ROS act as a key growth signal in root hairs and pollen tubes.
Abstract
Root hair cells and pollen tubes, like fungal hyphae, possess a typical tip or polar cell expansion with growth limited to the apical dome. Cell expansion needs to be carefully regulated to produce a correct shape and size. Polar cell growth is sustained by oscillatory feedback loops comprising three main components that together play an important role regulating this process. One of the main components are reactive oxygen species (ROS) that, together with calcium ions (Ca2+) and pH, sustain polar growth over time. Apoplastic ROS homeostasis controlled by NADPH oxidases as well as by secreted type III peroxidases has a great impact on cell wall properties during cell expansion. Polar growth needs to balance a focused secretion of new materials in an extending but still rigid cell wall in order to contain turgor pressure. In this review, we discuss the gaps in our understanding of how ROS impact on the oscillatory Ca2+ and pH signatures that, coordinately, allow root hair cells and pollen tubes to expand in a controlled manner to several hundred times their original size toward specific signals.
Polar growth occurs in root hair cells and pollen tubes, whereas coordinated multidirectional growth occurs in various other plant cells, such as puzzle-shaped pavement cells, trichomes, and stomata. Root hairs are single cells that absorb water and nutrients from the soil and have important roles in plant-microbe interactions, such as the legume-Rhizobium symbiosis, which involves nodule formation and N2 fixation (Oldroyd, 2001; Oldroyd and Dixon, 2014). Pollen tubes are cells that carry sperm nuclei to the ovule, thus facilitating fertilization and seed production. Polar growth is guided by specific external signals, such as nutrient gradients and small attractant peptides. For instance, the LURE1 Cys-rich peptide, which is secreted by synergid cells, and two groups of plasma membrane receptor-like kinases with extracellular Leu-rich repeats and an intracellular kinase domain (namely MALE DISCOVERED1-MDIS1-INTERACTING RECEPTOR-LIKE KINASE1/2 and POLLEN-SPECIFIC RECEPTOR-LIKE KINASE6 [PRK6]) within the growing pollen tube are involved in pollen tube guidance (Takeuchi and Higashiyama, 2016; Wang et al., 2016). Both types of receptor kinases bind to LURE1, triggering downstream responses, such as activation of the core tip growth machinery (e.g. ROPGEF8, ROPGEF9, ROPGEF12, and ROPGEF13) and RHO OF PLANTS1 (ROP1), in the case of PRK6 (Takeuchi and Higashiyama, 2016). By contrast, little is known about the molecular mechanisms that guide the polar growth of root hairs. Root hair shape, cell length, and density are strongly modulated by environmental signals, such as the gradients of low-mobility mineral nutrients (e.g. phosphorus, iron, and manganese) or scarce elements (e.g. vanadium and boron) in the soil (Yi et al., 2010; Martín-Rejano et al., 2011; Niu et al., 2014; Lin et al., 2015). Other signals, such as water status, carbon monoxide, and carbon dioxide levels, also affect root hair development (Guo et al., 2009; Niu et al., 2011; Kwasniewski et al., 2016). Polar growth also is responsive to endogenous signals, such as ethylene and auxin (for review, see Lee and Cho, 2013; Velasquez et al., 2016). For instance, auxin activates the class I basic helix-loop-helix transcription factor ROOT HAIR DEFECTIVE6-LIKE4 (RSL4), thus modulating downstream gene expression to trigger root hair cell expansion (Yi et al., 2010; Datta et al., 2015).
During polar cell expansion, the apical zone is characterized by a gradient of cytoplasmic Ca2+ ions (cytCa2+) and apoplastic reactive oxygen species (apoROS) production. High levels of cytCa2+ in the tip zone trigger apoROS production, in a reaction catalyzed by NADPH oxidases (NOXs). Furthermore, high levels of reactive oxygen species (ROS) transiently elevate the concentration of cytCa2+ (Duan et al., 2014) by an unknown mechanism. NOXC (Bibikova et al., 1998; Foreman et al., 2003; Monshausen et al., 2007, 2008) and NOXH/NOXJ (Wu et al., 2010; Boisson-Dernier et al., 2013) were proposed previously to connect apoROS production with the transient activation of plasma membrane Ca2+ channels (CaCs; Table I) in growing root hairs and growing pollen tubes, respectively. A ROS burst is crucial for pollen tube rupture and sperm release (Duan et al., 2014). Oscillatory growth also is linked to changes in pH that are mostly regulated by membrane H+-ATPases (AHA; Falhof et al., 2016) and by cation (H+)/anion (OH−)-permeable channels and antiporters (Ca2+/H+ exchangers). AHA2 is highly expressed in growing root hairs, while the AHA presumed to be responsible for pumping H+ out of pollen tubes remains to be identified. AHA directly regulates apoplastic pH (apopH), which impacts the enzymatic machinery that modifies wall components during cell expansion. Polar growth involves the deformation and stretching of the existing primary wall in the apical zone, which is accompanied by the secretion of new cell wall materials (Altartouri and Geitmann, 2015). In addition, in vitro pollen tube tip growth and root hair expansion are coupled to the Ca2+ gradient that directs polar secretion, the arrangement of the actin cytoskeleton, the movement of organelles, and the biochemical activity of necessary enzymes (Sanders et al., 2002; Fan et al., 2004; Rounds and Bezanilla 2013; Huang et al., 2015). Oscillations in ROS, Ca2+, and pH are coupled to transient cell wall loosening to allow turgor-driven localized cell expansion (Braidwood et al., 2014; Spartz et al., 2014; Wolf and Höfte, 2014). In root hairs, the maxima of these oscillatory fluctuations in apoROS concentration and apoplastic/cytoplasmic pH precede cell growth peaks by 7 to 8 s, while cytCa2+ oscillations lag oscillations in cell growth by approximately 5 to 6 s (Monshausen et al., 2007, 2008). In pollen tubes, the oscillations in cytCa2+ concentration are delayed by approximately 11 s relative to cell expansion rates (Pierson et al., 1994). Thus, polar cell growth is preceded, and perhaps transiently repressed, by high levels of cytCa2+ and, subsequently, high apoROS concentrations and a more alkaline apopH.
