Summary
Background
The standard of care for myelodysplastic syndromes is hypomethylating agents such as azacitidine. However, responses to azacitidine are generally temporary, and outcomes after hypomethylating agent failure are dismal. Therefore, the development of more effective treatments is crucial to improve outcomes in patients with myelodysplastic syndromes. We aimed to assess azacitidine and lenalidomide in patients with high-risk myelodysplastic syndromes and acute myeloid leukaemia.
Methods
We did this single-arm phase 1/2 study at the University of Texas MD Anderson Cancer Center, TX, USA. Patients of any age were eligible for phase 1 and 2a if they had relapsed or refractory acute myeloid leukaemia or myelodysplastic syndrome with bone marrow blasts more than 10%. For phase 2b, eligible participants were previously untreated with myelodysplastic syndrome with an International Prognostic Scoring System (IPSS) score of intermediate-1 or higher with up to 30% blasts. All participants received 75 mg/m2 azacitidine once a day for days 1–5 for each 28 day cycle. We gave patients oral lenalidomide for 5 or 10 days starting on day 6. We assessed seven lenalidomide doses in a 3 + 3 phase 1 design (n=28). The primary endpoint in phase 1 was the maximum tolerated dose, and the primary endpoint in phase 2 was overall survival. Outcome analyses were by intention to treat. This study is registered with ClinicalTrials.gov, number NCT01038635.
Findings
Between Dec 30, 2009, and June, 17, 2013, we enrolled 88 patients (28 in phase 1 and 60 in phase 2). One patient unexpectedly died in the phase 1 study at the highest dose level, six more patients were recruited with no further serious adverse events. We recorded no dose-limiting toxic effects, and the maximum tolerated dose of lenalidomide in combination with azacitidine in patients with acute myeloid leukaemia and myelodysplastic syndrome was initially established at 50 mg per day for 10 days. In the first 20 patients in phase 2, we noted a high rate of myelosuppression and myelosuppression-related toxic effects; therefore, we amended the lenalidomide dose to 25 mg per day for 5 days. We also adjusted the inclusion criteria to include patients with less than 30% blasts to focus mainly on patients with myelodysplastic syndromes. Median overall survival was 75 weeks (IQR 25–not reached) for the 40 patients in phase 2b. The most common grade 3–4 adverse events overall were neutropenic fever (n=27) and pneumonia (n=18).
Interpretation
We have identified a safe and active sequential treatment combination of azacitidine and lenalidomide for patient with myelodysplastic syndrome and have preliminary evidence that this dose is also safe for patients with acute myeloid leukaemia.
Funding
MD Anderson Cancer Center and Celgene.
Introduction
Myelodysplastic syndromes are malignant clonal disorders characterised by aberrant and ineffective haemo poiesis, bone marrow dysplasia, peripheral cytopenias including thrombocytopenia, and a propensity to transform into acute myeloid leukaemia.1,2 Myelodysplastic syndrome is generally stratified according to prognosis by use of various classification schemes based on the number and type of cytopenias, cytogenetic and molecular aberrations, and the percentage of blast cells within the bone marrow.3,4 Irrespective of the classification, overall survival is poor in patients with myelodysplastic syndromes and new agents and effective treatment regimens are urgently needed.
Azacitidine, a hypomethylating agent, is recommended as first-line treatment for patients with high-risk myelodysplastic syndromes including up to 30% bone marrow blasts, showing improved overall survival compared with acute myeloid leukaemia-type treatment or supportive care options.5 However, true remission is rare in these patients and durable cures with hypomethylating agent monotherapy are unlikely. Although the duration of response to hypomethylating agents is variable, the median effective duration for responding patients is 12–17 months, and patients have universally poor survival after hypomethylating agent failure.6,7
Several combination strategies are under development to improve the results of hypomethylating agent monotherapy in patients with myelodysplastic syndrome and acute myeloid leukaemia. Combination therapies with histone deacetylase inhibitors have been tested, but findings of clinical trials have not shown improvements in survival with these combinations.8 Another approach for combination therapy is lenalidomide, an immunomodulatory drug with substantial single-agent activity that is mainly reported in patients with low-risk myelodysplastic syndromes with isolated deletion of chromo some 5q. In patients with myelodysplastic syndrome with del(5q), responses to lenalidomide monotherapy at the recommended dose of 10 mg per day include haematological improvement in roughly 60% of patients and complete response in 30% of patients.9,10 Lenalidomide also leads to 14–30% of patients with myelodysplastic syndrome and acute myeloid leukaemia without isolated del(5q) achieving an overall response, which suggests increased therapeutic activity.9,11,12 Lenalidomide’s mechanism of action in del(5q) myelo dysplastic syndrome is believed to include cytotoxicity via clonal suppression driven at the molecular level by stabilisation of MDM2 via inhibition of cell cycle co-regulators located on chromosome 5q, leading to enhanced P53 degradation.13–15 In patients with non-del(5q) myelodysplastic syndrome, alternate mechanisms of action include restored erythropoiesis via erythro poietin-induced activation of STAT5, impairment of tumour angiogenesis, and modulation of the bone marrow microenvironment.13–18
In view of the single-agent activity of both azacitidine and lenalidomide in patients with myelodysplastic syndrome and acute myeloid leukaemia, a combination strategy with both agents could potentially improve outcomes over those achieved with either monotherapy alone. Smaller previous studies of lenalidomide at doses ranging from 5 to 50 mg per day in 5 to 28 days per cycle have investigated the combination of azacitidine and lenalidomide in the high-risk myelodysplastic syndrome and acute myeloid leukaemia population with encouraging results.19–23 However, the optimum dose and schedule for this combination remains unknown, and whether activity is increased in particular subsets of patients with myelodysplastic syndrome and acute myeloid leukaemia has not been established. Because lenalidomide inhibits cell cycle progression and azacitidine effectiveness is dependent on cycling cells, sequential administration of azacitidine followed by lenalidomide might increase efficacy, and this sequential synergy has been shown in in- vitro models.19,24 To explore the possible usefulness of such a schedule, we did a phase 1/2 trial of the sequential combination of azacitidine followed by lenalidomide in high-risk patients with myelodysplastic syndrome and acute myeloid leukaemia with the goal of assessing the maximum tolerated dose and clinical activity of that dose and identifying any possible predictors of clinical effectiveness.
