Mitochondrial DNA (mtDNA) diseases may be the poster child for highly targeted, “personalized” medicine. These heterogeneous disorders, although rare individually, have well-defined genetic causes — more than 400 known pathogenic mutations or deletions in the 16,569-base-pair mitochondrial chromosome that contains only 37 genes. Affected persons may present at any age with some combination of severe, often progressive, and sometimes fatal neurologic, musculoskeletal, cardiac, gastrointestinal, renal, ophthalmologic, and audiologic involvement. No cures or therapies have been approved by the Food and Drug Administration (FDA) for any mtDNA disease, although symptom-based clinical management can be beneficial.
Despite their precisely defined causes, it's often difficult to predict the onset or severity of these diseases because of heteroplasmy: the culprit mtDNA mutation is commonly present in only a fraction of the body's mitochondria. Building on the principle that less is better, reducing mutant heteroplasmy loads below an often tissue-specific and difficult-to-define threshold presents a potential opportunity to improve health that is unique to these diseases. Research in animal and somatic cellular models has focused on this potential, using endonuclease, TALEN (transcription activator–like effector nuclease), or CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 technologies to selectively target and degrade genomes harboring a specific mtDNA mutation,1 with subsequent tissue repopulation by nonmutated mtDNA genomes to levels considered sufficient to support normal mitochondrial energy production and restore organ health. But we don't yet have safe delivery mechanisms to accomplish such “heteroplasmy shift therapy” in the organs of patients with mtDNA disease.
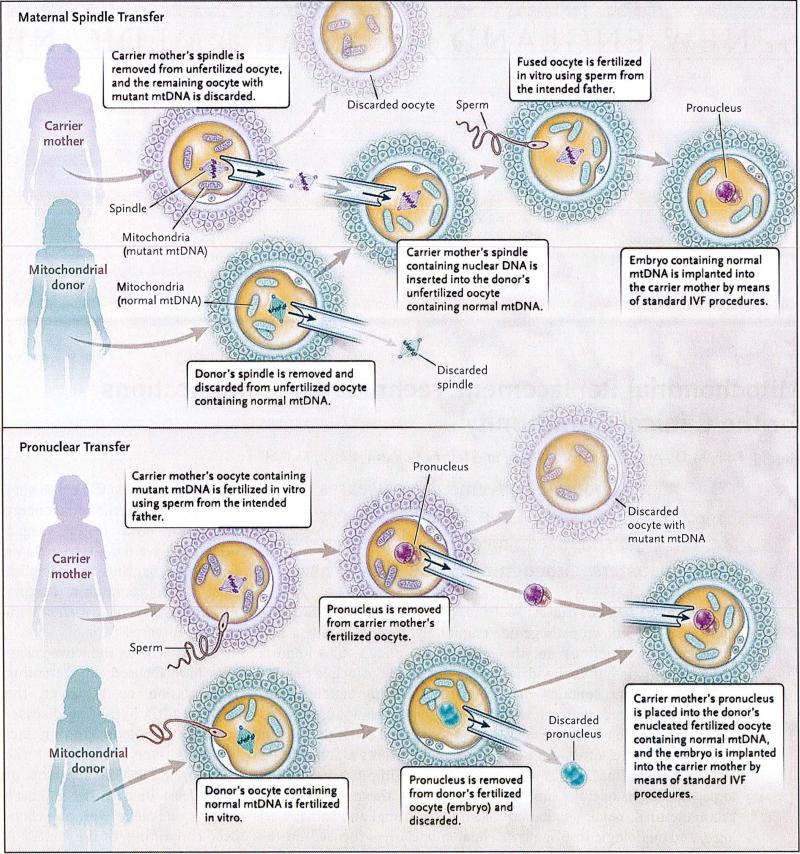
So instead, reproductive scientists have focused on preventing transmission by replacing the mitochondria harboring disease-causing mutations (and in fact replacing everything except the chromosome spindle apparatus or pronucleus) in a carrier mother's oocytes or zygotes with mitochondria containing healthy mtDNA genomes (see diagram). Since the mtDNA genome is quarantined within mitochondria, separated from the 20,000-plus genes residing in each cell's nucleus, it seems easiest to replace mutated mitochondria with healthy mitochondria from oocytes provided by another woman. Mitochondria are inherited only through the maternal germline, so those in an oocyte or zygote would theoretically need to be replaced only once to prevent the clinical sequelae of inherited mtDNA disease from manifesting in the child and, if that child was female, in her future offspring.
Two Mitochondrial Replacement Techniques — Maternal Spindle Transfer and Pronuclear Transfer.
In either procedure, some mutant mtDNA, estimated at 1 to 2%, might be carried over together with the spindle or pronucleus, but the levels are low enough to avoid disease risk. IVF denotes in vitro fertilization.
The technical feasibility of such mitochondrial replacement techniques (MRTs) has been demonstrated in animal models, and resulting nonhuman-primate offspring have been born without obvious disease.2 The MRT approach is generic: instead of targeting a specific mutation, MRTs replace en bloc nearly all mitochondria and their resident mtDNA and so could be applicable to reduce transmission in any inherited mtDNA disease.
