The maize protein kinase ZmCCaMK phosphorylates the ZmNAC84 transcription factor at Ser113 during abscisic acid-induced antioxidant.
Abstract
Calcium/calmodulin-dependent protein kinase (CCaMK) has been shown to play an important role in abscisic acid (ABA)-induced antioxidant defense and enhance the tolerance of plants to drought stress. However, its downstream molecular events are poorly understood. Here, we identify a NAC transcription factor, ZmNAC84, in maize (Zea mays), which physically interacts with ZmCCaMK in vitro and in vivo. ZmNAC84 displays a partially overlapping expression pattern with ZmCCaMK after ABA treatment, and H2O2 is required for ABA-induced ZmNAC84 expression. Functional analysis reveals that ZmNAC84 is essential for ABA-induced antioxidant defense in a ZmCCaMK-dependent manner. Furthermore, ZmCCaMK directly phosphorylates Ser-113 of ZmNAC84 in vitro, and Ser-113 is essential for the ABA-induced stimulation of antioxidant defense by ZmCCaMK. Moreover, overexpression of ZmNAC84 in tobacco (Nicotiana tabacum) can improve drought tolerance and alleviate drought-induced oxidative damage of transgenic plants. These results define a mechanism for ZmCCaMK function in ABA-induced antioxidant defense, where ABA-produced H2O2 first induces expression of ZmCCaMK and ZmNAC84 and activates ZmCCaMK. Subsequently, the activated ZmCCaMK phosphorylates ZmNAC84 at Ser-113, thereby inducing antioxidant defense by activating downstream genes.
Drought stress limits plant productivity worldwide. The phytohormone abscisic acid (ABA) plays important roles in regulating the adaptive response of plants to drought. One mode of ABA-enhanced drought tolerance is associated with the induction of antioxidant defense system. ABA can cause the generation of reactive oxygen species (ROS), which at low concentration are important signaling molecules that activate antioxidant defense to scavenge excess ROS, whereby plants can maintain ROS at a balanced level to avoid unwanted damage. Many signaling molecules, such as calcium ion (Ca2+) and nitric oxide, and protein kinases, such as mitogen-activated protein kinase (MAPK), calcium-dependent protein kinase, and calcium/calmodulin-dependent protein kinase (CCaMK), have been found to be involved in ROS response to ABA signaling (Ding et al., 2013; Ma et al., 2012; Sang et al., 2008; Zhang et al., 2006). However, the mechanism of ABA-induced antioxidant defense remains unclear.
CCaMK was first identified in flowers of lily (Lilium longiflorum) and subsequently isolated from tobacco (Nicotiana tabacum), maize (Zea mays), rice (Oryza sativa), wheat (Triticum aestivum), Lotus japonicus, Medicago truncatula, and Sesbania rostrata (Capoen et al., 2009; Chen et al., 2007; DeFalco et al., 2009; Godfroy et al., 2006; Harper et al., 2004; Hayashi et al., 2010; Lévy et al., 2004; Mitra et al., 2004; Patil et al., 1995; Tirichine et al., 2006; Yang and Poovaiah, 2003; Yang et al., 2011a; Zhang and Lu, 2003). CCaMK has a Ser/Thr kinase domain, a CaM-binding domain, and three EF-hand motifs. A model for the activation of CCaMK has been proposed based on work with CCaMK from lily (Sathyanarayanan et al., 2000): Ca2+ binding to the EF-hands of CCaMK promotes autophosphorylation, which in turn promotes CaM binding, relieving autoinhibition of the kinase and thus promoting substrate phosphorylation. This model includes a two-step activation of CCaMK by Ca2+ (Miller et al., 2013; Poovaiah et al., 2013). CCaMK is a central decoder of Ca2+-spiking in the common symbiosis pathway for regulating root nodules and arbuscular mycorrhizae (Gobbato, 2015; Miller et al., 2013; Shimoda et al., 2012). However, CCaMK is highly expressed outside of roots, indicating other roles besides the regulation of symbiosis. CCaMK has been shown to be involved in ABA signaling during abiotic stress response (Yang et al., 2011a) and ABA-induced antioxidant defense (Ma et al., 2012; Shi et al., 2012). ABA-induced H2O2 production activates CCaMK, which in turn induces antioxidant defense system by a largely unknown mechanism.
The NAC superfamily is one of the largest families of plant transcription factors (TFs) and widely distributed in plants (Olsen et al., 2005). The characteristic feature of this group of TFs is the presence of a highly conserved NAC domain at the N terminus, which serves as a platform for DNA binding and for homo- or heterodimerization with other NAC proteins. The C-terminal region, in contrast, is variable in sequence and length and serves as a transcriptional activator or repressor (Nuruzzaman et al., 2010), which reflects their numerous functions. NAC TFs regulate a diverse range of processes in plants, such as nutrient acquisition (He et al., 2015), root growth (Guo et al., 2005), plant height (Chen et al., 2015), leaf senescence (Guo and Gan, 2006), formation of secondary walls (Mitsuda et al., 2007), and hormone signaling (Fujita et al., 2004). Moreover, many members of the NAC TF family coordinate the response to biotic and abiotic stress including fungal infection (Wang et al., 2009), salt (Hu et al., 2006a), temperature (Fang et al., 2015), and drought (Huang et al., 2015; Mao et al., 2015). The diverse functions of NAC TFs possibly come from their multiple regulatory modes. They are regulated at the transcriptional level through numerous TF recognition sequences in their promoters, at the posttranscriptional level by microRNA-mediated cleavage of transcripts and at the posttranslational level by protein degradation, dimerization, interaction with other non-NAC proteins, and phosphorylation (Puranik et al., 2012).
In this study, using the full-length ZmCCaMK (GenBank ID DQ403196) from maize as bait in a yeast two-hybrid interaction screening approach, we identified a NAC transcription factor, ZmNAC84. We analyzed the interactions between ZmCCaMK and ZmNAC84, investigated the roles of ZmNAC84 in ABA-induced antioxidant defense, and identified the phosphorylation site of ZmNAC84 by ZmCCaMK in this process.
RESULTS
Identification of ZmNAC84 as a ZmCCaMK-Interacting Protein
To elucidate the molecular basis by which ZmCCaMK functions in ABA-induced antioxidant defense, we used a yeast two-hybrid screen of a maize leaf cDNA library with the full-length ZmCCaMK as bait. A total of 29 positive clones were isolated from the screen, corresponding to nine genes. Seven of 29 positive clones containing genes encoded a NAC-type transcription factor (GRMZM2G166721) belonging to a family with more than 100 members in the maize genome. GRMZM2G166721 (GenBank ID AFU81568.1) has previously been designated as ZmNAC84 (Fan et al., 2014). The interaction of ZmNAC84 with ZmCCaMK in the yeast two-hybrid system is shown in Figure 1A.
Figure 1.
