Abstract
Background
A major potential barrier for studying behavioral interventions for patients with Mild Cognitive Impairment (MCI) is the willingness and ability of people to enroll in and adhere to behavioral interventions, especially when the intervention involves dyads of patients with MCI and support partners. Details regarding recruitment strategies and processes (such as number of dyads screened) are often missing from reports of behavioral trials. In addition, reports do not detail the reasons a potentially eligible candidate opts out of participation in a research study.
Objective
To describe the challenges and successes of enrollment and retention in a behavioral trial for persons with MCI and their care partners, and to better understand barriers to participation from the patient’s point of view.
Design
Multi-site, randomized trial
Setting
Major medical centers
Participants
Our accrual target for the study was 60 participants. Potential candidates were patients presenting to memory evaluation clinics whose resulting clinical diagnosis was MCI. A total of 200 consecutive potential candidates were approached about participating in the study across the three sites.
Intervention
Detailed recruitment and retention data of a randomized trial comparing two behavioral interventions (memory notebook training versus computer training) provided in two separate training time frames (10 days versus 6 weeks).
Measurements
Structured interview with those declining to participate in the trial.
Results
Overall recruitment 37% with a range of 13%–72% across sites. Overall retention 86% with a range of 74%–94% across sites.
Conclusion
The primary barriers to enrollment from the patient’s perspective were distance to the treatment center and competing comprehensive behavioral programming. However, retention data suggest that those dyads who enroll in behavioral programs are highly committed.
Keywords: MCI, behavioral intervention, recruitment, retention
INTRODUCTION
Mild Cognitive Impairment (MCI), especially the memory or amnestic subtype, is considered a risk state for later development of dementia due to Alzheimer’s disease [1, 2]. Prevalence of MCI is high and increases with age. In the Cardiovascular Health Study Cognition Study, a multicenter population study, the overall prevalence of MCI was 19% with increasing prevalence with age (19% in those younger than 75 years, 29% in those older than 85 years; [3]. In a community-based sample followed longitudinally for 10 years, persons with MCI showed an increased risk of dementia over each 2 year evaluation interval (odds ratio = 3.9). Over time, Alzheimer’s disease has become more recognized as the etiology for a syndromic continuum from a pre-clinical stage to Mild Cognitive Impairment due to AD [4] [5]; [6]. With recognition of this disease continuum, there is increasing interest in secondary prevention strategies with the hope that treatment at the pre-clinical or MCI stage will delay or prevent progression to dementia.
Overall, medication trials for MCI have been disappointing. For example, in a randomized, double-blind, placebo-controlled, multi-center trial, there was no significant difference in the probability of progression from MCI to dementia between those treated with a placebo, Vitamin E or donepezil after three years of treatment [7]. In a recent meta-analysis of eight clinical trials examining the effects of medications classified as “cognitive enhancers” (donepezil, rivastigmine, galantamine, or memantine) on MCI, the authors concluded that cognitive enhancers did not improve cognition or function in patients with MCI, and further, were associated with greater risk of gastrointestinal symptoms [8]. Thus, there are no current FDA-approved medications for treatment of MCI.
To date, much of the behavioral treatment research in Alzheimer’s disease and other neurodegenerative conditions has focused on patients who had already progressed to dementia [9]; [10]. Given the lack of substantial medication options for people with MCI and increasing understanding of the risk state of MCI, there is increasing interest in behavioral approaches that may provide benefit in patients with MCI. This broadly includes cognitive rehabilitation, cognitive exercise, physical exercise, and nutritional wellness. The two behavioral approaches included in our randomized trial are cognitive rehabilitation approaches, involving use of a memory notebook for compensation for memory loss, as well as computerized cognitive exercise.
Memory notebooks are a form of compensation with validated efficacy in the treatment of memory impairment in traumatic brain injury patients [11]. Our early work has shown that patients diagnosed with MCI can learn to use a memory notebook with structured training [12], and use of the memory notebook improves functioning and memory self-efficacy in individuals with MCI, improves partners’ mood, and decreases partners’ sense of burden compared to an untreated control group [13].