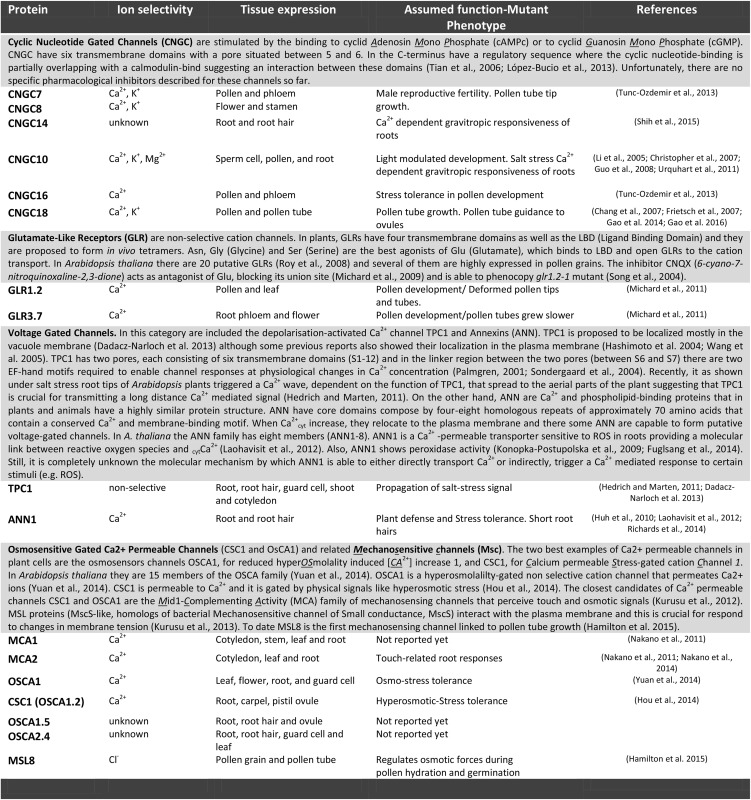
Table I. Selected examples of putative plasma membrane CaCs present in Arabidopsis and expressed preferentially in polar-expanding cells (pollen tubes or root hairs).
 |
Studies using molecular biosensors that detect rapid changes in the concentrations of several small molecules have emerged recently and provided insight into the molecular mechanism underlying tip growth (Uslu and Grossmann, 2016). Examples of these include biosensors that detect concentrations of ROS (Belousov et al., 2006; Costa et al., 2010), Ca2+ (Horikawa et al., 2010; Bardgett et al., 2014; Keinath et al., 2015), and H+ (Monshausen et al., 2007, 2011; Gjetting et al., 2012) at high spatiotemporal resolution in real time in different subcellular compartments. Specifically, a dynamic view of cytoplasmic reactive oxygen species (cytROS) oscillations during growth is provided by a genetically encoded HyPer sensor and by roGFP (for redox-sensitive GFP) coupled to OXIDANT RECEPTOR PEROXIDASE1 (Orp1), both of which are sensitive to hydrogen peroxide (H2O2; Mishina et al., 2013). HyPer consists of a circularly permuted yellow fluorescent protein (cpYFP) molecule coupled to a regulatory domain of the Escherichia coli H2O2 sensor OxyR. When exposed to H2O2, the excitation peak of cpYFP shifts from 420 to 500 nm, while the emission peak remains unchanged (at 516 nm; Belousov et al., 2006), thus allowing this probe to be used as a ratiometric biosensor (Costa et al., 2010; Hernández-Barrera et al., 2015). Importantly, HyPer and various other biosensors (e.g. the Ca2+ sensor R-GECO1) are sensitive to cellular pH, whereas others, such as roGFP2, are not. R-GECO1 has been engineered to contain dithiol/disulfide switches that act on a few Cys residues in the protein. As the oxidized and reduced forms of R-GECO1 have characteristic fluorescent spectra, this probe can be used ratiometrically to report on the thiol-disulfide status of the cell (Schwarzländer et al., 2008). When roGFP2 is fused directly to the human glutaredoxin Grx1 (Grx1-roGFP2), it detects changes in glutathione redox potential (Yu et al., 2013), while an Orp1-roGFP2 fusion can be used to monitor H2O2 (Gutscher et al., 2009). These probes may provide standardized response kinetics based on defined reaction mechanisms that are independent of the local intracellular environment of the sensor (Wagner et al., 2015).
It remains unclear how oscillatory gradients of cytCa2+, ROS, and cytoplasmic/apoplastic pH gradients are maintained during polar growth in pollen tubes and root hairs (Wudick and Feijó, 2014). Figure 1 shows possible links between ROS and other molecules in the cell surface-plasma membrane space of the growing tip. Below, we discuss recent exciting developments in our understanding of how ROS control polar growth in root hairs and pollen tubes. For discussions of the roles of ROS, Ca2+, and pH signaling in plant development and physiology, we refer the reader to other recent reviews (Swanson et al., 2011; Kurusu et al., 2013; Gilroy et al., 2014; Haruta et al., 2015; Kärkönen and Kuchitsu, 2015).
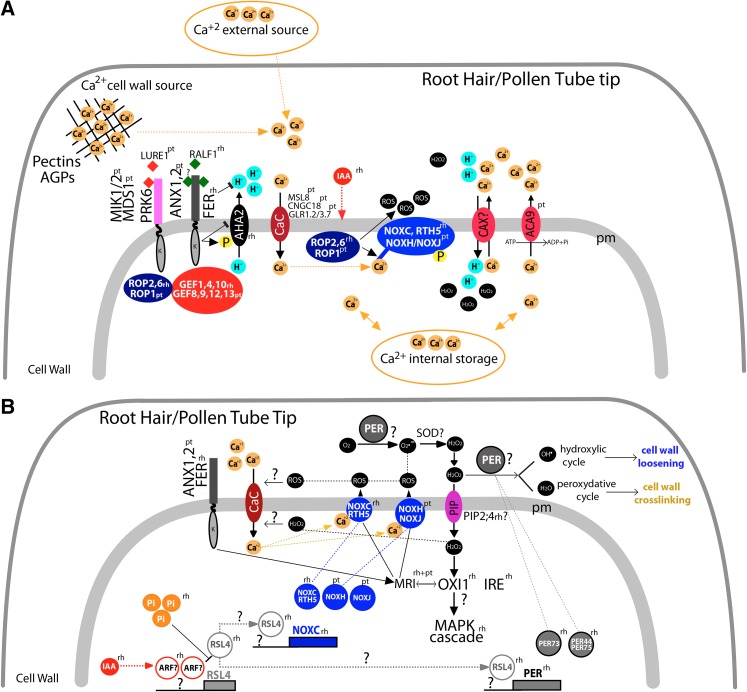
Figure 1.
