Supplemental Digital Content is available in the text.
Keywords: apoptosis, hexokinase, Lactobacillus casei, metabolism, mitochondria, peptide, peptidoglycan, tumour cells
Abstract
In a previous study, we reported the cytotoxic activity against various tumour cells of the peptidoglycan of Lactobacillus casei. To isolate the most active components, we performed column-chromatography separation of the peptidoglycan complex and tested the related fractions for their cytotoxic activity. The most active fractions were then lyophilized and the residue was analysed by gas chromatography for its amino acid content and composition. On the basis of the known chemical formula of the basic peptidic component of the peptidoglycan complex of L. casei, a peptide was then synthesized [Europ. (CH-DE-FR-GB) Patent number 1217005; IT number 01320177] and its cytotoxicity was tested against tumoural and normal cells. The synthetic peptide was found to impair the entire metabolism of cultured tumour cells and to restore the apoptotic process. By contrast, normal cells appeared to be stimulated rather than inhibited by the peptide, whereas primary mouse embryo fibroblasts behaved similarly to tumour cells. On the basis of these results, L. casei peptidoglycan fragments and their constituent basic peptide might be applicable as potent antitumour agents.
Introduction
The Lactobacillus casei, a Gram-positive, nonpathogenic lactic acid bacterium, is a natural host of the human microbiotic environment. Many reports have shown that L. casei has considerable antitumoural activity both in vivo and in vitro 1–3. Its anticancer activity has been related to several mechanisms involving activated macrophages, modulation of the host’s immune response and regulation of cell apoptosis. Although many antitumoural mechanisms of L. casei were initially believed to be host mediated, other in-vitro studies have shown that L. casei shows a direct antitumoural activity on cancer cells 2–5.
We previously reported a direct in-vitro anticancer activity of the peptidoglycan complex of L. casei and showed that the cytotoxic events, that is, the rapid and substantial decrease in the intracellular ATP level and, subsequently, the reduction in the succinate dehydrogenase activity, start briefly after the cells have been exposed to peptidoglycan, suggesting that the compound acts through a rapid and critical disturbance of cellular metabolism 6. On the basis of these findings, now confirmed by the action of the isolated synthetic, small peptide, further research was directed towards identifying the presumptive enzymatic target whose impairment could be responsible for the cytotoxic events observed in the tumour cells.
In this context, it must be noted that one of the most conspicuous properties of rapidly growing cancer cells is their high rate of glycolysis. Today, there are two well-documented phenomena of aberrant glucose metabolism in cancer cells. The first is the high overexpression of hexokinase type II, which is essential (a) to support the elevated request for energy and (b) to provide through glycolytic steps the anabolic precursors for the biosynthetic pathways. The second is the tendency of this enzyme to bind to the outer mitochondrial membrane, with the consequential well-known implications for the antiapoptotic process that confer stability to the tumoural condition. Among the various glycolytic enzymes present in tumour cells, hexokinase, and particularly isomer II, is the only one that can bind to mitochondria, the other glycolytic enzymes being generally localized in the cytosol 7. Moreover, it has been suggested that the binding of hexokinase to the surface of tumour mitochondria involves porin, a mitochondrial outer membrane protein also referred to as voltage dependent anion channel (VDAC). By analogy to bacterial porins of known three-dimensional structure, the porin molecule possesses a transmembrane β-barrel structure that, in its dimeric form, is believed to form a hydrophilic pore in the outer mitochondrial membrane, allowing neutral molecules (up to 5 kDa) to diffuse into and out of the mitochondria. In this way, the porins establish a regulated gate between the cytosol and intramitochondrial compartments for the exchange of metabolites. The hexokinase–porin interaction leads to a preferential interaction of hexokinase with ATP synthesized in the inner mitochondrial compartment 7. Further studies 8–12 were fundamental in clarifying the details of this capability of hexokinase to bind to the mitochondrial surface and its numerous functional consequences: first, much greater amounts of hexokinase are bound to mitochondria in tumour than in normal cells; second, hexokinase bound to mitochondria is much less sensitive to inhibition by glucose-6-phosphate, an important regulator of hexokinase in normal cells; and, third, the inhibition of the apoptotic process. The consequence of these three differences between tumour and normal cells is that in the former glycolysis is markedly enhanced and less inhibited by the product of phosphotransferase activity, whereby in both cases, the same reaction product is the fundamental precursor for the biosynthesis of nucleic acids and phospholipids. Under such conditions, tumour cells have the maximal energetic and structural prerequisites for abnormal proliferation and, even more, the presence of the VDAC-bound hexokinase II in the outer mitochondrial membrane inhibits Bax-induced apoptosis 13–15.
The present paper describes the identification and isolation steps of the active and the cytotoxic process that is determined from this small peptide that is the minimal peptidoglycan component responsible for the cytotoxic events observed in tumour cells. Moreover, evidence is presented to show that the observed metabolic blockage of glycolysis, secondary to this peptide’s activity, lies, as experimentally established, in its capacity to bind, and displaces the hexokinase. In addition, these events are dependent on the various metabolic phases of the cells that favour the antiapoptotic activity and thereby restore the antitumoural sequences of apoptosis.
Materials and methods
Bacterial strain
L. casei ATCC 25180 cells were kindly provided by Nestlè Co. (Orbe, Switzerland) and cultured in MRS medium (Oxoid GmbH, Wesel, Germany) for 20 h at 30°C. After cultivation, the cells were collected by centrifugation at 3000g for 10 min at room temperature, washed several times with MRS medium and finally lyophilized. Before use, the lyophilized bacterial preparation was washed in sterile saline (0.5% NaCl) and then cultured in MRS broth.
Animals
CD-1 Swiss mice, male, aged 7–8 weeks, weight ∼20 g, were kept at the animal facility of the Pathology Department at the University of Catania. The mice were kept under standard laboratory conditions (nonspecific pathogen free) with free access to food and water. The animal studies were carried out in accordance to local guidelines and approved by the local Institutional Animal Care and Use Committee.
Cells
Murine YAC-1 lymphoma (ATCC TIB 160), human K562 erythroleukaemia (ATCC CCL 243) and murine P815 mastocytoma (ATCC TIB 64) cells were cultured in RPMI 1640 medium (ICN Biomedicals GmbH, Meckenheim, Germany) supplemented with 10% fetal calf serum (FCS) (Gibco-BRL, Life Technologies GmbH, Eggenheim, Germany). Mouse Ehrlich ascites tumour (EAT; kindly provided by Dr A. Shatkin, Center Advanced Biotech/Med, Piscataway, New Jersey, USA), human KB epidermoid mouth carcinoma (ATCC CCL 17), mouse mammary tumour MMT 060562 (ATCC CCL 51), rat glioma C6 (ATCC CCL 107) and mouse neuroblastoma Neuro-2A (ATCC CCL 131) cells were grown in Dulbecco’s modified minimum essential Eagle medium (ICN Biomedicals, Inc., Aurora, Ohio, USA) with 10% FCS. Cells in the logarithmic growth phase were used in all assays. In all tests, viable cells were counted using the trypan blue exclusion method. Normal lymphocytes were isolated from human blood and purified by Ficoll gradient centrifugation according to standard procedures.
