Reactive oxygen species (ROS) play a crucial role in senescing tissues, with some similarities but also important differences in leaves and flowers.
Abstract
Reactive oxygen species (ROS) play a key role in the regulation of many developmental processes, including senescence, and in plant responses to biotic and abiotic stresses. Several mechanisms of ROS generation and scavenging are similar, but others differ between senescing leaves and petals, despite these organs sharing a common evolutionary origin. Photosynthesis-derived ROS, nutrient remobilization, and reversibility of senescence are necessarily distinct features of the progression of senescence in the two organs. Furthermore, recent studies have revealed specific redox signaling processes that act in concert with phytohormones and transcription factors to regulate senescence-associated genes in leaves and petals. Here, we review some of the recent advances in our understanding of the mechanisms underpinning the production and elimination of ROS in these two organs. We focus on unveiling common and differential aspects of redox signaling in leaf and petal senescence, with the aim of linking physiological, biochemical, and molecular processes. We conclude that the spatiotemporal impact of ROS in senescing tissues differs between leaves and flowers, mainly due to the specific functionalities of these organs.
The role of reactive oxygen species (ROS) in plants has emerged in the last two decades as a key research topic mainly due to the recent achievements in this field demonstrating that ROS can play a role in signaling (for review, see Foyer and Noctor, 2013; Munné-Bosch et al., 2013; Mignolet-Spruyt et al., 2016). ROS play a dual role in plants, both as key regulators of growth, development, and defense pathways and as toxic by-products of aerobic metabolism (Mittler et al., 2004). ROS participate in a number of redox processes in plants, being part of a complex, sometimes redundant, but at the same time highly coordinated and flexible redox signaling network (Mittler et al., 2004; Foyer and Noctor, 2011, 2013; Zechmann, 2014). Importantly, ROS create a localized oxidative environment that facilitates signaling by other pathways, such as calcium mobilization, thiol-disulfide exchange, protein-protein interactions, and transcription factor binding (Foyer and Noctor, 2013). However, how the balance between ROS production and elimination is regulated, and how signals are transmitted to the cell machinery to trigger senescence processes in plant organs, are still being unveiled. In discussing the role of ROS during senescence, it is important to remember that different ROS types have distinct properties: singlet oxygen (1O2) and the superoxide (O2.−) and hydroxyl (OH·) radicals are very reactive and relatively unstable. In contrast, hydrogen peroxide (H2O2) is more stable and diffusible (Dat et al., 2000). Where these ROS types are generated, which ones predominate, and where they diffuse to within the cell may have different consequences. Of particular interest in this context is how ROS and redox signaling contribute to senescence in different organs.
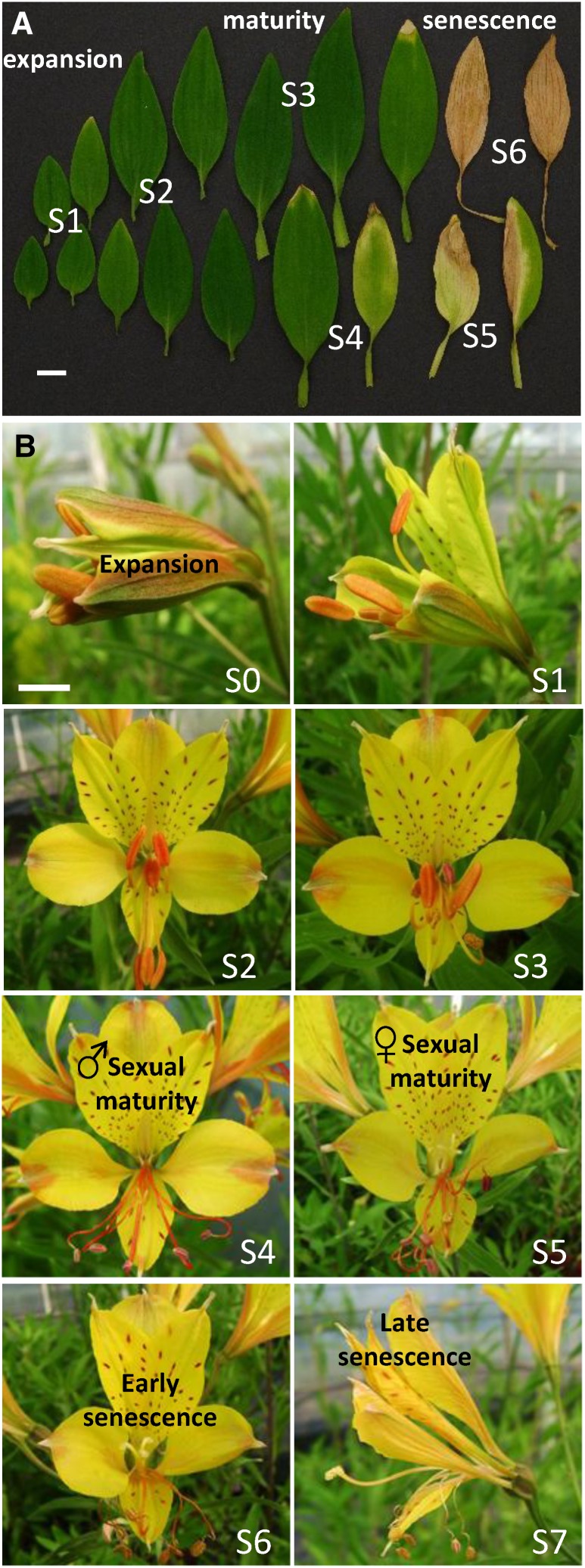
Senescence is the final stage in the development of a plant organ, usually leading to programmed death of all the cells. Previously, there has been discussion on the use of these terms (Thomas et al., 2003). Thomas (2013) makes a clear distinction between the active process of senescence, which requires live cells, and the irreversible and terminal phase of programmed cell death, although the two processes may be concurrent in different cells of the same organ. Although senescence is often considered as a distinct process, it is in fact part of the continuum of normal development in both leaves and petals. As seen in Figure 1A, in leaves, the first sign of senescence is yellowing due to chlorophyll degradation, which often begins at the tip of the leaf. In petals (Fig. 1B), the first visible signs are often wilting, changes in coloration, and rolling, which are closely coordinated with the sexual maturation of the flower and the production of scent to attract pollinators (Aros et al., 2012). Although senescence is often considered as a degenerative process, it is in fact an active stage of development in which the expression of many genes is drastically altered and new biochemical processes are activated (Breeze et al., 2011). Some of the activated mechanisms imply a conflict between initiating cell death and keeping cellular functions active long enough to enable efficient remobilization (Guiboileau et al., 2010). As a result, some patterns of gene expression during senescence resemble those activated during stress and defense responses (Guo and Gan, 2012). An interesting question, however, is whether ROS and redox signaling are involved in regulating the balance between life and senescence-associated cell death and whether the role of ROS and redox signaling in senescence is common to different plant organs.