Proposed scheme to describe how ROS are interconnected with Ca2+-pH signaling components in polar growing cells. A, Ca2+ ions are taken up by the cell from external sources or stored in the cell wall and released by changes in apopH controlled mainly by plasma membrane AHA. In addition, Ca2+ can be stored in subcellular compartments (e.g. vacuoles and ER-Golgi) and released into the cytosol. Still poorly characterized, plasma membrane CaCs transport free Ca2+ ions from the apoplast into the cytoplasm (Table I). To maintain physiologically low concentrations of cytCa2+, ACAs transport Ca2+ ions back to the apoplast. In addition, the H+/Ca2+ antiporter activity of CAX translocates Ca2+ back to the apoplast and, simultaneously, imports H+ into the cytoplasm. apoROS production is catalyzed by the NOX proteins, NOXC and RTH5 (in maize) in root hairs and NOXH/NOXJ in pollen tubes. These NOX proteins are regulated by complex partners, including Ca2+ ions, ROPs, and kinases. NOX produces apoplastic ion superoxide (O2−), which mostly is converted by superoxide dismutases (SODs) to H2O2. In root cells, the CrRLK1L kinase FER, which contains a malectin-like extracellular domain, binds to RALF1 and inhibits growth. In growing root hairs, FER forms a complex with the guanine nucleotide-exchange factor ROP-GEF1, and possibly with other ROP-GEFs (such as ROP-GEF4 or ROP-GEF10), to recruit and then activate the plant RHO GTPase ROP2. In pollen tubes, ANX1 and ANX2 are the CrRLK1L proteins that regulate ROS-linked cell growth. In addition, pollen tube guidance to the ovule relies on two groups of Leu-rich receptor-like kinases (MDS1-MIK1/2 and PRK6) binding to the Cys-rich peptide LURE1 (see text for details) and downstream ROPGEFs (ROPGEF8, ROPGEF9, ROPGEF12, and ROPGEF13) and ROP1 (at least demonstrated for PRK6). Then, ROP1 possibly targets the N-terminal domain of NOXH/NOXJ to trigger ROS production. In root hairs, spatially active ROP2 triggers ROS production, since it is able to bind directly to the N terminus of NOXC and enhance its enzymatic activity. Auxin (as indole acetic acid [IAA]) enhances ROP2 activity to generate ROS by triggering NOXC activation. B, In root hairs, high auxin levels (IAA), possibly attained through the activation of unknown ARF proteins, up-regulate the expression of the transcription factor basic helix-loop-helix RSL4 (in addition to activating ROP2) and promote ROS production. It is proposed here that RSL4 controls (directly or indirectly) the transcription of NOXC/RTH5 and NOXH/NOXJ as well as secreted type III peroxidases (PER) in root hairs. By contrast, high levels of Pi act as a repressor of RSL4 expression. High concentrations of apoROS enhance or reduce the stiffness of the cell wall, thereby either promoting or restricting cellular extension. PER uses H2O2 as an oxidant to convert cell wall phenolic compounds and structural proteins into free radicals that can subsequently come together to form covalent linkages (in the peroxidative cycle), thus restricting growth. Also, apoH2O2 and oxygen (O2) can be used to generate hydroxyl radicals (in the hydroxylic cycle), including •OH, which mediates the nonenzymatic cleavage of polysaccharides. This would induce transient relaxation of the cell wall, allowing tip growth. ROS also could promote Ca2+ transport by triggering the activation of CaCs (Table I) by unknown mechanisms, possibly in the apoplast, cytoplasm, or on both sides. Not all ROS remains in the apoplast, since some of the H2O2 may be transported back to the cytoplasm by aquaporins (plasma membrane intrinsic proteins [PIPs]) and trigger a signal cascade response (e.g. OXI1-mediated MAPK cascade). OXI1, an AGC2 kinase, could activate MARIS (MRI), a receptor-like cytoplasmic kinase present in growing root hairs and pollen tubes. MRI would be a downstream component of FER in root hairs and of ANX1/ANX2 in pollen tubes. Other root hair-specific AGC kinases in the VIII subfamily are IRE and AGC2-1. Solid arrows indicate a signaling pathway, a downstream step, or transport across the plasma membrane; solid lines indicate a close relationship between proteins or ions. P, Phosphorylated site; pm, plasma membrane; pt, pollen tubes; rh, root hairs.
apoROS HOMEOSTASIS IS REGULATED BY PLASMA MEMBRANE NOXS AND APOPLASTIC TYPE III SECRETED PEROXIDASES
Although ROS are produced in several intracellular compartments (e.g. chloroplasts, mitochondria, and peroxisomes; Foyer and Noctor, 2003; Overmyer et al., 2003), we will focus exclusively on the apoROS that are generated on the extracellular face of the plasma membrane. While the term ROS includes a variety of small molecules, we will concentrate our attention on the most discussed, including the hydroxyl radical (•OH), the superoxide radical (O2−•), H2O2, and singlet oxygen. Singlet oxygen is an excited state molecule with a short half-life (100 ns) that can travel only a short distance in cells (less than 100 nm; Niedre et al., 2002). Consequently, it reacts directly with molecules in close proximity to its site of production (Triantaphylidès and Havaux, 2009). O2−• is a highly unstable ion that is unable to diffuse through membranes. By contrast, H2O2 is relatively stable (half-life of approximately 1 ms) and moves through membranes via aquaporins. H2O2 reacts selectively with Cys residues in signaling proteins (e.g. peroxiredoxins, thioredoxins, and Cys-rich receptor-like kinases; Paulsen and Carroll, 2010), and its concentrations are tightly controlled by antioxidant systems. These features make H2O2 a good signaling candidate among the various ROS.
