Abstract
Nanoparticles have shown promise as both drug delivery vehicles and direct antitumor systems, but they must be properly designed in order to maximize efficacy. Computational modeling is often used both to design new nanoparticles and to better understand existing ones. Modeled processes include the release of drugs at the tumor site and the physical interaction between the nanoparticle and cancer cells. In this article, we provide an overview of three different targeted drug delivery methods (passive targeting, active targeting and physical targeting), compare methods of action, advantages, limitations, and the current stage of research. For the most commonly used nanoparticle carriers, fabrication methods are also reviewed. This is followed by a review of computational simulations and models on nanoparticle-based drug delivery.
Keywords: targeted drug delivery, self-assembly of nanoparticles, nanoparticle design, cancer, multiscale modeling
1. Introduction
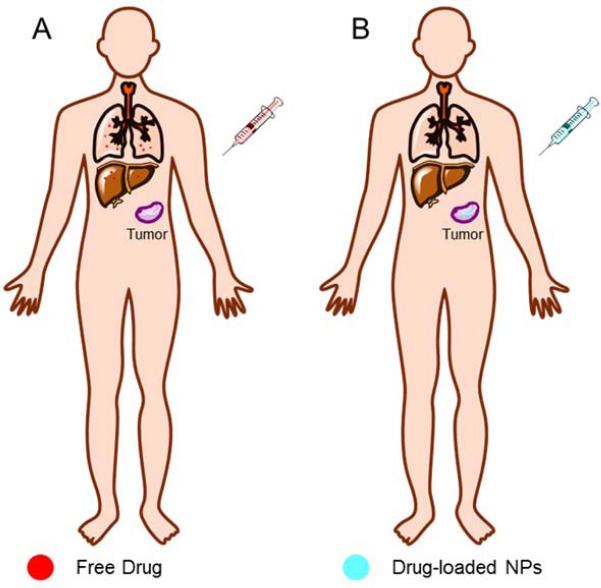
Nanoparticles (NPs) often exhibit different magnetic, thermal, optical and electrical properties due to their high surface area and limited quantum-mechanical effects 1. NPs are often developed and used as drug carriers, as they can deliver chemotherapeutics targeted to the tumor tissue without damaging normal organs (Fig. 1). The ideal NP carriers should be biodegradable, stable, non-immunogenic, easy to fabricate, cost effective, and able to release their payloads only at the target site 2.
Fig. 1.
Schematic contrast of drug biodistribution after injection of free drug (A) and drug-loaded NPs (B).
Medical NPs are often manufactured with a guided bottom-up method, in which engineered macromolecular components are guided by external stimuli to interact with each other and self-assemble into complex structures that otherwise would not be possible 3. Drugs can either be encapsulated within the nanoparticle or attached to surface.
A typical drug delivery nanoparticle starts with a nanoplatform class, which include liposomes, polymeric micelles, drug conjugated polymers, and dendrimers, among others 4–7. There are three main methods to transport drug-loaded nanoparticles to diseased sites: passive targeting, active targeting, and physical targeting. Passive targeting works through the increased permeability and retention (EPR) effect, which makes tumor cells preferentially absorb NP-sized bodies 8,9. In active targeting, NPs are functionalized with ligands such as antibodies, proteins, and peptides 10, which interact with receptors overexpressed at the target site 11. Physical targeting uses external sources or fields to guide NPs to the target site and to control the release process, for example in photothermal and magnetic hyperthermia therapy. For all targeting types, drug release can be triggered by a change in pH, temperature, or a combination of both.
In order to design an effective NP, one needs to understand the combinatorial effects of size, shape, surface chemistry, patient-specific information, and other parameters. Optimizing all of these parameters through experiments is both time and resource-intensive, and so computational modeling is used to shrink this possibility space. Simulations have been used to model the continuum of NP transport and the quantum mechanical interactions of ligand receptors. Mesoscale modeling and Monte-Carlo simulations are also often used when certain values are uncertain 12.
The aim of this paper is to review fabrication methods for the most common nanoparticle types (specifically self-assembly), targeted drug delivery processes, and the current state of NP computational modeling. Directions for future research are also discussed.
2. Self-assembled nanoparticles as delivery vehicles
Medical nanoparticles, despite their name, are far from the smallest things that scientists and engineers work with. Rather, they occupy a manufacturing blind spot located between large and small structures. Objects and materials with features at, and larger than, the microscale are now readily fabricated through “top-down” approaches like lithography and precision machining. Objects smaller than nanoparticles are generally easily synthesized through “bottom-up” methods in which individual chemicals essentially assemble themselves under the influence of intermolecular forces. Nanoparticles are too large and complex to be made by simply mixing their molecular components in a test tube, but much too small to be assembled with even the highest precision lithographic device. The solution is a guided bottom-up approach, in which macromolecular components are engineered to interact with each other and often external stimuli fields and self-assemble into structures more complex than would otherwise be possible 3.
While there are many types of self-assembled nanoparticles, the most studied can broadly be sorted by structure. Below is an introduction, with examples, to amphiphilic NPs (the most common type of drug carrying NP), followed by a brief explanation of other novel NP structures: dendrimers, polyrotaxanes, functionalized carbon nanotubes, graphene, and metal solid-core nanoparticles. It is important to note that all of these NPs can be functionalized to actively target specific sites in and on tumor cells.
2.1 Amphiphilic Nanoparticles
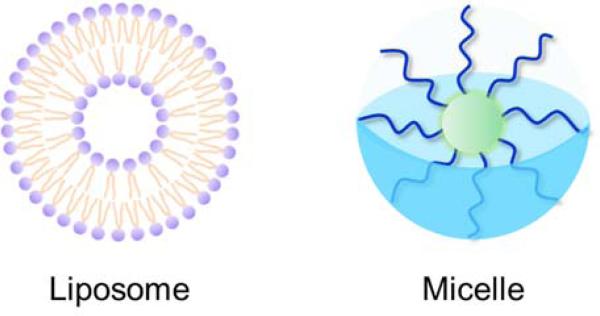
The earliest drug NPs were biomimetic in nature, mimicking the micelles and liposomes already present in the body. These NPs contain macromolecules with both hydrophobic and hydrophilic regions. In an aqueous environment such as blood or cellular fluid, the hydrophobic regions will cluster together in order to be shielded from the surrounding polar water, forming a micelle. Synthetic micelles have hydrophobic cores, while more complex amphiphiles can form full liposomes with a double-layered macromolecular wall and a hydrophilic core with the same or different properties as the surrounding fluid (Fig. 2). Drugs can be captured either in the core or on the surface of these particles, depending on their hydrophobicity. In either case, the drug is shielded from removal by the immune system and from other in vivo hazards.
Fig. 2.
Schematic of two types of amphiphilic nanoparticles, liposomes and micelles. Liposomes have a double layer and a hydrophilic core, while the core of micelles is hydrophobic.
An early example of micelle-forming particles involved poly(ethylene oxide)-poly(aspartic acid) block copolymers (PEO-PAA) 13. These polymers were used to create a self-assembling micelle containing the chemotherapy drug doxorubicin (DOX). DOX is commonly used to test the efficacy of self-assembling carriers, and comes in both hydrophobic and hydrophilic HCl formulations – it can be assumed to be hydrophobic unless stated otherwise. In this case, DOX was bound to the hydrophobic PAA polymer in organic solvent and then pushed through a membrane into an aqueous solution, a technique known variously as membrane dialysis or diafiltration. This transition from hydrophobic to hydrophilic solution induces the formation of micelles and leaves DOX encapsulated at the PAA center. The resulting nanoparticle was extremely soluble and stable in water, increasing the half-life of the payload drug.