Methods
Patients and study design
In this single-arm phase 1/2 study, we enrolled patients at The University of Texas MD Anderson Cancer Centre, TX, USA, following institutional guide lines. Patients (of any age) were eligible for phase 1 if they had relapsed or refractory myelodysplastic syndrome or acute myeloid leukaemia with bone marrow blast percentages higher than 10%, and for phase 2a, if they had relapsed, refractory, or treatment-naive acute myeloid leukaemia or myelodysplastic syndromes with bone marrow blast percentages higher than 10%. Previously untreated patients with myelodysplastic syndrome with an International Prognostic Scoring System (IPSS) score of intermediate-1 or higher25 with up to 30% blasts were eligible for phase 2b, thus including individuals with oligoblastic acute myeloid leukaemia (20–30% blasts) as defined by WHO criteria.26 All participants were registered into the RevAssist program. Additional inclusion criteria for both phases were ECOG performance status of 2 or less and adequate renal and hepatic function (bilirubin ≤34·2 μmol/L, serum glutamic-pyruvic aminotransferase ≤2·5 times the upper limit of normal, and creatinine ≤176·8 μmol/L). We excluded breastfeeding or pregnant women, people with known or suspected hypersensitivity to study drugs, and people unwilling or unable to comply with RevAssist. All patients provided written informed consent. Approval was obtained from the MD Anderson institutional review board.
Procedures
All patients received a fixed dose of 75 mg/m2 intravenous azacitidine once a day on days 1–5 of each 28 day cycle. Starting on day 6 of each 28 day cycle, oral lenalidomide was given every day for 5 or 10 days. In phase 1, we used a 3 + 3 dose escalation strategy to study seven oral lenalidomide doses: 10 mg, 15 mg, 20 mg, 25 mg, 50 mg, and 75 mg once a day for 5 days, and 75 mg per day for 10 days. In phase 2, lenalidomide was initially given at 50 mg per day orally for 10 days (n=20), but because of adverse events, we changed the dose to 25 mg per day orally for 5 days (n=40) starting on day 6 of each cycle. There was no limit to the number of cycles given if the participant continued to benefit from treatment. Bone marrow assessments were done at baseline (within 14 days of study start), at completion of cycle one, and then every one to three cycles as clinically indicated. Cytogenetic analysis was done in the MD Anderson clinical cytogenetics laboratory. Baseline cytogenetics data were obtained before treatment and with each subsequent bone marrow assessment in patients with baseline abnormal cytogenetics. Starting in October, 2012, the clinical molecular diagnostics laboratory at MD Anderson did molecular analysis with next-generation sequencing to detect somatic mutations on the baseline bone marrow (appendix).
In phase 1, we entered cohorts of three patients at each dose level. If one patient developed a grade 3 or higher non-haematological toxic effect, we accrued three more patients at that dose level. If two or more patients developed a grade 3 or higher non-haematological toxic effect, that dose level was deemed toxic. The maximum tolerated dose was defined as the dose lower than the one producing dose-limiting toxic effect in two or more patients. We graded toxic effects with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 3.0).
In phase 2, we assessed the activity of the azacitidine and lenalidomide combination and had a planned accrual of 40 patients at the phase 2 dose amount if the combination showed activity. It was designed with an early futility rule to stop the trial if the probability was less than 10% at least 30% of patients had an overall response; this was assessed after the first cycle of every cohort of ten patients. A second stopping rule for toxic effects defined an early stop if the posterior probability of risk higher than 0·3 was 75% or higher, with toxic effects defined as any drug-related grade 3 or greater toxic effect.
Outcomes
The primary study objectives were to establish the maximum tolerated dose of lenalidomide in combination with azacitidine in patients with acute myeloid leukaemia and myelodysplastic syndrome and to establish the clinical activity of the combination of azacitidine and lenalidomide in these patients. We assessed response with International Working Group (IWG) criteria for myelodysplastic syndrome.27,28 Complete response was defined as bone marrow with 5% or lower bone marrow blasts and normalisation of peripheral blood counts, including an absolute neutrophil count of 109 cells per L or higher and platelet count of 100 × 109 platelets per L or higher. Complete response with incomplete haematological recovery was defined as a complete response with incomplete recovery of peripheral blood counts.28 We calculated remission duration from date of first response until relapse, and overall survival from the start of treatment until date of death from any cause.
This trial is registered with ClinicalTrials.gov, number NCT01038635.
Statistical analysis
We summarised patient characteristics with medians and ranges for continuous variables and frequencies and percentages for categorical variables. We compared categorical variables with the χ2 or Fisher’s exact test and continuous variables with the Wilcoxon rank-sum test. All p values were two sided, with statistical significance defined as a p value of 0·05 or less. Overall survival was based on the Kaplan-Meier method, with differences compared with the log-rank test and confirmed by additional statistical methods (Gehan’s Wilcoxon, Cox’s F test, Cox-Mantell and Peto and Peto’s Wilcoxon; appendix) in the instance of intersecting survival curves. We assessed the predictive effects of various covariates with univariate and multivariate Cox proportional hazards models. Analyses were intention to treat. We used Statistica (version 12) and Stata (version 12.1) for analyses.
