 0.0001, F = 52.613). ANOVA, analysis of variance; MDA, malondialdehyde.
Comparative Effects of EPA, Fenofibrate, Niacin, Gemfibrozil, and Vitamin E on Human sdLDL, LDL, and VLDL Oxidation
EPA was further examined against other triglyceride-lowering agents (fenofibrate, niacin, and gemfibrozil) as well as vitamin E, each at 10.0 μM, regarding their comparative effects on sdLDL, LDL, and VLDL oxidation (Fig. 3). EPA was found to significantly inhibit sdLDL lipid oxidation compared with vehicle, any of the triglyceride-lowering agents, or vitamin E (all P < 0.001). No significant antioxidant activity was observed with any of the other triglyceride-lowering agents or vitamin E, whereas EPA inhibited sdLDL oxidation by >90% (P < 0.001), compared with vehicle-treated controls. Similar results were observed with LDL and VLDL.
FIGURE 3.
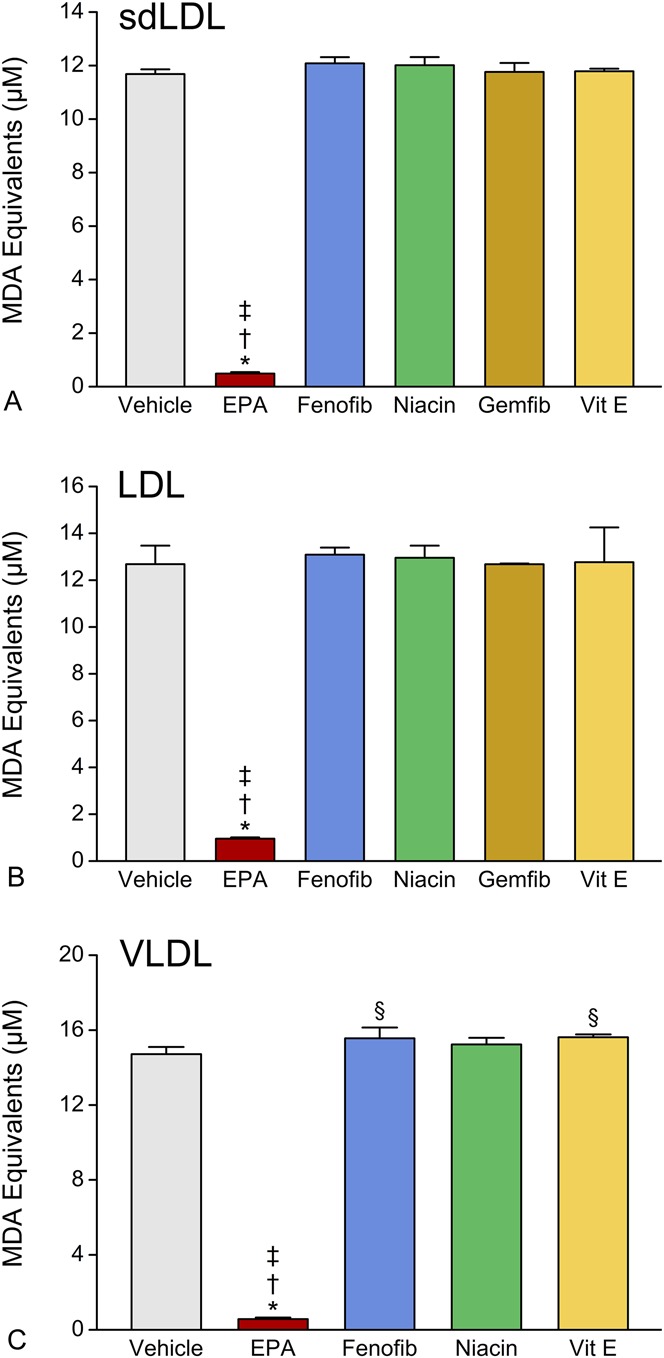
Comparative effects of EPA, fenofibrate, niacin, gemfibrozil, and vitamin E on human sdLDL (A), LDL (B), and VLDL (C) oxidation. All agents were tested at 10 μM. Data were collected after 1 hour (sdLDL and LDL) and 4 hours (VLDL) exposure to oxidative conditions. Values are mean ± SD (N = 3) and are expressed as molar equivalents of MDA. *P < 0.001 versus vehicle-treated control; †P < 0.001 versus fenofibrate, niacin, or gemfibrozil; ‡P < 0.001 versus vitamin E; §P < 0.05 versus vehicle-treated control (Student–Newman–Keuls multiple comparisons test; overall ANOVA—sdLDL data: P < 0.0001, F = 1268.1; LDL data: P < 0.0001, F = 132.38; VLDL data: P < 0.0001, F = 1031.4). ANOVA, analysis of variance; Fenofib, fenofibrate; Gemfib, gemfibrozil; MDA, malondialdehyde.