Isolation of soluble peptidoglycan fragments
Briefly, L. casei cells were grown in 2 l of MRS broth at 32°C in the exponential phase. The cells were then collected by centrifugation at 7000g for 15 min at room temperature. The bacterial pellet was resuspended in sterile PBS and washed four times by centrifugation at 7000g to remove any trace of culture medium. The washed pellet was then resuspended in 500 ml of PBS containing 2.5 g of d-glucose and 5 mg of penicillin G, and incubated under agitation at 32°C for 30 min. The supernatant containing soluble peptidoglycan fragments was then harvested by centrifugation at 12 000g for 30 min at 4°C, then heated at 65°C for 15 min and finally concentrated at 4°C by flash evaporation to 1/10 of volume and lyophilized.
Determination of mitochondrial succinate dehydrogenase activity
The method used (reagent kit from Roche Molecular Biochemicals, Mannheim, Germany) is based on the cleavage of the tetrazolium salt XTT (sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis-(4-methoxy-6-nitro) benzene sulphonic acid hydrate), which is metabolically reduced in viable cells by succinate dehydrogenase into an orange-coloured, water-soluble formazan product (see Supplementary file, Supplemental digital content 1, http://links.lww.com/ACD/A157).
Evaluation of hexokinase activity
In this method, glucose is first phosphorylated by ATP in a reaction catalysed by hexokinase. The glucose-6-phosphate formed is oxidized to 6-phosphogluconate under reduction of nicotinamide adenine dinucleotide phosphate (NADP) to NADPH, a reaction catalysed by glucose-6-phosphate dehydrogenase. The NADPH formed reduces phenazine methosulphate (PMS) to PMSH, which, in turn, reduces iodonitrotetrazolium chloride (INT) to INTH, which is finally measured colorimetrically at 520 nm. In the presence of a fixed amount of glucose, the reaction varies with the efficiency of the enzymatic factors. The hexokinase assay in the absence and presence of the synthetic pentapeptide was performed using the glucose assay kit purchased from Sigma (Deisenhofen, Germany), following the manufacturer’s instructions. The concentration of the pentapeptide in the peptide test mixture was 8 µg/ml.
Statistical analysis
Statistical analysis was carried out using Student’s t-test.
Results
Cytotoxic activity of peptidoglycan fragments
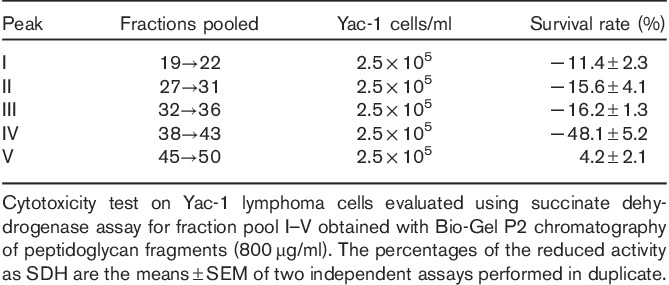
Soluble peptidoglycan fragments that accumulated in the supernatant of a penicillin G-treated L. casei cell culture were fractionated in distilled water on a Sephadex G-50 column and successively in a Bio-Gel P2 column (see Supplementary file, Supplemental digital content 1, http://links.lww.com/ACD/A157). They showed a remarkable inhibiting activity when subjected to the mitochondrial succinate dehydrogenase assay on Yac-1 lymphoma cells and Ehrlich tumour cells using the tetrazolium salt XTT as a test substance which is then reduced to the water-soluble formazan in the reaction medium (Table 1).
Table 1.
Cytotoxic activity of the gel chromatographic fractions
To obtain information on the dose and time dependency of the cytopathic effect, the peptidoglycan fragments of fraction IV that showed the maximum inhibitory activity were additionally analysed at the same concentration for their effect on cell membrane integrity in a flow cytometric assay using propidium iodide as a dead cell marker. A summary of the data obtained with Yac-1 lymphoma and Ehrlich tumour cells over a wider dose and time range of substance application is presented in Fig. 1.
Fig. 1.
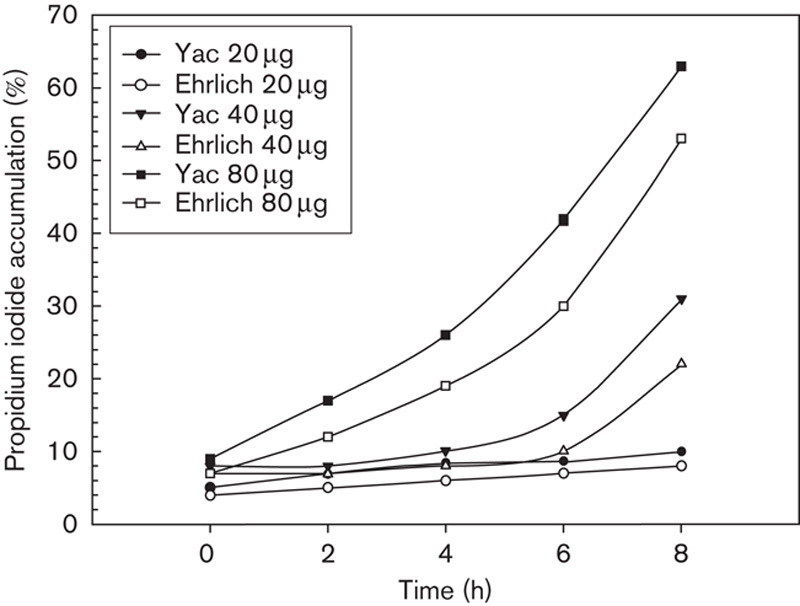
Flow cytometric assay of (25×105/ml) Yac-1 lymphoma cells and Ehrlich tumour cells treated at various periods of time and different amounts with peptidoglycan fraction IV and labelled with propidium iodide for the measurement of cell viability.
A significant cytotoxic effect on the Yac-1 lymphoma tumour cells was observed already after 4 h for fraction IV with a concentration of 80 µg/100 µl culture medium. Although 40% of the cell population were propidium iodide positive after 6 h, this percentage increased to 65% after 8 h. These results show that Yac-1 tumour cells respond very early to the mixture of peptidoglycan fragments of fraction IV with a decrease in the cell membrane integrity, and that this effect is time and dose dependent. A similar but weaker response was found with Ehrlich tumour cells.
Unexpectedly, no marked modifications in cell viability were observed in assays with XTT performed on normal human fibroblasts when treated with the same peptidoglycan fragments, whereas a similar cytotoxic effect was observed on primary mouse embryo fibroblasts and Yac-1 lymphoma cells (Fig. 2).
Fig. 2.