This study is registered with ClinicalTrials.gov, number NCT01038635.
Role of the funding source
MD Anderson investigators designed the study and did all data collection, data analysis, data interpretation, and wrote the manuscript. The funder Celgene had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
We enrolled 88 patients between Dec 30, 2009, and June, 17, 2013 (28 patients in phase 1 and 60 in phase 2). We assessed six additional patients for eligibility, but they did not fit inclusion and exclusion criteria and were not enrolled. All 88 patients were assessable for toxic effects and efficacy. Table 1 shows clinical and disease-specific characteristics for the treated patients. Median age was 67 years (range 32–88). Pre-treatment cytogenetic characteristics consisted of 24 (27%) patients with diploid cytogenetics and 11 (13%) patients with a cytogenetic abnormality including chromosome 5q; nine of those 11 patients in the setting of a complex karyotype (table 1). Figure 1 shows baseline molecular analysis in 25 patients enrolled on or after Oct 15, 2012, in the phase 2b cohort.
Table 1.
Clinicopathological characteristics of total patient cohort (n=88)
| All patients (n=88) | Phase 1 (n=28) | Phase 2 (n=60) | Phase 2a (n=20) | Phase 2b (n=40) | |
|---|---|---|---|---|---|
| Median age (years) | 67 (32–88) | 65 (32–79) | 68 (36–88) | 72 (36–88) | 66 (38–85) |
|
| |||||
| Patients with MDS and CMML | 45 | 10 | 35 | 8 | 27 |
|
| |||||
| Patients with AML/RAEB-T | 43 | 18 | 25 | 12 | 13 |
|
| |||||
| Previous lines of treatment | 0 (0–3) | 1 (1–3) | 0 (0–3) | 0 (0–3) | 0 (0) |
|
| |||||
| Median white blood cell count × 109/L | 2·4 (0·1–32·0) | 2·0 (0·1–19·1) | 2·6 (0·6–32·0) | 2·6 (0·6–28·6) | 2·6 (1·0–32·0) |
|
| |||||
| Median platelets × 109/L | 45 (1–742) | 61 (4–328) | 39 (1–742) | 32 (7–174) | 44 (1–742) |
|
| |||||
| Median bone marrow blast (%) | 16% (4–85) | 25% (11–84) | 15% (4–85) | 30% (8–85) | 14% (4–29) |
|
| |||||
| Cytogenetics | |||||
| Diploid, -Y | 24 | 8 | 16 | 4 | 12 |
| Misc non-complex | 17 | 2 | 15 | 6 | 9 |
| Misc complex | 9 | 1 | 8 | 4 | 4 |
| -5/5q- | 11 | 8 | 3 | 0 | 3 |
| Complex | 9 | 6 | 3 | 0 | 3 |
| -7/7q- | 7 | 3 | 4 | 1 | 3 |
| Complex | 2 | 1 | 1 | 1 | 0 |
| -5/5q- and -7/7q- | 15 | 5 | 10 | 4 | 6 |
| Complex | 14 | 4 | 10 | 4 | 6 |
| Insufficient metaphases | 5 | 1 | 4 | 1 | 3 |
|
| |||||
| International Prognostic Scoring System | |||||
| Intermediate-1 | 7 | 0 | 7 | 2 | 5 |
| Intermediate-2 | 20 | 3 | 17 | 3 | 14 |
| High | 38 | 12 | 26 | 5 | 21 |
| N/A (AML >30% blasts) | 23 | 13 | 10 | 10 | 0 |
|
| |||||
| Revised International Prognostic Scoring System | |||||
| Low | 2 | 0 | 2 | 1 | 1 |
| Intermediate | 9 | 3 | 6 | 1 | |
| High | 22 | 9 | 13 | 2 | 11 |
| Very high | 48 | 13 | 35 | 15 | 20 |
| N/A | 7 | 3 | 4 | 1 | 3 |
|
| |||||
| WHO classification | |||||
| RAEB-1 | 1 | 0 | 1 | 1 | 0 |
| RAEB-2 | 41 | 8 | 33 | 7 | 26 |
| RAEB-T | 20 | 5 | 15 | 2 | 13 |
| CMML-2 | 3 | 2 | 1 | 0 | 1 |
| AML | 23 | 13 | 10 | 10 | 0 |
Data are n (range) or % (range) unless otherwise stated. MDS=myelodysplastic syndromes. CMML=chronic myelomonocytic leukaemia. AML=acute myeloid leukaemia. RAEB-T=refractory anaemia with excess blasts in transition.
Figure 1. Molecular and cytogenetic characteristics in 25 patients.
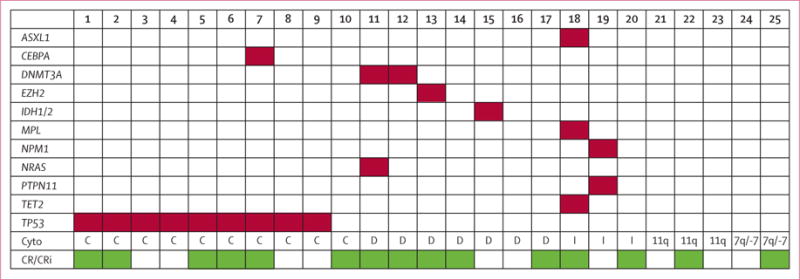
Cyto=cytogenetics. C=complex. D=diploid/-Y. I=intermediate-risk cytogenetics. 11q=Del(11q). 7q/-7=Del(7q)/-7. CR=complete response. CRi=complete response with incomplete platelet recovery.