A similar experiment was carried out with an amphiphilic pullulan acetate polymer 14. Cancer cells have been shown to overexpress vitamin H, so the polymer was functionalized with it in order to actively target these cancer cells. After DOX was loaded into the micelles using membrane dialysis, the authors found that the amount of vitamin H expressed on the surface of the nanoparticle correlated with its uptake by cancer cells, indicating successful active targeting.
More complex micelles can be engineered to release their payloads in response to external stimulation. Bae et al. 15 had designed pH-sensitive nanoparticle carriers which follows previous reports on poly(ethylene glycol)-poly(aspartate-hydrazone) copolymers. DOX was bound to the hydrazone group and micelles were again formed through membrane dialysis. Hydrazone bonds are easily cleaved in acidic conditions, so this micelle was designed to expose DOX in response to low pH. Micelles are taken up by cells through endocytosis and subsequently engulfed by lysosomes, which have a pH of around 5 and thus trigger drug release. The authors found that DOX concentration decreased as a function of pH, with around 30% of the drug released at a pH of 5 and the entire payload released at lower pH.
A particularly interesting trigger-based nanoparticle was created by combining the pH responsive polymer poly(acrylic acid) (PAA) with the heat sensitive poly(N-isopropylacrylamide) (PNIPAM) 16. PNIPAM becomes hydrophobic above its lower critical solution temperature of around 32°C, while PAA becomes hydrophobic at a pH below 4.8. The copolymer will thus form a micelle with a PNIPAM core at high temperature and pH, but flip to a PAA core at low temperature and pH. PAA also binds to DOX in aqueous solution, eliminating the need for organic solvents or membrane dialysis. When it binds at low temperature and high pH, the PAA-DOX complex becomes hydrophobic and forms micelles. This nanoparticle was shown to exhibit drug release both with an increase in temperature (which causes the micelle to flip inside-out and expose DOX to the environment) and a drop in pH (which causes PAA to protonate and release the less positive DOX). PNIPAM has also been used to synthesize nanoparticles that can shrink in volume in response to temperature 17.
Polymer nanoparticles have also been made with hybrid polymer-lipid amphiphiles, which allow for a broader range of potential polymers. This system was used in a nanoparticle able to hold both drugs and DNA using a cationic core-shell system 18. The main polymer chain is hydrophilic poly(N-methyldietheneamine sebacate) (PMDS) grafted with the hydrophobic N-(2-bromoethyl) carbarmoyl cholesterol lipid to form an amphiphilic copolymer. The antitumor drug Paclitaxel (PTX) was encapsulated through membrane dialysis, and luciferase-coding DNA was bound to the nanoparticle in order to detect fluorescence. It was found that cancer cells successfully expressed luciferase, indicating successful endocytosis. Lipids can also be used as a molecular shield to increase drug half-life in blood 19.
It is also possible to change the chemistry of certain polymers to distort the resulting nanoparticle 20. Micelles can be created in a variety of non-spherical shapes using poly[oligo(ethyleneglycol)methacrylate]-block-[poly(styrene)-copoly(vinyl benzaldehyde)] block polymers. The shape of the micelles changed from sphere to rod as the degree of polymerization for the P(ST-co-VBA) blocks increased. DOX was able to be loaded into the micelles as normal.
Huang et al. 21 designed a poly(lactide-co-glycolic acid) nanoparticle coated with sgc8 aptamer capable of carrying both a hydrophobic and hydrophilic cancer drug. After self-assembly, hydrophilic DOX is located in the poly(ethylene-glycol) shell and hydrophobic paclitaxel is located in the PLGA core. The sgc8 aptamer causes the nanoparticle to be internalized by cancer cells specifically, an example of active targeting. The authors show that the multidrug combo was more effective than either drug individually at reducing cell viability, and the addition of a second drug had an insignificant effect on the viability of normal cells. It is also possible to use transferrin on the nanoparticles to increase uptake by cancer cells 22.
Xia et al. 23 designed silk-elastin protein polymer nanoparticles. Silk-elastin-like proteins are synthetic genetically engineered proteins designed to mimic the properties of both silk and elastin. The proteins are temperature-dependent amphiphiles and will form micelles. In this case, it was shown that DOX actually triggered micelle formation in some cases by increasing the hydrophobicity of the silk-end chains. The authors reason that this polymer and method of self-assembly, since it is formed biologically in non-extreme environments, will generate fewer toxins and be a safer alternative to traditional methods.
2.2 Novel Nanoparticle Structures
An example of a novel nanoparticle structure is a dendrimer, which is a repeatedly branching nanostructure that mimics the structure of tree branches and blood vessels 7. This shape is shown to both generate large surface areas and disperse a surface more evenly throughout the structure. Dendrimers are commonly made of poly(amidoamine), but can also be made from other polymers. It has been shown that dendrimers can be used to target cancer cells with high accuracy 26. Lam et al reported a novel class of micelles made of linear PEG and dendritic cholic acids (CA) block copolymers (called telodendrimers) 27. By crosslinking the boronate esters at the core-shell interface, the stability of these micelles can be improved (Fig. 4) 28. As the crosslinking reactants, boronic acid and catechol were added to the polymers through step-wise peptide chemistry.
Fig. 4.
Schematic representation of the disulfide cross-linked micelles formed by oxidization of self-assembled thiolated telodendrimer PEG5k-Cys4-L8-CA8 27. Schematic representation of the telodendrimer pair [PEG5k-(boronic acid or Catechol)4-CA8] and the resulting boronate crosslinked micelles (BCM) triggered by mannitol and/or acidic pH values 28.
Rotaxanes are molecular linkages in which a cyclic molecule encircles a dumbbell-shaped one – the cyclic molecule can rotate but cannot slide off of the dumbbell. Polyrotaxanes can be a useful tool for drug delivery 29. Liu et al. attached cyclic cyclodextrin to poly(ethylene glycol) chains in order to form a nanoparticle. Hydrophobic cinnamic acid was attached to the ends of the PEG chains in order to increase the space between the cyclodextrin rings, providing space for DOX to bind. This system can also be used to transport the drug methotrexate 24.
Dendrimers and Rotaxanes are used primarily for drug delivery, but the next three structures are also often used in thermal therapy, where a localized temperature increase is used to destroy a tumor. Graphene is a single atom-thick hexagonal allotrope of carbon with novel electrical, thermal, and mechanical properties. Because of its high surface area, it is useful as a drug carrier, and its structure makes it efficient at converting infrared light into heat. It is, however, relatively toxic, and must be stabilized and shielded through the addition of polymers to its surface 25,30. Carbon nanotubes are rolled tubes of graphene that exhibit many of the same properties. Nanotubes have been used as hybrid drug carries, where the antitumor drug is released only in a specific site when bombarded with near-infrared radiation and the NPs are heated up. This can help to limit drug release to the tumor site and protect healthy tissue 31,32.
Metal-core nanoparticles can be used photothermally, like graphene and carbon nanotubes, but they can also be used for magnetic hyperthermia, where a magnetic NP oscillates and heats up in response to an external magnetic field. Most NPs are made of magnetite or maghemite cores, and are relatively biocompatible. Successful clinical trials have also been done with a magnetic fluid composed of dispersed NPs in water 33.
