Abstract
Objectives
First objective was better understanding of the indications of chemotherapy in elderly with advanced cancer, tolerability and toxicity of chemotherapy in this age group. The second objective was to define current practice in chemotherapy for elderly people with advanced cancer for a selected group of patients treated in Institute of Oncology Bucharest (IOB).
Materials and Methods
The study makes a clinical analysis of medical records of 27 patients from the archive of Institute of Oncology Bucharest treated by the same doctor. Patients were selected according to: age ≥ 65 years, ECOG performance status 0–1, normal blood counts and blood biochemistry, histological confirmation of the diagnosis of cancer, patients should received at least 3 cycles of chemotherapy. We extract characteristics of the patients to see if they were a homogeneous group of patients and to compare them with data from the literature. Overall survival was calculated by the Kaplan Meyer curve.
Results
295 patients more then 65 years were treated in our site in 2 years 2011, 2012. 93 patients received chemotherapy and only 27 patients were enrolled in this study following inclusion criteria. Common sites of cancer were lung and breast. The most used cytostatics for lung cancer was gemcitabine and carboplatine and cyclophosphamide, metotrexat and 5 fluorouracil for breast cancer. Toxicity was mild with the prevalence of hematologic toxicity. Overall survival without taking into account the type of cancer was 27.7 month.
Conclusions
For selected patients, chemotherapy was well tolerated and appears to prolong survival regardless of the location of cancer. The relatively small number of elderly patients who received chemotherapy is probably due to lack of compliance to treatment, the increased number of co-morbidities and evaluation of performance status only by the ECOG index known not to be good enough to establish the indication of chemotherapy.
Keywords: Chemotherapy, geriatric oncology, palliative care
INTRODUCTION
Chemotherapy in elderly patients with advanced cancer has become an important issue for oncologists around the world along with the aging population. However, geriatric oncology in Romania is underdeveloped due to lack of concern in this respect at the university level and with the health policy makers.
Incidence of cancer in elderly people worldwide and in Romania
Prestigious statistics reveals an increased incidence of cancer in general. Given that the proven malignant tumors occur most often in people over 60 years (with some exceptions) reveals the importance of the study of advanced cancer treatment in this population.
In Europe in 2008 there were 2.4 million cases of cancer (Ferlay et al., 2010). The risk of cancer at the age of 75 is of 26.5%, which means that about one in four adults who reach this age is at risks to get cancer. Europe’s population is an aging population and the risk of cancer is increasing.[1]
Standardized cancer incidence by age group rose in Europe particularly in the age group over 75 years. Thus, from 1669–100,000 (1975–1977), it reached to 2350–100,000 (1990–2000). This value maintaining approximately the same extent also in the last decade, while the standardized incidence of cancer in persons aged 60 to 74 years increased from 1028 (1975–1977) to 1409 (2008–2010 ).[2]
The highest incidence of cancer in men and women locations is as follows: Men: 1,095,200 lung cancer, prostate cancer 903,500, colon cancer and rectum 640,600, gastric cancer 663,600, liver cancer 522,400. In women breast cancer 138,350, colon and rectum 570,100, cervix uteri 529,800, lung cancer 513,600, stomach 349,000.[3]
In Romania, the incidence of cancer is not very well studied. Data in this study come from GLOBOCAN website and publications from Central Statistical Institute of Oncology, Cluj, Napoca.
Thus, in men over 65 years the incidence of cancer is as follows: 184–100,000 gastric cancer, lung cancer 164–100,000, prostate cancer 92–100,000, 38–100,000 bladder cancer, rectal cancer 22–100,000.
In women over 65 years the incidence of cancer is stomach cancer 85–100,000, 52–100,000 breast cancer, lung cancer 36–100,000 and 35–100,000 cervix.
This incident is quite different than the recorded incidence in adults aged 35 to 64 years. For these persons we have the following incidence data: Men: Pulmonary 67–100,000, stomach 33–100,000, 10–100,000 larynx, pancreas 8–100,000, 7–100,000 oral cavity. Women: 28–100,000 breast, cervical uterine 20–100,000, 100,000 stomac13, lung 11–100,000.[4]
MATERIALS AND METHODS
The study makes a clinical analysis of medical records of 27 patients from the archive of the Institute of Oncology Bucharest treated by the same doctor. Patients were selected according to: Age ≥65 years, ECOG performance status 0–1, normal blood counts and blood biochemistry, histological confirmation of the diagnosis of cancer, three or more cycles of chemotherapy and survival of more than 1 year. We extract characteristics of the patients to see if they cause a homogeneous group of patients and to compare them with data from the literature.
