Pathogenic variants in the SACS gene (OMIM #604490) cause autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). ARSACS is a neurodegenerative early-onset progressive disorder, originally described in French Canadians, but later observed elsewhere.1 Whole-exome sequencing of a large group of patients with unclassified progressive myoclonus epilepsies (PMEs) identified 2 patients bearing SACS gene mutations.2 We detail the PME clinical features associated with SACS mutations and suggest the inclusion of the SACS gene in diagnostic screening of PMEs.
Case reports.
Patient 1 is a 25-year-old woman from Canada, and patient 2 is a 36-year-old woman from Italy. Key clinical features of these 2 patients are summarized in table e-1 at Neurology.org/ng.
Patient 1 started walking at 18 months of age and had mild learning difficulties. Multifocal myoclonus began at the age of 13 years and progressively worsened throughout her life. Her first generalized tonic-clonic seizure (GTCS) occurred at age 15 years; valproate therapy resulted in good control for 3 years. Subsequently, she developed additional seizure types, sometimes elicited by photic stimuli, which became refractory to multiple antiepileptic drugs—lamotrigine, acetazolamide, levetiracetam, primidone, topiramate, clobazam, and clonazepam—as well as ketogenic diet. Her cognition progressively deteriorated. In addition, she had pyramidal signs, cerebellar ataxia, and 2 self-limiting episodes of visual hallucinations. Currently, at the age of 25 years, she has approximately 1 GTCS per month and persistent multifocal myoclonus, spontaneous and stimulus-induced. She uses a wheelchair because of ataxia and myoclonus.
Patient 2 started walking at 25 months of age and had mild learning disability. Absence seizures began, often in clusters and associated with an atonic component, at the age of 3 years. She had multifocal myoclonus from the age of 14 years. The myoclonus progressively worsened over the years, as did her cognition. In addition, she had pyramidal signs, cerebellar ataxia, and minor behavioral problems. Currently, at the age of 36 years, she continues to have absence seizures and myoclonus despite treatment with valproate, piracetam, and levetiracetam. She has difficulty ambulating (because of ataxia and myoclonus), but can walk unassisted.
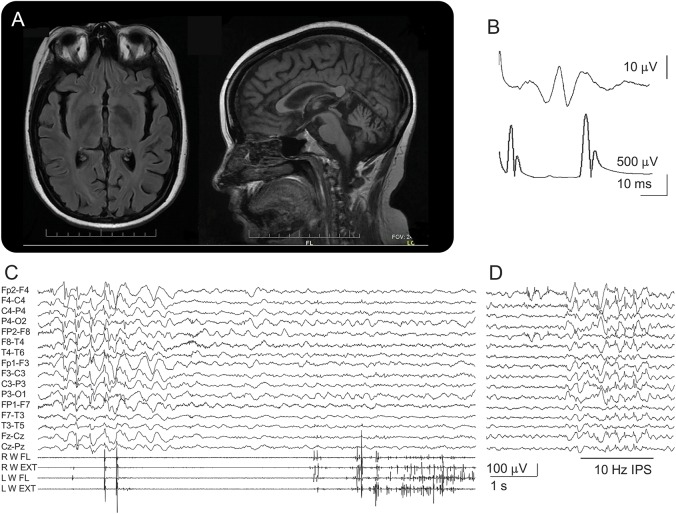
Both patients underwent repeated brain MRIs, which showed mild cerebral and moderate cerebellar and brainstem atrophy (figure, A). Magnetic resonance spectroscopy (not shown) was normal. Furthermore, in both patients, at the last follow-up, EEG studies showed slow background activity (5 Hz) and diffuse spikes and waves (SW). Patient 2 had abnormally increased somatosensory evoked potentials (SEPs) and enhanced long-loop reflexes (figure, B); investigation with SEPs was not performed in patient 1. Repeated EEG polygraphies performed in patient 2 documented the presence of spontaneous myoclonus associated with irregular SW discharge and of movement-activated myoclonus (figure, C). Moreover, patient 2 showed SW discharges induced by eye closure and a photoparoxysmal response to 10–20 Hz (figure, D).
Figure. Investigations of the reported patients.
(A) Axial and sagittal sections of 3 T brain MRI performed in patient 1 showing diffuse brain atrophy, pronounced infratentorially and involving the corpus callosum. (B) Somatosensory evoked potentials performed in patient 2 (35 years) showing enlarged P25-N33 component (upper trace); long-loop reflex (bottom trace; latency 45 ms) was also enhanced. (C) EEG polygraphy in patient 2 (23 years) showing irregular spike-and-wave discharge associated with spontaneous myoclonus (left part of the traces) and action myoclonus elicited by the extension of both hands (right part). (D) Photoparoxysmal response at 10-Hz photic stimulation (patient 2, 13 years).
Investigations aiming to determine the etiology of PMEs included skin and muscle biopsies, biochemical testing, and genetic sequencing of genes known to cause PMEs. All were negative.
Our patients finally underwent whole-exome sequencing within a cohort of 84 unrelated PME patients,2 which revealed compound heterozygous missense variants (in trans) in the SACS gene (NM_014363.5). Both patients had c.8393C>A (p.Pro2798Gln)—a mutation previously reported in homozygosity in ARSACS.3 Patient 1 also had c.1373C>T (p.Thr458Ile)—a mutation previously reported in compound heterozygosity or in homozygosity in 2 patients with atypical ARSACS (late-onset ataxia in both, absence of pyramidal features and polyneuropathy in one).1 Patient 2 had a novel c.2996T>C (p.Ile999Thr). All 3 mutations were assigned as likely pathogenic.2
Discussion.