Although multiple enzymes (e.g. oxalate oxidases, amine oxidases, lipoxygenases, and quinone reductases) contribute to the production and accumulation of apoROS (Passardi et al., 2004; Cona et al., 2006), most apoROS are produced by NOX proteins, which also are known as RBOHs (for respiratory burst oxidase homologs). In addition to NOX, we also consider the apoplastic type III peroxidases (apoPERIII) in this review, since, as discussed below, these enzymes may influence the pool of apoROS. Other reactive oxidizing species, such as nitric oxide, nitric dioxide, dinitrogen tetroxide, and nitrous acid, also may play roles in tip growth. Nitric oxide is a well-studied signal that has an important effect on polar growth (Lombardo and Lamattina, 2012; Domingos et al., 2015). However, the focus of our discussion will be on the roles of ROS.
Plant NOXs are complex plasma membrane proteins with six transmembrane domains, an N-terminal regulatory domain that includes a ROP GTPase-binding region, two EF hands, and several potential phosphorylation sites (Takeda et al., 2008) as well as a C-terminal FAD/NADP(H)-binding domain. All NOXs produce apoplastic ion superoxide, which is mostly converted chemically or enzymatically by superoxide dismutases to oxygen and H2O2. H2O2 production and transport need to be fine-tuned with high precision. Arabidopsis (Arabidopsis thaliana) NOX proteins (AtNOXA–AtNOXJ) have been implicated in ROS signaling linked to several developmental and abiotic/biotic stress responses (Torres and Dangl, 2005; Müller et al., 2009; Dubiella et al., 2013; Lee et al., 2013; Xie et al., 2014). Two NOXs (AtNOXH and AtNOXJ) have been implicated in pollen tube tip expansion and fertilization (Boisson-Dernier et al., 2013; Kaya et al., 2014; Lassig et al., 2014) and one NOX (AtNOXC, named ROOT HAIR DEFECTIVE [RHD2]) in root hair tip growth (Fig 1; Foreman et al., 2003; Takeda et al., 2008). Recently, a monocot NOX named RTH5 (from maize [Zea mays]), with high sequence similarity to AtNOXH and AtNOXJ, was identified as an important player in the transition between root hair bulge formation and tip growth (Nestler et al., 2014).
NOX proteins contain several EF hand motifs in their N-terminal domains, and they are activated by Ca2+ and by Ca2+-mediated protein phosphorylation. Both modifications (Ca2+ binding and phosphorylation) have synergistic effects on NOX activity, although phosphorylation seems to be a prerequisite for Ca2+-mediated NOX activation (Wong et al., 2007; Kimura et al., 2012; Takahashi et al., 2012). Experiments carried out in HEK293T (human embryonic kidney) cells showed that induced mutations in the EF hand domains of NOXC and in NOXH/NOXJ proteins partially or completely suppressed ROS production (Kaya et al., 2014). Ser residues that are crucial for activity have been identified as phosphorylation sites in the N-terminal regions of several NOXs, including Ser-318/Ser-322 in NOXC (Kobayashi et al., 2007; Nühse et al., 2007; Takeda et al., 2008; Sirichandra et al., 2009; Dubiella et al., 2013; Gao et al., 2014). In NOXH and NOXJ sequences, Ser-318 also is conserved and Ser-322 is replaced with a potential Thr phosphosite. This suggests the existence of a putative common activation mechanism mediated by Ca2+ and the phosphorylation of both NOXC and NOXH/NOXJ in growing pollen tubes and root hairs.
Little is known about the transcriptional regulation of NOXC and NOXH/NOXJ during polar growth. In root hairs, RSL4, which is downstream of the morphogenetic RHD6/RSL1 program, is thought to be a master regulator of growth (Datta et al., 2015). RSL4 is activated by low concentrations of inorganic phosphate (Pi; an environmental signal) and high concentrations of endogenous auxin, by an unknown mechanism (Yi et al., 2010; Datta et al., 2015). RSL4 controls the expression of root hair-specific genes, including several that encode cell wall proteins (e.g. extensins [EXT] and Pro-rich proteins [PRPs]) and cell wall enzymes (e.g. apoPERIII, expansins, xyloglucan-endotransglucosylase/hydrolase [XTH], and pectate lyases). In addition, it is possible that RSL4 directly or indirectly activates the transcription of NOXC during root hair growth. It is tempting to speculate that auxin and low-Pi conditions both up-regulate RSL4 expression to trigger ROS-linked polar growth in root hairs (Fig. 1B). However, the molecular links between auxin-mediated regulation of root hair growth and RSL4 activation (Yi et al., 2010; Datta et al., 2015) and between RSL4 expression and ROS production remain to be elucidated. Furthermore, the overexpression of RSL proteins in Brachypodium spp., rice (Oryza sativa), and wheat (Triticum aestivum) promotes root hair growth, and MpRSL1 in the liverwort Marchantia polymorpha (Proust et al., 2016) and PpRSL1 and PpRSL2 in the moss Physcomitrella patens (Jang and Dolan, 2011) are regulated by auxin and trigger cell expansion. Consequently, the auxin-RSL module is thought to have been conserved during plant evolution as a master regulator of polar growth and may have been derived from the first land plants that lived almost 500 million years ago (Pires et al., 2013; Proust et al., 2016). It is unclear if the auxin-RSL module controlled polar cell expansion by triggering ROS production in the early plant lineages. In pollen tubes, no transcription factor has hitherto been described as a central regulator of growth linked to NOXH/NOXJ-mediated ROS production.
In addition to NOX proteins, apoPERIII has a direct impact on apoROS levels (Fig. 1B) and can be positively modulated by Ca2+ (Plieth and Vollbehr, 2012). Depending on the substrate, peroxidases can perform peroxidative, hydroxylic, and oxygen reduction (Passardi et al., 2004). In the first reaction, apoPERIII use H2O2 as an oxidant to convert cell wall phenolic compounds and structural proteins (e.g. extensins) to free radicals that can subsequently come together to form covalent linkages. In the hydroxylic cycle, apoplastic hydrogen peroxide (apoH2O2) and oxygen are converted by apoPERIII into radicals, including •OH, that cleave polysaccharides in a nonenzymatic fashion (Dunand et al., 2007). In addition, apoPERIII generates O2−• from oxygen reduction that can subsequently be dismutated to apoH2O2. Overall, apoPERIII either augments or reduces the pool of apoH2O2 (Francoz et al., 2015). An increase in apoH2O2 concentration leads to rigidification of the cell wall, triggering an immediate cessation of cell expansion (Monshausen et al., 2007). Thus, some of these apoPERIII proteins could act to cross-link, at least transiently, specific cell wall components.