Figure 1.
Senescence is a continuum in both leaves and flowers, here shown in Alstroemeria spp. ‘Sweet Laura’. A, Leaf development from expansion through yellowing and full senescence, divided into six stages: S1 and S2, expanding leaves; S3, mature leaves that have reached full expansion; S4, early senescence, showing the first signs of yellowing at the tip; S5, later senescence, with yellowing across more than 50% of the leaf area; and S6, fully senesced leaf. B, Floral development showing the coordination between petal opening and wilting with sexual maturation. Floral stages are according to Wagstaff et al. (2010). Bars = 1 cm.
Leaves and petals have common evolutionary origins (Friedman et al., 2004) but perform very different functions in the plant, acting both as sources and sinks for photoassimilates and available nutrients depending on their developmental stage (Gan and Fischer, 2007; Juvany et al., 2013). Very young, growing leaves act as sinks, shifting to a source role early in development when photosynthesis-derived sugars surpass those needed for maintenance. Later in development, leaves can enter senescence, in which photosynthesis is reduced and the export of photoassimilates and available nutrients is possible due to catabolic processes. Developmental senescence can be associated with reproduction, as is seen in monocarpic plants such as cereal crops (Distelfeld et al., 2014), where the leaves enter senescence coincident with flowering and fruit ripening, or in perennial plants, where it may be linked to resource allocation or organ turnover (Thomas, 2013). The senescence of individual leaves also can be seen as a stress-mitigating strategy at the whole-plant level, providing sugars and nutrients to other plant parts (Munné-Bosch and Alegre, 2004). In contrast, both young and mature petals are sinks, and only after pollination, when fertilization and fruit set occur, can catabolism in senescing petals convert these organs to sources of nutrients (Jones, 2013; Rogers, 2013). Furthermore, the abundance of chloroplasts in leaves and their absence in most petals lead to different sites and mechanisms of ROS generation in their cells. Despite the shared role of some of the phytohormones involved in triggering their senescence, the induction factors, the underlying mechanisms of their regulation, the extent and speed of nutrient remobilization, and the ultimate goal of the senescence syndrome may differ markedly between leaves and petals. Therefore, a comparison of the role of ROS and redox signaling in this context is useful to assess common and divergent signaling pathways that may help us to better understand and manipulate these physiological processes, with important implications for agriculture and the agrobiotechnological industry.
ROS GENERATION AND SCAVENGING: SPATIOTEMPORAL EFFECTS
In plant cells, ROS are mainly generated in four cellular locations: chloroplasts, peroxisomes, mitochondria, and the apoplast, although microbodies, the cytosol, and the endoplasmic reticulum also can contribute to ROS production (Apel and Hirt, 2004; Mittler et al., 2004). In leaves, the main source of ROS is the chloroplasts, from which large amounts of nitrogen are mobilized during leaf senescence (Ishida et al., 2014). During senescence, chlorophyll degradation is tightly regulated (Hörtensteiner, 2006), as is the disassembly of the whole chloroplast (Ishida et al., 2014; Xie et al., 2015). During disassembly of the photosynthetic apparatus, CO2 fixation decreases and excess energy in chloroplasts can result in the formation of O2.− and 1O2 (Krieger-Liszkay, 2005; Pintó-Marijuan and Munné-Bosch, 2014). Activation of the antioxidant machinery is then required to avoid the risk of toxic levels of ROS production, which, if not properly regulated, would prevent slow chloroplast dismantling and, therefore, nutrient remobilization from being fully accomplished (Juvany et al., 2013). The paramount importance of chloroplasts in the induction and speed of leaf senescence also is illustrated by regreening experiments. Redifferentiation of gerontoplasts (i.e. senescing chloroplasts) and regreening of senescing tobacco (Nicotiana tabacum) leaves were observed after the application of cytokinins (Zavaleta-Mancera et al., 1999a, 1999b). Although regreening capacity can deteriorate at advanced stages (Zavaleta-Mancera et al., 1999b), thus leading to the so-called point of no return (van Doorn and Woltering, 2004), these experiments demonstrate the paramount importance of cytokinins in leaf senescence and that this process occurs slowly and is potentially reversible. Nutrient remobilization is less obvious in petals and begins well before the appearance of phenotypic signs of petal wilting and deterioration (Jones, 2013). Petals are fairly nutrient poor compared with leaves, and previous experiments indicate that leaves remobilize a much greater amount of nutrients than petals during senescence (Himelblau and Amasino, 2001; Verlinden, 2003; Chapin and Jones, 2007, 2009; Jones, 2013). Pollination-triggered senescence of petals allows the degradation of energy-costly, nonfunctional petals in pollinated flowers, with a modest contribution of nutrient salvage to other plant parts (Jones, 2013). Petal senescence is not reversible, and in many species, pollination-triggered senescence occurs rapidly, in a process mediated by an increase in endogenous ethylene production, which coordinates rapid cellular events within the different floral organs (Stead and van Doorn, 1994; Jones and Woodson, 1997). In ethylene-insensitive species, however, senescence can occur more slowly in a process associated with a rise in abscisic acid (e.g. Narcissus spp.; Hunter et al., 2004) and reductions in cytokinins (e.g. Iris and Lilium spp.; Arrom and Munné-Bosch 2012; van Doorn et al., 2013). This process appears to resemble that occurring during leaf senescence; indeed, petal senescence in Lilium spp. starts with chlorophyll degradation and chloroplast dismantling, exactly as it occurs in leaves (Arrom and Munné-Bosch 2010, 2012).