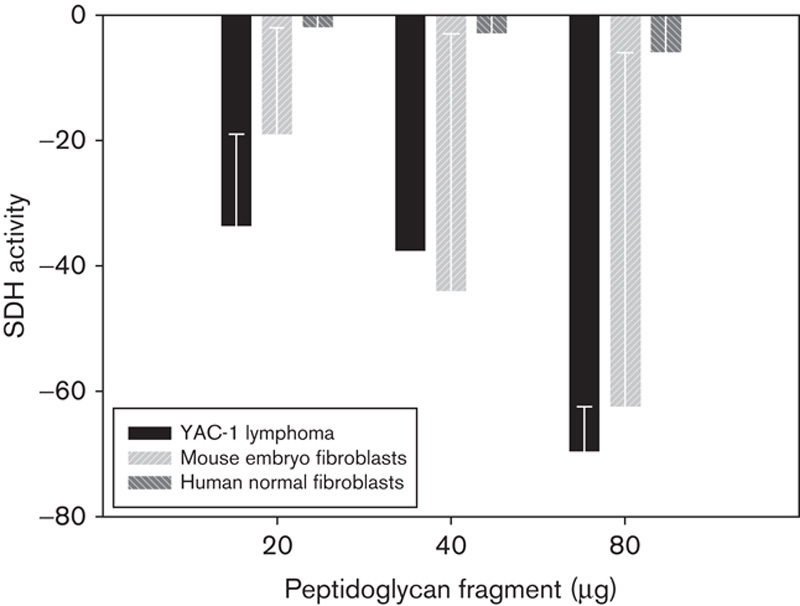
Differential peptide cytotoxic activity against primary mouse embryo fibroblasts, Yac-1 lymphoma cells and human normal fibroblasts. SDH, succinate dehydrogenase.
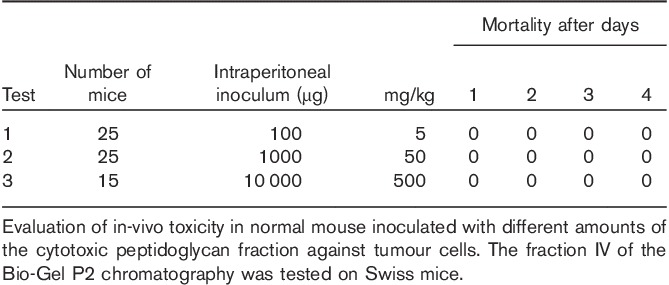
Toxicity test of peptidoglycan fragments in-vivo
Groups of Swiss albino mice, about 20 g in weight, were challenged by intraperitoneal inoculation with varying amounts of the active peptidoglycan fraction in different trials. The results of these assays are summarized in Table 2. In addition, no toxicity, evaluated in terms of general physical appearance, health and feeding behaviour of the animals, was found. An even more positive effect was observed in rabbits treated with the entire, original peptidoglycan complex for immune serum production as they responded with improved health and a significant gain in weight (data not shown).
Table 2.
No toxicity of peptidoglycan fragments in normal mice
Fractionation and characterization of peptidoglycan cytotoxic fraction
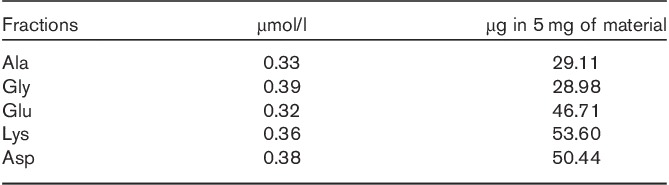
The most active fractions (IV) were those containing fragments in the molecular weight range 1000–600. These were lyophilized and the residue was analysed by gas chromatography (see Supplementary file, Supplemental digital content 1, http://links.lww.com/ACD/A157) for its amino acid content and composition. As listed in Table 3, 5 mg of the material contained 210 µg of the amino acids Ala, Gly, Glu, Lys and Asp in equimolar ratios, that is, those amino acids that are known to make up the peptidic fraction of the muropeptides of L. casei and Enterococcus faecium, both of which belong to subgroup A4 16. According to published amino acid analyses 17, the amino acids Glu and Asp very likely exist in their amide forms, Gln and Asn, in the peptidoglycan complex. The amino acid glycine represents the residue among the present amino acids that constitutes the glycinic interpeptide bridge joining the peptidic moieties of two different muropeptides in the entire peptidoglycan complex.
Table 3.
Amino acid analysis of peptidoglycan fragments (IV) gas-chromatographic analysis
Because of its width, it had to be assumed that the peak fractions (IV) are still rather heterogeneous in their peptidoglycan fragment composition. This could indeed be shown by MALDI-TOF mass spectrometry (see Supplementary file, Supplemental digital content 1, http://links.lww.com/ACD/A157), which indicated a complex mixture of presumably disaccharide moieties interconnected with amino acid sequences of two, three and four amino acid residues. These findings are similar to those reported by Billot-Klein et al. 17. All these compounds are within the 1500 Da range.
Cytotoxic activity of d-amino acids
A point of interest was whether, in the light of the occurrence of d-amino acids in the muropeptides of L. casei and E. faecium, such residues play a particular role in the cytotoxic activity of the peptides.
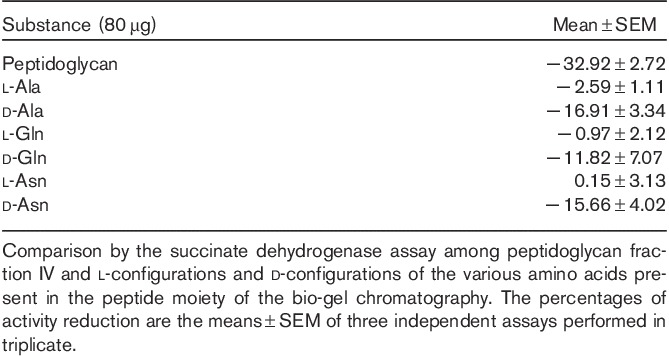
To clarify this issue in a first step, the l-configurations and d-configurations of the various amino acids present in the peptide moiety of the peptidoglycan fragments were tested for their capacity to affect the succinate dehydrogenase activity in Yac-1 lymphoma cells. Table 4 shows that the d-configurations of the amino acids Ala, Gln and Asn exerted a significant, inhibitory effect on the mitochondrial enzyme activity, which, on a mass basis, was approximately half as great as that of the peptidoglycan fragments (IV) of Bio-Gel P2 chromatography. The l-configurations of the three amino acids had almost no effect on the enzymatic activity. The same observations were made on other tumour cell lines (EAT, neuroblastoma and KB cells). The difference in cytotoxic activity between the individual d-amino acids and peptidoglycan fragments IV suggests that the individual d-amino acids act synergistically within the peptidic complex.
Table 4.