Oligopeptides have been extensively studied as nanocarriers due to their intrinsic pH sensitivity resulting from amino acids. Zhang et al. developed a liposome system based on zwitterionic oligopeptide lipids as nanocarriers 34. The amino acid-based lipids, 1,5-dioctadecyl-l-glutamyl 2-histidylhexahydrobenzoic acid (HHG2C18) and 1,5-distearyl N-(N-α-(4-mPEG2000) butanedione)-histidyl-l-glutamate (PEGHG2C18), have a multistage pH-response to the tumor microenvironmental pH (pH 6.8) then the endo/lysosomal pH (pH 4.5) successively.
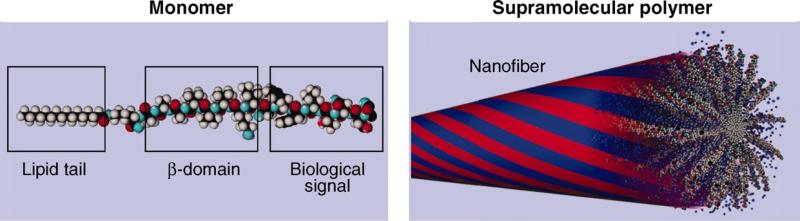
Supramolecular polymers have significant potential application in drug delivery due to their reversible monomer-to-polymer transitions. Fig. 5 shows the molecular unit designed to form a supramolecular architecture 35. The pair is a peptide amphiphile monomer composed of three segments: a biological signal-bearing sequence, an amino acid-contained domain, and a hydrophobic alkyl tail. These monomers can form a cylindrical aggregate where twisted β sheets (red) collapse through hydrophobic interactions among alkyl chains, resulting in high signal densities. The blue regions represent water domains present in the assembly interior.
Fig. 5.
Molecular representation of monomer and the corresponding supramolecular polymer formed after their aggregation through specific interactions 35.
2.3 Challenges in Nanoparticle Development
There are many challenges that arise in the early and late stage development of medical nanoparticles that are either nonexistent or minimal in non-nanoparticle based therapies 36. The primary driver of these issues is the hierarchical and non-uniform nature of nanoparticles, which means that a small change in a single property can have outsized effects on the pharmacokinetics or therapeutic efficacy of the particle. As an example, one common issue is maintaining a narrow distribution of particle size. For most applications, a particle size under 200nm is desirable. With a broad normal distribution of sizes, this means that often the average particle must be too small to be useful in order to limit the number of particles over 200nm. It is thus desirable to have a manufacturing process that ensures a narrow size distribution.
In addition, many unique challenges can arise during the trial and production stages of the nanoparticle. Oftentimes, a procedure for nanoparticle formation that works in a lab setting will not work in a factory, and the synthesis steps must be completely reworked. In a factory, the variation in nanoparticle structure must be smaller, the yield must be higher, and the synthesis must be more sterile than what is acceptable in a lab. All of these things can make a particle unviable to produce even if it works. If a particle is sold, it must be shelf-stable, which means that it both will not degrade in solution and that it will not clump over time, as nanoparticles often do. Finally, nanoparticles face extra regulatory challenges as their toxicity is much more difficult to determine than that of a small molecule. This greatly increases the time and cost of clinical trials. These challenges combined mean that it is always prudent to consider questions of scalability and reproducibility even at the earliest stages of development to prevent failure at a later stage.
3. Cancer cell targeted drug delivery
3.1 Mechanism of cancer cell targeted drug delivery
Cancer cells are otherwise normal cells with unique mutations in genes regulating growth, which cause them to divide uncontrollably and give them the ability to metastasize 37. Cancer cells successfully compete with normal cells for oxygen, glucose, and amino acids for division and growth, but a tumor can only grow to about 2mm3 without forming blood vessels (angiogenesis) 38–40. There are more than one hundred types of cancer, more than 85% of which are solid 40. Current treatment includes surgery, radiotherapy, chemotherapy, hormone therapy, and immunotherapy 40. However, the inability of drugs to specifically target cancer cells hinders most treatment 2,41,42. It is often quicker and cheaper to design a more effective way to better target an existing drug than to develop an entirely new one. Drug delivery targeting is classified as passive, active, or physical, and can target organs, cells, or organelles. Organelle targeting is an especially promising field of research, as many cancers specifically affect a single one, and certain organelles provide alternative paths for drug localization.
3.2 Passive targeting nanocarrier systems
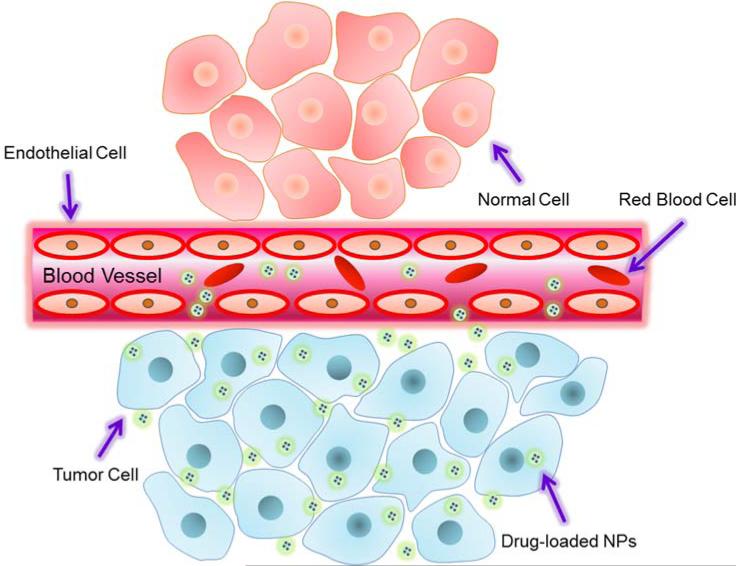
Drugs delivered intravenously tend to evenly disperse throughout the body. However, tumor cells tend to take up particles of a certain size to a greater degree than healthy cells due to a combination of leaky tumor blood vessels and faulty particle screening. This is known as the enhanced permeation and retention (EPR) effect (Fig. 6) and is the mechanism behind passive targeting 43.
Fig. 6.
Schematic illustration of enhanced permeation and retention (EPR) effect.
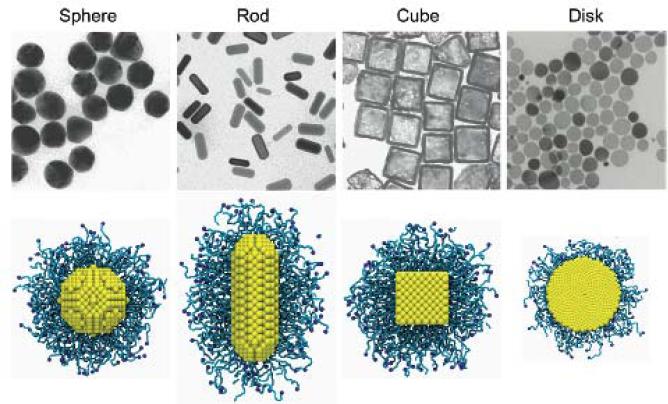
The EPR effect is influenced by NP properties including particle size, shape, and surface charge, and it in turn influences circulation time, penetration speed, and intracellular internalization 44-45. For example, phagocytic cells favor the uptake of larger particles, while non-phagocytic cells favor the uptake of smaller particles 46. It has been consistently shown that PEGylated nanoparticles smaller than 100nm have reduced plasma protein adsorption on their surface and reduced hepatic filtration 47. NPs with a negative surface charge will circulate longer in blood, but positively charged NPs are more readily taken up by cancer cells (which have negative surface charge) 8–10,48–50. In order to clarify the influence of shape on the cellular uptake of PEGylated NPs, Liu et al, performed large scale molecular simulations to study differently shaped NPs with identical surface area, ligand-receptor interaction strength, and PEG grafting density (Fig. 7) 51. They found that spheres exhibited the fastest internalization rate, followed by cubes, while rods and disks were the slowest.