On the other hand, cognitive training, especially via computer-based exercise programs, has also increased in popularity and interest given epidemiological evidence that those most cognitively active are less likely to develop cognitive impairment with aging [14]. Interventional trials in older subjects with normal cognitive functioning suggest improvement in cognitive abilities as well as a protective effect of cognitive training [15]; [16, 17]; [18].
Whether studies involve cognitive training or memory compensation, a major potential barrier for studying behavioral interventions is the willingness and ability of people to enroll in and adhere to behavioral interventions, especially when the intervention involves dyads of MCI patients and support partners [19]. For example, in a study involving comparison of cognitive training and physical activity or a combination of both, the researchers screened 343 potential subjects in order to enroll just 73 participants, a 21% enrollment rate [20]. Most were excluded as they were ineligible after further screening related to study exclusion criteria, but it appears a portion were also merely unwilling to participate in the research program. However, the authors reported good to excellent retention rates, with 76% of patients completing the physical activity arm, 96% completing the cognitive training arm, and 90% of patients completing the combination arm.
Describing screen failures based on investigator defined exclusion criteria in descriptions of study enrollment provides good information about the participants that researchers feel would be unlikely to benefit from a study or perhaps would be at increased risk for adverse effect. However, information on the potential participant’s point of view and specifics about why they decline to participate in research is generally missing. Yet some of the reasons otherwise eligible patients decline to participate in behavioral research trials may present surmountable barriers that could be overcome by researchers if better understood. In addition, some of the reasons eligible participants decline to enroll may be related to research specifically and would not be present if the intervention were available as a part of a clinical program (e.g., concern about randomization results). Trevedi, et al.[19]noted that details regarding recruitment strategies and processes (such as number of dyads screened) are often missing from reports of behavioral trials. In addition, to our knowledge, even if the number of screened dyads is included, reports do not detail the reasons a participant opts out of participation in a research study. Our report is meant as a step in that direction by reporting such enrollment and retention details for our behavioral trial, both from the researcher perspective (i.e., screen failures) as well as the participant’s perspective.
With funding from the National Institute of Nursing Research (NINR), we undertook a pilot project to better understand whether persons with MCI would enroll in and remain in an intensive trial comparing two behavioral interventions (memory notebook training versus computer training) provided in two separate training time frames (10 days versus 6 weeks). We intend to report the efficacy outcomes from that randomized comparison trial in a separate report. The goal of this report is to describe the challenges and successes of enrollment and retention in a behavioral trial for persons with MCI and their care partners, and to better understand barriers to participation from the patient’s point of view.
METHODS
Interventions
Memory Support System (MSS)
The MSS is a two-page-per-day calendar and note-taking system small enough to fit in a breast pocket or purse. The system and our training curriculum are described in detail in prior reports [12, 13]. Briefly, the MSS includes three sections: 1) events that happen at a particular time, i.e., appointments, 2) events that can happen anytime, i.e., daily “to do” items, and 3) a journaling section, i.e., important thoughts or events that happened that day.
The MSS training curriculum utilizes three training stages from learning theory outlined by Sohlberg and Mateer [11]: 1) an acquisition phase in which participants learn the sections of the MSS and their intended uses, 2) an application phase in which a participant is taught to apply MSS use to his/her daily life, and 3) an adaptation phase in which a participant practices incorporating the MSS into daily activities so as to make its use habitual.
Each training session provided orientation, modeling, practice use, and homework assignments. A typical agenda for a MSS training session included: 1) review and discussion of Intervention Plan/Questions related to the training phase (acquisition, application, or adaptation), 2) review of homework, 3) learning phase-appropriate instruction of MSS, and 4) assignment of next session’s homework.
Computer Training (Posit)
Those randomized to the computer training arm received copies of the MSS but without training. Each dyad completed computer activities on the same schedule as those receiving MSS training. Posit Science has developed a computer-based training program built on the principles of positive brain plasticity and designed for use by mature individuals. The training program (“Brain Fitness”) is focused on speech reception to strengthen an individual’s memory for speech. It has 6 modules name: Hi-Lo, Tell Us Apart, Match It, Listen and Do, Sound Replay and Story Teller.
Research to date has found: 1) participants with limited or no computer experience were capable of learning to perform the training exercises, 2) the training was safe and well tolerated by participants, 3) participants with MCI and cognitively normal older adults who trained on Brain Fitness also showed on average a 1/3 standard deviation improvement on memory and cognitive function [17, 21].