Patients received a median of three cycles on study (range 1–16 cycles), with a median follow-up of 57 weeks (range 10–172; IQR 14·1–73·6). All patients received 75 mg/m2 per day intravenous azacitidine on days 1–5 of every 28 day cycle. In phase 1, we assessed seven dose levels of daily oral lenalidomide starting on day 6: 10 mg for 5 days (n=5), 15 mg for 5 days (n=3), 20 mg for 5 days (n=3), 25 mg for 5 days (n=3), 50 mg for 5 days (n=4), 75 mg for 5 days (n=3), and 75 mg for 10 days (n=7). We identified no dose-limiting toxic effects, and therefore, the maximum tolerated dose was not reached. Neither stopping rule was met. In the group that received 75 mg lenalidomide for 10 days, one patient died unexpectedly, and six additional patients were treated, with no further serious adverse events recorded.
On the basis of data from phase 1, we defined the optimum dose and schedule of phase 2 as 75 mg/m2 intravenous azacitidine on days 1–5 followed by 50 mg oral lenalidomide on days 6–15 of every 28 day cycle. 20 patients were treated in this original phase 2 dose (phase 2a). Although we recorded no dose-limiting toxic effects in the first cycle, we noted prolonged myelosuppression, with an increased incidence of infection-related complications and a dose reduction in five (25%) patients for cycle two (table 2). Therefore, after discussion between the principal investigator (GG-M) and our institution’s Department of Biostatistics, the phase 2 dose was formally amended to 25 mg oral lenalidomide per day for 5 days on the basis of the dose with the highest proportion of overall responses in phase 1, and the inclusion criteria were modified to include only patients with less than 30% blasts to focus mainly on myelodysplastic syndrome. 40 additional patients were treated with the modified combination schedule (phase 2b). The median number of cycles received in the phase 2a dose level was two (range 1–12). The median number of cycles received in the phase 2b dose schedule was five (range 1–16). Table 2 summarises the most frequent non-haematological toxic effects recorded with the combination treatment in all patients.
Table 2.
Non-haematological toxic effects (irrespective of attribution) in all patients
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
| Neutropenic fever | 0 | 0 | 27 | 0 | 0 |
| Pneumonia | 0 | 0 | 18 | 0 | 0 |
| Other infection | 0 | 1 | 8 | 0 | 1 |
| Shortness of breath | 0 | 0 | 6 | 0 | 0 |
| Cellulitis | 0 | 1 | 5 | 0 | 0 |
| Sepsis | 0 | 0 | 2 | 0 | 3 |
| Fungal pneumonia | 0 | 0 | 6 | 0 | 0 |
| Urinary tract infection | 1 | 0 | 4 | 0 | 0 |
| Haemorrhage | 0 | 1 | 3 | 0 | 1 |
| Constipation | 24 | 4 | 0 | 0 | 0 |
| Pain/bone pain | 1 | 5 | 1 | 0 | 0 |
| Bacteraemia | 0 | 0 | 3 | 0 | 0 |
| Fatigue | 36 | 8 | 2 | 0 | 0 |
| Fever | 17 | 3 | 2 | 0 | 0 |
| Rash | 12 | 3 | 3 | 0 | 0 |
| Diarrhoea | 7 | 1 | 1 | 0 | 0 |
| Confusion or altered mentation | 0 | 1 | 3 | 0 | 0 |
| Myalgias or muscle spasms | 2 | 1 | 2 | 0 | 0 |
| Coronary artery disease | 0 | 0 | 2 | 0 | 0 |
| Fluid retention | 0 | 0 | 1 | 0 | 0 |
| Syncope | 0 | 0 | 1 | 0 | 0 |
| Hypotension | 0 | 0 | 2 | 0 | 0 |
| Pruritus | 14 | 2 | 0 | 0 | 0 |
| Nausea | 9 | 2 | 0 | 0 | 0 |
Grade 1–2 events affecting more than 10% of participants included and all grade 3 and higher events included.
All 88 patients were assessable for response (table 3). In the combined phase 1/2 cohort, we recorded an overall response in 31 (35%) patients, including 15 patients with complete response and 16 with complete response with incomplete haematological recovery. One patient with partial response consisting of bone marrow blast reduction, and one patient with haematological improvement of platelets were not classified as responders. Overall survival for each phase is shown in figure 2. Because of the differences between the phase 1 (ie, previously treated myelodysplastic syndrome or acute myeloid leukaemia) and phase 2 (ie, treatment-naive acute myeloid leukaemia or myelodysplastic syndrome with less than 30% blasts) cohorts, and the increased tolerability of the amended phase 2 (2b) dose compared with the original phase 2 (2a) dose during different cycles, we analysed responses separately for each phase and cohort (table 3 and figure 2). In the phase 1 cohort, four (14%) patients achieved an overall response for previously treated patients with a median overall survival of 17 weeks. None of the four responding patients in the phase 1 cohort had previously received hypomethylating agent treatment.
Table 3.