Comparative Effects of EPA and DHA on Human sdLDL, LDL, and VLDL Oxidation
Given the considerable interest in understanding potential differences among long-chain omega-3 fatty acids, we also tested the comparative antioxidant effects of EPA and DHA at equimolar concentrations in the low micromolar range of 2.5–10.0 μM depending on particle size (Fig. 4). EPA significantly inhibited sdLDL oxidation consistently over 4 hours compared with either vehicle or DHA (P < 0.001 at 4 hours). DHA was observed to have antioxidant properties early in the experimental time course but became neutral in effect by 4 hours. The differences in activity between EPA and DHA were significant as early as 1 hour and became more pronounced over the remainder of the time course of the experiment in a manner that was consistent over all particle sizes (Fig. 4).
FIGURE 4.
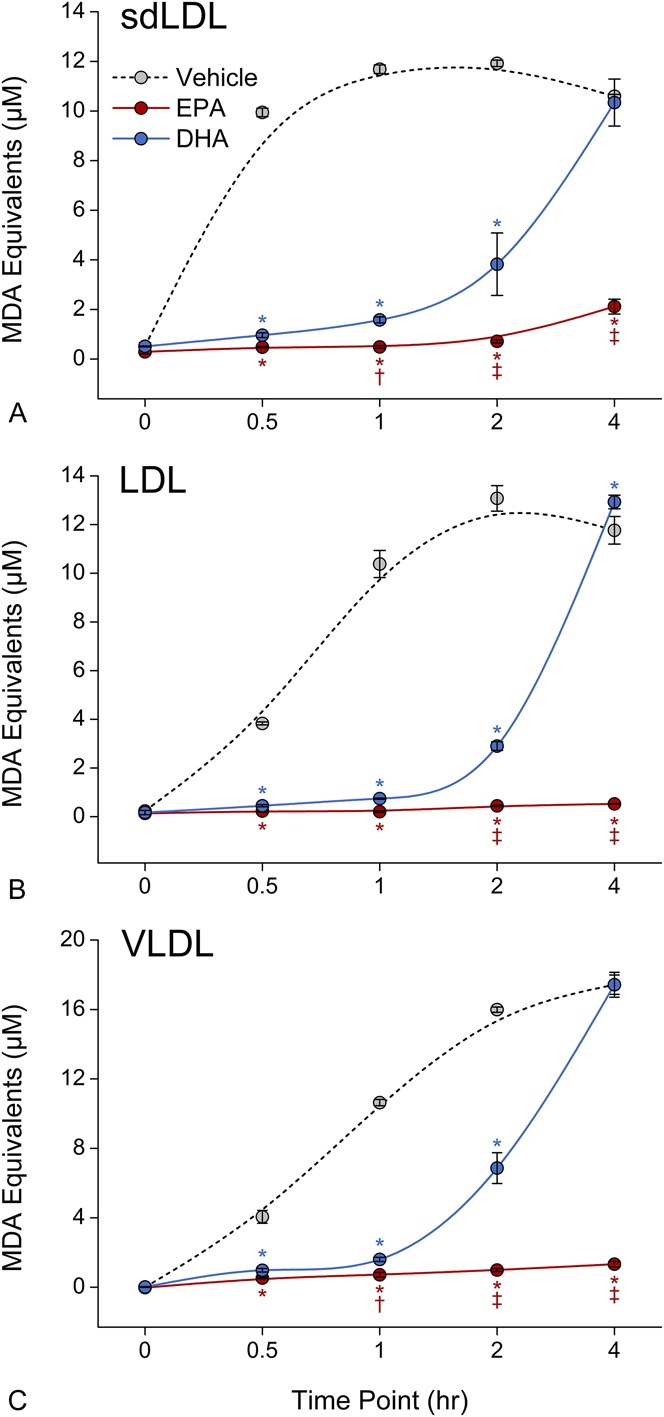
Comparative effects of EPA and DHA on human sdLDL (A), LDL (B), and VLDL (C) oxidation. EPA and DHA were tested at 10 μM (sdLDL) and 2.5 μM (LDL and VLDL). Data were collected at various time points up to 4 hours following initial exposure to oxidative conditions. *P < 0.001 versus vehicle-treated control; †P < 0.05 and ‡P < 0.001 versus DHA (Student–Newman–Keuls multiple comparisons test; overall ANOVA—sdLDL data: P < 0.0001, F = 391.88; LDL data: P < 0.0001, F = 1191.3; VLDL data: P < 0.0001, F = 1074.8). ANOVA, analysis of variance; MDA, malondialdehyde.