ROS concentrations rise at flower opening and then again once senescence symptoms become evident (Rogers, 2012). This has been shown in several unrelated species, both ethylene sensitive (e.g. carnation [Dianthus caryophyllus]; Zhang et al., 2007) and ethylene insensitive (e.g. daylily [Hemerocallis spp.]; Panavas and Rubinstein, 1998). It is possible that different ROS types are involved: in Chrysanthemum spp., H2O2 concentration rose earlier and the increase was associated with flower opening, whereas O2.− only rose later coincident with early senescence (Chakrabarty, et al., 2007). In petals of most species, few active chloroplasts remain by the stage of full bloom; most are converted to chromoplasts (Ljubesić et al., 1991; Marano et al., 1993). The main sources of ROS production in petals are thus likely to be peroxisomes, mitochondria, and the apoplast. Mitochondria also contribute to ROS production in both organs, mainly through electron transfer from respiration chain components; however, they are much greater contributors to cellular ROS in nongreen tissues such as petals (Rhoads et al., 2006). The intracellular origins and distribution of the different ROS types remain controversial due to questions regarding the absolute specificity of fluorescent markers that are commonly used as reporters (Jajic et al., 2015).
Important factors in regulating ROS homeostasis during senescence are changes in the activity of ROS-scavenging enzymes, the production of antioxidant compounds, and increases in metal-chelating proteins and peptides. The interconversion between oxidized and reduced forms of glutathione and ascorbic acid is central to maintaining the redox homeostasis of the cell (Foyer and Noctor, 2005, 2011). Ascorbic acid is then required for the removal of H2O2 through its conversion to water and oxygen by the action of ascorbate peroxidase. In several species, nutrient resorption in senescing leaves occurs in parallel with ascorbic acid degradation, partly due to ascorbate oxidation in chloroplasts during leaf senescence (García-Plazaola et al., 2003; Palma et al., 2006). Interestingly, alterations in ascorbate biosynthesis lead to elevated levels of some senescence-associated gene transcripts as well as increased salicylic acid levels, thus leading to accelerated senescence in Arabidopsis (Arabidopsis thaliana; Barth et al., 2004). Notably, the fall in ascorbate is one of the earliest events in the senescence of petals from several species and may even be part of a mechanism triggering the onset of programmed cell death (Rogers, 2012). In contrast, glutathione levels in chloroplasts remained unaltered or increased slightly, depending on nitrogen availability, in senescing leaves, thus suggesting a differential antioxidant behavior between ascorbate and glutathione in senescing leaves (Palma et al., 2006). This is further supported by enhanced glutathione levels in chloroplasts and vacuoles as a result of increased intracellular H2O2 production in catalase2 mutants (Queval et al., 2011).
Enzymatic scavenging of ROS, which acts in concert with ascorbate and glutathione, and calcium signaling (Davletova et al., 2005; Costa et al., 2010) also are important in regulating the progression rate of leaf and petal senescence. The location of the scavenging enzymes is also of interest in understanding the contribution of different subcellular compartments to ROS during senescence. For example, superoxide dismutase activity remained high in carnation peroxisomes during senescence compared with mitochondria, where the activity fell dramatically (Droillard and Paulin, 1990). This suggests that peroxisomes may be more important in buffering ROS levels during petal senescence or that they are the major ROS producers at this stage of development. Furthermore, it has been shown that the manipulation of ROS levels in the cytosol appears to have a greater impact on leaf senescence than peroxisomal H2O2 and that the cytosolic ascorbate peroxidase plays a central role in redox control (Ye et al., 2000; Davletova et al., 2005; Bieker et al., 2012).