It is also important to highlight that cell wall chemistry is quite different in root hairs and in pollen tube cells (for details, see Gu and Nielsen, 2013). In root hairs, the walls are composed mostly of (xylo)glucans, pectins, and O-glycoproteins (Galway et al., 2011; Velasquez et al., 2011; Peña et al., 2012), while in pollen tubes, the walls are enriched in pectins and also contain glycoproteins and xyloglucans/cellulose (Dardelle et al., 2010). Cell wall deficiencies in any of these polymers inhibit polar cell elongation in root hairs and pollen tubes, indicating that these polymers operate together to modulate controlled expansion (Bernhardt and Tierney, 2000; Favery et al., 2001; Pang et al., 2010; Ringli, 2010; Park et al., 2011; Velasquez et al., 2011; Zabotina et al., 2012; Wang et al., 2014). Specifically, Hyp-rich glycoproteins of the EXT type (Velasquez et al., 2011) and PRP (Bernhardt and Tierney, 2000) are Tyr cross-linked by an unidentified apoPERIII to facilitate the formation of a cell wall glycoprotein network (Cannon et al., 2008). This was suggested to occur in growing root hairs (Velasquez et al., 2015). By contrast, the apoROS produced by the activity of apoPERIII (in hydroxylic cycles) would enhance polysaccharide cleavage and act as a wall-loosening agent that promotes growth (Fry, 1998; Schopfer et al., 2002; Dunand et al., 2007; Macpherson et al., 2008) independently of Ca2+ signaling. Recently, two PER proteins (PER44 and PER75) were found to be repressed at the transcriptional level under low-gravity conditions. Indeed, the root hairs of the corresponding transfer DNA mutants (per44 and per75) exhibited tip rupturing and other defects under normal growth conditions (Kwon et al., 2015). Also, the PER inhibitor salicylhydroxamic acid drastically represses root hair growth. Both per mutants and salicylhydroxamic acid treatment cause root hairs to burst, possibly because the highly relaxed cell wall at the root hair tip cannot withstand the turgor pressure (Kwon et al., 2015). So far, no per mutants have been reported to have defective polar growth in pollen tubes, probably due to the high degree of genetic redundancy between the 71 encoded apoPERIII proteins in Arabidopsis (Francoz et al., 2015). Some apoPERIII proteins appear to harden the cell wall structure, while others impact cell wall loosening during plant development (Passardi et al., 2006; Jin et al., 2011; Pedreira et al., 2011; Herrero et al., 2013; Kunieda et al., 2013; Lee et al., 2013; Shigeto et al., 2013; Manzano et al., 2014). It is unclear how these two opposite effects on cell wall polymers that are mediated by apoPERIII are coordinated during the oscillatory growth cycles of these cells.
apoROS in the form of H2O2 (and other reactive species) could have a direct impact on the redox state of Cys-rich proteins, such as Cys-rich receptor-like kinases (CRKs; Chen, 2001). CRKs represent one of the largest groups of receptor-like kinases (RLKs), with 44 members in Arabidopsis (Wrzaczek et al., 2010). The extracellular domain of CRK comprises three well-conserved Cys residues (C-X8-C-X2-C) that form disulfide bridges and are potential targets for thiol-redox regulation. CRK may sense extracellular ROS and amplify the initial ROS signal. Recently, a large-scale broad phenotyping assessment of a complete crk transfer DNA insertion line collection showed that CRK controls several aspects of plant development (Bourdais et al., 2015), although direct evidence that links CRK to polar growth remains to be presented.
It is possible that polar growth processes are regulated by mechanisms involving the oscillatory changes in pH, which are controlled by the activation/deactivation of H+ pumps (AHA), H+/OH−-permeable channels, and calcium-exchanger (CAX) protein-type antiporters. Oscillating apoplastic H+ fluxes produce transient alkaline or more acidic environments on the growing tip apoplast that would activate or repress the activity of the peroxidative or oxidative cycle of apoPERIII. In addition, the activation/inactivation of many other cell wall-modifying enzymes, such as expansins (e.g. EXP7, EXP12, and EXP18 in root hairs and maize ZmEXPB1 and ZmEXPB11 in pollen tubes), pectin methyl esterases (e.g. PME1 and PME48 in pollen tubes), pectate lyases (e.g. ROOT HAIR-SPECIFIC14 in root hairs and PECTATE LYASE-LIKE in pollen tubes), and XTH (e.g. XTH12 and XTH26 in root hairs and XTH29 and XTH30 in pollen), is thought to trigger either localized wall rigidification or cell wall loosening. The regulation of polar growth by the pH microenvironment is consistent with the observed total cessation of growth in alkaline medium (pH 8) and root hair bursting, resulting from uncontrolled growth coupled to cell wall breakdown, on acidic medium (pH 4.5; Monshausen et al., 2007).
Specifically, PMEs, which demethylesterify carboxyl residues on the pectin backbone, affect not only cell wall viscosity but also Ca2+ dynamics. PME activity renders carboxyl residues present in pectins capable of binding to Ca2+ ions, which results in pectin cross-linking and likely cell wall stiffening at the pollen tube tip (Bosch and Helper, 2005; Palin and Geitmann, 2012). On the other hand, PME inhibitor proteins (e.g. PMEI-2) block this activity and retard deesterification reactions, thereby preventing excessive cross-linking of pectins in the pollen tube tip (Röckel et al., 2008). The coregulation of PME-PMEI interactions has been characterized in several biological processes (e.g. plant pathogen interactions and inflorescence meristem development; Wolf et al., 2009). Such interactions also are involved in regulating pollen tube growth, but evidence for similar interactions in root hair cells is lacking.