DISCUSSION
In this study, EPA inhibited the oxidation of different-sized apoB-containing lipoprotein particles in a dose-dependent manner at pharmacologically relevant treatment levels.42 The ability of EPA to interfere with oxidation in these particles may be attributed to quenching of reactive oxygen species following its intercalation into the target lipid environment.19 The inhibitory effects of EPA on sdLDL oxidation were not observed with other triglyceride-lowering agents or vitamin E under identical conditions. These findings suggest that EPA preferentially intercalates into various-sized apoB-containing lipoprotein particles, where it can provide optimal benefit. DHA also interfered with lipoprotein oxidation, but the effects were limited to a relatively short time period compared with EPA. These disparate effects may be the result of differences in the number of double bonds and carbon chain lengths, both of which may influence the location and orientation of these 2 distinct omega-3 fatty acids within the various-sized particles. The greater antioxidant activity of EPA in VLDL may be attributed to higher incorporation rates, or to more optimal orientation of EPA within the larger and therefore less curved outer lipid monolayer of VLDL compared with LDL or sdLDL.
Patients with severe hypertriglyceridemia are often treated with a potent statin (such as atorvastatin) and may potentially benefit with statin-EPA combination therapy. In the present study, we demonstrated that the antioxidant effects of EPA in the different-sized apoB-containing lipoproteins could be enhanced in combination with atorvastatin metabolite at certain concentrations. When combined at lower doses, EPA and atorvastatin metabolite inhibited LDL oxidation to an extent that was greater than the sum of their separate effects. This indicates potentially synergistic actions for the 2 agents and suggests the possibility of discrete physicochemical interactions that may enhance their separate electron trapping and stabilization mechanisms or facilitate electron sharing between the molecules. In particular, the metabolite form of atorvastatin used in this study has a phenoxy group that can donate a proton while also stabilizing an unpaired electron in its conjugated ring structure. Additional research will be valuable and necessary to determine the exact mechanism(s) for the potentially synergistic benefits associated with these agents.
HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase inhibitors (statins) interfere with LDL oxidation by several mechanisms, including inhibition of p21 Rac isoprenylation and reduction in the expression of NADPH subunits.43 The active hydroxy metabolite of atorvastatin, in particular, is capable of directly inhibiting LDL, sdLDL, and cell membrane lipid oxidation at pharmacologic levels.40,44 The chain-breaking antioxidant mechanism for atorvastatin metabolite is specifically attributed to its phenoxy moiety and is not evoked by other antioxidants such as vitamin E.38,39 Clinical support for an antioxidant benefit with atorvastatin has also been reported from independent laboratories in randomized trials.45,46 The antioxidant activity of atorvastatin metabolite may contribute to its anti-inflammatory benefits and account for differences in benefit beyond LDL lowering even as compared with other statins.47,48 Based on the findings from this study, we hypothesize that these important benefits may be enhanced in combination with EPA.