After the original identification of genetic defects leading to ARSACS,3 subsequent reports described subjects with atypical features, which include delayed onset, unexpectedly mild ataxia or mild pyramidal signs, and absence of peripheral neuropathy.1,4 However, myoclonus was reported only once5 and seizures (not further classified) were rarely noted.5–7
Our patients showed obvious signs of cortical hyperexcitability, including myoclonus and seizures, in addition to the ARSACS features of pyramidal abnormalities and ataxia. Although these cases could be considered “atypical ARSACS,” the progressively worsening seizures and myoclonus, as well as the enlarged SEPs, enhanced long-loop reflexes, and mild photosensitivity (in addition to ataxia), are typical of PMEs (table e-2). Therefore, our patients were clinically diagnosed with PME and investigated for mutations in PME-associated genes—but none was found. It was only through whole-exome sequencing of a large cohort of PME cases without etiology that mutations in the SACS gene were identified. This report describes the clinical features of PMEs caused by SACS gene variants and suggests the inclusion of SACS screening in the investigation of PMEs.
Supplementary Material
Footnotes
Supplemental data at Neurology.org/ng
Author contributions: Fábio A. Nascimento and Laura Canafoglia: study concept and design; acquisition of data; data analysis and interpretation; drafting manuscript; accepts responsibility for conduct of research. Danah Aljaafari and Mikko Muona: acquisition of data; data analysis and interpretation; accepts responsibility for conduct of research. Anna-Elina Lehesjoki: study concept and design; acquisition of data; data analysis and interpretation; drafting and revising manuscript; accepts responsibility for conduct of research. Samuel Berkovic: study concept and design; acquisition of data; data analysis and interpretation; revising manuscript; accepts responsibility for conduct of research. Silvana Franceschetti: study concept and design; acquisition of data; data analysis and interpretation; drafting manuscript; accepts responsibility for conduct of research. Danielle M. Andrade: study concept and design; study supervision; data analysis and interpretation; revising manuscript; final approval; accepts responsibility for conduct of research.
Study funding: D.M.A. has TWGF funding #579064750710.
Disclosure: Dr. Nascimento reports no disclosures. Dr. Canafoglia has received research support from the Italian Ministry of Health. Dr. Aljaafari reports no disclosures. Mr. Muona has been an employee of Blueprint Genetics and has received research support from the Emil Aaltonen Foundation, the Epilepsy Research Foundation, University of Helsinki Funds, Doctoral Programme in Biomedicine University of Helsinki, the Finnish Brain Foundation, the Paulo Foundation, and the Biomedicum Helsinki Foundation. Dr. Lehesjoki has received research support from the Academy of Finland (grants 137950, 141549, 037315, and 281234), the Sigrid Juselius Foundation, the Liv och Hälsa Foundation, and the Folkhälsan Research Foundation. Dr. Berkovic has served on the editorial boards of Brain, Epileptic Disorders, and Lancet Neurology; co-holds a patent on Diagnostic testing of using the SCN1A gene (International publication number WO2006/133508, filed 16/06/2006) and pending patent WO61/010176: Therapeutic compound that relates to discovery of PCDH19 gene as the cause of familial epilepsy with mental retardation limited to females; and has received research support from UCB, Janssen-Cilag, Sanofi Aventis, the National Health and Medical Research Council of Australia, and NINDS. Dr. Franceschetti has received research support from the Ministry of Health, EPICURE, and Fondazione Mariani. Dr. Andrade has served on the scientific advisory board of Esai; has received travel funding/speaker honoraria from UCB; and has received research support from the Ontario Brain Institute, the University of Toronto, the Krembil Neurosciences Centre, and the Toronto Western Hospital Foundation. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Synofzik M, Soehn AS, Gburek-Augustat J, et al. . Autosomal recessive spastic ataxia of Charlevoix Saguenay (ARSACS): expanding the genetic, clinical and imaging spectrum. Orphanet J Rare Dis 2013;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muona M, Berkovic SF, Dibbens LM, et al. . A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat Genet 2015;47:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engert JC, Berube P, Mercier J, et al. . ARSACS, a spastic ataxia common in northeastern Quebec, is caused by mutations in a new gene encoding an 11.5-kb. Nat Genet 2000;24:120–125. [DOI] [PubMed] [Google Scholar]
- 4.Baets J, Deconinck T, Smets K, et al. . Mutations in SACS cause atypical and late-onset forms of ARSACS. Neurology 2010;75:1181–1188. [DOI] [PubMed] [Google Scholar]
- 5.Stevens J, Murphy SM, Davagnanam I, et al. . The ARSACS phenotype can include supranuclear gaze palsy and skin lipofuscin deposits. J Neurol Neurosurg Psychiatry 2012;83:1–2. [DOI] [PubMed] [Google Scholar]
- 6.Pilliod J, Moutton S, Lavie J, et al. . New practical definitions for the diagnosis of autosomal recessive spastic ataxia of Charlevoix-Saguenay. Ann Neurol 2015;78:871–886. [DOI] [PubMed] [Google Scholar]
- 7.Tzoulis C, Johansson S, Haukanes BI, Boman H, Knappskog PM, Bindoff LA. Novel SACS mutations identified by whole exome sequencing in a Norwegian family with autosomal recessive spastic ataxia of Charlevoix-Saguenay. PLoS One 2013;8:e66145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.