Inhibitory effect of the amino acids with d-configuration and l-configuration
Role of hexokinase in the cytotoxic process
Further characterization of the cytotoxic effect of peptidoglycan fragments started out from the well-established fact that, compared with normal cells, in highly glycolytic and rapidly growing tumours, exokinase (ATP: d-hexose-6-phosphotransferase), the first glucose-consuming enzyme of the glycolytic pathway, is overproduced and shows a markedly elevated activity 18. This is particularly true for the isomer II of the enzyme and, to a lesser extent, for isomer I. The tendency of this tumour enzyme to bind to the outer mitochondrial membrane, together with the contribution from enzyme overproduction, results in elevated levels of glycolysis and energy production.
To substantiate this point, an assay similar to that shown in Table 4 was performed, but with the difference that the peptidoglycan fragments of chromatography fraction IV as well as various amino acids in their l-conformations and d-conformations were complexed by an appropriate amount of hexokinase 30 min before their application to Yac-1 lymphoma cells. The results (Fig. 3) clearly indicate that (a) the peptidoglycan fragments or d-amino acids were largely prevented from exerting their inhibitory effect on the mitochondrial succinate dehydrogenase activity when previously complexed by hexokinase and (b) only the d-amino acids could enter complexes with hexokinase.
Fig. 3.
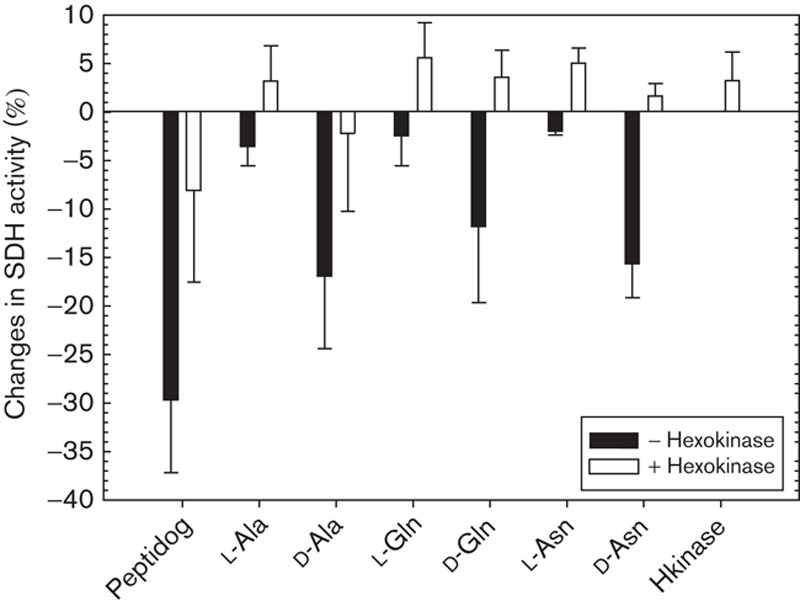
Cytotoxic properties of peptidoglycan-fraction IV an l- and d-amino acids (80 µg/100 µl). Influence of hexokinase. SDH, succinate dehydrogenase.
On the basis of the amino acid analysis of the peptidoglycan fragments of chromatography fraction IV in conjunction with the inhibitory effect of d-amino acids on the succinate dehydrogenase activity, the toxic principle of the biologically active material appears to consist of a small peptide with a certain content of amino acids with d-configuration, which are extremely rare in eukaryotic systems, but make up a part of the muropeptide moieties of bacterial peptidoglycans 16. Using this information and the known size and amino acid composition of the peptide moiety of L. casei peptidoglycan 17, a branched pentapeptide was synthesized (purchased from Eurogentec, Seraing, Belgium) whose chemical formula is presented in Fig. 4. Assays performed with lymphoma cells show an IC-50 index of 500 nmol/l (data not shown).
Fig. 4.
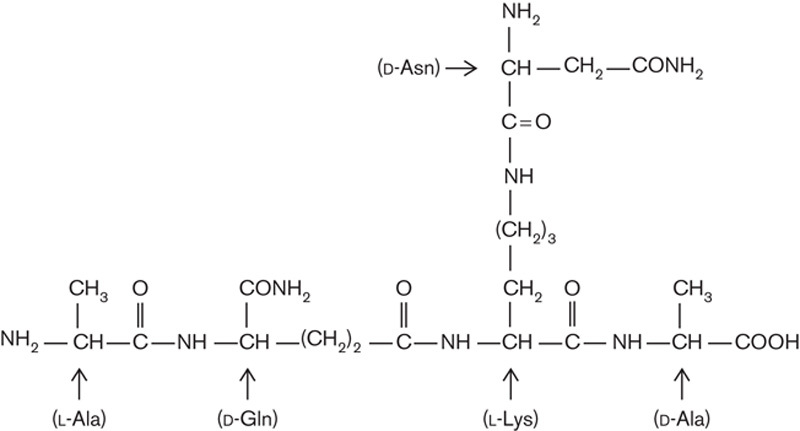
Structure of the basic pentapeptide of Lactobacillus casei peptidoglycan according to Billot-Klein et al. 17. After lyophilization, the peptide showed a white powder aspect with high solubility in water. Fundamental characteristics: molecular weight=529.56 Da; length=5 amino acid residuals; 1 µg=1888.349 pmol/l; molecular extinction coefficient=0±5; isoelectric point=9.00; charge at pH 7=0.91.
The biological activity of the peptide was tested on a series of normal and tumour cells with a particular focus on the dosage and time effects. To ascertain whether, besides the above-described time-dependent and dose-dependent cytotoxic activity, other factors were involved, further assays were performed to analyse the potential dependence of the cytotoxicity on different phases of cellular cycle. To this end, a cellular culture of Yac-1 lymphoma cells (5.75×105/ml) was synchronized with hydroxyurea (for detailed procedure, see the study by Cikes and Klein 19), treated with the synthetic pentapeptide (80 µg/100 µl) and, at successive intervals of hours, analysed for the succinate dehydrogenase activity to evaluate the cytotoxic activity (Fig. 5). The experimental results (Fig. 5) show that the susceptibility of tumour cells to the cytotoxic action of the peptide is not solely dose and time dependent but also correlate in the G2 phase with other processes of the cell life. Moreover, the synthetic peptide could block the glycolytic pathway with a much higher specific activity than the entire peptidoglycan complex. In contrast to cytotoxic effects on tumoural cells, the pentapeptide elicited a positive response in normal lymphocytes, as indicated by an increased vitality and a greater succinate dehydrogenase activity (Fig. 6).
Fig. 5.
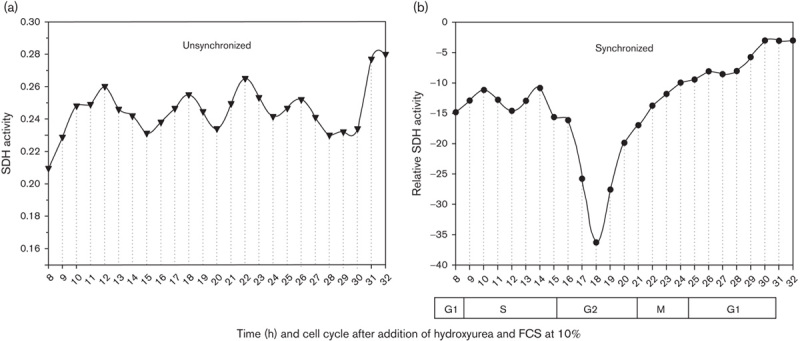
Test of modifications in cellular phases of cell cycle analysed in unsynchronized (a) and synchronized (b) cells of lymphoma culture treated with cytotoxic peptide. Each point in the graph represents the mean of three experiments conducted in triplicate. FCS, fetal calf serum; SDH, succinate dehydrogenase.