Oxidized lipids associated with lipoprotein particles are a major source of vascular inflammation during atherosclerosis.20,22,49,50 OxLDL is taken up by “scavenger receptors,” resulting in cholesteryl ester accumulation and macrophage foam cell formation.51 Oxidative modification also reduces the affinity of LDL particles for high-affinity LDL receptors, resulting in reduced hepatic clearance of oxLDL. Numerous studies have linked oxLDL to foam cell formation and plaque evolution and have recognized the potential role of antioxidants to reverse these effects.20,22,52 All these events lead to plaque destabilization and intracoronary thrombus formation through rupture of the fibrous cap. Indeed, levels of oxidized lipid, as measured by thiobarbituric acid reactive substances, lipid hydroperoxides, or monoclonal antibodies against oxLDL, have been shown to correlate with the severity of acute coronary syndromes and to predict cardiovascular events.23,53 Reduction of circulating levels of oxLDL has therefore become an important therapeutic consideration in the management of cardiovascular risk.
Circulating LDL particles are heterogeneous in size, density, and composition. There is evidence that increased levels of sdLDL, in particular, are associated with higher risk of CAD.28,30 This association is biologically plausible given the greater susceptibility of sdLDL to oxidation, diminished affinity for the LDL receptor, reduced turnover of sdLDL, and increased oxidative modification of apoB compared with large buoyant LDL.30,35 Levels of sdLDL are proportionately elevated in patients with cardiovascular risk factors or risk equivalents, such as type 2 diabetes and metabolic syndrome.54 Due to its antioxidant properties, EPA may influence sdLDL oxidation and clearance. This is supported by recent evidence from the MARINE (Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension) and ANCHOR trials in which oxLDL and LDL particle numbers (especially evident in sdLDL) were decreased with EPA treatment compared with placebo in patients with very high triglycerides and in statin-treated patients with high triglycerides, respectively, and from a subanalysis of patients with diabetes from the ANCHOR study in which treatment with EPA significantly reduced oxLDL compared with placebo.5,55–57 In addition, in a study of hemodialysis patients, EPA decreased circulating levels of oxLDL by 38%.58 These reductions in oxLDL could be occurring by decreases in oxidation, changes in particle release/clearance, or all 3, but they are suggestive that the antioxidant properties of EPA observed in the current studies may also be occurring clinically. The effects of EPA treatment on LDL and sdLDL particle levels could be clinically important given their atherogenic properties, especially when oxidized. There is also increased risk of CAD with elevated VLDL levels, which are common in patients with high triglyceride levels.37 In addition to the beneficial effects noted above, EPA treatment also decreased large VLDL particle concentration in the MARINE and ANCHOR studies.55,56
The experiments presented herein suggest a reduction in oxidation of apoB-containing lipoprotein particles, which is in agreement with the clinical data discussed above. In addition to antioxidant properties, other studies have suggested an improvement in lipoprotein functionality with the incorporation of EPA, such as an increase in the cholesterol efflux capacity of HDL from macrophages.59 Potential changes in apoB-containing lipoprotein functionality would be an interesting area for future research.
The ability of EPA to interfere with LDL oxidation has significant clinical implications. The potent antioxidant effects of EPA may help account for reduced cardiovascular events reported for hypercholesterolemic patients receiving statin treatment as observed in the Japan EPA Lipid Intervention Study (JELIS) and coronary plaque regression observed in statin-treated coronary heart disease patients as observed in the Combination Therapy of Eicosapentaenoic Acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography (CHERRY) study.11,60 The benefits of EPA treatment may be attributed, in part, to decreased inflammation and oxLDL compared with placebo.4–6 Further prospective studies are underway to assess the broader clinical benefits of EPA among high-risk cardiovascular patients. In particular, the ongoing Reduction of Cardiovascular Events with EPA–Intervention Trial (REDUCE-IT) will evaluate the ability of EPA to reduce cardiovascular outcomes in high-risk statin-treated patients with mixed dyslipidemia (NCT01492361).
CONCLUSIONS
EPA is a potent lipophilic antioxidant that was shown in this study to inhibit sdLDL, LDL, and VLDL oxidation at pharmacologic levels. We also observed that those effects were unique compared with other triglyceride-lowering agents, vitamin E, or DHA. These data provide new insight into the differential role of EPA with respect to other triglyceride-lowering agents in lipoprotein lipid oxidation, representing important events in atherosclerosis and CAD.
