To The Editor
Heart failure in the setting of a preserved ejection fraction (HFPEF) is increasing in incidence and disproportionately afflicts older adult women with multiple concomitant co-morbid conditions.1 Anemia is common in older adults with HFPEF2 and erythropoietin (EPO) has been proposed as a potentially effective therapy in such individuals.3 Recruiting and retaining older adults in randomized clinical trials of HFPEF has been challenging for several reasons including significant subject burden as the result of complex protocols and procedures that may require frequent hospital visits which are especially difficult for frail older adults with impaired mobility and sensory deficits. EPO requires weekly monitoring of hemoglobin to avoid rapid increases that can be associated with adverse events and careful monitoring of blood pressure, making the subject burden imposed by weekly clinic visits too great for many older adults. Accordingly, in the absence of a novel approach to clinical trial design the paradox exists that those most in need of being included in such trials will not be. As a remedy, in an ongoing randomized clinical trial focused solely on older adults with HFPEF and anemia, we sought to modify the clinical environment employed for a majority of the study visits from a traditional hospital based environment to an older adult subject’s home. To facilitate such an approach, we employed a point of care hemoglobin monitoring system that would provide quick, reliable and accurate results. Herein, we report the initial experience with this approach, and evaluate the agreement between the point of care system and a hospital laboratory measure of hemoglobin.
Eighteen subjects, age 77±11 years (range 55 to 96 years), predominately women (58%) enrolled in an ongoing RCT (NCT00286182) to evaluate the efficacy of erythropoietin in patients with heart failure, an ejection fraction ≥ 40%, and a hemoglobin ≤12gm/dl were included in this analysis. Several other co-morbid conditions were highly prevalent including hypertension (100%), diabetes (84%), chronic renal insufficiency (78%), and coronary artery disease (74%). Mobility issues were common, mainly attributable to heart failure and arthritic complains with average score on the Western Ontario and McMaster Universities Osteoarthritis Index (36±33) compatible with mild to moderate osteoarthritis.4 All subjects provided written informed consent and the study was approved by the Columbia University Medical Center Institutional Review Board, and monitored by a data safety monitoring board.
After randomization, subjects had principle visits at baseline, 3 and 6 months performed in the Clinical Cardiovascular Research Laboratory for the Elderly, a hospital based facility and weekly visits between these visits were performed in the subject’s homes. During these visits, study personnel would evaluate for a change in the subject’s clinical status and perform a targeted physical exam. A sample of whole blood was obtained by venipuncture and hemoglobin was determined by two methods: hospital core laboratory (Sysmex XE 2100; Sysmex Corporation, Kobe, Japan) and Hemocue (Hemocue Inc., Sweden). A random effects model was used to account properly for the correlation due to repeated observations on the same subjects. The mean hemoglobin of the repeated weekly measures for each individual was calculated, and used to construct Bland-Altman plot.
At the time of this analysis, the 18 enrolled subjects were scheduled for 318 visits as part of the study protocol. Compliance with scheduled study visits was high (99.4%). The mean hemoglobin as determined by Hemocue and hospital lab did not differ among the 235 samples (10.7±1.2 vs. 10.7±1.0 gm/dl, p = NS). The intraclass correlation coefficient between the POC and core lab determined hemoglobin was high (0.948, p<0.0001). As shown in the first panel of Figure 1, the correlation between the two techniques was very high. As shown in the second panel by the Bland Altman plot, the limits of agreement were 0.5 gm/dl and −0.3 gm/dl with a mean difference of 0.1 gm/dl; 100% cases were within this range.
Figure 1.
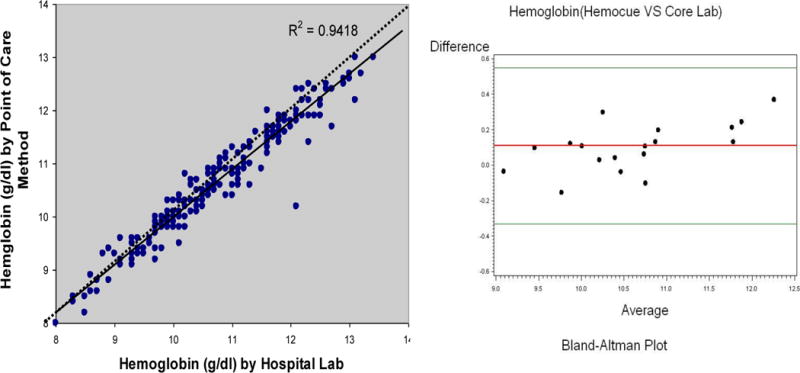
Correlation and Agreement between Methods to Measure Hemoglobin
The results of the analyses based on these data demonstrate excellent agreement between a point of care measure of hemoglobin and a hospital based lab. Accordingly, the approach employed in this ongoing randomized clinical trial (RCT) has resulted in adherence to study specified visits, which occur on a weekly basis, that are equivalent to those observed in other clinical trials in patients of a younger age and with less co-morbid conditions and on a less frequent basis.5–7 These home visits may provide a model for more easily including and retaining older adult subjects with complex medical problems in RCTs aimed at treating complex clinical syndromes, such as HFPEF.
Acknowledgments
This research was supported in part by a grant from the NIH/NIA (R01AG027518-01A1, Dr. Maurer).
Reference List
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006 Jul 20;355(3):251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RS, Mubashir A, Wajahat R, Mani S, Hummel S, Maurer MS. The cardio-renal-anemia syndrome in elderly subjects with heart failure and a normal ejection fraction: a comparison with heart failure and low ejection fraction. Congest Heart Fail. 2006 Jul;12(4):186–91. doi: 10.1111/j.1527-5299.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- 3.Van der MP, Groenveld H, Januzzi JL, van Veldhuisen DJ. Erythropoietin Treatment in Patients with Chronic Heart Failure: a meta-analysis. Heart. 2009 Jan 23; doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- 4.Hamel MB, Toth M, Legedza A, Rosen MP. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008 Jul 14;168(13):1430–40. doi: 10.1001/archinte.168.13.1430. [DOI] [PubMed] [Google Scholar]
- 5.Adherence to behavioral and pharmacological interventions in clinical research on older adults. Proceedings of a conference. May 1998, Winston-Salem, North Carolina, USA. Control Clin Trials. 2000 Oct;21(5 Suppl):155S–247S. [PubMed] [Google Scholar]
- 6.Bell RL, Curb JD, Friedman LM, McIntyre KM, Payton-Ross C. Enhancement of visit adherence in the national beta-blocker heart attack trial. Control Clin Trials. 1985 Jun;6(2):89–101. doi: 10.1016/0197-2456(85)90114-x. [DOI] [PubMed] [Google Scholar]
- 7.Shulman N, Cutter G, Daugherty R, et al. Correlates of attendance and compliance in the hypertension detection and follow-up program. Control Clin Trials. 1982 Mar;3(1):13–27. doi: 10.1016/0197-2456(82)90016-2. [DOI] [PubMed] [Google Scholar]