Training Schedules
In addition to comparing these two cognitive rehabilitation interventions, we were also interested in evaluating different training schedules. Each schedule provided 10 hours of intervention conducted either over 6 weeks or in 10 days.
Education
All participants (whether receiving MSS or Posit) in each scheduled program (6-week or 10-day) were convened for educational group at each session. The education component is an adaption and synthesis of the Savvy Caregiver psychoeducational program [22] and the “Memory Club” educational program [23, 24]. The education program in this study offered ten 45-minute group sessions with topics including Introduction to the Program, Living with MCI, Changes in Roles and Relationships, Sleep Hygiene, Steps to Healthy Brain Aging, Preventing Dementia, MCI and Depression, Nutrition and Exercise, Assistive Technologies, Participating in Research, Safety Planning, and Community Resources. As the 6-week program has 12 meeting dates but only 10 education sessions, dyads in the 6-week program did not have an educational session for the last two sessions of the program.
Booster Sessions
After completion of their 6-week or 10-day training, each participant was also seen at 3 months and 6 months for a follow-up visit and booster session. Upon arrival for each follow-up time point, the participant completed an MSS Adherence measure to determine their ongoing use of the memory support tool. For the MSS training group, if they scored 100% (10/10 points) on the Adherence measure they were merely encouraged to continue their use. If they scored less than 100%, they received a formal booster session involving training on the section of the calendar that they had trouble with on the adherence measure. If they received a booster, they returned again 1 week later for repeat assessment of their MSS use. The POSIT group automatically completed one booster session with the POSIT software at each follow-up point.
Recruitment methods
Our accrual target for the study was 60 participants. Potential candidates were identified from consecutive patients presenting to memory evaluations clinics at the evaluating institution (Emory University, Atlanta, GA; Mayo Clinic Rochester, MN; Mayo Clinic Scottsdale, AZ). Those whose resulting clinical diagnosis was amnestic MCI (single domain or multi-domain) were approached about participation in the study. Potential participants were asked to take part in a study to determine if individuals with MCI benefit from memory support training or computerized brain fitness exercises to compensate for their memory loss. If the candidate was not interested, they were asked if they would be willing to answer questions about the reasons they did not wish to participate (See Appendix A). It is from this brief, structured interview that the information for this report was gathered. If the participant was interested, they were seen for an in-person eligibility visit to confirm eligibility.
Eligibility Criteria
Dementia Rating Scale-2nd Edition (DRS-2, [25]) score of 115 or greater
Functional Activities Questionnaire (FAQ, [26]; [27]) total score below 6
Program partner with a Folstein Mini Mental Status Exam [28] of 24 or greater
Participant and partner free of severe depression suggesting more pressing need for psychiatric care [defined as Center for Epidemiological Studies-Depression (CES-D; [29]) total score less than 21]
Either not taking or stable on nootropic(s) for at least 3 months
English as primary language
Participants
A total of 200 consecutive potential candidates were approached about participating in the study across the three sites. Of those 74 were agreeable to the study and completed an eligibility visit (Of those, 64 participants passed eligibility screening, consented to participation, and were randomized to one of the four arms of the treatment protocol (6-week MSS = 16; 6-week Posit = 14; 10-day MSS = 18; 10-day Posit = 16). An additional 10 subjects were found to be ineligible for the protocol after the formal eligibility visit. The majority of subjects were ineligible for multiple reasons (n=6). Four (4) were ineligible due to DRS-2 total score and FAQ total score. One (1) was ineligible due to DRS-2 total score as well as partner MMSE total score. One (1) was ineligible due to DRS-2 total score, FAQ total score, and partner CES-D total score. The remaining were ineligible due to DRS-2 total score (n=1), FAQ total score (n=1), partner MMSE total score (n=1), and partner CES-D total score (n−1).
The details and outcomes of those participants who completed the study will be presented in a separate outcomes report as the focus of this report is details of enrollment and retention. The remaining 126 (63%) were approached but declined to participate in the study. It is those 126 participants on whom this detailed report is based (See Figure 1).
Figure 1.