apoROS IS CHANNELED INTO THE CYTOPLASM TO TRIGGER DOWNSTREAM RESPONSES
Although the ROS associated with polar growth are produced in the apoplast by NOX and other enzymes, most of the ROS occurs in the cytoplasm, close to the growing tip, during polar growth (Monshausen et al., 2007). It is not known how the ROS signal is transmitted to the cytoplasm or how the different subcellular compartments that contribute to the cytROS pool are connected. It is postulated that apoH2O2 is actively transported into the cytoplasm by a subgroup of plasma membrane aquaporins of the PIP type (Dynowski et al., 2008; Hooijmaijers et al., 2012). Recently, it was shown that AQUAPORIN8 is able to translocate apoH2O2 into the hyphae of the plant pathogen Botrytis cinerea (An et al., 2016). In addition, the in vivo influx of apoH2O2 triggered by PIP1;4 in Arabidopsis was recently shown to occur in plant cells during pathogen infection and pathogen-associated molecular pattern recognition (Tian et al., 2016). The transport of H2O2 by several AtPIP proteins, including PIP2;4 and PIP2;7, which are highly expressed in root hairs, was demonstrated in uptake and/or toxicity H2O2 assays in heterologous systems (e.g. yeast cells). In addition, a pip2;4 mutant was reported to have longer root hairs than the wild type when grown under Pi starvation or under normal Pi conditions (Lin et al., 2011). Still, loss-of-function mutants for these PIPs remain to be characterized with regard to ROS levels at the root hair/pollen tube growing tip, and an in vivo function in ROS transport during the growth of these cells has yet to be demonstrated. ROS production also could originate from several enzymes located in intracellular compartments, such as chloroplasts, vacuoles, mitochondria, and the endoplasmic reticulum (ER).
It is unclear how transiently elevated cytROS concentrations are transduced in a downstream signaling response. OXIDATIVE STRESS INDUCIBLE1 (OXI1), which belongs to AGC kinase (for protein kinase A/protein kinase G/protein kinase C) family, was shown to be required for ROS-mediated events in root hair elongation. Thus, OXI1 (also named as AGC2-1; At3g25250) appears to connect oxidative burst signals with downstream responses (Anthony et al., 2004; Rentel et al., 2004). Recently, it was postulated that OXI1 activates MRI, a receptor-like cytoplasmic kinase of the VIII subfamily. MRI present in growing root hairs and pollen tubes is postulated to act downstream of the Catharanthus roseus RLKs FERONIA (FER) and ANXUR1 (ANX1)/ANX2 (Boisson-Dernier et al., 2015). Recently, it was shown that MRI interacts with OXI1, and in an vitro assay it was phosphorylated by OXI1 (Liao et al., 2016). In leaves, OXI1, which relays ROS signals, phosphorylates MITOGEN-ACTIVATED PROTEIN KINASE3 (MAPK3), MAPK4, and MAPK6, which activate genes involved in the ROS response (Moon et al., 2003). The OXI1 kinase activity on MAPKs is induced by H2O2, although the molecular mechanism underlying root hair growth is still unclear (Rentel et al., 2004). Several mutants of the MAPK signaling cascade exhibit defects in root hair growth (Nakagami et al., 2006; Qiu et al., 2008; Beck et al., 2010), suggesting that several components of this signaling cascade are positive regulators of root hair tip growth. By contrast, the MAPK6 mutant (mapk6wb/ir) developed extra-long root hairs, suggesting that MAPK6 is a negative regulator of root hair elongation (López-Bucio et al., 2014). It is uncertain whether these components of the MAPK cascade are linked to cytH2O2-OXI1 activation, as reported for wounding, cell wall damage, or pathogen attack. In addition, other signals, such as Ca2+ ions, hormones (e.g. auxin and ethylene), or nutrient status (e.g. low Pi), also could be linked to a MAPK-mediated response. INCOMPLETE ROOT HAIR ELONGATION (IRE; Oyama et al., 2002), which is an AGC kinase (like OXI1), also promotes root hair growth. It is unclear how all of these components are interconnected to orchestrate polar growth in root hairs.
DOES A ROS-INDEPENDENT, pH-DEPENDENT MECHANISM UNDERLIE POLAR CELL EXPANSION?
Analysis of growth and surface pH in real time established that growth accelerates after apoplastic acidification and slows upon alkalinization. An alkaline environment (approximately pH 8) stopped tip growth in root hairs, whereas an acidic medium (approximately pH 4.5) triggered uncontrolled expansion of root hairs and cell bursting (Monshausen et al., 2007). Surprisingly, root hairs of the noxC mutant (rhd2-1; Takeda et al., 2008) were rescued by increasing the extracellular pH from 5 to 6. This mutant still showed reduced cytROS levels but a normal tip-focused Ca2+ gradient (Monshausen et al., 2007). This finding suggests that physiological levels of ROS are not absolutely critical for root hair development or for gating the Ca2+ channels needed to generate the tip-focused gradient. It is unclear why a shift in pH to a more neutral environment is able to restore Ca2+ signatures and polar growth in a ROS-deficient mutant. The apopH is thought to be regulated by proton fluxes generated by AHA, which also would regulate the release of Ca2+ ions stored in the cell wall (Fig. 1A). AHA, as a member of the P-type superfamily of ATPases, transports H+ out of the cell and regulates the membrane potential and intracellular pH homeostasis (Haruta et al., 2015). Of the 11 functional AHA proteins present in Arabidopsis (Palmgren, 2001; Haruta et al., 2015), AHA1 and AHA2 are the most highly expressed isoforms. Accordingly, aha2 mutants showed reduced cell elongation in hypocotyls. A number of environmental factors that regulate plant growth target the autoinhibitory domain of AHA, and several RLKs that phosphorylate specific sites of AHA have been identified (Falhof et al., 2016). Some of them are able to activate or repress AHA2 (Fuglsang et al., 2014; Haruta et al., 2014). The C. roseus RECEPTOR-LIKE KINASE1-LIKE (CrRLK1L) kinase FER binds to RAPID ALKALINIZATION FACTOR1 (RALF1). Upon binding, FER-RALF1 inhibits root growth by phosphorylating Ser-899 in AHA2 by an unknown kinase (Haruta et al., 2014). FER forms a protein complex with the guanine nucleotide-exchange factor ROP-GEF1 (ROP-GEF4 or ROP-GEF10 may be additional components of the complex) to recruit and activate the plant RHO GTPase ROP2 (Duan et al., 2010). The active form of ROP2 (and possibly ROP6) enhances the enzymatic activity of NOXC (Foreman et al., 2003; Takeda et al., 2008; Oda et al., 2010). In a similar way, it is expected that the CrRLK1L proteins present in growing pollen tubes, ANX1 and ANX2, recruit ROP1 by an unknown ROPGEF, with any of the ROPGEFs that interact with the PRK6 receptor (i.e. ROPGEF8, ROPGEF9, ROPGEF12, or ROPGEF13) being the best candidates. Then, ROP1 would bind to NOXH/NOXJ and promote ROS production in growing pollen tubes (Fig. 1A). In addition, active ROP1 and ROP2 proteins that are targeted to the plasma membrane of tip-growing cells have a direct impact on actin dynamics and vesicle trafficking. ROP-interactive CRIB motif-containing proteins control pollen tube growth by regulating F-actin dynamics (Gu et al., 2003, 2005), and similar effector components might regulate actin homeostasis and vesicle trafficking in growing root hair tips. A more general CrRLK1L-NOX-GEF-ROP signaling module was proposed recently as a conserved system that controls cell expansion throughout the plant (Nissen et al., 2016). Another member of the CrRLK1L family, CAP1 (for cytCa2+-associated protein kinase), was found recently to regulate root hair growth by maintaining cytCa2+ gradients (Bai et al., 2014). Although CAP1 is localized in the root hair vacuolar membrane, it is responsible for maintaining an alkaline cytpH by modulating the cytoplasmic NH4+ levels. CAP1 may sense the cytoplasmic NH4+ status and regulate its homeostasis by modulating the cytCa2+ and cytpH gradients (Bai et al., 2014).