Fig. 7.
Differently shaped NPs: sphere, rod, cube and disk. The top shows the transmission electron microscopy images of these NPs.52,53 The bottom shows the PEGylated NPs with grafting density 1.6 chains per nm2 in molecular simulations 51.
Delivery platforms include liposomes 4, polymeric micelles 5,6, targeted polymer drug conjugates 7, and dendrites. They all consist of macromolecule collections in which drugs are dissolved, entrapped or conjugated to the surface 54. Several liposomal drug delivery systems have received clinical approval, including ones for doxorubicin and daunorubicin. An albumin-bound nanoparticle carrying paclitaxel, abraxane, was also approved by FDA for breast cancer treatment 55.
Despite the EPR effect, more than 95% of passively targeted NPs fail to reach the tumor when administered intravenously 48. Targeting can be greatly improved by locally controlling drug release at the site of the tumor. This can be triggered through changes in the microenvironment (pH, temperature, or enzymatic) or through external stimuli (light, electric fields, magnetic fields, or ultrasound) directed at the tumor site 56–58.
An alternative way to improve the uptake of NPs, both passive and active, has been to functionalize their surfaces with cell-penetrating peptides (CPPs). It has been found that certain short (~30 amino acids) peptide sequences can pierce cell membranes and transport drug cargo into a cell. These CPPs can be attached to micelles, liposomes, and other types of NPs 59. Certain CPPs can also act as drug carriers on their own, carrying small molecules and short stretches of DNA into cells 59. Most CPPs are amphiphilic, with a net positive charge. Because they are shaped similar to lysing peptides, certain CPPs can exert unwanted toxic side effects.
3.3 Active targeting
Active targeting uses ligands bound to the NP surface to improve their uptake selectivity. These ligands can react with target cells and will often protect NPs from enzyme destruction. Ligands with a high binding affinity to the target cell will strongly increase delivery efficiency. The most basic form of active targeting involves functionalizing a NP with a ligand that binds to a molecule overexpressed on cancer cells. The issue with this, of course, is that healthy cells still express the same molecule, and as healthy cells greatly outnumber tumor cells most of the NPs miss their target. This issue can be mitigated by using multiple ligands, or by using ligands of different types.
Approaches to identify potential receptors in and on cancer cells include in vivo phage screening and aptamer screening 60. Using in vivo phage screening, F3 was discovered to bind well with nucleolin 61, which is present at tumor cell surfaces and in tumor endothelial cells. The cytoplasmic proteins annexin1 62, plectin-1 63, and p32 protein 64 were also found through in vivo phage screening. By studying the expression of the known cell surface receptors in tumor vessels, other molecular markers can be detected. For example, ∂vß3, ∂vß5 integrins, and ED-B were discovered in angiogenic vessels using this principle 65–68. Gene expression analysis has also been used to discover overexpression of collagen in tumor endothelial cells 69 A detailed review on various markers and their discovery methods was given by Ruoslahti 60.
Many antibodies have been approved for use in clinical treatment by the FDA, such as rituximab, Ipilimumab, and trastuzumab 70. Antibodies are among the most studied ligands because of their high specificity and availability. An antibody conjugated dendrimer was found to bind exclusively to human prostate adenocarcinoma (LNCaP) cells that express PSmA(J591) 26. Although antibodies have many merits, they are difficult to conjugate to NPs, result in a short circulation time, and are expensive. Peptides are a promising alternative, as they are smaller, simpler, more stable, and easier to produce. Among peptides, RGD is often used due to its strong binding with αvβ3 integrin receptors. Nucleic acid base aptamers combine the advantages of both antibodies and peptides, but they degrade quickly. Other small molecules can also be used as ligands, such as folic acid for folate receptors 71. Such molecules are small, stable, and easy to produce. Unfortunately, ligand detection for relevant substrates is challenging. Even with proper binding ligands and receptors, binding incompatibility can limit therapeutic efficiency. Multiple ligands with different charges can increase overall the binding affinity, but the limited binding ability and capacity of receptors will govern the quantity and quality of the binding. For instance, overly strong binding can actually reduce tumor penetration, hinder selectivity, and lead to an overdose of carriers 72.
Active targeting alters the natural distribution patterns of a carrier, directing it to a specific organ, cell, or organelle. In contrast, passive targeting relies on the natural distribution of the drug and the EPR effect. Both of these processes depend on blood circulation and the location of initial drug delivery. However, no actively targeted NPs are commercially available currently.
3.4 Physical targeting
Physical targeting navigates drugs to cancer cells using external stimulation, such as radiation or magnetic fields. Photothermal therapy is often used because of its relatively few side effects. Photothermal therapy uses NPs that once delivered, efficiently convert near infrared light energy to heat, killing cancer cells. Currently, most research is being carried out using gold nanoparticles because they can be well controlled and have low toxicity. Their SPR effects can be tuned by shape, size, and thickness to maximize excitation and focus on a specific wavelength. According to a previous report, gold nanoparticles are already being tested in animals 73.
Photothermal agents other than gold have also been explored. Carbon nanotubes show strong absorbance in near infrared region 31, and it has been demonstrated that photothermal hyperthermia using them can inhibit G2-M cell cycles 32. Graphene has also been used in photothermal drug delivery. Functionalized graphene oxide with polymer conjugates is pH sensitive and its photothermal effect can cause cell death 30. Silica-coated graphene nano-sheets functionalized with hydrophilic polyethylene glycol have also been used to deliver doxorubicin 25. In the cases of carbon nanotubes and graphene oxide, pH and heat are used to initiate drug release.
One limitation to photothermal therapy is that cancer cells are often tolerant to environmental stress, for example with heat shock proteins that prime the cell against further damage 74. Nanoparticle-free radiation therapies are quite common, however. High-energy x-ray or gamma radiation is cytotoxic and can kill cells in specific regions 75. The details of radiation therapy have been reviewed by Stacy 75.
Magnetic hyperthermia uses the heat energy produced by magnetic nanoparticles oscillating in a magnetic field. The distance between magnetic nanoparticles and target cells, sensitivity to magnetic fields, and magnetic field strength all affect heat energy production and correlate to therapeutic effects. Magnetic NPs typically consist of four parts: NPs, protective agents, biomolecules, and surface agents. They are usually synthesized based on magnetite (Fe3O4), maghemite, cobalt, or nickel. Among them, iron oxides are most used due to its biocompatibility and shape controllability.
Magnetic NPs are usually coated with functional polymers like carbohydrates and proteins to protect against corrosion and potential toxin release. However, some polymers’ mechanical strength and selectivity are not easy to control, so organic linkers are often used to create electrostatic interaction. Replacing ions and changing the pH of the immediate environment can also modify the binding strength of magnetic particles. Both photothermal therapy and magnetic hyperthermia can be done in vivo and in vitro, and several clinical trials have been performed 76. Unlike active targeting or physical targeting alone, this combination can effectively promote NP internalization by tumor cells 77.