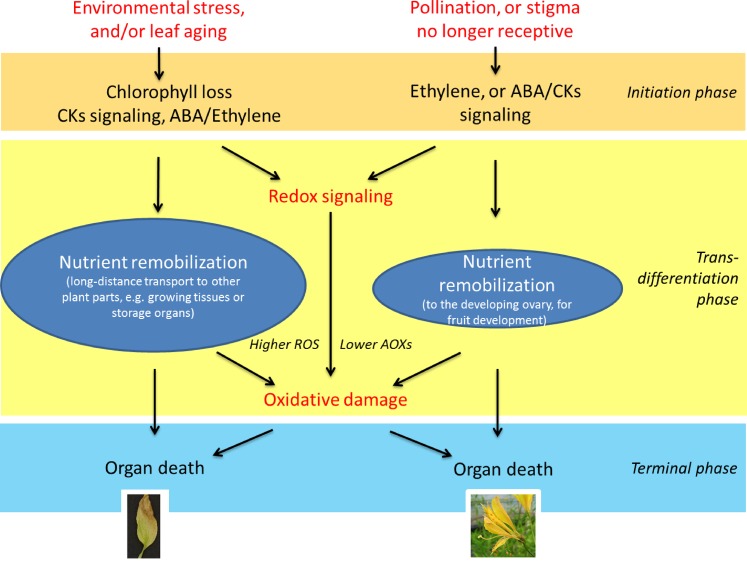
In both petals and leaves, ROS levels also are buffered by lipophilic antioxidant compounds: tocopherols and carotenoids. In leaves, carotenoids, located in both the light-harvesting antennae (mainly xanthophylls) and the thylakoid matrix of chloroplasts (β-carotene and also zeaxanthin in light-exposed leaves), are important for photosynthesis and photoprotection (Havaux et al., 2005; Caliandro et al., 2013). Tocopherols, associated with thylakoid membranes, protect the cell against oxidative stress both by eliminating ROS, mainly 1O2, and inhibiting the propagation of lipid peroxidation (Munné-Bosch and Alegre, 2002; Falk and Munné-Bosch, 2010). In petals, carotenoids and tocopherols tend to increase in early senescence and then decline (Cavaiuolo et al., 2013). The same trend has been reported in leaves for carotenoids, particularly zeaxanthin (Prochazkova et al., 2001) and tocopherols (Juvany et al., 2012). Thus, it would seem that, in early senescence of both organs, these antioxidant compounds likely perform a very similar role to delay the accumulation of ROS, which at the latest stages of both leaf and petal senescence will inevitably lead to oxidative damage and cell death (Fig. 2). Furthermore, the disassembly of the chloroplast electron transport chain in leaves and the breakdown of enzymes during senescence-associated proteolysis release metal ions, including Fe2+ and Cu2+ (Buchanan-Wollaston, 1997). These can then result in the production of OH· through the Fenton/Haber Weiss reactions between H2O2 and O2.−. One way to reduce this effect is the production of metal-chelating proteins. In many transcriptomes from petals (e.g. Alstroemeria spp. [Wagstaff et al., 2010] and Narcissus spp. [Hunter et al., 2004]) and leaves (e.g. Arabidopsis; Guo and Gan, 2004), metallothioneins are highly up-regulated during senescence. This family of proteins can bind to a wide range of monovalent and divalent metal ions and plays an important role in metal homeostasis (Leszczyszyn et al., 2013). During senescence in both tissues, therefore, they are likely to be relevant in delaying or reducing ROS production.
Figure 2.
Mechanisms involved in the regulation of leaf (left) and petal (right) senescence, including the role of nutrient remobilization in both cases. The amount of nutrients remobilized during leaf senescence, however, is higher than during petal senescence, in part due to the functional role of the process in each organ and the speed with which this occurs. ABA, Abscisic acid; AOXs, antioxidants; CKs, cytokinins.
REDOX SIGNALING
ROS, such as 1O2, H2O2, O2.−, and OH·, are molecules that are considered to be both signaling and potentially damaging molecules (Mittler et al., 2004). Antioxidants, such as ascorbate, glutathione, carotenoids, and tocopherols, as well as enzymes, such as superoxide dismutase, ascorbate peroxidase, catalase, peroxiredoxins, and glutathione peroxidase, play essential roles in ROS-scavenging mechanisms (Apel and Hirt, 2004; Dietz, 2011; Foyer and Noctor, 2011; Munné-Bosch et al., 2013; Zechmann, 2014), as has been discussed previously. Extracellular ROS, such as the diffusible H2O2, also can transmit intracellular signals rapidly to the nucleus and/or amplify signals passing from the chloroplast and/or mitochondria to the cell nucleus (Munné-Bosch et al., 2013; Mignolet-Spruyt et al., 2016). A loss of redox control ultimately leads to cell death, but we know that the cell death associated with senescence is tightly regulated. So a useful question to ask is whether the changes in ROS seen during senescence act as a signal that is required for senescence itself (i.e. the delay in cell death, which allows remobilization) and/or whether a programmed or consequential loss of redox control is only associated with cell death.
The transcription factors regulated by ROS result in the transcription of a large number of genes (Maxwell et al., 2002; Miller et al., 2010; Smykowski et al., 2010; Shaikhali et al., 2012; Munné-Bosch et al., 2013). Recently, there has been a growing interest in understanding the regulation of senescence at the level of systems biology, using bioinformatics tools to build models for transcription factor networks (Penfold and Buchanan-Wollaston, 2014). NAC and WRKY transcription factors have emerged as key regulators, and as these network models are tested, the role of ROS in their regulation is defined. The induction of leaf senescence by dark treatment resulted in changes in the expression of 39 ROS-induced transcription factors (Rosenwasser et al., 2011), including seven from the NAC and 11 from the WRKY gene families. This suggests that ROS indeed act as signals in early senescence. This is supported by the early induction of ROS-responsive genes during developmental leaf senescence (Breeze et al., 2011).