ZmNAC84 directly interacts with ZmCCaMK in vitro and in vivo. A, Yeast two-hybrid assay of interaction between ZmNAC84 and ZmCCaMK. BD-Krev1/AD-RalGDS-wt was used as a positive control, and BD-Krev1/AD-RalGDS-m2 was used as negative control. B, Pull-down assay of ZmNAC84 interaction with ZmCCaMK. GST-ZmCCaMK fusion protein or GST alone was incubated with His-ZmNAC84 in GST beads. His-ZmNAC84 was then detected by western blot using anti-His antibody. C, Phosphorylation of ZmNAC84 by ZmCCaMK in vitro. Protein extract from maize leaves was immunoprecipitated with ZmCCaMK antibody. Recombinant His-ZmNAC84 or maltose binding protein (MBP) was used as substrate and subjected to in-gel kinase assay. Asterisks indicate phosphorylated contaminants. WB, western blot. D, Interaction of ZmNAC84 and ZmCCaMK in onion epidermal cells. YFP signals were detected by confocal laser scanning microscope. E, In vivo Co-IP assay of ZmCCaMK and ZmNAC84 interaction. Maize mesophyll protoplasts were transfected with ubi:ZmCCaMK-myc and ubi:ZmNAC84-His simultaneously or ubi:ZmNAC84-His alone. Crude protein was immunoprecipitated with anti-myc and anti-His antibodies.
To validate the yeast two-hybrid data, both in vitro and in vivo experiments were performed to test the ZmNAC84-ZmCCaMK interaction. Recombinant full-length ZmCCaMK and glutathione S-transferase (GST) fusion protein and the full-length ZmNAC84 tagged with poly-His were produced in Escherichia coli and purified. The His-tagged ZmNAC84 was retained on beads with immobilized GST-ZmCCaMK but not with immobilized GST alone (Fig. 1B). The in vitro interaction between ZmCCaMK and ZmNAC84 was further confirmed by immunocomplex kinase assay using His-tagged ZmNAC84 as substrate. ZmCCaMK was found to phosphorylate His-ZmNAC84 in vitro (Fig. 1C).
To further confirm the interaction, bimolecular fluorescence complementation (BiFC) assays were performed in onion (Allium cepa) epidermal cells. YFP was reconstituted when the coding sequences of ZmCCaMK and ZmNAC84 were coexpressed (Fig. 1D, a–c). In contrast, coexpression of the YFP-N terminus and ZmNAC84-YFP C terminus, or the ZmCCaMK-YFP N terminus and YFP-C terminus, did not result in fluorescence (Fig. 1D, d–i), which confirmed that the ZmCCaMK-ZmNAC84 interaction is specific. Moreover, the BiFC experiments revealed that ZmCCaMK-ZmNAC84 interacted in nucleus (Fig. 1D, a–c; Supplemental Fig. S1).
Coimmunoprecipitation (Co-IP) assays were performed to confirm the interaction between ZmCCaMK and ZmNAC84 in maize mesophyll protoplasts. ZmCCaMK-myc and ZmNAC84-His were coexpressed or ZmNAC84-His was expressed alone transiently in maize protoplasts. Crude protein extracts of protoplasts were immunoprecipitated by anti-myc antibody and then analyzed by immunoblotting with anti-His antibody. As shown in Figure 1E, ZmNAC84-His was detected, further confirming the in vivo interaction between the two proteins.
ZmNAC84 Displays a Partially Overlapping Expression Pattern with ZmCCaMK after ABA Treatment and H2O2 Is Required for ABA-Induced ZmNAC84 Expression
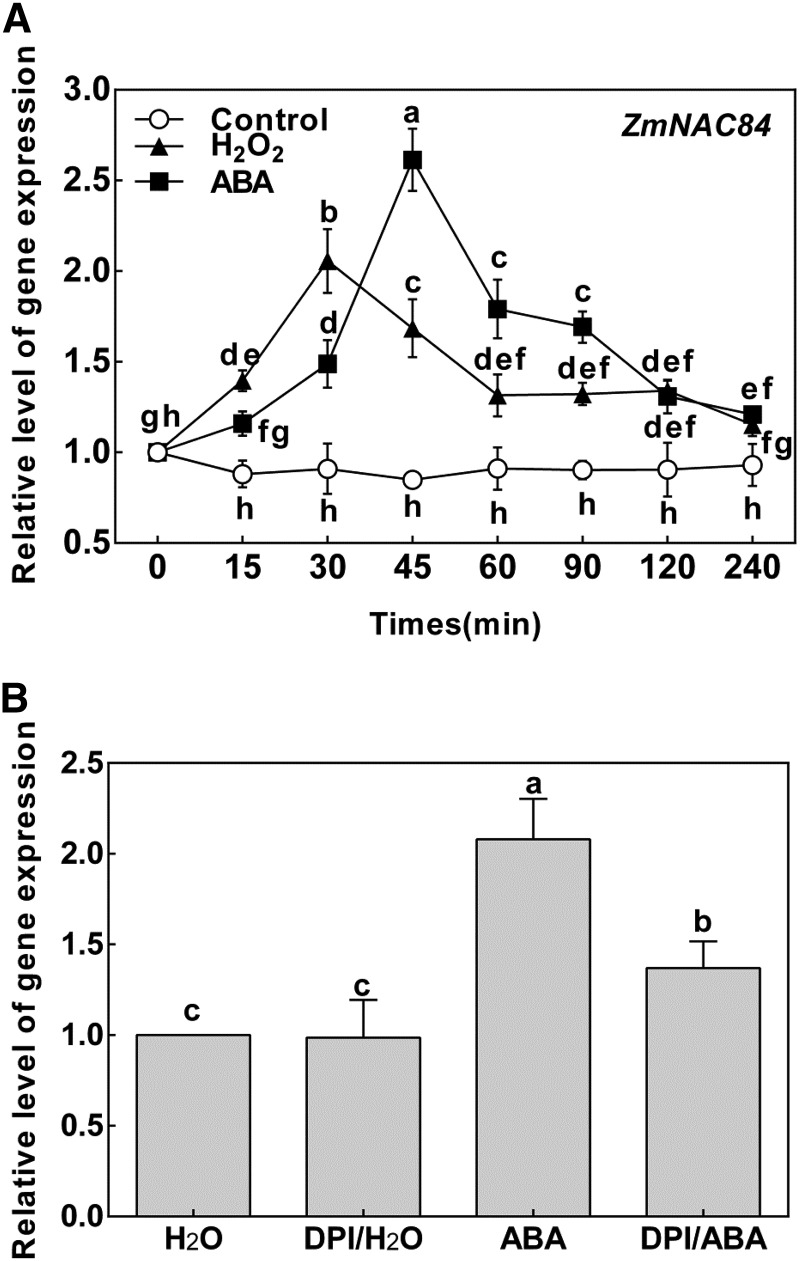
Using quantitative RT-PCR (qRT-PCR), we first examined ZmNAC84 mRNA accumulation in various organs/tissues at different developmental stages. ZmNAC84 was broadly expressed with the highest level in female flower and a higher level in leaf compared with male flower, root, and stem (Supplemental Fig. S2). To gain insight into the functional significance of the interaction between ZmNAC84 and ZmCCaMK, we further explored the expression pattern of ZmNAC84 after ABA treatment in maize leaves. A significant response in the expression of ZmNAC84 was observed after 15 min ABA treatment, was maximal at 45 min, and then decreased (Fig. 2A). This expression pattern overlapped with that of ZmCCaMK but displayed some differences, as ZmCCaMK expression peaked 30 min after ABA treatment (Supplemental Fig. S3).
Figure 2.