Response assessment
| N | CR | CRi | ORR | Median overall survival (95% CI; weeks) | Median courses (range) | Median response duration, weeks (range) | |
|---|---|---|---|---|---|---|---|
| Phase 1 | |||||||
| Lenalidomide dose | |||||||
| <25 mg | 11 | 0 | 0 | 0% | 12·4 (12·0–12·8) | 3 (1–6) | N/A |
| 25 mg | 3 | 2 | 0 | 67% | NR | 6 (6–12) | 81 (35–127) |
| >25 mg | 14 | 2 | 0 | 14% | 15·6 (15·2–16·0) | 2·5 (1–12) | 81 (78–85) |
| All patients | 28 | 4 | 0 | 14% (p=0·01) | 16·7 (16·4–17·0) | 3 (1–12) | 81 (35–127) |
| Phase 2 | |||||||
| Lenalidomide dose | |||||||
| 50 mg | 20 | 2 | 3 | 25% | 20·5 (20·2–20·8) | 2 (1–12) | 18 (2–98) |
| 25 mg | 40 | 9 | 13 | 55% | 74·5 (73·9–75·0) | 4 (1–16) | 27 (2–71) |
| All patients | 60 | 11 | 16 | 45% (p=0·03) | 59·7 (59·6–59·8) | 3 (1–16) | 24 (2–98) |
| Phase 1 and 2 | 88 | 15 | 16 | 35% | 32·8 (32·7–32·9) | 3 (1–16) | 29 (2–127) |
CR=complete response. CRi=complete response with incomplete haematological recovery. ORR=overall response rate. OS=overall survival. NR=no response.
Figure 2.
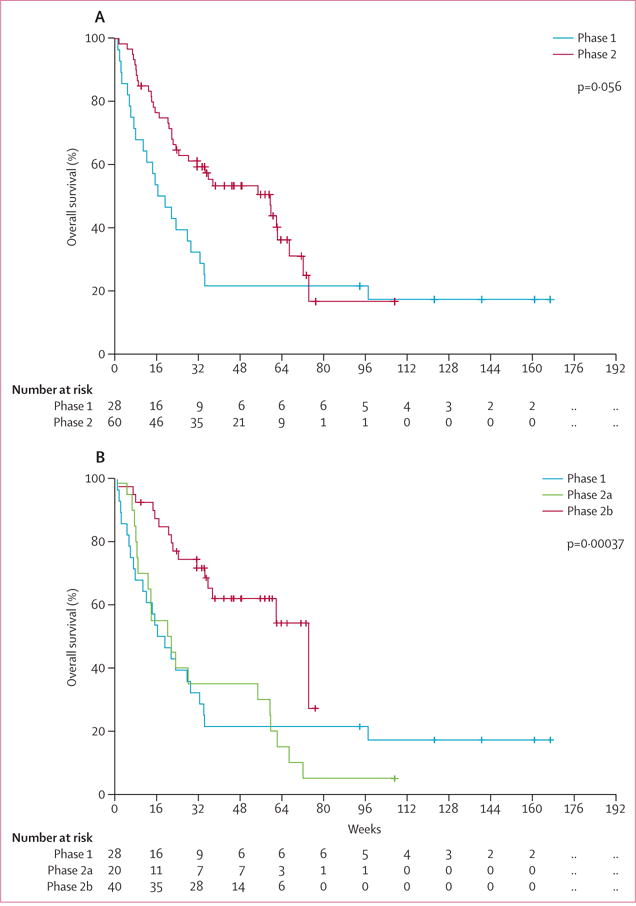
Overall survival for phase 1 and phase 2 (A) and phase 1, phase 2a, and phase 2b (B)
In the phase 2 part of treatment-naive patients, overall responses were noted in 27 (45%) patients, with a median overall survival of 60 weeks (IQR 6–34). In the optimum phase 2b cohort of 40 patients with myelodysplastic syndrome, 22 (55%) patients overall had a response (nine had complete response and 13 had complete response with incomplete haematological recovery) with a median survival of 75 weeks (IQR 25–not reached). The survival difference between cohorts was significant (p<0·0001) by log-rank test (figure 2). The appendix shows additional post-hoc statistical analyses comparing the cohorts.
In responding patients (n=31), the median response duration was 54 weeks (IQR 9–not estimable), with a median overall survival that has not been reached (figure 3) and a median follow-up of 57 weeks (IQR 14–74). 13 (42%) of the 31 responding patients proceeded with a stem-cell transplant, and ten of those 13 patients continue to have sustained remission.
Figure 3.
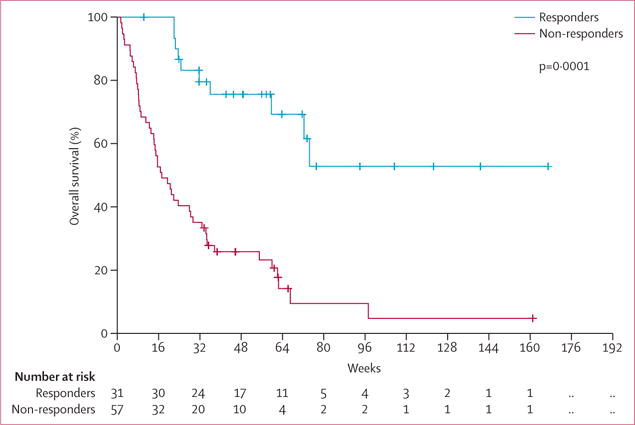
Overall survival by response
When analysed by IPSS category (including up to 30% bone marrow blasts), an overall response was achieved in five (71%) of seven patients with Int-1 disease, 13 (65%) of 20 with Int-2 disease, 12 (32%) of 38 with high-risk myelodysplastic syndrome, including RAEB-T (p=0·04). We noted only one responding patient in the 23 patients with acute myeloid leukaemia (>30% blasts) treated in phase 1. Findings of the IPSS-R classification showed similar prognostic importance, with overall responses achieved in both patients with low-risk disease, six (67%) of the nine patients with intermediate-risk disease, six (27%) of the 22 patients with high-risk disease, and 15 (31%) of the 48 patients with very high-risk disease (p=0·038). Overall survival was also significantly associated with both IPSS (p=0·0001) and IPSS-R score (p=0·0069; figure 4).