Ca2+ TRANSPORT AND HOMEOSTASIS AFFECT POLAR GROWTH
Like ROS, Ca2+ ions act as secondary messengers in various signaling transduction processes, including polar growth. Ca2+ ions can be transported from external sources into the cell or can be stored in the cell wall polymers, mostly in negatively charged pectins and arabinogalactan proteins (Tian et al., 2006; Lamport and Várnai, 2013). Changes to a more acidic pH environment that are controlled mainly by plasma membrane AHAs could produce a significant release of Ca2+ ions within the apoplast space. Also, Ca2+ can be made available in the cytosol from subcellular compartments (e.g. vacuoles, ER, and Golgi). To date, little is known about the plant plasma membrane CaCs that account for the focused cytCa2+ gradient in the tip of polar growing cells, although more information is available for pollen tubes than for root hair cells (Table I). This could be explained by redundancies of multiple CaC members or as a consequence of the lethal nature of the corresponding CaC mutants. In addition, it is possible that CaCs act as nonselective Ca2+-permeable channels.
Five types of Ca2+-transporting systems have been characterized to date in plant cells (Table I): osmosensitive gated Ca2+-permeable channels (CSC1 and OsCA1) and the related mechanosensitive channels; the cyclic nucleotide-gated channel (CNGC) family; glutamate like-receptor (GLR) genes; and voltage-gated channels (TWO PORE CHANNEL11 and annexins [ANNs]). Specific members of these families are expressed in root hairs or pollen tubes. Indeed, loss-of-function lines for some of these proteins display defects in pollen tube germination and reproduction and several other pleiotropic phenotypes (Table I; Konrad et al., 2011; Hou et al., 2014; Richards et al., 2014; Yuan et al., 2014). One of the best examples of the biological roles of CaCs was recently reported for CNGC18, GLR1.2/GLR3.7, and MSL8 channels. Based on careful characterization using mutant analysis, in vivo fertilization experiments, Ca2+ imaging, and electrophysiology, CNGC18 was clearly shown to be the main CaC involved in pollen tube guidance to the ovule (Gao et al., 2014, 2016). Previously, it was proposed that GLR1.2 and GLR3.7 are important components of the Ca2+ transport system in growing pollen tubes (Michard et al., 2011). These two pollen GLRs are activated specifically by d-Ser, and the enzyme SERINE RACEMASE1 (which converts l-Ser into d-Ser) also was localized close to the entry point of ovules (the micropyle). In the case of the MscS-like protein MSL8, a homolog of the bacterial mechanosensitive channel of small conductance (MscS) is required to maintain an optimal osmotic potential to ensure the cellular integrity required to drive germination during pollen germination and tube growth (Hamilton et al., 2015). This shows that several CaCs may act in the same cell type in response to different stimuli or in the same biological process, but at different steps.
Active efflux pumping of Ca2+ is a requirement to restore low concentrations of cytCa2+ (around 100–200 nm) after the gradient has triggered a signaling event. The removal of Ca2+ from the cytosol to the apoplast as well as to the endomembrane system requires active transport against its electrochemical gradient. Autoinhibitory P-type IIB Ca2+-ATPases (ACAs) as well as the Ca2+/H+ antiporters of CAX proteins are the best candidates to catalyze this movement. In addition, both ACA and CAX as well as ECA proteins (for P-type IIA Ca2+-ATPases) also all are present in the endomembrane system, so they can contribute to balancing the pool of cytCa2+ after a signal transduction event. Of the 10 ACA pumps present in Arabidopsis, only ACA9 was shown to be targeted to the pollen tube plasma membrane, where it had a tremendous impact on Ca2+ homeostasis and seed production (Schiøtt et al., 2004), while ACA13, which is expressed in the papilla cells of the stigma, exported Ca2+ to support compatible pollen grain germination (Iwano et al., 2014). No ACA has been reported thus far to act on root hair cells. In the case of the CAX antiporters, the counter ion (H+) movement provides the driving force for Ca2+ transport. While several members of CAX are encoded in the Arabidopsis genome, only CAX4 and CAX9 are highly expressed in pollen tubes (Pina et al., 2005; Wang et al., 2008), suggesting that their main role is in tube growth; however, their molecular characterization is pending. So far, no CAX members have been reported to exist in root hairs.