ACKNOWLEDGMENTS
The authors thank Rebecca Juliano, PhD, and Sephy Philip, RPh, PharmD, for helpful scientific discussions.
Footnotes
Supported by Amarin Pharma Inc, Bedminster, NJ. Editorial assistance was provided by Peloton Advantage, LLC, Parsippany, NJ and was funded by Amarin Pharma Inc.
R. P. Mason has received grant/research support from Amarin Pharma Inc, Pfizer and Novartis; provides speaking and consultancy services (including receipt of honoraria) for Novartis and Amarin Pharma Inc. The other authors report no conflicts of interest.
REFERENCES
- 1.Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–1053. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56:1073–1080. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984–992. [DOI] [PubMed] [Google Scholar]
- 5.Bays HE, Ballantyne CM, Braeckman RA, et al. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108:682–690. [DOI] [PubMed] [Google Scholar]
- 7.Braeckman RA, Manku MS, Bays HE, et al. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study). Prostaglandins Leukot Essent Fatty Acids. 2013;89:195–201. [DOI] [PubMed] [Google Scholar]
- 8.Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30:144–146. [DOI] [PubMed] [Google Scholar]
- 9.Satoh-Asahara N, Shimatsu A, Sasaki Y, et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35:2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18:99–107. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 12.Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191:162–167. [DOI] [PubMed] [Google Scholar]
- 13.Takaki A, Umemoto S, Ono K, et al. Add-on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: a randomized controlled study. J Atheroscler Thromb. 2011;18:857–866. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki J, Miwa T, Odawara M. Administration of highly purified eicosapentaenoic acid to statin-treated diabetic patients further improves vascular function. Endocr J. 2012;59:297–304. [DOI] [PubMed] [Google Scholar]
- 15.Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 16.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson MH, Rosenson RS, Maki KC, et al. Effects of fenofibric acid on carotid intima-media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo-controlled study (FIRST). Arterioscler Thromb Vasc Biol. 2014;34:1298–1306. [DOI] [PubMed] [Google Scholar]
- 19.Mason RP, Jacob RF. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim Biophys Acta. 2015;1848:502–509. [DOI] [PubMed] [Google Scholar]
- 20.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg D. Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. [DOI] [PubMed] [Google Scholar]
- 22.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. [DOI] [PubMed] [Google Scholar]
- 23.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–1960. [DOI] [PubMed] [Google Scholar]
- 24.Holvoet P, Kritchevsky SB, Tracy RP, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53:1068–1073. [DOI] [PubMed] [Google Scholar]
- 25.Walter MF, Jacob RF, Jeffers B, et al. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol. 2004;44:1996–2002. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo M, Berneis K. Low-density lipoprotein size and cardiovascular risk assessment. QJM. 2006;99:1–14. [DOI] [PubMed] [Google Scholar]
- 27.Ai M, Otokozawa S, Asztalos BF, et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 29.Hirano T, Ito Y, Koba S, et al. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol. 2004;24:558–563. [DOI] [PubMed] [Google Scholar]
- 30.Koba S, Hirano T, Ito Y, et al. Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis. 2006;189:206–214. [DOI] [PubMed] [Google Scholar]
- 31.Anber V, Griffin BA, McConnell M, et al. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis. 1996;124:261–271. [DOI] [PubMed] [Google Scholar]
- 32.Galeano NF, Al-Haideri M, Keyserman F, et al. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res. 1998;39:1263–1273. [PubMed] [Google Scholar]
- 33.Krauss RM. Heterogeneity of plasma low-density lipoproteins and atherosclerosis risk. Curr Opin Lipidol. 1994;5:339–349. [DOI] [PubMed] [Google Scholar]
- 34.Nigon F, Lesnik P, Rouis M, et al. Discrete subspecies of human low density lipoproteins are heterogeneous in their interaction with the cellular LDL receptor. J Lipid Res. 1991;32:1741–1753. [PubMed] [Google Scholar]