H2O2 is required for ABA-induced expression of ZmNAC84 in maize leaves. A, Expression analysis of ZmNAC84 in leaves of maize plants exposed to ABA or H2O2 treatment. The maize seedlings were treated with ABA (100 μM) or H2O2 (10 mM) for various times as indicated. B, Effects of pretreatment with the NADPH oxidase inhibitor DPI on the expression of ZmNAC84. The maize seedlings were treated with 100 μM DPI for 4 h and then exposed to 100 μM ABA for 45 min. Relative expression level of ZmNAC84 was analyzed by qRT-PCR. Values are means ± se of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
H2O2 is required for ABA-induced ZmCCaMK activation and gene expression (Ma et al., 2012). To study the role of H2O2 in ABA-induced ZmNAC84 expression, we treated maize plants with exogenous H2O2. qRT-PCR results showed that H2O2 treatment also induced a significant increase in the expression of ZmNAC84 at 30 min, i.e. faster than after ABA treatment (Fig. 2A), which suggests that ABA-induced ZmNAC84 expression might be via H2O2. To test this hypothesis, the NADPH oxidase inhibitor, diphenylene iodonium (DPI), which blocks the ABA-induced H2O2 production (Hu et al., 2005, 2006b), was used. We treated maize plants with DPI before ABA treatment. Results showed that pretreatment with DPI significantly blocked ABA-induced ZmNAC84 expression (Fig. 2B). These data suggest that H2O2 is required for ABA-induced ZmNAC84 expression.
ZmNAC84 Is Involved in ABA-Induced Antioxidant Defense
To explore the possible function of ZmNAC84 in ABA-induced antioxidant defense, we transiently expressed or silenced ZmNAC84 in maize mesophyll protoplasts (Sheen, 2001; Zhai et al., 2009) and determined the effect on activities of ascorbate peroxidase (APX) and superoxide dismutase (SOD), which are key enzymes in antioxidant defense. As shown in Figure 3A, transient expression of ZmNAC84 resulted in significant increases in the activities of APX and SOD when compared with control, which were further enhanced by ABA treatment. Conversely, RNAi-mediated transient silencing of ZmNAC84 decreased the activities of APX and SOD, which could not be restored to the level of controls by ABA treatment (Fig. 3B). Given the functional redundancy of NAC family, we used the chimeric repressor silencing technology to confirm the function of ZmNAC84 (Hiratsu et al., 2003; Lin et al., 2013; Mitsuda et al., 2005). We fused the exogenous EAR motif repression domain SRDX to the C terminus of ZmNAC84 (ubi:ZmNAC84-SRDX-mCherry) and then transfected it into protoplasts. Transient expression of ZmNAC84-SRDX decreased the activities of APX and SOD in ABA-treated and -untreated protoplasts (Fig. 3C), similarly to that of ZmNAC84 transiently silencing analysis. These results clearly indicated that ZmNAC84 is involved in ABA-induced antioxidant defense.
Figure 3.
Role of ZmNAC84 in ABA-induced increases in activities of APX and SOD in maize protoplasts. A, The activities of APX and SOD in protoplasts transiently expressing ZmNAC84. The protoplasts were transfected with constructs carrying ubi:ZmNAC84-mCherry (ZmNAC84) or empty vector (Control). B, The activities of APX and SOD in protoplasts transiently silencing ZmNAC84. The protoplasts were transfected with dsRNA against ZmNAC84 (RNAiZmNAC84) or distilled water (Control). C, The activities of APX and SOD in protoplasts transiently expressing ZmNAC84-SRDX. The protoplasts were transfected with constructs carrying ubi:ZmNAC84-SRDX-mCherry (ZmNAC84-SRDX) or empty vector (Control). The protoplasts (A–C) were treated with culture medium (−ABA) or 10 μM ABA (+ABA) for 10 min. The activities of APX and SOD were measured as described in “Materials and Methods.” Values are means ± se of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
ZmNAC84 Interacting with ZmCCaMK Affects ABA-Induced Antioxidant Defense
Both ZmNAC84 (Fig. 3) and ZmCCaMK (Ma et al., 2012) were involved in ABA-induced antioxidant defense. To further dissect the biological relationship of ZmNAC84 and ZmCCaMK in regulating ABA-induced antioxidant defense, we then transiently silenced both of them in maize mesophyll protoplasts. Silencing both ZmNAC84 and ZmCCaMK (RNAiZmNAC84/ZmCCaMK) resulted in a more severe inhibition of the activities of APX and SOD compared with silencing ZmNAC84 (RNAiZmNAC84) or ZmCCaMK (RNAiZmCCaMK) alone (Fig. 4A), suggesting that the two genes could act together during ABA-induced antioxidant defense. To further examine this interaction hypothesis, we transiently silenced ZmNAC84 and expressed ZmCCaMK (RNAiZmNAC84/ZmCCaMK) simultaneously. As shown in Figure 4B, RNAiZmNAC84/ZmCCaMK protoplasts exhibited higher activities of APX and SOD than ZmNAC84 transiently silencing (RNAiZmNAC84) and lower activities than ZmCCaMK transiently expressing (ZmCCaMK) protoplasts, suggesting that increased expression of ZmCCaMK could partially complement the ZmNAC84 silencing. These data provide further evidence that ZmNAC84 is a functional partner of ZmCCaMK in ABA-induced antioxidant defense.
Figure 4.
Effects of ZmNAC84 interacting with ZmCCaMK on regulating ABA-induced antioxidant enzyme activity. A, The activities of APX and SOD in protoplasts transiently silencing ZmNAC84 alone (RNAiZmNAC84), ZmCCaMK alone (RNAiZmCCaMK), or ZmNAC84 and ZmCCaMK simultaneously (RNAiZmNAC84/ZmCCaMK). B, The activities of APX and SOD in protoplasts transiently silencing ZmNAC84 alone (RNAiZmNAC84), transiently expressing ZmCCaMK alone (ZmCCaMK), or transiently silencing ZmNAC84 and transiently expressing ZmCCaMK simultaneously (RNAiZmNAC84/ZmCCaMK). The protoplasts were treated with culture medium (−ABA) or 10 μM ABA (+ABA) for 10 min. The activities of APX and SOD were measured as described in “Materials and Methods.” Values are means ± se of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Phosphorylation of Ser-113 of ZmNAC84 by ZmCCaMK Is Required for the Effect on Antioxidant Defense
To identify the phosphorylation sites of ZmNAC84 in regulating antioxidant enzyme activity, we predicted eight Ser/Thr residues as potential phosphorylation sites (Fig. 5A) and generated multiple versions of ZmNAC84 by site-directed mutagenesis (S113A, T120A, S211A, T226A, S240A, S281A, T328A, and T420A) to eliminate phosphorylation at these residues. These mutant versions of ZmNAC84 were coexpressed with ZmCCaMK in maize mesophyll protoplasts and examined for their ability to affect antioxidant enzyme activity. Remarkably, only the S113A mutation blocked the increases in APX and SOD activities (Fig. 5B). These results suggest that Ser-113 is an important site of ZmNAC84 phosphorylation by ZmCCaMK in regulating antioxidant enzyme activity. To confirm that Ser-113 is the phosphorylation site of ZmNAC84 by ZmCCaMK, we performed mass spectrometry (liquid chromatography-tandem mass spectrometry [LC-MS/MS]) analysis. His-tagged truncations of ZmNAC84 containing the Ser-113 site, Asn-186 (1–186 amino acids) and Asn-118 (1–118 amino acids), were generated, and GST pull-down analysis showed that both Asn-186 and Asn-118 could interact with ZmCCaMK (Fig. 6A). Further in vitro kinase assay showed that ZmCCaMK phosphorylated Asn-118 (Fig. 6B). Then, we enriched Asn-118 peptides and analyzed them by LC-MS/MS. LC-MS/MS analysis revealed that Ser-113 is the phosphorylation site of ZmNAC84 by ZmCCaMK (Fig. 6C). To further confirm the phosphorylation of Ser-113 in ZmNAC84 by ZmCCaMK, we mutated Ser-113 to Ala or Asp and generated ZmNAC84S113A (phosphor-ablative mutant) or ZmNAC84S113D (phosphor-mimicking mutant). The mutation of S113A and S113D did not affect the subcellular localization of ZmNAC84 (Supplemental Fig. S4) but substantially blocked the phosphorylation of ZmNAC84 by ZmCCaMK (Fig. 6D). Taken together, these data indicate that Ser-113 is a crucial phosphorylation site of ZmNAC84 by ZmCCaMK in regulating antioxidant enzyme activity.