The files examined were selected from 295 cases of patients over 65 years old with different localizations of cancer, admitted to the Oncology Institute of Bucharest in day care or impatient departments. The period studied was: January 2011-December 2012. 93 patients from the whole lot received chemotherapy. From the evaluation were excluded patients who did not qualify for the inclusion criteria. We watched availability of cytostatic treatment of older people suffering from cancer, number of cycles of chemotherapy administered, and tolerability to the used cytostatics type. Overall survival of the entire group was estimated based on the Kaplan Meyer curve. Toxicity was appreciated under the WHO classification.
In this study assessing functional status of patients was made clinically without a standardized test due to the actual conditions of work in the IOB (lack of specialists in geriatrics, shortage of nurses trained in the care of the elderly, the short time that medical oncologists can give patient for consultation).
So have proved simple questions to define ECOG performance status (do you have disease symptoms at rest: Do you have symptoms of illness during exercise? Do you wait for more than 50% of the waking time of the day in bed? Can you do daily activities without the help of other people?); one single question about the mental state: Do you think [name of patient] has been more confused lately?[5] Questions about family relationships: Living with husband (wife) or other person in the family? Can you rely on the help of a person in the family or another person who is close?
RESULTS
In 2011 were treated 420 patients (of which 140 elderly) respectively 430 in 2012 (of which 155 elderly).
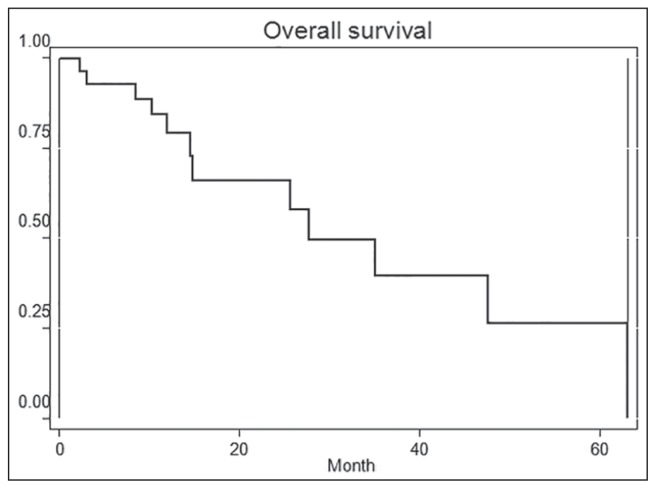
Patient characteristics: Tables 1–3. Most patients had two to three co-morbidities. The number of cycles of chemotherapy was in average 12 (3–24). The majority of patients had two to three line of chemotherapy. Anticancer drugs used: Carboplatin, epirubicin, gemcitabine, paclitaxel, docetaxel, 5-fluorouracil, cyclophosphamide, irinotecan, capecitabine. Overall survival was of 27.7 months of the group of patients not taking into account the type of cancer [Figure 1]. Toxicity was assessed according to the WHO classification [Table 4].
Table 1.
Patient characteristics
| Average age: 73.4 (65–80) |
| Sex: 16 women/11 man |
| TNM: 70% St IV, 25% StIII, 5% StII |
Table 2.
Patient characteristics, cancer localizations
| Type of cancer | No. of cases |
|---|---|
| Lung | 9 |
| Breast | 8 |
| Bladder | 3 |
| Ovarian | 3 |
| Colon | 2 |
| Soft tissue sarcoma | 1 |
| Gastric | 1 |
Table 3.
Patient characteristics, co-morbidity
| 59% of patients have co morbidities |
| The main concomitant disease are |
| Arterial hypertension |
| Ischemic cardiac disease |
| Arteriopathy |
| Chronic hepatitis |
| Renal stones |
| Degenerative rheumatism |
| Hypertiroidy |
| Prostate adenoma |
Figure 1.
Median survival: 27.7 month (The Kaplan-Meier estimation of overall survival)
Table 4.
Toxicity
| Only 2 cases: Degree 3 |
| 25 cases had degree I and II toxicity |
| Anemia: 37.5% |
| Nausea: 40% |
| Mucositis: 20% |
| Asthenia: 60% |
| Dispnea: 25.9% |
| Neurological toxicity: 5% |
| Diarrhea: 35 |
| Intercurrent infections: 18.5% |
| Necessary hospitalization: 10% |
DISCUSSION
Approximately 60% of cancers occur in people aged over 65. We also know that the population of this age is much less introduced in studies that make it have less evidence on efficacy and tolerability of patients of this age to chemotherapy. Our study had the intention to reveal characteristics of patients who tolerated chemotherapy well and had a clinical benefit. Other objectives were the estimated overall survival and toxicities for commonly used chemotherapy. Median overall survival of 27.7 months refers to the whole group of patients. For this reason survival data have no great relevance. Relevance is low due to the small number of patients studied and different analyzed types of cancer that have specific features for survival. Many patients were still alive at the time of analysis of survival which increases the possibility of error. However, global survival calculated value means that in well-selected groups of patients older than 65 years, chemotherapy may result in an improvement in survival.