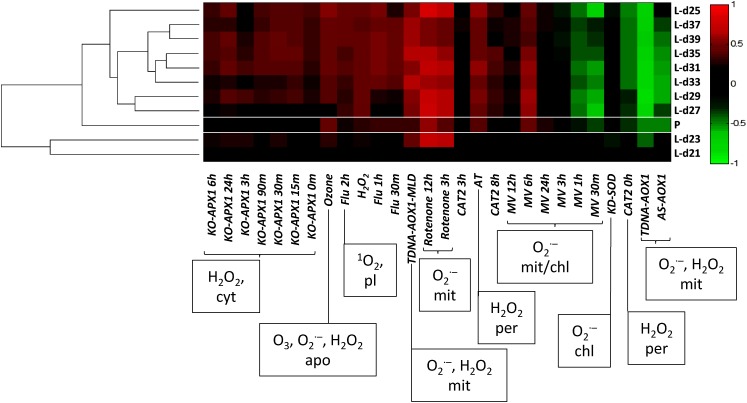
In petals, the role of ROS in senescence is as yet less defined. The key problem lies in the lack of detailed data for gene expression during senescence across the whole transcriptome. The development of Arabidopsis flowers has been divided into 17 stages, from primordial development to full abscission of the floral organs (Smyth et al., 1990). However, available data for Arabidopsis on The Arabidopsis Information Resource (TAIR) compare only two petal stages (stages 12 and 15), which correspond to unopened flower and fully open flower, respectively. Nevertheless, this is likely to represent the transition into early senescence, since already from flower opening, changes in cellular structure indicative of senescence and cell death are evident in many species (e.g. Alstroemeria spp.; Wagstaff et al., 2003). The correlation of ROS-elicited gene expression with expression in Arabidopsis petals and the very well-defined stages of Arabidopsis leaf senescence (Breeze et al., 2011) was made using ROSMETER (Rosenwasser et al., 2013; Fig. 3). It shows a clustering of age-related expressed petal sequences with leaf time points after day 25, which was 2 d after bolting and coincides with the first drop in photosynthetic activity. However, the expressed petal sequences group distinctly from the leaf-expressed genes, indicating differences in ROS responses between the two tissues. There is a positive correlation with a number of ROS-related genes in both leaf and petal senescence. In particular, there is a significant positive correlation with the production of O2.− in mitochondria following rotenone treatment in both tissues, although the effect in petals seems less marked (Garmier et al., 2008). There are also negative correlations to both antisense and insertional mutants of AOX1 linked to the production of O2.− and H2O2, again in mitochondria (Umbach et al., 2005; Giraud et al., 2008), suggesting that mitochondria may play a role in ROS signaling during senescence in both tissues. Peroxisomal ROS also may act as a signal in petal senescence, as a good correlation was obtained to amidotriazole treatment (Gechev et al., 2005), thought to induce the production of H2O2 in peroxisomes. Out of the 44 ROS-induced transcription factors analyzed by Rosenwasser et al. (2011), approximately half (23) were up-regulated in one or more stages of dark-induced senescence in leaves. Of these, 21 showed the same trend or no change in expression pattern in senescing petals, while only two showed the opposite trend (AtHSFA2 [At2g26150] and HSF7/HSFB2B [At4g11660]; data from eFP browser [Winter et al., 2007]). More detailed expression data on these genes and functional data from mutants would be of interest, since in at least some cases (e.g. ANAC042/JUB1; Wu et al., 2012), expression does increase in older flowers, which are not represented in TAIR array data.
Figure 3.
ROSMETER analysis of leaf developmental senescence. This program allows for changes in gene expression related to ROS treatments from microarray experiments as described by Rosenwasser et al. (2013). Major ROS types and proposed subcellular locations for the different experiments are indicated: apo, apoplast; chl, chloroplast; cyt, cytoplasm; mit, mitochondria; per, peroxisome; pl, plastid. L rows are data from Breeze et al. (2011), averaged from each day; P indicates petal senescence data from TAIR, analyzed by Wagstaff et al. (2009), comparing changes in gene expression between stage 12 (oldest bud with protruding white petal tips) and stage 15 (fully open flower). Arabidopsis lines/treatments are as follows: KO-APX1 are transfer DNA (T-DNA) insertion mutants of cytosolic APX1 exposed to high-light treatment (250 µmol m–2 s–1); Ozone are wild-type plants treated with 200 nL L−1 ozone for 1 h; Flu are conditional fluorescent mutants subjected to a dark-to-light shift (80–100 µmol m–2 s–1); H2O2 are wild-type plants treated with H2O2 (20 mm for 1 h); TDNA-AOX1 are T-DNA insertion mutants of ALTERNATIVE OXIDASE1A (AOX1A); -MLD indicates treatment with moderate light (250 μE m–2 s–1) and drought; AS-AOX1 are antisense lines of the same gene grown under standard conditions; Rotenone are wild-type cell suspension cultures treated with 40 µm (v/v) rotenone; CAT2 are antisense lines in which the peroxisomal CATALASE2 gene is down-regulated: plants were subjected to a high-light shift (140–1,800 µmol m–2 s–1); MV are wild-type plants treated with methyl viologen (10 µmol); KD-SOD are T-DNA knockdown insertion mutants of thylakoid-attached copper/zinc superoxide dismutase (KD-SOD). Time points refer to time after treatments. For details, see Rosenwasser et al. (2013).
ROS also could act as a more direct, terminal signal for cell death during senescence. In both leaves and petals, ion leakage often is used as a measure of senescence progression. Unlike wilting or loss of chlorophyll, ion leakage tends to occur rapidly once initiated and is presumably linked to a rapid increase in the number of dead or dying cells (Rogers, 2012). Prior to cell death, in both organs, there are changes in membrane structure. Lipid biosynthesis is down-regulated and lipolytic activity increases, leading to a loss of membrane stability (Thompson et al., 1998). Membrane instability results in the release of free fatty acids, which are rapidly oxidized either enzymatically through the action of lipoxygenases or nonenzymatically. This lipid peroxidation generates ROS types, including 1O2 and lipid derivatives such as the alkoxy and peroxy radicals. These then amplify the lipid peroxidation autocatalytically, resulting in further membrane damage. It was suggested that lipoxygenase might act as a senescence induction signal in petals (Rubinstein, 2000). Lipid peroxidation (measured by the release of malondialdehyde) increases during petal senescence in both ethylene-sensitive and -insensitive species (e.g. Rosa spp. [Fukuchi-Mizutani et al., 2000] and Lilium spp. [Arrom and Munné-Bosch, 2010]), sometimes also associated with increases in lipoxygenase activity. However, it seems unlikely that an increase in lipoxygenase is a primary signal, given that in some species (e.g. Alstroemeria spp.) lipoxygenase activity levels actually fall during petal senescence (Leverentz et al., 2002). It seems more likely, therefore, that the effects of ROS via lipid peroxidation are downstream effects, perhaps due to the deregulation of redox homeostasis once senescence has been initiated.
Despite differences in senescence induction factors and the extent and speed of nutrient remobilization in leaves and petals, downstream senescence events largely converge. For example, in wallflower (Erysimum linifolium) and Arabidopsis, over 50% of genes differentially expressed during senescence were in common between the two organs (Price et al., 2008; Wagstaff et al., 2009). Furthermore, cell death in both organs proceeds by a similar mechanism, which bears some similarities but also dissimilarities with classical autophagy, and has been termed vacuolar cell death (van Doorn et al., 2011). Indeed, Arabidopsis mutants deficient in components of the autophagic machinery senesce earlier than wild-type plants (Liu and Bassham, 2012), supporting the concept of a prosurvival role for autophagy to enable efficient nutrient remobilization during senescence.