4. Modeling applications in drug delivery and nanoparticle device design
4.1 Models and their applications in the field
Drug delivery NPs become complex because the pharmacokinetic properties of the drug itself must be fully understood as well as all parts of the delivery system. NPs can be affected by many parameters, including platform (liposomes, polymeric micelles, polymer drug conjugates, dendrites), physical parameters (size, shape), and surface chemistry. On its way to the target, the NP must pass through and interact with multiple biological barriers, including those in the bloodstream, at the site of the tumor, at the surface of the cell, and several within the cell. In addition, the efficacy of a NP is highly dependent on individual patient conditions.
Because nanoparticle research is complex, with many variables and costly experiments, it is an excellent candidate for computer simulations. Simulations can both screen drug and carrier candidates and provide design insights towards entirely new NPs. Theoretical and computational modeling can be used for any of the drug delivery processes previously reviewed to provide solutions for optimized geometry, surface chemistry, or other properties. For instance, continuum based modeling is used to study transport and dissolution, molecular dynamics are used for cell interaction, and stochastic approaches such as Monte Carlo simulations can be used to calculate random variables and take uncertainties into account for each patient.
Continuum Modeling
Transport modeling of NPs through the vascular network is challenging due to vastly different blood vessel diameters, which range from centimeters for larger vessels to microns for capillaries. Advection-diffusion models are used for transport in the larger vessels, where blood is modeled as a simple Newtonian fluid 78-79. In the microvascularture, a convection-diffusion-reaction model was developed for nanoparticle concentration studies. G. Fullstone 80 used flexible large-scale agent based modeling (FLAM) coupled with computational fluid dynamics (CFD) to study NP distribution in capillaries. In the microvasculature, red blood cells aid NP dispersion to the vessel walls and modify the EPR effect. It was reported that larger NPs (submicron size) are more likely to be pushed to the vessel walls than smaller NPs, which in turn gives them a greater chance of permeating through diseased vasculatures to reach the target cells 80. NP adhesion with endothelial cells modeled with IMEFEM suggests that NP shape affect adhesion, with spherical NPs having a lower lateral drift velocity compared with ellipsoids. A NPs binding probability can be simulated by randomly assigning initial positions of nanoparticles at the channel inlet, applying a Brownian adhesion dynamics model, conducting a number of independent trials, calculating the average of number of bonded nanoparticles, and then normalizing the total number of nanoparticles for a certain depletion layer thickness and shear rate. Nanoparticle deposition and distribution patterns inside the blood vessel network can also be simulated using a continuum model 12. Saltzman and Radomsky 81 developed a diffusion based kinetics model to study drug release problems in brain tissue. Their prediction was validated with drug distribution profiles gained through in vitro experiments.
Pressure, velocity, and blood chemistry will all affect the transport of NPs and can lead to premature deformation or release. An accurate prediction of these parameters is thus critical to design stable NPs. Factors that affect NP deformation and release include blood flow velocity, shear rate, bonding energy, and porosity. It has been shown that NPs can be modeled using a combination of diffusion, swelling, and erosion 79,82,83. Because of possible swelling and erosion during circulation, one must assume a dynamic boundary for the continuum models during the release process 12. Finite element analysis can be used to solve these models.
Molecular Modeling
Molecular modeling can be used to understand the size, shape, and charge distribution within a system. It is based on interactions between atoms and molecules for a fixed time period. Free energy minimizations of the system can generate a numerical solution for complex system properties. Hence, it can be used in animating molecular motion and elucidating both the uptake process and the influence of molecular structure, NP size, shape, and surface chemistry. Depending on how potential energies are calculated, various molecular modeling methods, including empirical methods, ab initial quantum mechanical methods, classic molecular dynamics, and coarse grain molecular dynamics can be used to understand drug-carrier and carrier-medium interactions 84.
Empirical molecular descriptors relying heavily on experimental data fitting can be used to predict physical properties such as drug solubility, and the diffusion coefficient, which often results in complicated artificial parameters. By deriving the theoretical molecular descriptors from compound chemical structures, limitations of experiment data can be resolved. Quantum mechanical ab initio method is a useful tool for property calculations and higher-level model calibrations by considering the electronic degrees of freedom. It uses quantum mechanical methods such as the density functional theory to determine information about electronic behavior. It can simulate a few hundred atoms without any experimental input and output information otherwise unavailable, such as the electronic state. Adhikari et al. 85 studied RGD-4C peptide electronic structure, partial surface charge distribution, and dielectric response with ab initio quantum mechanical methods and shed light on the peptide-∂vß3 integrin receptor interaction. It is particularly useful for the study of bond breaking and formation. Unfortunately this method is very complex and computationally intensive to simulate large systems, or to simulate for significant lengths of time.
Classical molecular mechanical simulations are less time consuming compared with the ab initial quantum mechanical methods. The potential energy is relatively easy to calculate, so they can be used for larger systems and a longer period of time. Coarse-grained molecular dynamics are used for large systems and for simulations that run on a timescale of larger than 1ms. Instead of calculating all the atoms in the system, subunits are selected for system energy calculations. Model parameters need to be fitted with experimental data, so the accuracy is limited by the availability of data. Loverde, et al. used coarse grain molecular dynamics to study the shape effect of Worm-like PEG-PCL micelles in drug delivery. It was found that PEG and the PEG-PCl interface play am important role in drug release 86. Coarse grain MD can also be used for cellular uptake process simulations with multi-wall CNT. Dr. Gao et al. have modeled the uptake process with CGMD by assuming immobile ligands and diffusive receptors 87. They predicted a critical NP size for endocytosis consistent with experimental results 87. The studies of Yang and Ma 88 and Ying Li 89 both show that shape and initial orientations affect the endocytosis process. Endocytosis is governed by the bending energy of the cell membrane and the ligand-receptor binding energy. Bao and Bazilevs 90 also used large-scale coarse-grained MD to understand how PEGylation affects NP endocytosis. They found that the repulsive interaction energy between grafted chains and cell membranes is larger than the membrane bending energy through endocytosis. Optimal grafting density is also predicted for ligand-receptor interactions.
Stochastic Modeling
Each patient's physical and pathological conditions are different, including blood flow rates, red blood cells, vascular networks and receptor densities on the tumor cell surface. Even for the same person, these parameters can change over time. It is thus crucial to consider uncertainties for the parameters in models. Simply using average values can lead to unacceptable errors in the modeling and design of a NP platform. Monte Carlo methods are particularly good options when dealing with these parameters uncertainties 12.