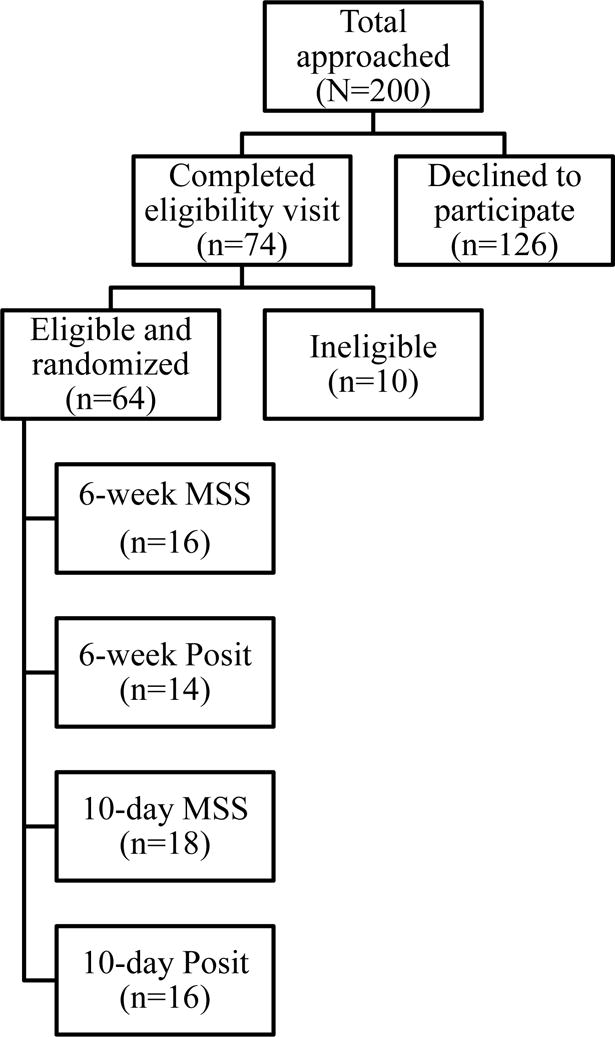
Enrollment details
RESULTS
The mean age of those who declined to participate was similar to those enrolled in the study [75.7(8.4) vs 76.7(7.1); t=.827(184), p=.41; d=.13], but those who declined were less likely to have completed college, (enrolled=67% with college degree, declined=48% with a college degree, p=.01). There was no difference in gender (enrolled = 61% male, declined = 56% male; p=.48) or minority ethnicity status (enrolled = 90% white, declined= 98% white, p =.08). Excluding those decliners for whom lack of a program partner was the issue, there was also a higher tendency for decliners to have a non-spouse partner (e.g., adult child, friend), as a program partner (23%) than those who enrolled (9%; p=.03). Similar to the participant comparisons, there was no difference in identified program partner age from the partners of those who enrolled [70.2(12.5) vs 71.6(10.8); t=.487(141), p=.49; d=.12], but decliner program partners had fewer years of education than partners of those who enrolled [14.6(2.2) vs. 15.7(2.6); t=2.53(131), p=.01; d=0.44].
Of the 126 who declined to participate in the study, 7 (5.6%) declined to provide any further information about their reasons and therefore did not complete the standardized interview on that topic. Of the remaining 119, the most common reason for declining to participate was the distance required to travel to the center (n=39, 31%). The remaining reasons are listed in Table 1.
Table 1.