CONCLUSION AND PROSPECTS
Molecular genetic, biochemical, and physiological studies have been pivotal in dissecting specific redox signaling processes that act in concert with phytohormones and transcription factors to regulate senescence-associated genes in leaves and petals. Photosynthesis-derived ROS, nutrient remobilization, and the reversibility of senescence are distinct key features of the progression of senescence in these organs and mark important differences in the spatiotemporal impact of ROS in senescing leaves and flowers. Chlorophyll degradation and photooxidative stress are essential hallmarks of senescence in leaves due to their declining photosynthetic capacity during senescence, providing an important avenue for ROS production and the induction of leaf senescence. Furthermore, the large amount of nitrogen stored in chloroplasts, which is remobilized to other plant parts during the progression of leaf senescence, determines the speed of the process. This process takes place slowly in leaves, so that this function can be accomplished before severe oxidative damage occurs concomitant with cell death. If the point of no return has not been surpassed, cytokinin application allows leaf regreening, another hallmark of leaf senescence. In contrast, pollination is an important trigger for petal senescence, in which some nutrient remobilization often occurs to provide nutrients to the ovary for fertilization and fruit set, but the amounts of nutrients moved are smaller. This process is irreversible and is triggered by ethylene or other phytohormones such as abscisic acid in ethylene-sensitive or -insensitive flowers, respectively. Pollination-triggered petal senescence occurs swiftly, so oxidative damage follows rapidly in some flowers prior to cell death. In the absence of pollination, petal senescence follows an often slower, predetermined developmental pathway, still involving a rise in ROS and ending with programmed cell death.
There are still, however, many gaps in our knowledge of the production and scavenging of ROS and the cross talk between redox and hormonal signaling in the regulation of senescence in leaves and flowers. In flowers, this is mainly due to a lack of adequate models for studying the different developmental programs with sufficient resources of gene sequences and mutants. For leaf senescence, there is a lack of studies with double and triple mutants affected in these processes, even in model species such as Arabidopsis, and more studies are needed to address the cross talk between ROS from various organelles. It would also be very helpful to find good models to represent both leaf and petal senescence in the same species, enabling more direct comparisons. Answers to these remaining questions are important to increase our understanding of leaf and flower senescence, and the use of combined omics approaches will undoubtedly help us better understand and manipulate these physiological processes with important applications in agriculture and the agrobiotechnological industry.
Acknowledgments
We thank Lyndon Tuck for growth and Basmh Al Harbi and Danilo Aros for photographs of Alstroemeria spp. leaf and flower senescence, respectively; Dan Pass for assistance in the analysis of the transcriptomic data for Arabidopsis petals; and Shilo Rosenwasser and Haya Friedman for help in producing Figure 3.
Glossary
- ROS
reactive oxygen species
- 1O2
singlet oxygen
- O2.−
superoxide radical
- OH·
hydroxyl radical
- H2O2
hydrogen peroxide
- TAIR
The Arabidopsis Information Resource
Footnotes
This work was supported by the Spanish Government (grant no. BFU2012–32057) and the Generalitat de Catalunya (ICREA Academia award to S.M.-B.).
Articles can be viewed without a subscription.
References
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Aros D, Gonzalez V, Allemann RK, Müller CT, Rosati C, Rogers HJ (2012) Volatile emissions of scented Alstroemeria genotypes are dominated by terpenes, and a myrcene synthase gene is highly expressed in scented Alstroemeria flowers. J Exp Bot 63: 2739–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrom L, Munné-Bosch S (2010) Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci 179: 289–295 [Google Scholar]
- Arrom L, Munné-Bosch S (2012) Hormonal changes during flower development in floral tissues of Lilium. Planta 236: 343–354 [DOI] [PubMed] [Google Scholar]
- Barth C, Moeder W, Klessig DF, Conklin PL (2004) The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol 134: 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker S, Riester L, Stahl M, Franzaring J, Zentgraf U (2012) Senescence-specific alteration of hydrogen peroxide levels in Arabidopsis thaliana and oilseed rape spring variety Brassica napus L. cv. Mozart. J Integr Plant Biol 54: 540–554 [DOI] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim YS, Penfold CA, Jenkins D, et al. (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23: 873–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. (1997) The molecular biology of leaf senescence. J Exp Bot 48: 181–199 [Google Scholar]
- Caliandro R, Nagel KA, Kastenholz B, Bassi R, Li Z, Niyogi KK, Pogson BJ, Schurr U, Matsubara S (2013) Effects of altered α- and β-branch carotenoid biosynthesis on photoprotection and whole-plant acclimation of Arabidopsis to photo-oxidative stress. Plant Cell Environ 36: 438–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaiuolo M, Cocetta G, Ferrante A (2013) The antioxidants changes in ornamental flowers during development and senescence. Antioxidants (Basel) 2: 132–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty D, Chatterjee J, Datta SK (2007) Oxidative stress and antioxidant activity as the basis of senescence in chrysanthemum florets. Plant Growth Regul 53: 107–115 [Google Scholar]
- Chapin LJ, Jones M (2007) Nutrient remobilization during pollination-induced corolla senescence in Petunia. Acta Hortic 755: 181–190 [Google Scholar]