Monte Carlo method use random generators from certain probability distributions to artificially produce samples repetitively and calculate mean and variance of the samples. The In fact, Monte Carlo simulations have been used to study the interaction between ligand-bound NPs with both tumor cells and healthy cells. Many parameter values have been considered as inputs in order to understand their effects 91, and it was found that multiple weak reversible ligand receptors binding is the most important variable in selective targeting. Liu et al. used Monte Carlo simulation to understand the effect of surface functionalization of NPs on its binding to endothelium. They found that antibody coverage is the key parameter for binding process to occur.92 Recently, Ying Li, etc. quantified the uncertain dispersion coefficient of NPs during microvascular drug transport process with Bayesian calibration method. 89
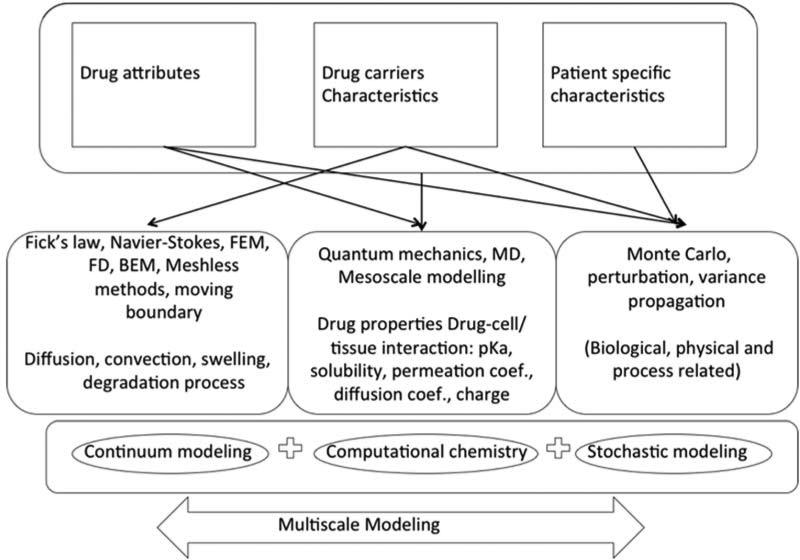
Linking of Multiple Length Scales in Modeling
Drug delivery takes place across various time and spatial scales. NP circulation, endocytosis, and drug release can each be modeled with appropriate computational methods mentioned above. However, to simulate the entire system from drug to patient remains a challenging problem. There are some reports on multiscale model frameworks shown in (Fig.8). The input of these systems tends to be things like drug attributes, delivery system characteristics and patient specific information. Monte Carlo methods and atomic calculations such as molecular dynamics can be used to predict physical parameters, diffusivity, and solubility. At the patient scale, MRI images taken from experiments can be used to provide the geometry of the vascular network. After providing initial concentration and location of injected NPs, the concentration profile of NPs in the vascular system can be simulated with immersed FEM analysis. Coarse-grained MD can use the concentration information together with the drug carrier architecture to simulate the interaction between NPs and target cell membranes. When the information is passed from one model to another, uncertainties are also passed along. Ying Li provided a good example of how to deal with error propagation when connecting models together. By coupling different scales together, the drug delivery optimization problem can get much closer to the right solution 89.
Fig. 8.
A multiscale-modeling framework for drug delivery processes. Reorganized with permission from 12.
4.2 Challenges and Limitations in Modeling
Computational modeling has not been widely used in pharmaceutical industry for drug delivery design due to its limited predictability and accuracy. Most current approaches are agent-based empirical models. Mechanism based models are unable to realistically describe biological and chemical interactions and delivery processes. The fundamental mechanisms of each process needs to be better understood, and can be aided by smarter experimental design. Each of the computational models has its limitations. Most of the current mechanistic models focus on a particular process instead of coupling with each other to simulate the entire process from drug to patient. Linking models of different temporal and spatial scales is challenging but will provide the greatest benefit in terms of accuracy.
Mechanistic models require high quality data for parameter estimation, model calibration, validation, and error analysis. To track molecules and nanoparticles in living cells and tissues over time is challenging, so in vitro data for more physiologically realistic simulations is also needed. Repeated experiments are needed to build physiological and delivery system variable statistical distributions. Uncertainty quantification and updating is required for connecting lower scale level atomistic models with higher scale level continuum models as error from one part of the simulation can propagate leading to an unacceptable solution.
5 Conclusion and Future Works
NPs are good candidates for targeted drug delivery carriers. They are widely available, easily functionalized with good biocompatibility and stability. Concentrated doses of toxic drugs can be encapsulated and delivered by the nanoparticles directly to the tumor site. Currently there are three delivery strategies: Passive targeting, which relies on the EPR effect; Active targeting, which uses ligand-receptor interactions for more selective drug delivery; and next-generation photothermal and magnetic hybrid NPs, where drugs release is controlled in both time and location using heat generated by the photothermal or magnetic nanoparticle. Physical targeting is still at the clinical trial stage.
NPs can self-assemble into different structures, including amphiphilic micelles and liposomes, as well as more exotic rotaxanes, dendrimers, and metal core particles. Each of these particles has specific strengths and weaknesses and is suitable for the delivery of different therapies to different regions. The choice of a nanoparticle has much to do with the application in mind- there is not yet a gold standard. Most typical NPs used as drug delivery carriers are micelles and liposomes, but there is increasing research into other particles. Rotaxanes can release cargo in response to a tailored stimulus, while dendrimers can sometimes maximize surface area and cargo capacity when compared with simple spherical structures. In addition, metal-core particles are a good choice for physical and combination physical-active or physical-passive targeting.
Experimental development of these NPs is challenging, as size, shape, and surface charge must all be controlled to carry drugs to the target site. Inappropriate physical parameters not only can compromise drug delivery efficacy but also cause serious side effects. Developed NPs have to be tested in both living cells and tissue first before they can be moved to in vivo tests. Environmental differences can also impact drug delivery efficacy. Due to the complexities of the drug delivery process and the large amount of uncertainty involved, computational modeling is mostly applied for NP design optimization. Currently, they are not used widely in the pharmaceutical industry due to their poor predictability. Most models only focus on specific processes in drug delivery instead of full simulations from the drug to the patient scale. Multiscale modeling across different temporal and spatial scales is rare.
In the future, NP design can be better guided by multiscale modeling. However, experiments also provide needed data for model calibration, validation, and help to understand the mechanisms unique to each drug delivery process. Multiscale models that take in personalized data, such as MRI scanned vascular network image, genome, family history, targeted cell receptor density, and the physical-chemical properties of NPs should be further improved and validated in clinical settings. Collaborative work of computation and experiment is already used for more efficient nanocarrier design. Shi, et al 93 used molecular docking/MD simulation to screen building blocks for nanocarrier synthesis. Their models were validated with experimental synthesis and evaluations of nanocarrier library. Jiang, et al 94 employed multiscale modeling together with experiments to understand the building blocks’ role in telodendrimer self-assembly process. Recently, microchip device development has become another direction to simulate a tumor's microenvironment and test the effectiveness of NPs for targeted drug delivery 95,90. Device design can also be facilitated by computational models, which will further advance the development of NPs for drug delivery.
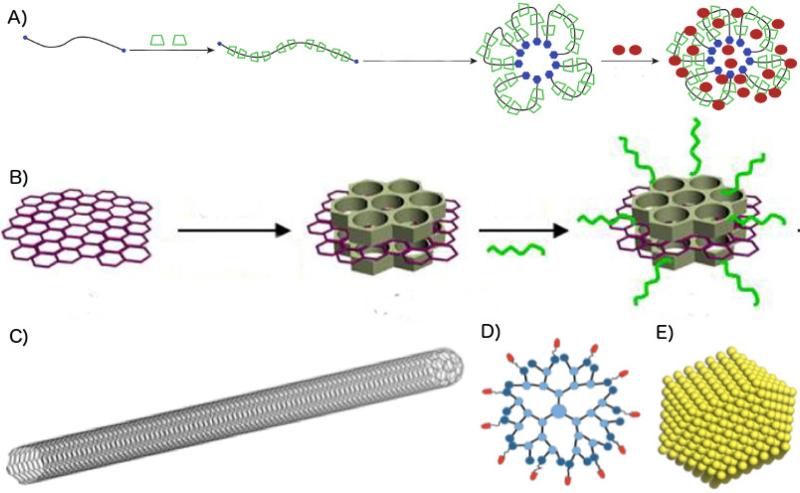
Fig. 3.