Figure 5.
Prediction of phosphorylation sites of ZmNAC84 and functional analysis of these sites in regulating antioxidant enzyme activity. A, Prediction of phosphorylation sites of ZmNAC84. Potential phosphorylation residues of ZmNAC84 were predicted according to common calcium-dependent kinase phosphorylation motifs: [MVLIF]-x-R-x(2)-[ST]-x(3)-[MVLIF]. B, The activities of APX and SOD in protoplasts transiently expressing various mutants of ZmNAC84 and ZmCCaMK. In B, values are means ± se of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Figure 6.
Identification of Ser-113 as a phosphorylation site of ZmNAC84. A, GST pull-down analysis of ZmCCaMK interaction with truncations of ZmNAC84. GST-ZmCCaMK fusion protein or GST alone was incubated with His-tagged full-length (ZmNAC84) or its truncations (Asn-186, N-terminal amino acids 1–186; Asn-118, N-terminal amino acids 1–118) in GST beads. ZmNAC84 or truncations with His-tag were then detected by western blot using anti-His antibody. B, Phosphorylation of the Asn-118 polypeptide by ZmCCaMK in vitro. Protein extract from maize leaves was immunoprecipitated with ZmCCaMK antibody. Recombinant His-Asn-118 or MBP was used as substrates and subjected to in-gel kinase assay. C, Phosphorylation analysis of Asn-118 by LC-MS/MS. D, Phosphorylation of ZmNAC84 mutant versions (ZmNAC84S113A and ZmNAC84S113D) by ZmCCaMK in vitro. Protein extract from maize leaves was immunoprecipitated with ZmCCaMK antibody. Recombinant His-ZmNAC84, His-ZmNAC84S113A, or His-ZmNAC84S113D was used as substrates and subjected to in-gel kinase assay. CBB, Coomassie Brilliant Blue.
Ser-113 Is a Crucial Phosphorylation Site of ZmNAC84 Functioning in ABA-Induced Antioxidant Defense
In order to determine the significance of ZmNAC84 Ser-113 phosphorylation in ABA-induced antioxidant defense, we transiently expressed ZmNAC84 mutant (ZmNAC84S113A or ZmNAC84S113D) alone or expressed it with ZmCCaMK simultaneously in maize mesophyll protoplasts. The results showed that mutation of S113A (ZmNAC84S113A) led to complete loss of the ZmNAC84-dependent increases of APX and SOD activities in response to ABA (Fig. 7). Moreover, ABA treatment significantly enhanced the activities of APX and SOD in coexpressed ZmNAC84 and ZmCCaMK protoplasts, whereas the activities in coexpressed ZmNAC84S113A and ZmCCaMK were the same as with expression of ZmCCaMK alone (Fig. 7). Expressed ZmNAC84S113D alone and coexpressed with ZmCCaMK increased the activities of APX and SOD compared with control (Fig. 7). These data clearly suggested that ZmCCaMK phosphorylating ZmNAC84 at Ser-113 regulates ABA-induced antioxidant defense.
Figure 7.
The effects of Ser-113 mutation on ABA-induced antioxidant defense. The protoplasts transiently expressing ZmNAC84 alone, ZmCCaMK alone, ZmNAC84S113A alone, ZmNAC84S113D alone, ZmNAC84 and ZmCCaMK simultaneously, ZmNAC84S113A and ZmCCaMK simultaneously, or ZmNAC84S113D and ZmCCaMK simultaneously were treated with culture medium (−ABA) or 10 μM ABA (+ABA) for 10 min. The activities of APX and SOD were measured as described in “Materials and Methods.” Values are means ± se of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
ZmNAC84 Overexpression in Tobacco Confers Seedling Drought Tolerance
To further confirm the role of ZmNAC84 in ABA-induced antioxidant defense, we generated transgenic tobacco overexpressing the coding sequence of ZmNAC84 under control of the cauliflower mosaic virus 35S promoter. The recombinant vector and empty vector were transferred to Agrobacterium tumefaciens and then were used to transform tobacco. Three independent lines (#7, #9, and #12) with ZmNAC84 expression (Supplemental Fig. S5) were selected for analysis. As shown in Figure 8A, the ZmNAC84 transgenic tobacco (ZmNAC84) displayed higher activities of APX and SOD compared with the empty vector control plants (vector). One mode of plant tolerance to drought stress is inducing antioxidant defense system, so we next investigate the response of ZmNAC84 to drought stress. We first analyzed the expression of ZmNAC84 under polyethylene glycol (PEG) treatment. Results showed that PEG treatment strongly induced ZmNAC84 expression (Supplemental Fig. S6). Moreover, compared with the control plants, the ZmNAC84 transgenic tobacco displayed enhanced drought tolerance, whereas no morphological changes were observed under normal growth conditions (Fig. 8B). Fresh weight and relative water content (RWC) of the transgenic plants were significantly higher than those of control after drought treatment (Fig. 8C). The transgenic tobacco also showed less oxidative damage in response to drought as indicated by the content of malondialdehyde (MDA) and the percentage of electrolyte leakage compared with control (Fig. 8C). These data clearly indicated that ZmNAC84 could enhance tobacco seedling tolerance to drought stress.
Figure 8.
Drought tolerance of ZmNAC84 transgenic tobacco. A, The activities of APX and SOD in transgenic tobacco of ZmNAC84. Plants were exposed to 20% PEG for 6 h. B, Phenotype of ZmNAC84 transgenic tobacco. Photographs were taken under well-watered conditions (Control) and drought treatment for 14 d. Vector-transformed (vector #1 and #3) and ZmNAC84-transformed (ZmNAC84) transgenic plants are shown C, Fresh weight, RWC, the content of MDA, and the percentage of leakage of electrolyte in ZmNAC84 transgenic tobacco. Plants were exposed to drought treatment (fresh weight, RWC) for 14 d or 20% PEG (the content of MDA and the percentage of leakage of electrolyte) for 12 h. The experiments were repeated at least three times. Means denoted by the same letter did not significantly differ at P < 0.05 according to Duncan’s multiple range test.