Primary reasons for declining study participation overall and by study site (n=126)
| Reason | Frequency | Percent |
|---|---|---|
| Overall study (126/200) | ||
| Distance | 39 | 31% |
| Lack of program partner | 21 | 17% |
| Preferred HABIT | 17 | 14% |
| Length of study | 14 | 11% |
| Number of sessions | 13 | 10% |
| Other medical issues | 9 | 7% |
| Did not provide reason | 7 | 6% |
| Wouldn’t help | 3 | 2% |
| In another research trial | 2 | 2% |
| Concern about randomization | 1 | <1% |
| Emory University (n=10/36) | ||
| Distance | 5 | 50% |
| Lack of program partner | 3 | 30% |
| Other medical issues | 1 | 10% |
| Concern about randomization | 1 | 10% |
| Mayo Clinic, Scottsdale, AZ (n=57/96) | ||
| Distance | 17 | 30% |
| Lack of program partner | 9 | 16% |
| Number of sessions | 8 | 14% |
| Other medical issues | 7 | 12% |
| Did not provide reason | 6 | 11% |
| Length of the study | 5 | 8% |
| Wouldn’t help | 3 | 5% |
| In another research trial | 2 | 3% |
| Mayo Clinic, Rochester, MN (n=59/68) | ||
| Preferred HABIT program | 17 | 29% |
| Distance | 17 | 29% |
| Lack of program partner | 9 | 15% |
| Length of the study | 9 | 15% |
| Number of sessions | 5 | 9% |
| Other medical reason | 1 | 1% |
| Did not provide reason | 1 | 1% |
Note. HABIT = Healthy Action to Benefit Independence and Thinking, a clinical intervention program for individual with MCI offered only at Mayo Clinic Rochester.
There was a site effect for tendency to decline to participate in the study. Emory University had the lowest rate of declining to participate (28%) while Mayo Clinic Rochester had the highest rate (87%; p>.001). Mayo Clinic Scottsdale had a 59% decline rate (p=.002 with Emory; p>.001 with Mayo Clinic Rochester). The reasons for declining are detailed by site in Table 1.
At Mayo Clinic, Rochester it is noted that there was a unique, competing, multi-component behavioral intervention clinical program being offered: Mayo Clinic’s Healthy Action to Benefit Independence and Thinking program (HABIT). HABIT is a 5-hour-per-day, 5-day-per-week, 2-week multi-component behavioral program for individuals diagnosed with MCI and a partner. The 5 components are 1 hour each of 1) daily physical exercise, 2) computer-based cognitive exercise (brain fitness), 3) patient and family education, 4) separate support groups for MCI patients and partners, and 5) memory support system compensation training (cognitive rehabilitation). That program was placed on hiatus while this research trial was being conducted. However, 30% of those approached for the study at Mayo Clinic Rochester expressed a desire to wait until the HABIT program was restarted rather than enroll in the research trial. These patients expressed a desire for the more comprehensive program despite the fact that the clinical program would come with significant out-of-pocket financial cost borne by the patient.
When this reason for declining to participate is removed from the equation, the remaining reasons for declining are very similar across sites: Distance to travel and lack of a program partner were the most prominent concerns. Of note, very few reported concerns about participating in research or in the randomization process for this trial. It is noted, however, that this is not a placebo-controlled trial, but rather an active-controlled trial of comparison of two different cognitive rehabilitation strategies.
Despite this variable rate of declining to participate in the trial across sites, retention of those who were eligible was consistently high. Eighty-six percent (55/64) of those who enrolled in the study completed the study intervention. At Mayo Clinic Rochester one participant was enrolled and remained interested in the study, but had to be withdrawn as the site was unable to recruit additional participants to fill that intervention session. The remaining enrolled participants at Mayo Clinic Rochester completed the intervention (6/6, 100%). Retention was slightly lower at Emory University (17/23, 74%) compared to Mayo Clinic Scottsdale (32/34, 94%) and Mayo Clinic Rochester (6/6, p=.03). Reasons for withdrawal at Emory University included: Feeling the intervention was not helpful (n=1), disappointment with the time format randomization (6 weeks vs. 10 day, n=1), withdrawal prior to intervention due to personal/family emergency (n=1), withdrawal during the intervention due to unrelated medical issue requiring immediate treatment (n=1), and unexpected scheduling conflict before the intervention even began (n=2). At Mayo Clinic Scottsdale both participants withdrew prior to the start of the intervention: one due to an unexpected scheduling conflict and one due to feeling the distance would be too far to travel after thinking about it more. There were no significant differences between those who withdrew from the study and those who completed the intervention on age (p=.32), patient gender (p=.13), FAQ total score (p=.73), DRS-2 total score (p=.65), MCI participant CES-D total score (p=.46) and partner CES-D total score (p=.15). There was a trend toward those who withdrew having fewer years of education than those who completed the intervention [14.6 (2.7) vs. 16.3(2.4); t=1.794(61), p=.08; d=0.68 (CI=.04 to 2.60)].