Structures of novel nanoparticles. A) Polyrotaxane NPs are assembled from cyclical molecules threaded around a long polymer chain. Hydrophilic ends are added to the chain in order to induce self-assembly. Drugs are then added to the finished NP. Adapted with permission from 24. B) Graphene functionalized with shielding molecules and ligands. Adapted with permission from 25. C) Carbon nanotube schematic. D) Dendrimer schematic. E) Metal-core photothermal NP schematic.
Acknowledgement
Z.C. acknowledges support from the Society in Science Branco Weiss Fellowship, administered by ETH Zürich. The research reported in this paper was in part supported by the National Cancer Institute of the National Institutes of Health under Award no. U01CA202123. The authors thank S.J. Huang for comments.
References
- 1.Ochekpe N, Olorunfemi P, Ngwuluka N. Nanotechnology and Drug Delivery Part 1: Background and Applications. Trop. J. Pharm. Res. 2009;8 [Google Scholar]
- 2.Poste G, Kirsh R. Site–Specific (Targeted) Drug Delivery in Cancer Therapy. Bio/Technology. 1983;1:869–878. [Google Scholar]
- 3.Whitesides G, Kriebel J, Mayers B. Nanoscale Assem. 2005:217–239. doi:10.1007/0-387-25656-3_9. [Google Scholar]
- 4.Igarashi E. Factors affecting toxicity and efficacy of polymeric nanomedicines. Toxicol. Appl. Pharmacol. 2008;229:121–34. doi: 10.1016/j.taap.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud A, Xiong X-B, Aliabadi HM, Lavasanifar A. Polymeric micelles for drug targeting. J. Drug Target. 2007;15:553–84. doi: 10.1080/10611860701538586. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004;61:2549–59. doi: 10.1007/s00018-004-4153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv. Drug Deliv. Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]
- 9.Yuan F, et al. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 10.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruoslahti E. Specialization of Tumour Vasculature. Nat. Rev. Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 12.Haddish-Berhane N, Rickus JL, Haghighi K. The role of multiscale computational approaches for rational design of conventional and nanoparticle oral drug delivery systems. Int. J. Nanomedicine. 2007;2:315–331. [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon GS, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Biodistribution of micelle-forming polymer-drug conjugates. Pharm. Res. 1993;10:970–974. doi: 10.1023/a:1018998203127. [DOI] [PubMed] [Google Scholar]
- 14.Na K, et al. Self-assembled nanoparticles of hydrophobically-modified polysaccharide bearing vitamin H as a targeted anti-cancer drug delivery system. Eur. J. Pharm. Sci. 2003;18:165–173. doi: 10.1016/s0928-0987(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 15.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chemie - Int. Ed. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Song S, Guo L, Ma S. Self-Assembly of Thermo- and pH-Responsive Poly(acrylic acid)-b-poly(N-isopropylacrylamide) Micelles for Drug Delivery. J. Polym. Sci. Part A Polym. Chem. 2008;46:5028–5035. [Google Scholar]
- 17.Rahikkala A, Aseyev V, Tenhu H, Kauppinen EI, Raula J. Thermoresponsive Nanoparticles of Self-Assembled Block Copolymers as Potential Carriers for Drug Delivery and Diagnostics. Biomacromolecules. 2015;16:2750–2756. doi: 10.1021/acs.biomac.5b00690. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Gao S, Ye W-H, Yoon HS, Yang Y-Y. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat. Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagoz B, et al. Polymerization-Induced Self-Assembly (PISA) – control over the morphology of nanoparticles for drug delivery applications. Polym. Chem. 2014;5:350–355. [Google Scholar]
- 21.Huang F, et al. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chem. Commun. 2014;50:3103. doi: 10.1039/c3cc49003c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, et al. Biospecific Self-Assembly of a Nanoparticle Coating for Targeted and Stimuli-Responsive Drug Delivery. Adv. Funct. Mater. 2015;25:1404–1417. [Google Scholar]
- 23.Xia XX, Wang M, Lin Y, Xu Q, Kaplan DL. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules. 2014;15:908–914. doi: 10.1021/bm4017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Su T, He B, Gu Z. Self-assembly Polyrotaxanes Nanoparticles as Carriers for Anticancer Drug Methotrexate Delivery. Nano-Micro Lett. 2014;6:108–115. [Google Scholar]
- 25.Wang Y, et al. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013;135:4799–804. doi: 10.1021/ja312221g. [DOI] [PubMed] [Google Scholar]
- 26.Patri AK, et al. Synthesis and in vitro testing of J591 antibody-dendrimer conjugates for targeted prostate cancer therapy. Bioconjug. Chem. 2004;15:1174–81. doi: 10.1021/bc0499127. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. Well-defined, Reversible Disulfide Cross-linked Micelles for On-demand Paclitaxel Delivery. Biomaterials. 2011;32:6633–6645. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, et al. Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to Acidic pH Values and cis-Diols. Angew. Chemie. 2012;124:2918–2923. doi: 10.1002/anie.201107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R, et al. Supramolecular nanoparticles generated by the self-assembly of polyrotaxanes for antitumor drug delivery. Int. J. Nanomedicine. 2012;7:5249–5258. doi: 10.2147/IJN.S33649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu A, Choi W, II, Lee JH, Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34:6239–48. doi: 10.1016/j.biomaterials.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 31.Estrada AC, Daniel-da-Silva AL, Trindade T. Photothermally enhanced drug release by κ-carrageenan hydrogels reinforced with multi-walled carbon nanotubes. RSC Adv. 2013;3:10828. [Google Scholar]
- 32.Zhang H, et al. Targeting and hyperthermia of doxorubicin by the delivery of single-walled carbon nanotubes to EC-109 cells. J. Drug Target. 2015;21:312–319. doi: 10.3109/1061186X.2012.749880. [DOI] [PubMed] [Google Scholar]
- 33.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int.J Hyperth. 2008;24:467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 34.Mo R, Sun Q, Li N, Zhang C. Intracellular delivery and antitumor effects of pH-sensitive liposomes based on zwitterionic oligopeptide lipids. Biomaterials. 2013;34:2773–2786. doi: 10.1016/j.biomaterials.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science (80−. ) 2012;335:813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–95. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzakos AG, Briasoulis E, Thalhammer T, Jäger W, Apostolopoulos V. Novel oncology therapeutics: targeted drug delivery for cancer. J. Drug Deliv. 2013;2013:918304. doi: 10.1155/2013/918304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossfeld GD, et al. Thrombospondin-1 expression in patients with pathologic stage T3 prostate cancer undergoing radical prostatectomy: association with p53 alterations, tumor angiogenesis, and tumor progression. Urology. 2002;59:97–102. doi: 10.1016/s0090-4295(01)01476-5. [DOI] [PubMed] [Google Scholar]
- 39.Jones A, Harris AL. New developments in angiogenesis: a major mechanism for tumor growth and target for therapy. Cancer J. Sci. Am. 4:209–17. [PubMed] [Google Scholar]
- 40.Zhang JIN, et al. Design of Nanoparticles as Drug Carriers for Cancer Therapy. Cancer Genomics Proteomics. 2006;158:147–157. [PubMed] [Google Scholar]
- 41.Poste G. Experimental systems for analysis of the malignant phenotype. Cancer Metastasis Rev. 1982;1:141–99. doi: 10.1007/BF00048224. [DOI] [PubMed] [Google Scholar]