Figure 4.
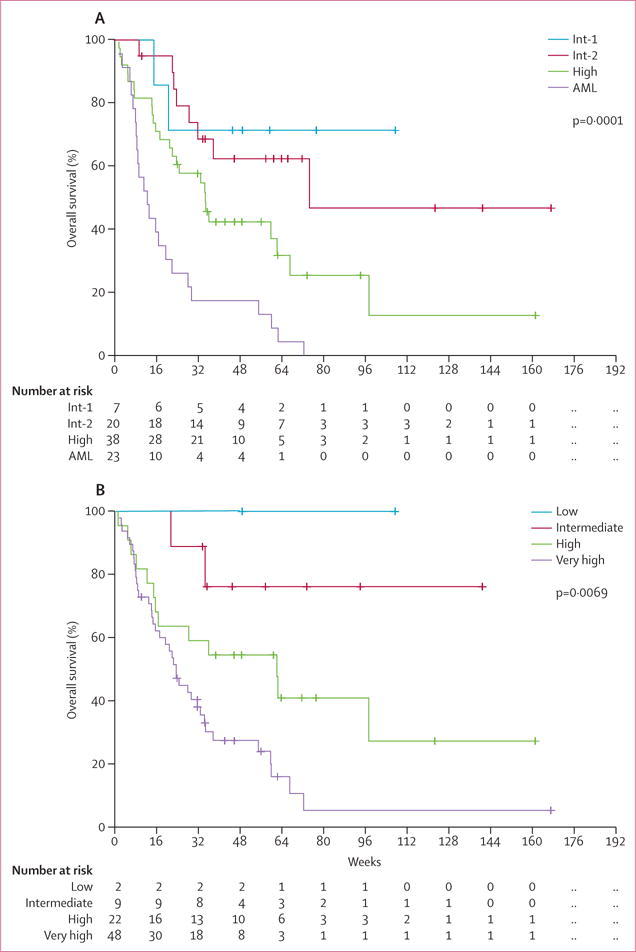
Subgroup analyses of overall survival by IPSS and IPSS-R score
We did a post-hoc analysis of outcomes by cytogenetic group and paid particular attention to patients with abnormalities in chromosome 5 because of the distinct activity of lenalidomide in low-risk patients with myelodysplastic syndrome with 5q deletion. 25 of 26 patients with abnormalities in chromosome 5 had other cytogenetic abnormalities as well; four (16%) of 26 patients had complete response or complete response with incomplete haematological recovery in this group. Although not significant, we noted trends toward improved response in certain cytogenetic risk groups, including an overall response in 13 (54%) of 24 patients with a diploid or isolated–Y karyotype and four (80%) of five patients with 20q deletion (p=0·071). Of the 15 patients who had complete response or complete response with incomplete haematological recovery with clonally abnormal metaphases at the study’s start, seven (47%) had a cytogenetic response during treatment, three with a partial cytogenetic response, and four complete cytogenetic responses according to IWG criteria.27
In univariate analyses of the phase 2 cohort, decreased overall survival was significantly associated with an oral lenalidomide dose greater than 25 mg per day (p=0·001) and complex cytogenetics (p=0·02). Platelet counts of 50 × 109 platelets per L or lower at diagnosis weakly associated with inferior overall survival (p=0·06). We recorded no significant associations based on other factors within the IPSS systems. Of note, bone marrow blast percentage at diagnosis was not associated with overall response (appendix). By multivariate analysis, treatment with lenalidomide and azacitidine was significantly associated with worse overall survival in patients with complex cytogenetics (HR 2·5, 95% CI 1·14–5·64; p=0·02) and those receiving lenalidomide doses greater than 25 mg (HR 2·7, 95% CI 1·34–5·49; p=0·005; appendix).
Of the 40 patients treated in phase 2b, we put the bone marrow samples of 25 patients in a next-generation sequencing panel for somatic molecular testing (figure 1 and appendix). All nine patients with somatic TP53 mutations had a complex karyotype, and five (56%) of the nine patients with TP53 mutations achieved a complete response or complete response with incomplete haematological recovery. Both patients with DNMT3A mutations and the patient with the EZH2 mutation achieved a complete response or complete response with incomplete haematological recovery, and the patient with the IDH2 mutation had stable disease for seven cycles and then discontinued treatment at the time of disease progression.
Discussion
This is the largest study so far of sequential azacitidine and lenalidomide treatment for patients with myelodysplastic syndrome and acute myeloid leukaemia; we have defined a safe and active combination schedule with 75 mg/m2 oral azacitidine once a day for 5 days followed by 25 mg oral lenalidomide once a day for 5 days (days 6–10 of each 28 day cycle; panel). More than half of patients with myelodysplastic syndrome on this schedule achieved complete response or complete response with incomplete haematological recovery. The 5 day azacitadine regimen is consistent with both community and academic experience, and several combination trials of azacitidine and lenalidomide that have used 5 day azacitidine dosing.20–23 Our findings also compare favourably with other studies examining azacitidine monotherapy, for which haematological improvement is recorded in roughly 50% of patients, but complete responses generally occur in less than 20% of patients. The responses in our study are also durable, with a median overall survival in responders that has not been reached after more than 1 year of follow-up.
Results of multivariate analysis confirmed an optimum lenalidomide dose of 25 mg and non-complex cytogenetics as predictive of response. Additionally, by both the IPSS and R-IPSS scoring systems, most patients with intermediate-risk disease responded well to this combination treatment by both overall response to treatment and improved overall survival.