- Chapin LJ, Jones ML (2009) Ethylene regulates phosphorus remobilization and expression of a phosphate transporter (PhPT1) during petunia corolla senescence. J Exp Bot 60: 2179–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Lo Schiavo F (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J 62: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57: 779–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ. (2011) Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal 15: 1129–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Avni R, Fischer AM (2014) Senescence, nutrient remobilization, and yield in wheat and barley. J Exp Bot 65: 3783–3798 [DOI] [PubMed] [Google Scholar]
- Droillard MJ, Paulin A (1990) Isozymes of superoxide dismutase in mitochondria and peroxisomes isolated from petals of carnation (Dianthus caryophyllus) during senescence. Plant Physiol 94: 1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Munné-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61: 1549–1566 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17: 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2013) Redox signaling in plants. Antioxid Redox Signal 18: 2087–2090 [DOI] [PubMed] [Google Scholar]
- Friedman WE, Moore RC, Purugganan MD (2004) The evolution of plant development. Am J Bot 91: 1726–1741 [DOI] [PubMed] [Google Scholar]
- Fukuchi-Mizutani M, Ishiguro K, Nakayama T, Utsunomiya Y, Tanaka Y, Kusumi T, Ueda T (2000) Molecular and functional characterization of a rose lipoxygenase cDNA related to flower senescence. Plant Sci 160: 129–137 [DOI] [PubMed] [Google Scholar]
- Gan S, Fischer AM (2007) Nutrient remobilization during leaf senescence. In S Gan, ed, Senescence Processes in Plants. Annual Plant Reviews, Vol 26. Blackwell Publishing, Oxford, pp 87–107 [Google Scholar]
- García-Plazaola JI, Hernández A, Becerril JM (2003) Antioxidant and pigment composition during autumnal leaf senescence in woody deciduous species differing in their ecological traits. Plant Biol 5: 557–566 [Google Scholar]
- Garmier M, Carroll AJ, Delannoy E, Vallet C, Day DA, Small ID, Millar AH (2008) Complex I dysfunction redirects cellular and mitochondrial metabolism in Arabidopsis. Plant Physiol 148: 1324–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Minkov IN, Hille J (2005) Hydrogen peroxide-induced cell death in Arabidopsis: transcriptional and mutant analysis reveals a role of an oxoglutarate-dependent dioxygenase gene in the cell death process. IUBMB Life 57: 181–188 [DOI] [PubMed] [Google Scholar]
- Giraud E, Ho LHM, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH, et al. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147: 595–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiboileau A, Sormani R, Meyer C, Masclaux-Daubresse C (2010) Senescence and death of plant organs: nutrient recycling and developmental regulation. C R Biol 333: 382–391 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27: 521–549 [Google Scholar]
- Guo Y, Gan SS (2012) Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ 35: 644–655 [DOI] [PubMed] [Google Scholar]
- Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17: 3451–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau E, Amasino RM (2001) Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol 158: 1317–1323 [Google Scholar]
- Hörtensteiner S. (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57: 55–77 [DOI] [PubMed] [Google Scholar]
- Hunter DA, Ferrante A, Vernieri P, Reid MS (2004) Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus “Dutch Master”). Physiol Plant 121: 313–321 [DOI] [PubMed] [Google Scholar]
- Ishida H, Izumi M, Wada S, Makino A (2014) Roles of autophagy in chloroplast recycling. Biochim Biophys Acta 1837: 512–521 [DOI] [PubMed] [Google Scholar]
- Jajic I, Sarna T, Strzalka K (2015) Senescence, stress, and reactive oxygen species. Plants 4: 393–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML. (2013) Mineral nutrient remobilization during corolla senescence in ethylene-sensitive and -insensitive flowers. AoB Plants 5: plt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Woodson WR (1997) Pollination-induced ethylene in carnation: role of stylar ethylene in corolla senescence. Plant Physiol 115: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvany M, Müller M, Munné-Bosch S (2012) Leaves of field-grown mastic trees suffer oxidative stress at the two extremes of their lifespan. J Integr Plant Biol 54: 584–594 [DOI] [PubMed] [Google Scholar]
- Juvany M, Müller M, Munné-Bosch S (2013) Photo-oxidative stress in emerging and senescing leaves: a mirror image? J Exp Bot 64: 3087–3098 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56: 337–346 [DOI] [PubMed] [Google Scholar]
- Leszczyszyn OI, Imam HT, Blindauer CA (2013) Diversity and distribution of plant metallothioneins: a review of structure, properties and functions. Metallomics 5: 1146–1169 [DOI] [PubMed] [Google Scholar]
- Leverentz MK, Wagstaff C, Rogers HJ, Stead AD, Chanasut U, Silkowski H, Thomas B, Weichert H, Feussner I, Griffiths G (2002) Characterization of a novel lipoxygenase-independent senescence mechanism in Alstroemeria peruviana floral tissue. Plant Physiol 130: 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bassham DC (2012) Autophagy: pathways for self-eating in plant cells. Annu Rev Plant Biol 63: 215–237 [DOI] [PubMed] [Google Scholar]
- Ljubesić N, Wrischer M, Devidé Z (1991) Chromoplasts: the last stages in plastid development. Int J Dev Biol 35: 251–258 [PubMed] [Google Scholar]
- Marano MR, Serra EC, Orellano EG, Carrrillo N (1993) The path of chromoplast development in fruits and flowers. Plant Sci 94: 1–17 [Google Scholar]
- Maxwell DP, Nickels R, McIntosh L (2002) Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J 29: 269–279 [DOI] [PubMed] [Google Scholar]
- Mignolet-Spruyt L, Xu E, Idänheimo N, Hoeberichts FA, Mühlenbock P, Brosché M, Van Breusegem F, Kangasjärvi J (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot http://dx.doi.org/10.1093/jxb/erw080 [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2002) The function of tocopherols and toctrienols in plants. Crit Rev Plant Sci 21: 31–57 [Google Scholar]
- Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31: 203–213 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JM, Jiménez A, Sandalio LM, Corpas FJ, Lundqvist M, Gómez M, Sevilla F, del Río LA (2006) Antioxidative enzymes from chloroplasts, mitochondria, and peroxisomes during leaf senescence of nodulated pea plants. J Exp Bot 57: 1747–1758 [DOI] [PubMed] [Google Scholar]