Fig. 6.
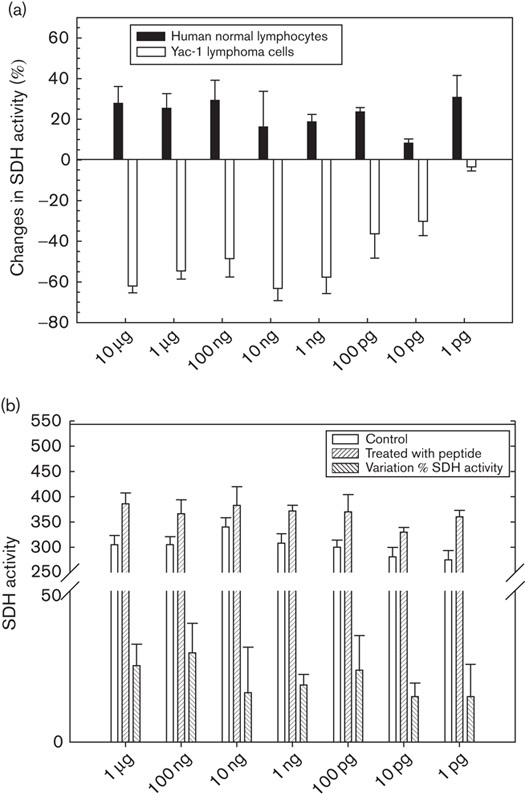
Effect of the synthetic pentapeptide on succinate dehydrogenase (SDH) activity on (a) Yac-1 lymphoma cells (2.5×105/ml) and normal lymphocytes treated with the pentapeptide and (b) variation of enzymatic activity between treated and untreated normal lymphocytes (2.5×105/ml). Each data point represents the mean±SEM of three independent experiments conducted in triplicate.
Similar results in normal and tumoural cells were obtained in assays with human bone marrow cells treated with the synthetic pentapeptide. We evaluated, using a colony growth assay, a significant productive activity of this new agent against normal bone marrow progenitors and a differential cytotoxicity for the leukaemic cell lines (Fig. 7).
Fig. 7.
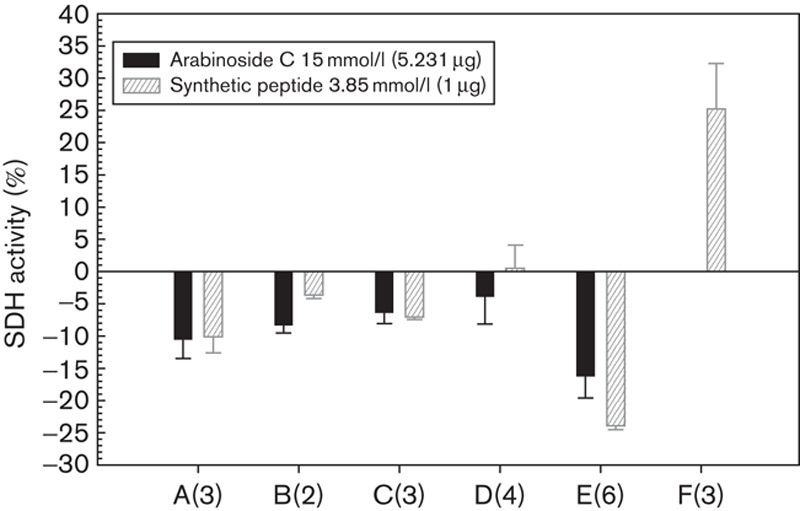
Selective cytotoxicity valued as SDH of the arabinoside C and the synthetic pentapeptide towards leukaemic cells and normal lymphocytes. Mean of colonies of normal bone marrow precursors in methylcellulose medium counted for the different leukaemic cells after treatment with arabinoside, peptide or without treatment are 0, 28 and 7, respectively. (A) Bone marrow cells from acute myeloid leukaemia patients at diagnosis; (B) bone marrow cells from chronic myeloid leukaemia in the chronic phase; (C) bone marrow cells from lymphoma patients with no evidence of neoplastic involvement; (D) bone marrow cells from normal donors (E) K562 cells; (F) Peripheral blood lymphocytes from normal donors. Figures between parentheses indicate the number of assays. SDH, succinate dehydrogenase.
To ascertain whether the peptidoglycan fragments (Fig. 8a) or synthetic pentapeptide (Fig. 8b) could bind to hexokinase, a two-step enzymatic reaction based on the phosphorylation of glucose by hexokinase and the successive oxidation of glucose-6-P by glucose-6-P dehydrogenase under simultaneous reduction of the cofactor NADP to NADPH was performed. The peptidoglycan fragments of chromatographic fraction caused a discreet reduction in the NADPH production, which can be considered an indirect proof of its binding to the hexokinase, suggesting their involvement in the complexation process (Fig. 8a). The results with the synthetic pentapeptide (Fig. 8b) showed an increase in absorbance, likely related to a purified active structure.
Fig. 8.
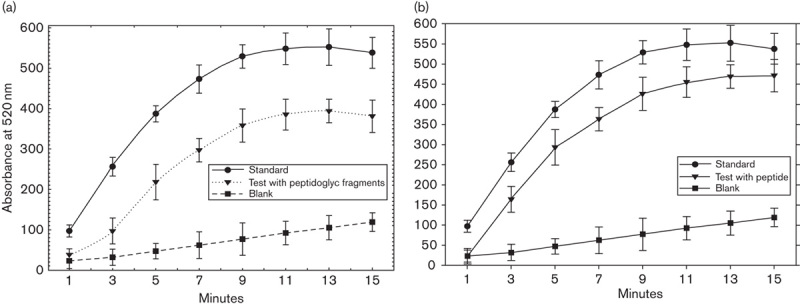
In-vitro binding of hexokinase with peptidoglican fragments (a) and derived synthetic peptide (b).
Cytotoxicity test on various tumour cell lines
The peptidoglycan fraction IV of the Bio-Gel P2 chromatography and the synthetic peptide were tested in parallel for their cytotoxic effects on several tumour cell lines. The results presented in Table 5 show only a slightly different response of the different tumour cell lines. The somewhat higher cytotoxic activity of the synthetic peptide might be because of its higher efficient concentration. A further indirect morphological evidence of cytotoxic activity of the synthetic pentapeptide was obtained using confocal fluorescence microscopy in C6 glioma cells (Fig. 9)
Table 5.