DISCUSSION
CCaMK is a central regulator of nodule organogenesis, rhizobia infection, and arbuscular mycorrhizal signaling (Gobbato, 2015; Miller et al., 2013; Shimoda et al., 2012). Recently, a growing body of evidence reveals additional functions of CCaMK, such as response to ABA signaling and stress tolerance in wheat, maize, and rice (Ma et al., 2012; Shi et al., 2012; Yang et al., 2011a). Hence, it is getting increasingly important to identify the downstream targets of CCaMK in order to understand the physiological role of CCaMK in different biological processes. So far, only two directly interacting proteins of CCaMK, IPD3/CYCLOPS and CIP73 in L. japonicus, M. truncatula, and rice, were identified (Chen et al., 2008; Horváth et al., 2011; Kang et al., 2011; Messinese et al., 2007; Yano et al., 2008). IPD3 is a member of the common symbiotic signaling pathway and is necessary for the invasion of the host cell by both rhizobial and fungal symbiotic partners (Horváth et al., 2011). CIP73 has also been shown to be essential for nodule formation (Kang et al., 2011). However, neither IPD3 nor CIP73 was involved in ABA-induced increases in the activities of antioxidant enzymes (Shi et al., 2014), and the mechanism and phosphorylation targets through which CCaMK mediates this process have been completely unclear. In this report, we identified a physically interacting protein of ZmCCaMK, ZmNAC84, which functions in ABA-induced antioxidant defense.
Using the full-length ZmCCaMK as bait in a yeast two-hybrid screen, we identified a NAC transcription factor, ZmNAC84, and we could confirm physical interaction between the two proteins in vitro and in vivo (Fig. 1). Therefore, ZmNAC84 is a new interaction protein of ZmCCaMK besides IPD3 and CIP73. Our previous study showed localization of ZmCCaMK in the nucleus (Ma et al., 2012), and ZmNAC84 was also located in the nucleus in maize protoplasts (Supplemental Fig. S1). The molecular mechanism by which ZmCCaMK and ZmNAC84 mediate biological processes presumably involves the activation of downstream genes, where ZmNAC84 functions either as a downstream effector, tethering ZmCCaMK to transcription regulatory complexes of target genes, as a substrate of this protein kinase, or both. The ZmNAC84 regulated downstream genes remain to be identified.
ZmNAC84 belongs to the NAC superfamily of TFs (Fan et al., 2014). The typical NAC proteins share a conserved N-terminal DNA binding domain but vary greatly in other regions, resulting in distinct functions of different proteins. The NAC family has been found to play pivotal roles in response to abiotic stress (Fang et al., 2015; Garapati et al., 2015; Hu et al., 2006a; Huang et al., 2015; Mao et al., 2015; Wu et al., 2012) and ABA signaling (Du et al., 2014; Fujita et al., 2004; Garapati et al., 2015; Hu et al., 2006a; Yang et al., 2011b). NAC TFs regulate abiotic stress in both ABA-dependent and ABA-independent manner (Xu et al., 2013). In this study, we demonstrated that ZmNAC84 is a positive component of ABA-induced antioxidant defense involved in tolerance to drought stress. This conclusion was based on the following results: First, expression analysis of ZmNAC84 after PEG treatment indicates that ZmNAC84 expression was positively correlated with adaptation to seedling drought stress (Supplemental Fig. S6), which suggested a positive regulatory role of ZmNAC84 in maize seedlings exposed to drought stress. Second, ABA treatment also up-regulated the expression of ZmNAC84 (Fig. 2). Third, ZmNAC84 was essential for ABA-induced antioxidant defense (Fig. 3). Finally, overexpression of the ZmNAC84 in transgenic tobacco could improve drought tolerance and alleviated oxidative damage in response to drought (Fig. 8).
Both exogenous H2O2 and ABA-produced endogenous H2O2 affected ZmNAC84 expression (Fig. 2). Hence, the increased expression of ZmNAC84 in response to ABA may be indirect via the ABA-induced increase in H2O2. Our previous studies demonstrated that ABA first induced initial H2O2 accumulation, the initially accumulated H2O2 activated CCaMK, and the activated CCaMK enhanced H2O2 production, forming a positive amplification loop of H2O2 in ABA signaling (Shi et al., 2012). In this report, ZmNAC84 interacting with ZmCCaMK performed an essential role in ABA-induced antioxidant defense (Fig. 4). Therefore, it is highly possible that initial produced H2O2-induced ZmNAC84, interacting with H2O2-activated ZmCCaMK, promotes H2O2 amplification, and thus activates antioxidant defense in ABA signaling. Moreover, silenced ZmNAC84 in overexpressed ZmCCaMK protoplasts could not completely block the effects of ZmCCaMK on ABA-induced antioxidant enzyme activities (Fig. 4B). Possibly, yet to be identified phosphorylation targets of CCaMK are able to, at least partially, substitute for the loss of ZmNAC84 in this process.
To further explain the mechanism of ZmCCaMK interaction with ZmNAC84 in ABA-induced antioxidant defense, we predicted the possible phosphorylation sites of ZmNAC84 and mutated them from Ser/Thr to Ala to eliminate phosphorylation of these sites. Only S113A mutagenesis of ZmNAC84 blocked the ZmCCaMK-enhanced and ABA-induced antioxidant enzyme activity (Figs. 5 and 7). LC-MS/MS analysis confirmed Ser-113 in ZmNAC84 as the phosphorylation site by ZmCCaMK (Fig. 6). Future studies will be required to determine if and how the Ser-113 phosphorylation affects DNA binding of this NAC TF. Phosphorylation is a prerequisite for nuclear localization of OsNAC4 (Kaneda et al., 2009) and phosphorylation of AFAF1 by SnRK1 kinase could modulate its subcellular colocalization (Kleinow et al., 2009). However, the nuclear localization of ZmNAC84 was not altered by the phosphorylation state of Ser-113 site (Supplemental Fig. S4).
In summary, our data showed that ZmNAC84 interacts with ZmCCaMK in modulating ABA-induced antioxidant defense, and the phosphorylation at Ser-113 of ZmNAC84 by ZmCCaMK is essential for its role in this process.
MATERIALS AND METHODS
Plant Materials and Treatments
Seeds of maize (Zea mays cv Nongda 108; from Nanjing Agricultural University, China) were sown in trays of sand in a growth chamber at a temperature of 22 to 28°C, photosynthetic active radiation of 200 µmol m−2 s−1, and a photoperiod of 14/10 h (day/night) and watered daily. For protoplast isolation, maize plants were grown at 25°C under dark conditions. When the second leaves were fully expanded, they were collected and used for investigations.
The plants were excised at the base of stem and placed in distilled water for 2 h to eliminate wound stress. Maize seedlings were placed in beakers wrapped with aluminum foil containing 100 μM ABA, 10 mM H2O2, or 10% (w/v) polyethylene glycol (PEG6000) solutions, respectively. In order to study the effects of inhibitor of ROS production, the detached plants were pretreated with 100 µM DPI for 4 h, then subjected to 100 μM ABA treatment for 45 min under the same conditions as described above. Detached plants were treated with distilled water under the same conditions for the whole period and served as controls for the above. After treatments of detached plants, the second leaves were sampled and immediately frozen under liquid N2 for further analysis.
Yeast Two-Hybrid Assay
Yeast two-hybrid assays were performed using the ProQuest two-hybrid system with Gateway technology according to the manufacturer’s instructions (Invitrogen). Saccharomyces cerevisiae strain MaV203 cotransformed with indicated constructs was grown on the SC/-Leu/-Trp or SC/-Leu/-Trp/-His+10 mM 3-amino-triazole media, and the transformants were used for LacZ assay using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside).