Analyzing the data of cancer incidence in Romania by age shows that our selected group is a balanced group of that proportion. Out of 850 patients of all ages who were treated in 2011 and 2012 in the study site by the same physician, 295 were over 65 years (36%). Only 27 patients of those over 65 have met the criteria for inclusion in the study 9.1%. Given that the most important criteria for selection of these patients was the number of series of chemotherapy, which should be more than 3 performance status 0–1. Other patients could not receive chemotherapy for various reasons. The causes are multiple: Multiple co-morbidities, lack of patients or family of the necessary budget to present to the hospital (transport and attendance cost), and altered performance status. But we consider that the unavailability of a comprehensive geriatric assessment resulted in lack of adequate treatment of this group of patients because the performance status ECOG in people more than 65 years old is not reliable enough to decide the indication or contra-indication of chemotherapy. Family and social issues of the elderly who have an important impact on therapeutic decision from both the patient and the doctor have been demonstrated in other studies.[6]
In discussing the case of the selected patient group, the number of cycles of chemotherapy was conducted between 3 and 24 with a mean of 12 cycles. This relatively large number of cycles of chemotherapy reveals a good tolerability. Although it was a group of patients with a good short- and medium-term forecast, their treatment required a more detailed assessment of the present state, good functionality of vital organs complexity of cases as a consequence of the fact that most have a complex-associated pathology.[7]
In our study, most cases were breast and lung cancers. We had seven cases of breast cancer who received the most frequent CMF chemotherapy (ciclophosphamide 500 mg/m2, methotrexate 25 mg/m2, 5 fluorouracil 500 mg/m2) with toxicities blood in particular. The higher frequency of such toxicity in the elderly is known from the previous practice experience, doses were lower than those commonly used. Chemotherapy of breast cancer in older patients was better studied even realizing meta-analysis for pertinent conclusions. Such a meta-analysis failed to establish a workable model of chemotherapy in older women and its effectiveness. However, external validation is expected to render final conclusions.[8]
Monochemotherapy dealt with another therapeutic modality in patients in our study. We used capecitabine or vinorelbine tablets recording the good tolerance and compliance to treatment. We have not administrated to patients in this study anthracyclines or taxanes having previous experience of some major toxicities confirmed by certain studies. Such a study of Chetan Shenoy et al. shows that cardiac toxicity in patients who have had antraciclines chemotherapy for breast cancer is significantly higher than those that were not being treated with anthracyclines but only with hormone therapy.[9]
In our study we had nine cases of lung cancer that were treated with the following chemotherapy regimens: Gemcitabine plus erlotinib for one patient, paclitaxel plus carboplatin for four patients, vinorelbine plus carboplatin for one patient, docetaxel alone for one patient, vinorelbine alone for one patient (for non-small-cell lung cancer — NSCLC — first line), Etoposide plus carboplatin (first line), topotecan with oral use (second line for small-cell lung cancer — SCLC). Only three patients had NSCLC treatment in second line: Two with erlotinib and one with gemcitabine). Many cases of lung cancer are treated only symptomatically because of poor general condition, of co-morbidities and probably the lack of adequate geriatric assessment to determine the actual risk of elderly patients with chemotherapy treatment. In the United States the median age of diagnosis of lung cancer is 72 years[10] (but in our practice in Romania we have many cases younger than 60 years old). It follows that most of the population suffering from lung cancer is 72 years and older. However, the authors conclude that it is a small number of trials that enrolled mostly patients with quoted ages and a small number of lung cancer patients aged over 65 years are generally treated. The mentioned study reveals that doublet chemotherapy is more effective than mono-chemotherapy. In previous studies of adjuvant chemotherapy in patients over 65 years found that it increases survival.[11]
In our study NSCLC chemotherapy in patients over 65 and even over 70 years proved feasible in the sense of tolerance similar to that of the adult population result confirmed in other studies.[12–14]
In regard to small-cell lung cancer (SCLC), we cannot have reliable conclusions about a single case being treated with chemotherapy, the event which had a similar trend to that of an adult up to 65 years, a trend confirmed by existing studies.[15]
We cannot draw conclusions on the behavior of chemotherapy in patients with other cancers included in the study because their number is very small and it should be only a case report. However, we can say that for the selected cases of cancer of the ovary, bladder, soft tissue sarcoma, chemotherapy is relatively well tolerated and may have a positive impact on patients’ symptoms and even survival.