Cytotoxicity test of peptidoglycan fraction pool IV and the synthetic pentapeptide on various tumour cell lines using the succinate dehydrogenase assay
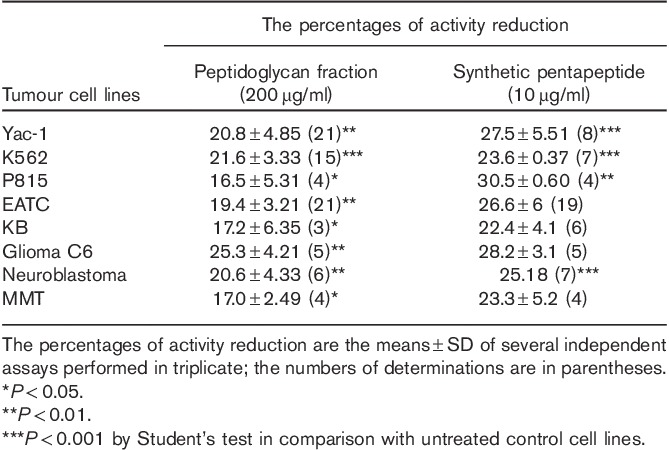
Fig. 9.
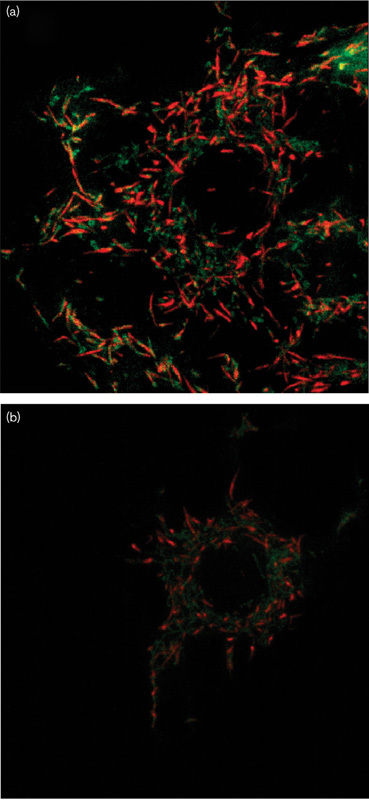
Confocal fluorescence microscopy showing that in control cells the mitochondrial membrane potential, as expected, is preserved and clearly shown by well-defined red fluorescence. In contrast, cells treated with the peptide show limited, weak red fluorescence and poor green fluorescence so that the mitochondrial structure is difficult to define. This situation indicates the general disturbance in the molecular organization of the mitochondria evidenced by a reduction in the membrane potential.
Indirect demonstration of the binding of the synthetic peptidoglycan pentapeptide to hexokinase
It was shown previously (Fig. 3) that succinate dehydrogenase activity is impaired (as a consequence of hexokinase binding) when the peptidoglycan fraction or d-amino acids are added to lymphoma cell suspension. As shown in Fig. 10, the same inhibitory behaviour towards hexokinase was found for the synthetic peptide. In this trial, the succinate dehydrogenase activity of the target cells (Yac-1 lymphoma) was inhibited by the synthetic peptide at concentrations as low as 10 pg/100 µl. Nevertheless, when the peptide was first mixed with certain amounts of hexokinase (1.74 U), no or weakly reduced cytotoxic activity was detected, whereas succinate dehydrogenase activity was unimpaired or slightly increased (Fig. 10). This trial confirmed the identical action of the synthetic peptide and peptidoglycan fraction on hexokinase, that is, the tendency to bind with the enzyme.
Fig. 10.
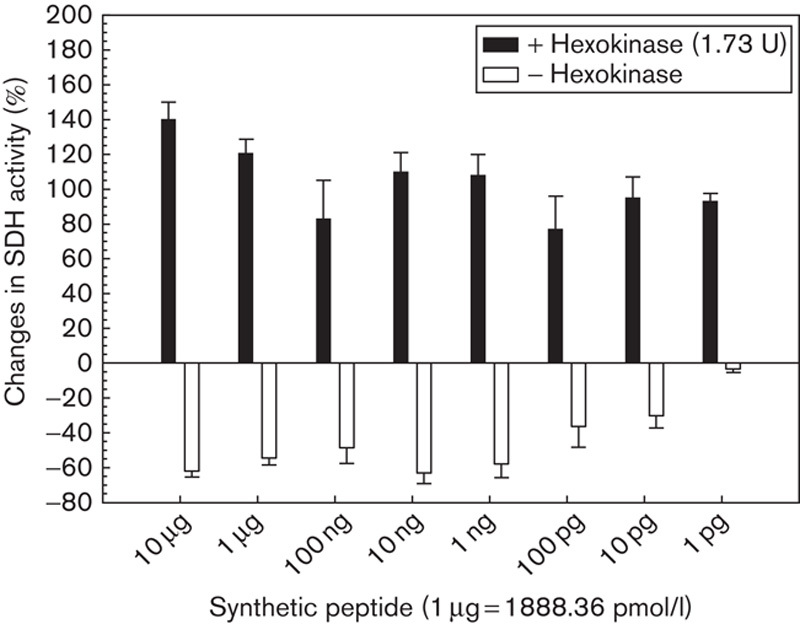
Changes in the succinate dehydrogenase (SDH) activity of Yac-1 lymphoma cells (2.5×105/ml) induced by the synthetic pentapeptide before and after its extracellular reaction with hexokinase (1.74 U).
Discussion
In the field of structures that are capable of showing cytotoxic properties against tumour cells, many substances have been proposed and analysed. In particular, curcumin and the 3-bromopyruvate have been discussed. Curcumin has been found to induce dissociation of HKII from mitochondria and favour apoptosis in tumour cells 20. However, other authors have reported that curcumin also undergoes extensive metabolism in the intestine and liver; consequently, high concentrations of curcumin cannot be achieved and maintained in plasma and tissues after oral ingestion. This is a major obstacle in the clinical development of this agent and suggests that the therapeutic potential of oral curcumin is limited. Accumulating data have shown since then that curcumin can induce DNA damage and chromosomal alterations both in-vitro and in-vivo at concentrations similar to those reported to exert beneficial effects. 3-Bromopyruvate is able to effectively target the energy metabolism of cancer cells. Biochemically, the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase has been identified as the primary target of 3-bromopyruvate. Its inhibition results in the depletion of intracellular ATP, causing cell death. Several other glycolytic inhibitors that target HKII have to be considered. The 2-deoxy-glucose, an analogue of glucose, functions as a competitor of glucose for entering the cell through GLUTs and phosphorylation by HKs. 2-Deoxy-glucose-6P accumulates in the cells and disrupts the binding of HKII to the mitochondria without inhibiting HKII kinase activity. Methyl jasmonate, a plant lipid derivative, which functions as a signalling molecule in the stress response of plants, disrupts the interaction between HKII and VDAC without inhibiting the kinase activity. These pharmacological inhibitors have been shown to exert antitumour activity by inhibiting glycolysis, depleting ATP, impairing cell cycle progression and inducing cell death. However, their molecular target specificity and the nonspecific inhibition of all isoforms of HKs may lead to unwanted side effects when used systemically in patients. Such a wide range of targets would also be expected to lead to severe toxicity in normal tissues, especially in those that are highly dependent on functional glucose metabolism and on mitochondrial oxidative phosphorylation, for example, in the heart, brain and kidney 21.