GST Pull-Down Assay
GST or GST-ZmCCaMK fusion proteins were kept immobilized on glutathione Sepharose 4B beads and then were incubated with His-tagged ZmNAC84 or its truncations (Asn-118 and Asn-186) in binding buffer (140 mM NaCl, 4.2 mM Na2HPO4, 2 mM KH2PO4, 10 mM KCl, and 10% bovine serum albumin, pH 7.2) at 4°C for 2 h. Subsequently, the beads were washed at least five times with washing buffer (400 mM NaCl, 4.2 mM Na2HPO4, 2 mM KH2PO4, and 10 mM KCl, pH 7.2). After extensive washing, the pulled down proteins were eluted by boiling and separated by 12% SDS-PAGE. Proteins were probed with rat monoclonal anti-His antibody (Sigma-Aldrich) followed by incubation with goat anti-rat HRP-conjugated secondary antibody (Sigma-Aldrich). Signals were visualized by x-ray film.
BiFC Assay
For generation of the BiFC vectors, the ZmNAC84 coding region without stop codon was amplified and cloned at the BamHI-KpnI sites in pSPYCE to form ZmNAC84-YFPC construct, and ZmCCaMK without stop codon was cloned into the BamHI-SalI sites of pSPYNE to generate ZmCCaMK- YFPN plasmid. Onion (Allium cepa) tissues were prepared, and two fusion proteins were transiently transfected into onion epidermal cells as described previously (Lee et al., 2008). YFP fluorescence in transformed onion tissues was detected and imaged using a confocal laser scanning microscope (TCS-SP2; Leica) after incubation for 16 h.
Co-IP Assay
The coding sequences of ZmNAC84 or ZmCCaMK were fused with His or myc tags and cloned into pXZP008 at BamHI-KpnI sites, respectively. Maize mesophyll protoplasts were transfected with ubi:ZmCCaMK-myc and ubi:ZmNAC84-His or ubi:ZmNAC84-His alone. After incubation for 16 h, protoplasts were harvested and homogenized in Co-IP buffer as described previously (Zhang et al., 2006). The solubilized proteins were incubated with anti-myc antibody bound to protein A beads for 2 h. The beads were washed three times with immunoprecipitation buffer, and the proteins were eluted by boiling in 1× SDS sample buffer for 5 min. After centrifugation, the supernatant fraction was analyzed by immunoblotting with anti-His antibody.
Immunoprecipitation Kinase Activity Assay
Protein were extracted and quantified as described previously (Ma et al., 2012). For immunocomplex kinase assay, protein extract (100 µg) was incubated with anti-ZmCCaMK antibody (7.5 µg) in immunoprecipitation buffer. Immunocomplex was incubated with 2 µg substrates in reaction buffer (25 mM Tris, pH 7.5, 5 mM MgCl2,1 mM DTT, 2.5 mM CaCl2, 2 µM CaM with 200 nM ATP plus 1 µCi of [γ-32P]ATP [3,000 Ci mM–1]) for 30 min. The reaction was stopped by adding SDS sample buffer, and the reaction mix was separated on SDS-PAGE. The unincorporated [γ-32P]ATP was removed by washing with 5% (w/v) trichloroacetic acid/1% (w/v) sodium pyrophosphate at least three times. The gel was dried onto Whatman 3 MM paper and exposed to Kodak XAR-5 film.
Isolation of Total RNA and qRT-PCR Analysis
Total RNA was isolated from leaves or protoplasts using an RNAiso Plus kit (TaKaRa) following the manufacturer’s protocol and treated with RNase-free DNase to remove contaminating DNA (TaKaRa). Approximately 2 µg of total RNA was reversely transcribed using an oligo(dT)16 primer and Moloney murine leukemia virus reverse transcriptase (TaKaRa). Transcript levels of several genes were measured by qRT-PCR using a DNA Engine Opticon 2 real-time PCR detection system (Bio-Rad) with SYBR Premix Ex Taq (TaKaRa) according to the manufacturer’s instructions. The expression level was normalized against that of ZmActin in maize or NtActin in tobacco (Nicotiana tabacum).
Vector Construction and in Vitro Synthesis of Double-Stranded RNA
The constructed vector pXZP008 with mCherry driven by ubiquitin promoter (the original 35S promoter was substituted by the ubiquitin promoter using HindIII and BamHI) was used for protoplast transient expression and subcellular localization assay. The full-length ZmNAC84 was amplified by PCR and cloned into pXZP008 at BamHI-KpnI sites. The exogenous EAR motif repression domain SRDX (5′-CTGGATCTAGAACTCCGTTTG-3′) was introduced into the C terminus of ZmNAC84 by PCR and then cloned into pXZP008.
For in vitro synthesis of double-stranded RNA (dsRNA), DNA templates were produced by PCR using primers containing the T7 promoter sequence (5′-TTAATACGACTCACTATAGGGAGG-3′) on both the 5′ and 3′ ends. The primers used to amplify DNA of ZmCCaMK or ZmNAC84 are listed in Supplemental Table S1. dsRNA of ZmCCaMK or ZmNAC84 was synthesized in vitro using the RiboMAX Large Scale RNA Production System-T7 (Promega) according to the manufacturer’s instructions. The purity and concentration of synthesized dsRNA were checked by 2% agarose gel electrophoresis and spectrophotometry.
Protoplast Preparation and Transfection with DNA Constructs or dsRNAs
Protoplast isolation and transfection with DNA constructs or dsRNAs were based on the protocol for maize mesophyll protoplasts provided online by J. Sheen’s laboratory (http://genetics.mgh.harvard.edu/sheenweb) with minor modifications. Maize protoplasts (1 mL, usually 5 × 105 cells mL–1) were transfected with 100 µg of fusion constructs (empty vector as control) or 150 µg dsRNAs (water as control) using a PEG-calcium-mediated method. The transfected protoplasts were then incubated in incubation solution overnight in the dark at 25°C. After that, protoplasts were collected and used for further analysis.
Site-Directed Mutagenesis
To mutate ZmNAC84, the Multi-Directed Mutagenesis Kit (Agilent Technologies) was used according to the manufacturer’s instructions. The DNA oligonucleotides used in mutagenesis were synthesized, and their sequences are listed in Supplemental Table S1. All of the mutated plasmids were confirmed by Sanger sequencing.
Localization
Protoplasts were transfected with ubi:ZmNAC84-mCherry, ubi:ZmNAC84S113A-mCherry, or ubi:ZmNAC84S113D-mCherry constructs and incubated for 16 h. The fluorescence was observed using a confocal laser scanning microscope (TCS-SP2; Leica). The nucleus was stained with 4′,6-diamidino-2-phenylindole (1 μg μL−1) dye.
Antioxidant Enzyme Assay
The protoplasts or tobacco leaves were homogenized in 0.6 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone, with the addition of 1 mM sodium ascorbate in the case of APX assay. The homogenate was centrifuged at 12,000g for 30 min at 4°C, and the supernatant was immediately used for the subsequent antioxidant enzyme assays. The total activities of antioxidant enzymes were determined as previously described (Zhu et al., 2013).
Mass Spectrometry Analysis
The truncated ZmNAC84 (Asn-118) was reacted with ZmCCaMK in vitro in kinase assay buffer with ATP (200 nM). Phosphorylated His tagged Asn-118 was enriched and subjected to trypsin digestion followed by LC-MS/MS analysis as described previously (Gampala et al., 2007).