- 42.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cnacer chemotherapy: mechanism of tumoritropic accumulatio of proteins and the antitumor agents Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 44.Chou LYT, Ming K, Chan WCW. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 45.Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 46.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 47.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oupicky D, et al. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol. Ther. 2002;5:463–72. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 50.Guo X, et al. Dual-Responsive Polymer Micelles for Target-Cell-Specific Anticancer Drug Delivery. Chem. Mater. 2014;26:4405–4418. [Google Scholar]
- 51.Li Y, Kröger M, Liu WK. Shape effect in cellular uptake of pegylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7:16631–16646. doi: 10.1039/c5nr02970h. [DOI] [PubMed] [Google Scholar]
- 52.Xia X, et al. Quantifying the coverage density of poly (ethylene glycol) chains on the surface of gold nanostructures. ACS Nano. 2011;6:512–522. doi: 10.1021/nn2038516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang B, et al. Kinetic effects of halide ions on the morphological evolution of silver nanoplates. Phys. Chem. Chem. Phys. 2009;11:10286–10292. doi: 10.1039/b912985e. [DOI] [PubMed] [Google Scholar]
- 54.Kreuter J. Nanoparticles--a historical perspective. Int. J. Pharm. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Saha RN, Vasanthakumar S, Bende G, Snehalatha M. Nanoparticulate drug delivery systems for cancer chemotherapy. Mol. Membr. Biol. 2010 doi: 10.3109/09687688.2010.510804. at < http://www.tandfonline.com/doi/abs/10.3109/09687688.2010.510804>. [DOI] [PubMed]
- 56.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Kale AA, Torchilin VP. Environment-responsive multifunctional liposomes. Methods Mol. Biol. 2010;605:213–42. doi: 10.1007/978-1-60327-360-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh KT, Yin H, Lee ES, Bae YH. Polymeric nanovehicles for anticancer drugs with triggering release mechanisms. J. Mater. Chem. 2007;17:3987. [Google Scholar]
- 59.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Deliv. Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christian S, et al. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003;163:871–8. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh P, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 63.Kelly KA, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008;5:e85. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fogal V, Zhang L, Krajewski S, Ruoslahti E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008;68:7210–8. doi: 10.1158/0008-5472.CAN-07-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brooks P, Clark R, Cheresh D. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science (80−. ) 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 66.Erdreich-Epstein A, et al. Integrins {{alpha}}v{beta}3 and {{alpha}}v{beta}5 Are Expressed by Endothelium of High-Risk Neuroblastoma and Their Inhibition Is Associated with Increased Endogenous Ceramide. Cancer Res. 2000;60:712–721. [PubMed] [Google Scholar]
- 67.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nilsson F, Kosmehl H, Zardi L, Neri D. Targeted Delivery of Tissue Factor to the EDB Domain of Fibronectin, a Marker of Angiogenesis, Mediates the Infarction of Solid Tumors in Mice. Cancer Res. 2001;61:711–716. [PubMed] [Google Scholar]
- 69.Carson-Walter EB, et al. Cell Surface Tumor Endothelial Markers Are Conserved in Mice and Humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 70.Gabizon AA. Pegylated Liposomal Doxorubicin: Metamorphosis of an Old Drug into a New Form of Chemotherapy. Cancer Invest. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 71.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008;41:120–9. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 72.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science (80−. ) 2012;338:903–10. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008;23:217–28. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 74.Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol. Ther. 2004;101:227–57. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Stacy DR, Lu B, Hallahan DE. Radiation-guided drug delivery systems. Expert Rev. Anticancer Ther. 2014 doi: 10.1586/14737140.4.2.283. at < http://www.tandfonline.com/doi/abs/10.1586/14737140.4.2.283>. [DOI] [PubMed]
- 76.McBain SC, Yiu HHP, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomedicine. 2008;3:169–80. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cole AJ, Yang VC, David AE. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDougall SR, Anderson ARA, Chaplain MAJ, Sherratt JA. Mathematical modelling of flow through vascular networks: implications for tumour-induced angiogenesis and chemotherapy strategies. Bull. Math. Biol. 2002;64:673–702. doi: 10.1006/bulm.2002.0293. [DOI] [PubMed] [Google Scholar]
- 79.Siepmann J, Peppas NA. Mathematical modeling of controlled drug delivery. Adv. Drug Deliv. Rev. 2001;48:137–138. doi: 10.1016/s0169-409x(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 80.Fullstone G, Wood J, Holcombe M, Battaglia G. Modelling the Transport of Nanoparticles under Blood Flow using an Agent-based Approach. Sci. Rep. 2015;5:10649. doi: 10.1038/srep10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saltzman WM, Radomsky ML. Drugs released from polymers: diffusion and elimination in brain tissue. Chem. Eng. Sci. 1991;46:2429–2444. [Google Scholar]
- 82.Langer R, Peppas N. Chemical and Physical Structure of Polymers as Carriers for Controlled Release of Bioactive Agents: A Review. J. Macromol. Sci. Part C. 2006;23:61–126. [Google Scholar]
- 83.Siepmann J. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001;48:229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 84.Neumann D, Lehr C-M, Lenhof H-P, Kohlbacher O. Computational modeling of the sugar–lectin interaction. Adv. Drug Deliv. Rev. 2004;56:437–457. doi: 10.1016/j.addr.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Adhikari P, et al. Electronic Structure, Dielectric Response, and Surface Charge Distribution of RGD (1FUV) Peptide. Sci. Rep. 2014;4 doi: 10.1038/srep05605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loverde SM, Klein ML, Discher DE. Nanoparticle Shape Improves Delivery: Rational Coarse Grain Molecular Dynamics (rCG-MD) of Taxol in Worm-Like PEG-PCL Micelles. Adv. Mater. 2012;24:3823–3830. doi: 10.1002/adma.201103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. 2005;102:9469–74. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang K, Ma Y-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010;5:579–583. doi: 10.1038/nnano.2010.141. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, et al. Multiscale modeling and uncertainty quantification in nanoparticle-mediated drug/gene delivery. Comput. Mech. 2014;53:511–537. [Google Scholar]
- 90.Bao G, et al. USNCTAM perspectives on mechanics in medicine. J. R. Soc. Interface. 2014;11:20140301. doi: 10.1098/rsif.2014.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncan G. a., Bevan M. a. Computational design of nanoparticle drug delivery systems for selective targeting. Nanoscale. 2015;7:15332–15340. doi: 10.1039/c5nr03691g. [DOI] [PubMed] [Google Scholar]
- 92.Liu J, et al. Computational model for nanocarrier binding to endothelium validated using in vivo, in vitro, and atomic force microscopy experiments. Proc. Natl. Acad. Sci. 2010;107:16530–16535. doi: 10.1073/pnas.1006611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang Shi, et al. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015;6:7449. doi: 10.1038/ncomms8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wenjuan Jiang, et al. Multiscale Approach to Investigate Self-Assembly of Telodendrimer Based Nanocarriers for Anticancer Drug Delivery. Langmuir. 2015;31:4270–4280. doi: 10.1021/la503949b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhise NS, et al. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release. 2014;190:82–93. doi: 10.1016/j.jconrel.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]