Up to now, the presence of complex cytogenetics or TP53 mutations has been associated with inferior response rates in patients with myelodysplastic syndrome receiving lenalidomide monotherapy,29,30 whereas initial response rates to hypomethylating agents can be recorded irrespective of cytogenetic risk or TP53 mutation status, with a median time to response of about four cycles.5,31 These findings have been corroborated by data from recent reports suggesting that TP53 expression is associated with higher overall response in patients treated with azacitidine but predicts shorter overall survival in patients with del(5q) treated with lenalidomide.32,33 This evidence and the complementary modes of action of azacitidine and lenalidomide suggest that the azacitidine and lenalidomide combination might be especially effective for patients with TP53 mutations or unfavourable cytogenetics.24 In support of this proposed synergy, sequencing has previously shown that within one cycle of combination azacitidine and lenalidomide treatment, TP53 clones can decrease and even disappear.24 We noted complete response or complete response with incomplete haematological recovery in five (56%) of the nine patients with TP53 mutations despite the concomitant presence of complex cytogenetics. These data suggest that this regimen might be especially appropriate for this subgroup of high-risk patients with few treatment options.
Repeated cycles with daily doses of oral lenalidomide higher than 25 mg might lead to increased toxic effects, as evidenced by our initial phase 2a findings related to myelosuppression and infection-related events. The improved responses reported with the first-line combination regimen of 25 mg lenalidomide for 5 days (complete response or complete response with incomplete haematological recovery [55%]) without dose-limiting toxicities or treatment delays for myelosuppression suggest that this might be an optimum treatment schedule. However, the efficacy of this regimen should be further confirmed in randomised controlled trials.
When compared with similar studies by Sekeres and colleagues,22 the activity reported here is slightly lower. However, our 5 day azacitidine treatment cycle followed by a 5 day lenalidomide cycle on days 6 to 10 is a shorter treatment course than any other combination study done up to now. This reduced treatment burden might be beneficial for some patients, especially if molecular or clinical markers for adverse events or response to combination therapy can be identified, such as the recent finding that TET2 mutations can identify patients more likely to respond to hypomethylating agent treatment.34
19 molecularly characterised patients treated with combination azacitidine and lenalidomide have been previously reported by Sekeres and colleagues,13 and those with TET2, DNMT3A, and IDH1/2 mutations were more likely to respond to the combination of azacitidine and lenalidomide in that trial than those without. In our cohort of 25 molecularly annotated patients, both patients with DNMT3A mutations achieved a complete response or complete response with incomplete haematological recovery, whereas our patient with IDH2 mutation had initially stable disease which progressed after seven cycles. One 71-year-old patient with a high-risk EZH2 mutation on the phase 2b dose level remains in sustained complete response after 1 year of continued treatment.
An additional limitation with regards to interpretation of our survival data is that many of our patients went on to receive allogeneic stem cell-transplants, a consolidation strategy that improves the chances of long-term cure, but at the expense of increased early morbidity and mortality. Although having the opportunity to transition to a stem-cell transplant is a favourable outcome in itself, it makes it more difficult to assess the survival rates strongly associated with our treatment regimen.
In conclusion, we have identified the combination of 75 mg/m2 intravenous azacitidine once a day on days 1–5 with 25 mg oral lenalidomide on days 6–10 over a 28 day cycle as an effective first-line regimen for high-risk patients with myelodysplastic syndrome and acute myeloid leukaemia with up to 30% blasts. Responses are rapid (with a median of two cycles for response) and durable, and treatment with this dosing schedule is well tolerated. Responses in certain subgroups, such as patients with TP53 mutations, warrant attention in dedicated prospective studies.
Supplementary Material
Panel: Research in context.
Systematic review
We did a systematic review of the scientific literature on the subject of myelodysplastic syndrome and acute myeloid leukaemia and their treatments. We searched PubMed for studies published in English up to June 1, 2014, using terms that included “MDS,” “AML”, “MDS treatment”, “AML treatment”, “MDS AML lenalidomide”, “MDS AML azacitidine”, and “azacitidine lenalidomide”. This information was supplemented with pre-existing knowledge of the scientific literature, from clinical researchers specialising in this area. These searches and this knowledge base made it evident that the sequential combination of azacitidine and lenalidomide was supported by evidence and was an appropriate treatment strategy to pursue in the context of a clinical trial.
Interpretation
We recorded a safe sequential combination strategy for azacitidine and lenalidomide in high-risk patients with myelodysplastic syndrome and acute myeloid leukaemia consisting of 75 mg/m2 intravenous azacitidine once a day for 5 days followed by 25 mg oral lenalidomide once a day for 5 days. Further, we have provided preliminary evidence that this dosing strategy is active in patients with myelodysplastic syndrome. Compared with other trial regimens, our sequential combination strategy is short and shows promising results. Our data suggest that TP53 might have a role in response to this combination, which is supported by findings of other recent studies; the mechanism and exact role of TP53 in response remains to be fully elucidated.
Acknowledgments
This study was funded by Celgene (Summit, NJ, USA) and partly by the MD Anderson Cancer Center (Support Grant P30 CA016672). GG-M is also supported by the Edward P Evans Foundation, the Fundacion Ramon Areces, and by generous philanthropic contributions to MD Anderson’s myelodysplastic syndrome and acute myeloid leukaemia Moon Shot programme. CDD is also supported by the Jeanne F Shelby Scholarship Fund, which has supported her R Lee Clark Fellow award. We thank our technical writer Zach Bohannan (ELS, Memphis, TN, USA), for editorial assistance.