DISCUSSION
In a study involving people with Mild Cognitive Impairment and their support partners where participants were randomized to intervention (memory compensation versus computerized cognitive training) and schedule (10 sessions over 10 days versus 6 weeks) our overall recruitment rate of 37% was slightly lower than the mean recruitment rate of 51% seen in the review by Trivedi et al[19]. However, the range of recruitment rates across this multi-site study is broad (13%–72%) and reflects the wide range also seen by those authors. On the other hand, our overall retention rate of 86% was higher than that found in that review. Our results support Trivedi et al.’s assertion that the primary challenge to behavioral trials involving dyads is in the recruitment phase of the study, rather than in the retention of those who enroll. Our high retention suggests that those who commit to intensive behavioral programs are highly committed. In our study, primary barriers to enrollment included distance to travel (31%), lack of a program partner (17%) and availability of a more comprehensive behavioral intervention for MCI at one of our institutions (30% at that site).
Regarding distance, it is noted that we cast a broad net when considering who to approach for the study. Each of our institutions attracts significant regional and national patient populations. However, we attempted to approach all persons in our practices diagnosed with MCI who lived regionally near our centers (e.g., Southeast Minnesota for Mayo Clinic Rochester, the greater Atlanta metropolitan areas and northern/central Georgia for Emory University, the greater Phoenix metropolitan area and surrounding suburbs for Mayo Clinic Scottsdale). We were desirous of over-inclusion of potential candidates given the goal of understanding recruitment barriers. Thus, it was not uncommon for us to approach patients who live an hour or more from our various institutions. This detailed analysis supports the fact that many evaluated at a tertiary care medical center from a distance will not find it feasible to return for such an intervention. However, we also note that we had some individuals in the study who did enroll in the program from such distances. It is also noted that the Mayo Clinic Rochester clinical HABIT program routinely has patients enroll who return to the area and pay for a 2-week hotel stay to participate in the program. Thus, although distance may continue to be a barrier, there are some patients who will bear the cost of a lengthy local stay. A way to boost this participation may be to offer financial support to those motivated patients who do not have the resources for a hotel stay, but would be willing to travel from a significant distance to participate in a behavioral intervention program. Developing mechanisms to allow temporary local housing for eligible candidates may entice that group of otherwise eligible and motivated participants. Alternatively, telemedicine continues to grow in popularity, and future studies may investigate the feasibility and efficacy of such a mode of intervention.
The second most common barrier was lack of a program partner. We find that the cognitive rehabilitation requires significant repetition given that the hallmark of MCI is short-term memory loss, with specific challenge retaining new information. In addition, a program partner with frequent contact with the patient also serves to encourage continued practice of the behavior post intervention. Finally, in research trials, specifically, many outcome measures involve the report of a collateral informant who observes how the patient is functioning pre and post intervention. Thus, strategies to overcome or reduce the barrier of lacking a study partner will involve both (1) strategies to help with the learning component of a behavioral intervention that are not dependent on a study partner and (2) strategies to measure outcomes that do not require an informant. For the former, programs could consider telephone follow-ups from the intervention provider or perhaps developing a pool of volunteer or professional partners who are trained to be paired with research participants to help complete learning trials (e.g. homework tasks, practice repetitions, real world trials).
Finally, it is noted that time commitment was a relatively low concern for this group. However, it was a concern for some. Paradoxically, the most common reason for declining to participate in our behavioral trial at Mayo Clinic Rochester was a desire to participate in a competing clinical program (HABIT) that actually required MORE time commitment and MORE out-of-pocket financial commitment than the research trial. We believe that the group of individuals who declined to participate in the study in favor of the HABIT program generally understood that the point of the research was to accumulate further evidence validating the effectiveness of the behavioral intervention under study. This group seems to have been willing to forego additional certainty regarding the value of the intervention in order to assure that they were receiving all available interventions. This speaks to the motivation of some MCI patients and their partners to do all they can to reduce the risk of progression to MCI no matter the strength of the evidence base. The prevalence of declining in order to receive the full HABIT program at Mayo Clinic Rochester also raises the dilemma in clinical research regarding how much evidence is sufficient before one begins to offer clinical services to people who are so strongly motivated. This is especially true when these behavioral interventions are associated with relatively low risk of adverse effects and have been shown to have benefit in other populations or with other outcomes (e.g., memory rehabilitation in TBI and stroke, group therapy and emotional wellness, physical exercise and physical health). However, there may be patient-centered study designs that can address patients’ desire to maximize treatment (or minimize under-treatment) including assignment to placebo [30]while specifically developing data on the behavioral intervention’s impact on MCI-relevant outcomes. We have recently been funded by the Patient Centered Outcomes Research Institute to conduct the Comparative Effectiveness of Behavioral Interventions to Prevent or Delay Dementia trial. In this study we will assign MCI patient/partner dyads to randomly receive 4 of the 5 components of the funded HABIT program, thereby assuring them they will receive ‘80% of the treatment’.