- Panavas T, Rubinstein B (1998) Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci 133: 125–138 [Google Scholar]
- Penfold CA, Buchanan-Wollaston V (2014) Modelling transcriptional networks in leaf senescence. J Exp Bot 65: 3859–3873 [DOI] [PubMed] [Google Scholar]
- Pintó-Marijuan M, Munné-Bosch S (2014) Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. J Exp Bot 65: 3845–3857 [DOI] [PubMed] [Google Scholar]
- Price AM, Aros Orellana DF, Salleh FM, Stevens R, Acock R, Buchanan-Wollaston V, Stead AD, Rogers HJ (2008) A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol 147: 1898–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161: 765–771 [Google Scholar]
- Queval G, Jaillard D, Zechmann B, Noctor G (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34: 21–32 [DOI] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN (2006) Mitochondrial reactive oxygen species: contribution to oxidative stress and interorganellar signaling. Plant Physiol 141: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HJ. (2012) Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ 35: 217–233 [DOI] [PubMed] [Google Scholar]
- Rogers HJ. (2013) From models to ornamentals: how is flower senescence regulated? Plant Mol Biol 82: 563–574 [DOI] [PubMed] [Google Scholar]
- Rosenwasser S, Fluhr R, Joshi JR, Leviatan N, Sela N, Hetzroni A, Friedman H (2013) ROSMETER: a bioinformatic tool for the identification of transcriptomic imprints related to reactive oxygen species type and origin provides new insights into stress responses. Plant Physiol 163: 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser S, Rot I, Sollner E, Meyer AJ, Smith Y, Leviatan N, Fluhr R, Friedman H (2011) Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol 156: 185–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. (2000) Regulation of cell death in flower petals. Plant Mol Biol 44: 303–318 [DOI] [PubMed] [Google Scholar]
- Shaikhali J, Norén L, de Dios Barajas-López J, Srivastava V, König J, Sauer UH, Wingsle G, Dietz KJ, Strand Å (2012) Redox-mediated mechanisms regulate DNA binding activity of the G-group of basic region leucine zipper (bZIP) transcription factors in Arabidopsis. J Biol Chem 287: 27510–27525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smykowski A, Zimmermann P, Zentgraf U (2010) G-box binding factor1 reduces CATALASE2 expression and regulates the onset of leaf senescence in Arabidopsis. Plant Physiol 153: 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead AD, van Doorn WG (1994) Strategies of flower senescence: a review. In Scott RJ, Stead AD, eds, Molecular and Cellular Aspects of Plant Reproduction. Cambridge University Press, Cambridge, UK, pp 215–238 [Google Scholar]
- Thomas H. (2013) Senescence, ageing and death of the whole plant. New Phytol 197: 696–711 [DOI] [PubMed] [Google Scholar]
- Thomas H, Ougham HJ, Wagstaff C, Stead AD (2003) Defining senescence and death. J Exp Bot 54: 1127–1132 [DOI] [PubMed] [Google Scholar]
- Thompson JE, Froese CD, Madey E, Smith MD, Hong Y (1998) Lipid metabolism during plant senescence. Prog Lipid Res 37: 119–141 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Fiorani F, Siedow JN (2005) Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol 139: 1806–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J, et al. (2011) Morphological classification of plant cell deaths. Cell Death Differ 18: 1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Çelikel FG, Pak C, Harkema H (2013) Delay of iris flower senescence by cytokinins and jasmonates. Physiol Plant 148: 105–120 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ (2004) Senescence and programmed cell death: substance or semantics? J Exp Bot 55: 2147–2153 [DOI] [PubMed] [Google Scholar]
- Verlinden S. (2003) Changes in the mineral nutrient concentrations in petunia corollas during development and senescence. HortScience 38: 71–74 [Google Scholar]
- Wagstaff C, Bramke I, Breeze E, Thornber S, Harrison L, Thomas B, Buchanan-Wollaston V, Stead AD, Rogers HJ (2010) A specific group of genes respond to cold dehydration stress in cut Alstroemeria flowers whereas ambient dehydration stress accelerates developmental senescence expression patterns. J Exp Bot 61: 2905–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff C, Malcolm P, Rafiq A, Leverentz M, Griffiths G, Thomas B (2003) Programmed cell death (PCD) processes begin extremely early in Alstroemeria petal senescence. New Phytol 160: 49–59 [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Yang TJW, Stead AD, Buchanan-Wollaston V, Roberts JA (2009) A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J 57: 690–705 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. (2012) JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24: 482–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Michaeli S, Peled-Zehavi H, Galili G (2015) Chloroplast degradation: one organelle, multiple degradation pathways. Trends Plant Sci 20: 264–265 [DOI] [PubMed] [Google Scholar]
- Ye Z, Rodríguez R, Tran A, Hoang H, de los Santos D, Brown S, Vellanoweth RL (2000) The developmental transition to flowering represses ascorbate peroxidase activity and induces enzymatic lipid peroxidation in leaf tissue in Arabidopsis thaliana. Plant Sci 158: 115–127 [DOI] [PubMed] [Google Scholar]
- Zavaleta-Mancera HA, Franklin KA, Ougham HJ, Thomas H, Scott IM (1999a) Regreening of senescent Nicotiana leaves. I. Reappearance of NADPH-protochlorophyllide oxidoreductase and light-harvesting chlorophyll a/b-binding protein. J Exp Bot 50: 1677–1682 [Google Scholar]
- Zavaleta-Mancera HA, Franklin KA, Ougham HJ, Thomas H, Scott IM (1999b) Regreening of senescent Nicotiana leaves. II. Redifferentiation of plastids. J Exp Bot 50: 1683–1689 [Google Scholar]
- Zechmann B. (2014) Compartment-specific importance of glutathione during abiotic and biotic stress. Front Plant Sci 5: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo WM, Chen SM, Han L, Li ZM (2007) The role of N-lauroylethanolamine in the regulation of senescence of cut carnations (Dianthus caryophyllus). J Plant Physiol 164: 993–1001 [DOI] [PubMed] [Google Scholar]