Footnotes
Contributors
HK and GG-M designed the study. ND, EJ, TK, GB, MK, NP, WW, JEC, FR, HMK, and GG-M enrolled and treated patients. CDD, ND, HY, SP, CB-R, KPP, and GG-M analysed and interpretated data. CDD and SP did the statistical analysis. CDD and GG-M wrote the report.
Declaration of interests
We declare no competing interests.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Albitar M, Manshouri T, Shen Y, et al. Myelodysplastic syndrome is not merely “preleukemia”. Blood. 2002;100:791–98. doi: 10.1182/blood.v100.3.791. [DOI] [PubMed] [Google Scholar]
- 3.Komrokji RS, Padron E, Lancet JE, List AF. Prognostic factors and risk models in myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 2013;13(suppl 2):S295–9. doi: 10.1016/j.clml.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:97–108. doi: 10.1002/ajh.23642. [DOI] [PubMed] [Google Scholar]
- 5.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. the International Vidaza High-Risk MDS Survival Study Group Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–27. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–34. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prebet T, Sun Z, Figueroa ME, et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905. J Clin Oncol. 2014;32:1242–48. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.List A, Dewald G, Bennett J, et al. the Myelodysplastic Syndrome-003 Study Investigators Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 10.Fenaux P, Giagounidis A, Selleslag D, et al. the MDS-004 Lenalidomide del5q Study Group A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–76. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 11.Fehniger TA, Uy GL, Trinkaus K, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117:1828–33. doi: 10.1182/blood-2010-07-297143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekeres MA, Gundacker H, Lancet J, et al. A phase 2 study of lenalidomide monotherapy in patients with deletion 5q acute myeloid leukemia: Southwest Oncology Group Study S0605. Blood. 2011;118:523–28. doi: 10.1182/blood-2011-02-337303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaballa MR, Besa EC. Myelodysplastic syndromes with 5q deletion: pathophysiology and role of lenalidomide. Ann Hematol. 2014;93:723–33. doi: 10.1007/s00277-014-2022-3. [DOI] [PubMed] [Google Scholar]
- 14.Wei S, Chen X, McGraw K, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene. 2013;32:1110–20. doi: 10.1038/onc.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padron E, Komrokji R, List AF. Biology and treatment of the 5q-syndrome. Expert Rev Hematol. 2011;4:61–9. doi: 10.1586/ehm.11.2. [DOI] [PubMed] [Google Scholar]
- 16.Hoefsloot LH, van Amelsvoort MP, Broeders LC, et al. Erythropoietin-induced activation of STAT5 is impaired in the myelodysplastic syndrome. Blood. 1997;89:1690–700. [PubMed] [Google Scholar]
- 17.Wobus M, Benath G, Ferrer RA, et al. Impact of lenalidomide on the functional properties of human mesenchymal stromal cells. Exp Hematol. 2012;40:867–76. doi: 10.1016/j.exphem.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Vallet S, Palumbo A, Raje N, Boccadoro M, Anderson KC. Thalidomide and lenalidomide: Mechanism-based potential drug combinations. Leuk Lymphoma. 2008;49:1238–45. doi: 10.1080/10428190802005191. [DOI] [PubMed] [Google Scholar]
- 19.Pollyea DA, Zehnder J, Coutre S, et al. Sequential azacitidine plus lenalidomide combination for elderly patients with untreated acute myeloid leukemia. Haematologica. 2013;98:591–96. doi: 10.3324/haematol.2012.076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platzbecker U, Braulke F, Kündgen A, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013;27:1403–07. doi: 10.1038/leu.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherman E, Malak S, Perot C, Gorin NC, Rubio MT, Isnard F. Interest of the association azacitidine-lenalidomide as frontline therapy in high-risk myelodysplasia or acute myeloid leukemia with complex karyotype. Leukemia. 2012;26:822–24. doi: 10.1038/leu.2011.284. [DOI] [PubMed] [Google Scholar]
- 22.Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–51. doi: 10.1182/blood-2012-06-434639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsingh G, Westervelt P, Cashen AF, et al. A phase 1 study of concomitant high-dose lenalidomide and 5-azacitidine induction in the treatment of AML. Leukemia. 2013;27:725–28. doi: 10.1038/leu.2012.214. [DOI] [PubMed] [Google Scholar]
- 24.Platzbecker U, Germing U. Combination of azacitidine and lenalidomide in myelodysplastic syndromes or acute myeloid leukemia—a wise liaison? Leukemia. 2013;27:1813–19. doi: 10.1038/leu.2013.140. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 26.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Bennett JM, Kopecky KJ, et al. the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–49. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 29.Möllgård L, Saft L, Treppendahl MB, et al. Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica. 2011;96:963–71. doi: 10.3324/haematol.2010.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burcheri S, Prebet T, Beyne-Rauzy O, et al. Lenalidomide (LEN) in INT 2 and high risk MDS with del 5q. Interim results of a phase II trial by the GFM Blood (ASH Ann Meet Abstr) 2007;110:820. [Google Scholar]
- 31.Kulasekararaj AG, Smith AE, Mian SA, et al. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol. 2013;160:660–72. doi: 10.1111/bjh.12203. [DOI] [PubMed] [Google Scholar]
- 32.Saft L, Karimi M, Ghaderi M, et al. p53 protein expression independently predicts outcome in patients with lower-risk myelodysplastic syndromes with del(5q) Haematologica. 2014;99:1041–49. doi: 10.3324/haematol.2013.098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller-Thomas C, Rudelius M, Rondak IC, et al. Response to azacitidine is independent of p53 expression in higher-risk myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica. 2014;99:e179–81. doi: 10.3324/haematol.2014.104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124:2705–12. doi: 10.1182/blood-2014-06-582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