The site specific differences in recruitment and retention rates may suggest further variables at play that were not measured specifically in this study. For example, Emory University had a very high recruitment rate compared to the other centers, but also had the lowest retention rate. Variables such as the skill/personality of the recruiter on the telephone, confidence/obedience of a patient to a well-liked referring physician in signing up for a research study may affect recruitment. On the other hand higher expectations after recruitment, the suitability/comfort of the facilities, or personalities of those delivering intervention may impact retention.
The only small demographic difference from those who enrolled in the trial and those who declined to participate was a slightly lower level of education in those who declined to participate the study. Overall, however, both groups were highly educated (some college) such that it is not clear entirely if this is a barrier. Nevertheless, it is worthy of consideration to determine the feasibility of and interest in such cognitive rehabilitation behavioral interventions for those with lower levels of educational exposure given this mild finding.
Future research should explore the impact of mitigating barriers to participation (e.g. subsidizing travel, alternatives for those without partners) on enrollment rates. In addition, these findings are informative for planning behavioral clinical trials. The 37% enrollment rate and 88% retention rate found in this study, suggest that any trial that requires x completers will need to have available a population of roughly x*3.1 candidates and enroll roughly x*1.14 participants. For example, a trial requiring 100 study completers will need to a pool of approximately 310 potential candidates to screen for the study and enroll 114 participants. These data may also have relevance to clinical practice in providing rough estimates for how many eligible clinical patients are likely to enroll in behavioral programs directed at MCI patients. These data also suggest that the vast majority of patients who enroll will complete the program.
Acknowledgments
FUNDING
This study was supported by NINR (R01 NR012419) for all sites. Additional Arizona support: NIA P30AG19610, NIA R01AG031581, and the Arizona Alzheimer’s Research Consortium; Additional Mayo Clinic Rochester support: Mayo Alzheimer’s Disease Research Center P50 AG16574; Additional Emory University support: AG025688.
APPENDIX A: Declined Enrollment Telephone Script
1. Introduction and Overview
Thank you for your time today and we also respect your decision not to enroll in our study. In order to better understand what types of supports individuals with MCI would like to be involved in, we would like to ask a few brief questions about your decision not to take part in the study. Your responses to these to questions are confidential and in no way affect your involvement with us as a patient or participant on other research activities. Are you willing to answer these questions?
If no:
Ok then. Thank you very much for your time.
If yes:
Thank you very much. I’d like to encourage you to give your honest answers to my questions. We are truly interested in what you think. I’ll write down what you say. We should be finished with the interview within the next 5 minutes. Do you have any questions before we get started?
Encourage both participant and partner (if available) to answer questions throughout.
2. Interview Questions and Question Probes
Question 1: Could you describe the reasons why you did not want to participate in the study?
Probe: Were you worried about the length of time in the study?
Probe: Were you concerned about the number of sessions in the study?
Probe: Do you feel you would not have a program partner to attend with you?
Probe: Were you worried that the intervention would not be helpful?
Probe: Were you worried about the distance you would have to travel?
Probe: Were you worried about which group you would be placed in?
If participant gives more than one reason, ask “You stated you were concerned about X, Y, and Z in participating in the study. Which was the primary reason?”
Question 2: In general, what types of research programs for MCI would you be likely to be interested in?
Probe: Would you be willing to be involved in a drug study?
Probe: Would you be willing to be involved in a study of brain activities, such as puzzles or other mentally stimulating activities?
Probe: Would you be willing to be involved in another type of study involving ways to help people use their memories better?