- 35.de Graaf J, Hak-Lemmers HL, Hectors MP, et al. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb. 1991;11:298–306. [DOI] [PubMed] [Google Scholar]
- 36.Tribble DL, Holl LG, Wood PD, et al. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93:189–199. [DOI] [PubMed] [Google Scholar]
- 37.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. 2014;13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mason RP, Walter MF, Day CA, et al. Active metabolite of atorvastatin inhibits membrane cholesterol domain formation by an antioxidant mechanism. J Biol Chem. 2006;281:9337–9345. [DOI] [PubMed] [Google Scholar]
- 39.Aviram M, Rosenblat M, Bisgaier CL, et al. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 1998;138:271–280. [DOI] [PubMed] [Google Scholar]
- 40.Jacob RF, Walter MF, Self-Medlin Y, et al. Atorvastatin active metabolite inhibits oxidative modification of small dense low-density lipoprotein. J Cardiovasc Pharmacol. 2013;62:160–166. [DOI] [PubMed] [Google Scholar]
- 41.Davies IG, Graham JM, Griffin BA. Rapid separation of LDL subclasses by iodixanol gradient ultracentrifugation. Clin Chem. 2003;49:1865–1872. [DOI] [PubMed] [Google Scholar]
- 42.Braeckman RA, Stirtan WG, Soni PN. Pharmacokinetics of eicosapentaenoic acid in plasma and red blood cells after multiple oral dosing with icosapent ethyl in healthy subjects. Clin Pharmacol Drug Dev. 2014;3:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner AH, Kohler T, Ruckschloss U, et al. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. [DOI] [PubMed] [Google Scholar]
- 44.Mason RP, Walter MF, Jacob RF. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109(21 suppl 1):II34–II41. [DOI] [PubMed] [Google Scholar]
- 45.Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–1412. [DOI] [PubMed] [Google Scholar]
- 46.Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–431. [DOI] [PubMed] [Google Scholar]
- 47.Mason RP, Walter MF, Day CA, et al. Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am J Cardiol. 2005;96:11F–23F. [DOI] [PubMed] [Google Scholar]
- 48.Kinlay S, Schwartz GG, Olsson AG, et al. Inflammation, statin therapy, and risk of stroke after an acute coronary syndrome in the MIRACL study. Arterioscler Thromb Vasc Biol. 2008;28:142–147. [DOI] [PubMed] [Google Scholar]
- 49.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 50.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein JL, Ho YK, Basu SK, et al. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parthasarathy S, Young SG, Witztum JL, et al. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986;77:641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walter MF, Jacob RF, Bjork RE, et al. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: the PREVENT study. J Am Coll Cardiol. 2008;51:1196–1202. [DOI] [PubMed] [Google Scholar]
- 54.Hulthe J, Hulten LM, Fagerberg B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism. 2003;52:1612–1614. [DOI] [PubMed] [Google Scholar]
- 55.Bays HE, Braeckman RA, Ballantyne CM, et al. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on lipoprotein particle concentration and size in patients with very high triglyceride levels (the MARINE study). J Clin Lipidol. 2012;6:565–572. [DOI] [PubMed] [Google Scholar]
- 56.Ballantyne CM, Braeckman RA, Bays HE, et al. Effects of icosapent ethyl on lipoprotein particle concentration and size in statin-treated patients with persistent high triglycerides (the ANCHOR Study). J Clin Lipidol. 2015;9:377–383. [DOI] [PubMed] [Google Scholar]
- 57.Brinton EA, Ballantyne CM, Bays HE, et al. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200–500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ando M, Sanaka T, Nihei H. Eicosapentanoic acid reduces plasma levels of remnant lipoproteins and prevents in vivo peroxidation of LDL in dialysis patients. J Am Soc Nephrol. 1999;10:2177–2184. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka N, Ishida T, Nagao M, et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis. 2014;237:577–583. [DOI] [PubMed] [Google Scholar]
- 60.Ando K, Watanabe T, Daidoji H, et al. Combination therapy of eicosapentaenoic acid and pitavastatin for coronary plaque regression evaluated by integrated backscatter intravascular ultrasonography: a randomized controlled trial. Circulation. 2015;132:A12007. [DOI] [PubMed] [Google Scholar]