Generation of Transgenic Plants
The full-length cDNA of ZmNAC84 was inserted into the SacI-KpnI sites of the binary vector super1300 driven by the cauliflower mosaic virus 35S promoter. The recombinant vector or empty super1300 (vector) was introduced into tobacco using Agrobacterium tumefaciens strain EHA105 via leaf disc transformation (Horsch et al., 1985). T0, T1, and T2 plants were grown in a greenhouse, and the presence of the transgene was determined in each generation by PCR analysis. The expression of ZmNAC84 in transgenic plants was determined by qRT-PCR, and three independent T2 lines, ZmNAC84#7, ZmNAC84#9, and ZmNAC84#12, were selected for further analysis.
Phenotype and Oxidative Damage Analysis
For the phenotype, fresh weight, and the RWC assays, 4-week-old seedlings grown in pots with soil were treated by withholding water for 14 d. The phenotype of seedlings was photographed, and the shoot fresh weight and the RWC were measured as described by Jiang and Zhang (2001). For the oxidative damage analysis, 4-week-old seedlings were treated with 20% PEG for 12 h. The content of MDA and the percentage of electrolyte leakage were determined as described by Shi et al. (2012).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ZmCCaMK DQ403196; ZmNAC84 AFU81568.1; ZmActin J01238; NtActin U60495.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Subcellular localization of ZmNAC84 in maize protoplasts.
Supplemental Figure S2. Expression analysis of ZmNAC84 in various tissues of maize.
Supplemental Figure S3. ABA-induced expression of ZmCCaMK in maize leaves.
Supplemental Figure S4. Subcellular localization of Ser-113 site-directed mutagenesis of ZmNAC84 in maize protoplasts.
Supplemental Figure S5. ZmNAC84 expression analysis in transgenic tobacco plants.
Supplemental Figure S6. PEG-induced expression of ZmNAC84 in maize leaves.
Supplemental Table S1. PCR primers used.
Supplementary Material
Glossary
- ABA
abscisic acid
- ROS
reactive oxygen species
- CCaMK
calcium/calmodulin-dependent protein kinase
- TF
transcription factor
- BiFC
bimolecular fluorescence complementation
- Co-IP
coimmunoprecipitation
- DPI
diphenylene iodonium
- APX
ascorbate peroxidase
- SOD
superoxide dismutase
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- PEG
polyethylene glycol
- RWC
relative water content
- MDA
malondialdehyde
- dsRNA
double-stranded RNA
Footnotes
Articles can be viewed without a subscription.
This study was supported by grants from the National Natural Science Foundation of China (31371547, 31071344, and J1210056), the Fundamental Research Funds for the Central Universities (KYZ201637, KYZ201402, and KYZ201157), the Program for New Century Excellent Talents in University (NCET-10-0498), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and as part of the DOE Joint BioEnergy Institute (http://www.jbei.org) by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy.
References
- Capoen W, Den Herder J, Sun J, Verplancke C, De Keyser A, De Rycke R, Goormachtig S, Oldroyd G, Holsters M (2009) Calcium spiking patterns and the role of the calcium/calmodulin-dependent kinase CCaMK in lateral root base nodulation of Sesbania rostrata. Plant Cell 21: 1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ané JM, Zhu H (2008) OsIPD3, an ortholog of the Medicago truncatula DMI3 interacting protein IPD3, is required for mycorrhizal symbiosis in rice. New Phytol 180: 311–315 [DOI] [PubMed] [Google Scholar]
- Chen C, Gao M, Liu J, Zhu H (2007) Fungal symbiosis in rice requires an ortholog of a legume common symbiosis gene encoding a Ca2+/calmodulin-dependent protein kinase. Plant Physiol 145: 1619–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lu S, Wang Y, Zhang X, Lv B, Luo L, Xi D, Shen J, Ma H, Ming F (2015) OsNAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice. Plant J 82: 302–314 [DOI] [PubMed] [Google Scholar]
- DeFalco TA, Bender KW, Snedden WA (2009) Breaking the code: Ca2+ sensors in plant signalling. Biochem J 425: 27–40 [DOI] [PubMed] [Google Scholar]
- Ding Y, Cao J, Ni L, Zhu Y, Zhang A, Tan M, Jiang M (2013) ZmCPK11 is involved in abscisic acid-induced antioxidant defence and functions upstream of ZmMPK5 in abscisic acid signalling in maize. J Exp Bot 64: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Zhai Q, Deng L, Li S, Li H, Yan L, Huang Z, Wang B, Jiang H, Huang T, et al. (2014) Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26: 3167–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K, Wang M, Miao Y, Ni M, Bibi N, Yuan S, Li F, Wang X (2014) Molecular evolution and expansion analysis of the NAC transcription factor in Zea mays. PLoS One 9: e111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Liao K, Du H, Xu Y, Song H, Li X, Xiong L (2015) A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J Exp Bot 66: 6803–6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran LS, Yamaguchi-Shinozaki K, Shinozaki K (2004) A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J 39: 863–876 [DOI] [PubMed] [Google Scholar]
- Gampala SS, Kim TW, He JX, Tang W, Deng Z, Bai MY, Guan S, Lalonde S, Sun Y, Gendron JM, et al. (2007) An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell 13: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapati P, Xue GP, Munné-Bosch S, Balazadeh S (2015) Transcription factor ATAF1 in Arabidopsis promotes senescence by direct regulation of key chloroplast maintenance and senescence transcriptional cascades. Plant Physiol 168: 1122–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbato E. (2015) Recent developments in arbuscular mycorrhizal signaling. Curr Opin Plant Biol 26: 1–7 [DOI] [PubMed] [Google Scholar]
- Godfroy O, Debellé F, Timmers T, Rosenberg C (2006) A rice calcium- and calmodulin-dependent protein kinase restores nodulation to a legume mutant. Mol Plant Microbe Interact 19: 495–501 [DOI] [PubMed] [Google Scholar]
- Guo HS, Xie Q, Fei JF, Chua NH (2005) MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell 17: 1376–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gan S (2006) AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J 46: 601–612 [DOI] [PubMed] [Google Scholar]
- Harper JF, Breton G, Harmon A (2004) Decoding Ca(2+) signals through plant protein kinases. Annu Rev Plant Biol 55: 263–288 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Banba M, Shimoda Y, Kouchi H, Hayashi M, Imaizumi-Anraku H (2010) A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J 63: 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qu B, Li W, Zhao X, Teng W, Ma W, Ren Y, Li B, Li Z, Tong Y (2015) The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiol 169: 1991–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M (2003) Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J 34: 733–739 [DOI] [PubMed] [Google Scholar]
- Horsch R, Fry J, Hoffmann N, Eichholtz D, Rogers S, Fraley R (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Horváth B, Yeun LH, Domonkos A, Halász G, Gobbato E, Ayaydin F, Miró K, Hirsch S, Sun J, Tadege M, et al. (2011) Medicago truncatula IPD3 is a member of the common symbiotic signaling pathway required for rhizobial and mycorrhizal symbioses. Mol Plant Microbe Interact 24: 1345–1358 [DOI] [PubMed] [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L (2006a) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103: 12987–12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Jiang M, Zhang A, Lu J (2005) Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 223: 57–68 [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang A, Zhang J, Jiang M (2006b) Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress. Plant Cell Physiol 47: 1484–1495 [DOI] [PubMed] [Google Scholar]