3. Examples of Additional General Probes
Would you please explain further?
Can you give me an example?
Is there anything else?
Please describe what you mean.
4. Closing and Dismissal
Thank you so much for sharing all of this valuable information with me. This is the end of our interview. Your information will be combined with the information shared by others and then we will review the information and look for themes that came up across the interviews. Thank you for your important contribution to this research project. Do you have any final questions for me?
References
- 1.Petersen R, et al. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Ganguli M, et al. Mild cognitive impairment, amnestic type an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 3.Lopez O, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 4.Albert M, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKhann G, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling R, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen R, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. The New England Journal of Medicine. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 8.Tricco AC, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185(16):1393–401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clare L, Woods RT. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD003260. [DOI] [PubMed] [Google Scholar]
- 10.Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer’s disease: a meta-analysis of the literature. Acta Psychiatr Scand. 2006;114(2):75–90. doi: 10.1111/j.1600-0447.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 11.Sohlberg MM, Mateer CA. Training use of compensatory memory books: a three stage behavioral approach. Journal of clinical and experimental neuropsychology. 1989;11(6):871–91. doi: 10.1080/01688638908400941. [DOI] [PubMed] [Google Scholar]
- 12.Greenaway MC, et al. A Behavioral Rehabilitation Intervention for Amnestic Mild Cognitive Impairment. American Journal of Alzheimers Disease and Other Dementias. 2008;23(5):451–461. doi: 10.1177/1533317508320352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenaway MC, Duncan NL, Smith GE. The memory support system for mild cognitive impairment: randomized trial of a cognitive rehabilitation intervention. Int J Geriatr Psychiatry. 2013;28(4):402–9. doi: 10.1002/gps.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geda YE, et al. Computer activities, physical exercise, aging, and mild cognitive impairment: a population-based study. Mayo Clin Proc. 2012;87(5):437–42. doi: 10.1016/j.mayocp.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kueider AM, et al. Computerized cognitive training with older adults: a systematic review. PLoS One. 2012;7(7):e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebok GW, et al. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. J Am Geriatr Soc. 2014 doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GE, et al. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. Journal of the American Geriatrics Society. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelinski EM, et al. Improvement in memory with plasticity-based adaptive cognitive training: results of the 3-month follow-up. Journal of the American Geriatrics Society. 2011;59(2):258–265. doi: 10.1111/j.1532-5415.2010.03277.x. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi RB, et al. Recruitment and retention rates in behavioral trials involving patients and a support person: a systematic review. Contemp Clin Trials. 2013;36(1):307–18. doi: 10.1016/j.cct.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Legault C, et al. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: the Seniors Health and Activity Research Program Pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatr. 2011;11:27. doi: 10.1186/1471-2318-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes D, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Disease and Associated Disorders. 2009;23(3):205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hepburn K, et al. The Savvy Caregiver program: the demonstrated effectiveness of a transportable dementia caregiver psychoeducation program. J Gerontol Nurs. 2007;33(3):30–6. doi: 10.3928/00989134-20070301-06. [DOI] [PubMed] [Google Scholar]
- 23.Gaugler JE, et al. The Memory Club: Providing support to persons with early-stage dementia and their care partners. Am J Alzheimers Dis Other Demen. 2011;26(3):218–26. doi: 10.1177/1533317511399570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarit SH, et al. Memory Club: a group intervention for people with early-stage dementia and their care partners. Gerontologist. 2004;44(2):262–9. doi: 10.1093/geront/44.2.262. [DOI] [PubMed] [Google Scholar]
- 25.Jurica PJ, Leitten CL, Mattis S. DRS-2: Dementia rating scale-2: professional manual. Lutz, FL: Psychological Assessment Resources; 2001. p. 47. [Google Scholar]
- 26.Pfeffer RI, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–9. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 27.Teng E, et al. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(4):348–53. doi: 10.1097/WAD.0b013e3181e2fc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein M, Folstein S, McHugh P. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 30.Welton AJ, et al. Is recruitment more difficult with a placebo arm in randomised controlled trials? A quasirandomised, interview based study. BMJ. 1999;318(7191):1114–7. doi: 10.1136/bmj.318.7191.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]