CONCLUSIONS
The study reaffirms that chronological age by itself it is not an appropriate factor for assessing the indication and especially tolerability to chemotherapy. For selected patients, chemotherapy was well tolerated and appears to prolong survival regardless of the location of cancer. Toxicity was moderate and not different from that seen in adults except for a slightly higher hematologic toxicity.
Many older patients with breast or lung cancer were receiving chemotherapy treatment with good tolerability, they were rated as ECOG 0 or 1.
The relatively small number of elderly patients with advanced cancer who received chemotherapy is probably due to lack of compliance to treatment, the increased number of co-morbidities and evaluation of performance status only by ECOG index which is known that is not good enough to establish the indication of chemotherapy.
A prospective study is needed to define the so-called reality map for practice of geriatric oncology in IOB.
ACKNOWLEDGMENTS
The study had no sponsorship. We thank the Bucharest Oncology Institute staff for administrative problems that it helped to solve.
Footnotes
Source of Support: NIL, Conflict of Interest: NIL.
REFERENCES
- 1.OECD/European Union. Health at a Glance: Europe 2010. OECD Publishing; 2010. Cancer Incidence. http://dx.doi.org/10.1787/9789264090316-18-en. [Google Scholar]
- 2.Cancer Research UK. Cancer incidence by age. [Last accessed on 2014 Nov 10]. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/age/
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Senciuc Adrian. Epidemiologia cancerelor umane. [Last accessed on 2014 Nov 10]. Available at: http://www.scientia.ro/biologie/37-cum-functioneaza-corpul-omenesc/4050-epidemiologia-cancerelor-umane.html.
- 5.Sands MB, Dantoc BP, Hartshorn A, Ryan CJ, Lujic S. Single Question in Delirium (SQiD): Testing its efficacy against psychiatrist interview, the Confusion Assessment Method and the Memorial Delirium. Palliat Med. 2010;24:561–5. doi: 10.1177/0269216310371556. [DOI] [PubMed] [Google Scholar]
- 6.Given B, Given CW. Older adults and cancer treatment. Cancer. 2008;113(12 Suppl):3505–11. doi: 10.1002/cncr.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olver Ian. Chemotherapy for elderly patients with advanced cancer: is it worth it? Aust Prescr. 2000;23:80–2. [Google Scholar]
- 8.Kumar A, Soares H, Balducci L, Djulbegovic B. Treatment tolerance and efficacy in geriatric oncology: A systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. J Clin Oncol. 2007;25:1272–6. doi: 10.1200/JCO.2006.09.2759. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy C1, Klem I, Crowley AL, Patel MR, Winchester MA, Owusu C, et al. Cardiovascular Complications of Breast Cancer Therapy in Older Adults. Oncologist. 2011;11:1138–43. doi: 10.1634/theoncologist.2010-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldhirsch A, Colleoni M, Coates AS, Castiglione-Gertsch M, Gelber RD. Adding adjuvant CMF chemotherapy to either radiotherapy or tamoxifen: Are all CMFs alike? International Breast Cancer Study Group (IBCSG) Ann Oncol. 1998;9:489–93. doi: 10.1023/a:1008236502420. [DOI] [PubMed] [Google Scholar]
- 11.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: Findings from medicare claims data. J Clin Oncol. 2001;19:1455–61. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 12.Quoix E, Zalcman G, Oster JP, Westeel V, Pichon V, Lavole A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–88. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 13.Gridelli C, Maione P, Comunale D, Rossi A. Adjuvant chemotherapy in elderly patients with non-small-cell lung cancer. Cancer Control. 2007;14:57–62. doi: 10.1177/107327480701400108. [DOI] [PubMed] [Google Scholar]
- 14.Chen YM, Perng RP, Shih JF, Tsai CM, Whang-Peng J. Chemotherapy for non-small cell lung cancer in elderly patients. Chest. 2005;128:132–9. doi: 10.1378/chest.128.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Janssen-Heijnen ML, Maas HA, van de Schans SA, Coebergh JW, Groen HJ. Chemotherapy in elderly small-cell lung cancer patients: Yes we can, but should we do it? Ann Oncol. 2011;22:821–6. doi: 10.1093/annonc/mdq448. [DOI] [PubMed] [Google Scholar]