The Institute of Medicine (IOM) Committee on the Ethical and Social Policy Considerations of Novel Techniques for Prevention of Maternal Transmission of Mitochondrial DNA Diseases, on which we served, considered whether and under what conditions undertaking initial clinical investigations of MRTs in humans would be ethically permissible. The committee concluded that “it is ethically permissible to conduct clinical investigations of MRT[s], subject to certain conditions and principles.”3
If and when initial investigations are undertaken, critical safety and efficacy questions will remain before regulatory approval or clinical use can occur. For instance, will these techniques reliably reduce mtDNA-mutation heteroplasmy in the oocyte or embryo to a level low enough to avert all symptoms and effects of mtDNA disease in the resulting child? Might unanticipated health, fertility, or developmental problems arise, along with questions about identity, for children conceived by mixing mitochondria organelles containing mtDNA genomes from one woman with the nuclear DNA of another? Are there potential health risks from the additional manipulations of in vitro embryos required by MRTs? Will any unforeseen, potentially irreversible problems be introduced in the resulting child and future descendants?
The IOM report concludes that safety for the child must remain paramount both in deciding whether to pursue clinical investigations of MRTs and in conducting any such studies.3 The committee argues that transferring only male embryos for implanting in initial studies would permit evaluation of initial safety considerations without transmitting to future descendants any potentially heritable risks related to manipulated mtDNA genomes. As experience is gained with MRTs and resulting offspring, the relative emphasis on precaution could change, and increasing knowledge could guide future decision making about expanding MRT research to include the transfer of female embryos, if clinical experience and international debates about the permissibility of heritable genetic modification result in accepted guidance.3
From the perspective of patients with mtDNA disease, their families and advocates, and physicians who study or treat mitochondrial diseases, complementary goals include preventing disease as well as devising effective treatments and, eventually, cures. MRTs would have no health benefits for people who already have mtDNA diseases, nor would they prevent the occurrence of newly arising mtDNA mutations causing serious diseases. However, surveys suggest that many patients with a known risk for transmitting a serious mtDNA disease to their offspring are highly motivated to prevent it from occurring.4
Without MRTs, people carrying mtDNA diseases had limited options: avoid having children, use egg donors, adopt, or conceive naturally and accept a high risk for clinically manifest mtDNA disease in offspring. MRTs might allow such people to pursue their reproductive preferences without passing on the risk of severe disease. Prospective parents with a known risk of transmitting nuclear gene disorders have options such as preimplantation genetic diagnosis (PGD), whereby embryos created through in vitro fertilization could be tested and unaffected embryos selected for implantation. Unfortunately, PGD without MRT is not uniformly reliable for mtDNA diseases, largely because of the biologic challenges of producing embryos with levels of mutant mtDNA low enough to reliably reduce the risk that the resulting children will manifest disease.5 Ironically, the same biologic factors that limit our ability to predict mtDNA disease in embryos (the location of mitochondrial chromosomes outside the nucleus, heteroplasmy with a varying threshold effect, and unpredictable replication of the mtDNA genome, unsynchronized with cell division) open a pathway for creating an embryo without mtDNA disease — an approach not open to parents carrying nuclear gene disorders.
The prospect of MRTs, however, raises questions about how the clinical community will manage the application of an emerging reproductive technology. Even if approved by the FDA, MRTs may be clinically applicable for relatively few women with known risk of passing on serious mtDNA diseases; if so, would it be appropriate to use substantial research and clinical resources for this endeavor? The possibility raises ethical and economic issues, such as the trade-off between using new techniques to avert disease and paying the costs of severe illness for individuals, families, and society. On the other hand, enterprising reproductive medicine practitioners could seek to offer MRTs as conception aids to older women, which raises questions about the appropriate dissemination of a new reproductive technology beyond its envisioned disease-specific applications. The IOM committee argued that the FDA should make clear policy, and professional societies should issue practice recommendations, limiting inappropriate and opportunistic applications of MRTs.3
Among the practical implications of the committee's recommendations is that specialized expertise will be needed both to clinically diagnose mtDNA diseases and to perform MRTs. Transparency in laboratory reporting will be essential for evaluating the efficacy of proposed methods for reducing the mutation-heteroplasmy load while yielding successful live births. For children conceived after PGD, follow-up is largely limited to confirmatory genetic diagnostic procedures prenatally or at birth; for MRTs, long-term monitoring would be needed throughout childhood, and probably well into adulthood, to determine efficacy over time and to identify any negative outcomes that change the risk–benefit balance.
The United Kingdom recently approved a process by which its Human Fertilisation and Embryology Authority could license MRT on a case-by-case basis. Since the United States lacks a comparable regulatory authority, the FDA, professional organizations and societies, and individual practitioners will have to work together to implement effective processes and practices to support the responsible use of MRTs and other related technologies. We hope that the medical community will embrace this challenge.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hashimoto M, Bacman SR, Peralta S, et al. MitoTALEN: a general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol Ther. 2015;23:1592–9. doi: 10.1038/mt.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tachibana M, Sparman M, Sritanaudomchai H, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–72. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Academies of Science, Engineering, and Medicine . Mitochondrial replacement techniques: ethical, social, and policy considerations. National Academies Press; Washington, DC: 2016. [PubMed] [Google Scholar]
- 4.Engelstad K, Sklerov M, Kriger J, et al. Attitudes towards prevention of mtDNA-related diseases through oocyte mitochondrial replacement therapy. Hum Reprod. doi: 10.1093/humrep/dew033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitalipov S, Amato P, Parry S, Falk MJ. Limitations of preimplantation genetic diagnosis for mitochondrial DNA diseases. Cell Rep. 2014;7:935–7. doi: 10.1016/j.celrep.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]