- Huang Q, Wang Y, Li B, Chang J, Chen M, Li K, Yang G, He G (2015) TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol 15: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42: 1265–1273 [DOI] [PubMed] [Google Scholar]
- Kaneda T, Taga Y, Takai R, Iwano M, Matsui H, Takayama S, Isogai A, Che FS (2009) The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J 28: 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Zhu H, Chu X, Yang Z, Yuan S, Yu D, Wang C, Hong Z, Zhang Z (2011) A novel interaction between CCaMK and a protein containing the Scythe_N ubiquitin-like domain in Lotus japonicus. Plant Physiol 155: 1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinow T, Himbert S, Krenz B, Jeske H, Koncz C (2009) NAC domain transcription factor ATAF1 interacts with SNF1-related kinases and silencing of its subfamily causes severe developmental defects in Arabidopsis. Plant Sci 177: 360–370 [Google Scholar]
- Lee MO, Cho K, Kim SH, Jeong SH, Kim JA, Jung YH, Shim J, Shibato J, Rakwal R, Tamogami S, et al. (2008) Novel rice OsSIPK is a multiple stress responsive MAPK family member showing rhythmic expression at mRNA level. Planta 227: 981–990 [DOI] [PubMed] [Google Scholar]
- Lévy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, Journet EP, Ané JM, Lauber E, Bisseling T, et al. (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364 [DOI] [PubMed] [Google Scholar]
- Lin H, Niu L, McHale NA, Ohme-Takagi M, Mysore KS, Tadege M (2013) Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants. Proc Natl Acad Sci USA 110: 366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Lu R, Liu H, Shi B, Zhang J, Tan M, Zhang A, Jiang M (2012) Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. J Exp Bot 63: 4835–4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Wang H, Liu S, Li Z, Yang X, Yan J, Li J, Tran LS, Qin F (2015) A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat Commun 6: 8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinese E, Mun JH, Yeun LH, Jayaraman D, Rougé P, Barre A, Lougnon G, Schornack S, Bono JJ, Cook DR, Ané JM (2007) A novel nuclear protein interacts with the symbiotic DMI3 calcium- and calmodulin-dependent protein kinase of Medicago truncatula. Mol Plant Microbe Interact 20: 912–921 [DOI] [PubMed] [Google Scholar]
- Miller JB, Pratap A, Miyahara A, Zhou L, Bornemann S, Morris RJ, Oldroyd GE (2013) Calcium/calmodulin-dependent protein kinase is negatively and positively regulated by calcium, providing a mechanism for decoding calcium responses during symbiosis signaling. Plant Cell 25: 5053–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GE, Long SR (2004) A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc Natl Acad Sci USA 101: 4701–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, Ohme-Takagi M (2007) NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 19: 270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17: 2993–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S (2010) Genome-wide analysis of NAC transcription factor family in rice. Gene 465: 30–44 [DOI] [PubMed] [Google Scholar]
- Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10: 79–87 [DOI] [PubMed] [Google Scholar]
- Patil S, Takezawa D, Poovaiah BW (1995) Chimeric plant calcium/calmodulin-dependent protein kinase gene with a neural visinin-like calcium-binding domain. Proc Natl Acad Sci USA 92: 4897–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah BW, Du L, Wang H, Yang T (2013) Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant Physiol 163: 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Sahu PP, Srivastava PS, Prasad M (2012) NAC proteins: regulation and role in stress tolerance. Trends Plant Sci 17: 369–381 [DOI] [PubMed] [Google Scholar]
- Sang J, Zhang A, Lin F, Tan M, Jiang M (2008) Cross-talk between calcium-calmodulin and nitric oxide in abscisic acid signaling in leaves of maize plants. Cell Res 18: 577–588 [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan PV, Cremo CR, Poovaiah BW (2000) Plant chimeric Ca2+/calmodulin-dependent protein kinase. Role of the neural visinin-like domain in regulating autophosphorylation and calmodulin affinity. J Biol Chem 275: 30417–30422 [DOI] [PubMed] [Google Scholar]
- Sheen J. (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127: 1466–1475 [PMC free article] [PubMed] [Google Scholar]
- Shi B, Ni L, Zhang A, Cao J, Zhang H, Qin T, Tan M, Zhang J, Jiang M (2012) OsDMI3 is a novel component of abscisic acid signaling in the induction of antioxidant defense in leaves of rice. Mol Plant 5: 1359–1374 [DOI] [PubMed] [Google Scholar]
- Shi B, Ni L, Liu Y, Zhang A, Tan M, Jiang M (2014) OsDMI3-mediated activation of OsMPK1 regulates the activities of antioxidant enzymes in abscisic acid signalling in rice. Plant Cell Environ 37: 341–352 [DOI] [PubMed] [Google Scholar]
- Shimoda Y, Han L, Yamazaki T, Suzuki R, Hayashi M, Imaizumi-Anraku H (2012) Rhizobial and fungal symbioses show different requirements for calmodulin binding to calcium calmodulin-dependent protein kinase in Lotus japonicus. Plant Cell 24: 304–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, Nakagawa T, Sandal N, Albrektsen AS, Kawaguchi M, et al. (2006) Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441: 1153–1156 [DOI] [PubMed] [Google Scholar]
- Wang X, Basnayake BM, Zhang H, Li G, Li W, Virk N, Mengiste T, Song F (2009) The Arabidopsis ATAF1, a NAC transcription factor, is a negative regulator of defense responses against necrotrophic fungal and bacterial pathogens. Mol Plant Microbe Interact 22: 1227–1238 [DOI] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. (2012) JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24: 482–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZY, Kim SY, Hyeon Y, Kim DH, Dong T, Park Y, Jin JB, Joo SH, Kim SK, Hong JC, Hwang D, Hwang I (2013) The Arabidopsis NAC transcription factor ANAC096 cooperates with bZIP-type transcription factors in dehydration and osmotic stress responses. Plant Cell 25: 4708–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li A, Zhao Y, Zhang Z, Zhu Y, Tan X, Geng S, Guo H, Zhang X, Kang Z, et al. (2011a) Overexpression of a wheat CCaMK gene reduces ABA sensitivity of Arabidopsis thaliana during seed germination and seedling growth. Plant Mol Biol Report 29: 681–692 [Google Scholar]
- Yang SD, Seo PJ, Yoon HK, Park CM (2011b) The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 23: 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW (2003) Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 8: 505–512 [DOI] [PubMed] [Google Scholar]
- Yano K, Yoshida S, Müller J, Singh S, Banba M, Vickers K, Markmann K, White C, Schuller B, Sato S, et al. (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc Natl Acad Sci USA 105: 20540–20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Sooksa-nguan T, Vatamaniuk OK (2009) Establishing RNA interference as a reverse-genetic approach for gene functional analysis in protoplasts. Plant Physiol 149: 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Jiang M, Zhang J, Tan M, Hu X (2006) Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 141: 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lu YT (2003) Calmodulin-binding protein kinases in plants. Trends Plant Sci 8: 123–127 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zuo M, Liang Y, Jiang M, Zhang J, Scheller HV, Tan M, Zhang A (2013) MAP65-1a positively regulates H2O2 amplification and enhances brassinosteroid-induced antioxidant defence in maize. J Exp Bot 64: 3787–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.