In contrast, the peptide analysed in this study has shown a stimulating rather than an inhibiting effect on normal cells as reported in human bone marrow (Fig. 7) and it may serve as a purging agent before autologous transplantation in leukaemia patients 22. The data presented in this research aim to define several aspects of the cytotoxic activity of L. casei peptidoglycan fragments and their basic peptide moiety towards tumour cells. First, this antitumour activity was found to be the highest in cells characterized by an abnormally high proliferating potential and also in embryonal cells. Second, the fundamental reaction underlying the cytotoxic processes is the binding of the peptidoglycan fragments to hexokinase by their basic peptide moiety, a synthetic version of the peptide showing the highest activity on a mass basis. It has been shown that all transformed cells contain either hexokinase I or hexokinase II and that both isoenzymes are expressed at increased rates. A correspondingly enhanced mRNA production indicates transcriptional control of hexokinase gene expression. During neoplastic transformation, the hexokinase isoenzymes are differentially expressed and distributed in the cell depending on the transformation stage 23,24.
Experimental results show (Fig. 8) that the derived synthetic pentapeptide can bind with the hexokinase and exerts a weak inhibitory effect on hexokinase enzymatic specific activity. This situation, irrespective of the exact mechanism, is probably because of the interaction of hexokinase with the VDAC on the mitochondrial membrane and suggests a presumable positive strong competition at the level of the VDAC–hexokinase complex of the described pentapeptide with the bound hexokinase. The mechanism of this competition remains unknown, but other authors 25 have reported that hexokinase binds effectively to VDAC-1 reconstituted in lipid membranes and affects its ion permeability characteristics, promoting a closed state of the channel. Both hexokinase I and II have a hydrophobic N-terminal sequence of 15 amino acids that has features compatible with an amphipathic α-helix and may be at least partly inserted into the lipid bilayer 26. The N-terminal domain is necessary and sufficient for mitochondrial binding and hexokinase I or II can be displaced from mitochondria by a peptide corresponding to the N-terminal domain 27. In the case of the present research, the pentapeptide and amino acid d-configurations are different, and the binding mechanism is consequently unknown. Nevertheless, the experimental findings clearly show the activity of the pentapeptide.
It is noteworthy that the peptide: (a) in extracellular mixtures, binds with hexokinase (Fig. 8a and b) and this linkage exerts a negligible effect on the catalytic function of the enzyme, implying that catalytic and binding functions are located in distinct portions of the molecule. The linking of the peptide with the hexokinase suggests that the peptide cannot bind with further amounts of hexokinase. At the cellular level, the peptide is no longer available to bind the hexokinase complexed with VDAC and in this manner the cytotoxicity is abolished and the antiapoptotic process is preserved and: (b) when directly assayed, the peptide is free and available to bind with the hexokinase complexed with VDAC, whereby the cytotoxic process remains operative.
In tumour cells, the hexokinase is found partially (∼30%) in the cytosol compartment, the remaining amount is bound to the VDAC structure of the outer mitochondrial membrane. This configuration in the cells mirrors what occurs in the experimental assays: a percentage of peptide is partially blocked by the cytosolic hexokinase and the remaining is available to bind with the mitochondrial-bound hexokinase. This binding triggers the Bax-induced proapoptotic process including a decrease in succinate dehydrogenase activity, cellular ATP and mitochondrial membrane potential, as well as changes in the VDAC structure on the outer mitochondrial membrane and cytochrome C release. As the final stage, the antiapoptotic process is suppressed, the cellular functions are altered and the cell is destroyed.
The experimental results obtained, especially with the synthetic pentapeptide, indicate that the percentage of tumour cells damaged not only depends on the peptide concentration tested but also on the extent of hexokinase bound to the mitochondrial surface. The varying percentage of dead cells after treatment with the peptide fraction, or with the synthetic peptide (Figs 1 and 6a), even at high concentrations, suggests that the activity is not absolute, but increases with the time of administration. There are two possible reasons of this time-dependent cytotoxicity. First, dependency on the various amounts of the mitochondrial hexokinase expressed, because of a quantitative difference in binding sites, and second, cytotoxic variability related to the cell cycle phase. This latter possibility, implying that hexokinase availability and receptivity are cell cycle phase dependent, has been shown by tests on peptide activity performed with tumour cells (EAT and YAC-1) synchronized by hydroxyurea treatment (Fig. 5). It was found that the cells were mostly damaged in the G2 phase of the cell cycle, suggesting that the optimal availability of hexokinase for complexation with the peptide is provided in the G2 phase.
However, the experimental data show, in addition, that succinate dehydrogenase activity is not totally blocked by elevated amounts of peptide. It has been shown 28 that in tumour cells, the amount of mitochondrial-bound hexokinase is not so much dependent on the structure (isoform) of porine (VDAC), but rather on the availability of sufficient numbers of binding sites for hexokinase on the mitochondrion. In any case, an amplification of porin expression with an enhanced binding of hexokinase to the mitochondria may lead to a more incisive cytotoxic activity of the peptide, which, through the interaction with the hexokinase, is the determining factor for apoptosis. Conversely, a decrease in binding sites may result in a lower cytotoxic activity. As a consequence of this dynamic condition of the presence of porine assumed to be cell cycle dependent (G2 phase), the level of bound hexokinase would be vary across the whole cellular population.
As a result, not all cells in a cellular population may be affected by the cytotoxic activity of the peptide, and the succinate dehydrogenase activity would not be impaired in all cells. Referring to the ability of the peptide, at certain concentrations, to bind with a certain amount of hexokinase, a percentage of peptide will remain linked because there is no porin available on the outer mitochondrial membrane to bind the enzyme. This could explain the partial impairment of the glycolytic process observed in the assays performed here.
A further question arises: why, as the experimental data suggest, does the peptide have a stimulating effect on the glycolytic pathway in normal cells and a cytotoxic one in tumour cells? The answer may arise indirectly from the considerations made above in terms of the peculiar localization of the hexokinase in tumour cells. The basic postulate of the localization of the enzyme in normal eukaryotic cells is that hexokinase, as the first enzyme of the anaerobic pathway of glycolysis, is present outside the mitochondria within the cytosol and that it binds preferentially to nonmitochondrial receptor sites. As glucose-6-phosphate is produced, the hexokinase enzyme becomes inhibited. This situation is altered in tumour cells, where, under the conditions of excited malignant proliferation, up to 80% of the overexpressed hexokinase shifts to the mitochondrial-bound condition. In normal cells, the hexokinase bound to the cytosolic receptors is also linked by peptide depriving the cell of the necessary metabolic energetic requirement. Under these conditions, the normal cells may operate a kind of compensating productive reaction that leads to an overexpression of cytosolic hexokinase available for the phosphorylation of glucose to glucose-6-phosphate. If this is the case, the glucose-6-phosphate obtained still possesses its normal inhibiting property, but the amount present, related to the greater hexokinase availability, is greater than that in the basal condition. In normal cells, the outcome is a quantitative enhancement of the entire normal glycolytic pathway and a greater physiological activity of cellular metabolism.
The experimental findings presented here and the consideration of the mechanism that could explain the cytotoxic effects on tumour cells lead to the conclusion that, for the peptide component isolated in this research, the main operating aspect is the efficient interaction in tumour cells with the mitochondrial-bound hexokinase that becomes decoupled from complexation with VDAC, leading to the availability of the structures that promote the proapoptotic process. In this light, the characterized peptidic formula could be used as an instrument to inhibit the malignant proliferation of tumour cells, without interfering in any way with normal cells, and thus offers a promising horizon for therapeutic benefits.
Supplementary Material
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.anti-cancerdrugs.com).
Acknowledgements
The authors are indebted to Dr Peter Traub for allowing use of the research facilities of the Max Planck Institute for Cell Biology, Ladenburg, bei Heidelberg – Germany, to Dr Günter Giese and Robert L. Shoeman for carrying out the cytofluorometric and MALDI-TOF mass spectrometric analyses, respectively; to Ulrike Traub and Annemarie Scherbarth for the provision of cultured cells and to Drs R.L. Shoeman, Genrich Tolstonog and P. Traub for many valuable and stimulating discussions, and to Dr Sebastiano Sciuto of the Chemistry Dept. University of Catania for the amino acid analysis.
The authors declare that the research and most of the experimental procedures were performed at the Max-Planck-Institut für Zellbiologie, Rosenhof, D-68526 Ladenburg bei Heidelberg, Germany.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kato I, Kobayashi S, Yokokura T, Mutai M. Antitumor activity of Lactobacillus casei in mice. Gann 1981; 72:517–523. [PubMed] [Google Scholar]
- 2.Han DJ, Kim JB, Park SY, Yang MG, Kim H. Growth inhibition of hepatocellular carcinoma Huh7 cells by Lactobacillus casei extract. Yonsei Med J 2013; 54:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, Aindelis G, Tompoulidou E, Lamprianidou EE, et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS One 2016; 11:e0147960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer 2012; 64:871–878. [DOI] [PubMed] [Google Scholar]
- 5.Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V, Russo F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer 2012; 64:1103–1111. [DOI] [PubMed] [Google Scholar]
- 6.Fichera GA, Giese G. Non-immunologically-mediated cytotoxicity of Lactobacillus casei and its derivative peptidoglycan against tumor cell lines. Cancer Lett 1994; 85:93–103. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima RA, Paggi MG, Scott LJ, Pedersen PL. Purification and characterization of a bindable form of mitochondrial bound hexokinase from the highly glycolytic AS-30D rat hepatoma cell line. Cancer Res 1988; 48:913–919. [PubMed] [Google Scholar]
- 8.Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem 1988; 263:17422–17428. [PubMed] [Google Scholar]
- 9.Rempel A, Mathupala SP, Perdersen PL. Glucose catabolism in cancer cells: regulation of the type II hexokinase promoter by glucose and cyclic AMP. FEBS Lett 1996; 385:233–237. [DOI] [PubMed] [Google Scholar]
- 10.Parry DM, Pedersen PL. Intracellular localization and properties of particulate hexokinase in the Novikoff ascites tumor. Evidence for an outer mitochondrial membrane location. J Biol Chem 1983; 258:10904–10912. [PubMed] [Google Scholar]
- 11.Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N’-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry 1986; 25:1015–1021. [DOI] [PubMed] [Google Scholar]
- 12.Arora KK, Filburn CR, Pedersen PL. Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase. J Biol Chem 1991; 266:5359–5362. [PubMed] [Google Scholar]
- 13.Arora KK, Fanciulli M, Pedersen PL. Glucose phosphorylation in tumor cells. Cloning, sequencing, and overexpression in active form of a full-length cDNA encoding a mitochondrial bindable form of hexokinase. J Biol Chem 1990; 265:6481–6488. [PubMed] [Google Scholar]
- 14.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells. Isolation, sequence, and activity of the promoter for type II hexokinase. J Biol Chem 1995; 270:16918–16925. [DOI] [PubMed] [Google Scholar]
- 15.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 2002; 277:7610–7618. [DOI] [PubMed] [Google Scholar]
- 16.Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 1972; 36:407–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billot-Klein D, Legrand R, Schoot B, van Heijenoort J, Gutmann L. Peptidoglycan structure of Lactobacillus casei, a species highly resistant to glycopeptide antibiotics. J Bacteriol 1997; 179:6208–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora KK, Parry DM, Pedersen PL. Hexokinase receptors: preferential enzyme binding in normal cells to nonmitochondrial sites and in transformed cells to mitochondrial sites. J Bioenerg Biomembr 1992; 24:47–53. [DOI] [PubMed] [Google Scholar]
- 19.Cikes M, Friberg S, Jr, Klein G. Quantitative studies of antigen expression in cultured murine lymphoma cells. II. Cell-surface antigens in synchronized cultures. J Natl Cancer Inst 1972; 49:1607–1611. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Fan H, Chen Q, Ma G, Zhu M, Zhang X, et al. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015; 26:15–24. [DOI] [PubMed] [Google Scholar]
- 21.Shoshan MC. 3-Bromopyruvate: targets and outcomes. J Bioenerg Biomembr 2012; 44:7–15. [DOI] [PubMed] [Google Scholar]
- 22.Milone G, Moschetti G, Tornello A, Indelicato F, Leotta F, Guido G, et al. Selective cytotoxicity of a new syntetic pentapeptide towards K 562 leukemic cell line but not towards normal hematopoietic precursors – A new purging agent. Bone Marrow Transplant 2002; 29 (Suppl 2):S232. [Google Scholar]
- 23.Rempel A, Bannasch P, Mayer D. Differences in expression and intracellular distribution of hexokinase isoenzymes in rat liver cells of different transformation stages. Biochim Biophys Acta 1994; 1219:660–668. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara Y, Ishida T, Yamazaki N, Baba Y, Terada H. Characterization of porin isoforms expressed in tumour cells. EurJ Biochem 2002; 67:6067–6073. [DOI] [PubMed] [Google Scholar]
- 25.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J 2004; 377 (Pt 2):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie GC, Wilson JE. Rat brain hexokinase: the hydrophobic N-terminus of the mitochondrially bound enzyme is inserted in the lipid bilayer. Arch Biochem Biophys 1988; 267:803–810. [DOI] [PubMed] [Google Scholar]
- 27.Arzoine L, Zilberberg N, Ben-Romano R, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with hexokinase to prevent its anti-apoptotic activity. J Biol Chem 2009; 284:3946–3955. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr 2008; 40:171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.anti-cancerdrugs.com).