SUMMARY AND FUTURE QUESTIONS
Recent studies provide insight into ROS as signaling molecules that have a great impact on the regulation of polar cell expansion. Apoplastic/cytoplasmic ROS homeostasis is tightly linked to Ca2+ transport and pH regulation and also to changes in cell wall dynamics during polar growth. Nonetheless, several links are missing between the signaling function of ROS and the molecular mechanisms that trigger this developmental process in response to internal and external signals. For example, the mechanism by which changes in apopH bypass low levels of apoROS (e.g. in the noxC rhd2 mutant) to trigger and sustain root hair growth remains to be determined. An important goal of future studies is to identify unknown components that are controlled by pH and to establish how these relate to cytCa2+ gradients. Furthermore, the molecular identity of the CaC(s) involved in polar cell growth and the mechanism by which ROS or other signals activate these channels remain to be established. Several CaC candidates are listed in Table I. It is likely that more than one type of CaC is active in these polar growing cells. It has been proposed that the ROS-mediated activation of several CaCs is triggered by signals in the apoplast (Foreman et al., 2003; Takeda et al., 2008; Duan et al., 2014), although the underlying mechanism is unclear. The H2O2-responsive Ca2+-permeable K+ channel SHAKER-LIKE STELAR K+ OUTWARD RECTIFIER (Garcia-Mata et al., 2010) and the ROS-stimulated ANN1 (Laohavisit et al., 2012) may function in tip growth. Based on current evidence, it cannot be discounted that ROS also activates CaCs from the cytoplasmic side. Another important aspect of polar growth that remains unclear is how ROS signals are transduced to a downstream signaling cascade. There is evidence that MRI, OXI1 (and possibly other AGC kinases), and the MAPK cascade are involved in the downstream responses to ROS; however, it remains unknown how these components are interconnected. pH homeostasis also plays an important role in cellular expansion, but it is unclear how apopH regulates Ca2+ fluxes, how H2O2 and other ions are transported, and what signals and activators trigger H+ pumping specifically in growing root hairs and pollen tubes. It is possible that ANX1/ANX2 in pollen tubes and FER in root hairs activate/repress the activity of AHA2 (and other AHA proteins) in response to specific signals (e.g. low Pi, high levels of auxin, and RALF peptide binding). Root hairs and pollen tube cells are excellent model systems for dissecting the cellular effectors required for polar growth. These systems are highly amenable to both genetic studies and sophisticated imaging approaches. Deciphering these processes is not only of scientific interest but potentially has important agronomic implications in developing more efficient crops in nutrient-deficient soils and enhancing their seed production.
Glossary
- cytCa2+
cytoplasmic Ca2+ ions
- apoROS
apoplastic reactive oxygen species
- ROS
reactive oxygen species
- CaC
Ca2+ channel
- apopH
apoplastic pH
- cytROS
cytoplasmic reactive oxygen species
- H2O2
hydrogen peroxide
- •OH
hydroxyl radical
- O2-•
superoxide radical
- apoPERIII
apoplastic type III peroxidase
- Pi
inorganic phosphate
- apoH2O2
apoplastic hydrogen peroxide
- ER
endoplasmic reticulum
Footnotes
This work was supported by ANPCyT (grant nos. PICT2013–0003 and PICT2014–0504 to J.M.E.).
Articles can be viewed without a subscription.
References
- Altartouri B, Geitmann A (2015) Understanding plant cell morphogenesis requires real-time monitoring of cell wall polymers. Curr Opin Plant Biol 23: 76–82 [DOI] [PubMed] [Google Scholar]
- An B, Li B, Li H, Zhang Z, Qin G, Tian S (2016) Aquaporin8 regulates cellular development and reactive oxygen species production, a critical component of virulence in Botrytis cinerea. New Phytol 209: 1668–1680 [DOI] [PubMed] [Google Scholar]
- Anthony RG, Henriques R, Helfer A, Mészáros T, Rios G, Testerink C, Munnik T, Deák M, Koncz C, Bögre L (2004) A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J 23: 572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Ma X, Zhang G, Song S, Zhou Y, Gao L, Miao Y, Song CP (2014) A receptor-like kinase mediates ammonium homeostasis and is important for the polar growth of root hairs in Arabidopsis. Plant Cell 26: 1497–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett RD, Mommer L, De Vries FT (2014) Going underground: root traits as drivers of ecosystem processes. Trends Ecol Evol 29: 692–699 [DOI] [PubMed] [Google Scholar]
- Beck M, Komis G, Müller J, Menzel D, Šamaj J (2010) Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell 22: 755–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S (2006) Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Tierney ML (2000) Expression of AtPRP3, a proline-rich structural cell wall protein from Arabidopsis, is regulated by cell-type-specific developmental pathways involved in root hair formation. Plant Physiol 122: 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova TN, Jacob T, Dahse I, Gilroy S (1998) Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development 125: 2925–2934 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Franck CM, Lituiev DS, Grossniklaus U (2015) Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc Natl Acad Sci USA 112: 12211–12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S, Grossniklaus U (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hepler PK (2005) Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17: 3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdais G, Burdiak P, Gauthier A, Nitsch L, Salojärvi J, Rayapuram C, Idänheimo N, Hunter K, Kimura S, Merilo E, et al. (2015) Large-scale phenomics identifies primary and fine-tuning roles for CRKs in responses related to oxidative stress. PLoS Genet 11: e1005373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidwood L, Breuer C, Sugimoto K (2014) My body is a cage: mechanisms and modulation of plant cell growth. New Phytol 201: 388–402 [DOI] [PubMed] [Google Scholar]
- Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, Chen L, Lamport DT, Chen Y, Kieliszewski MJ (2008) Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA 105: 2226–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Yan A, Zhao LN, Wu WH, Yang Z (2007) A putative calcium‐permeable cyclic nucleotide‐gated channel, CNGC18, regulates polarized pollen tube growth. J Integr Plant Biol 49: 1261–1270 [Google Scholar]
- Chen Z. (2001) A superfamily of proteins with novel cysteine-rich repeats. Plant Physiol 126: 473–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher DA, Borsics T, Yuen CY, Ullmer W, Andème-Ondzighi C, Andres MA, Kang BH, Staehelin LA (2007) The cyclic nucleotide gated cation channel AtCNGC10 traffics from the ER via Golgi vesicles to the plasma membrane of Arabidopsis root and leaf cells. BMC Plant Biol 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona A, Rea G, Angelini R, Federico R, Tavladoraki P (2006) Functions of amine oxidases in plant development and defence. Trends Plant Sci 11: 80–88 [DOI] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Lo Schiavo F (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J 62: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadacz-Narloch B, Kimura S, Kurusu T, Farmer EE, Becker D, Kuchitsu K, Hedrich R (2013) On the cellular site of two-pore channel TPC1 action in the Poaceae. New Phytol 200: 663–674 [DOI] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, Mollet JC (2010) Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol 153: 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Prescott H, Dolan L (2015) Intensity of a pulse of RSL4 transcription factor synthesis determines Arabidopsis root hair cell size. Nat Plants 1: 15138. [DOI] [PubMed] [Google Scholar]
- Domingos P, Prado AM, Wong A, Gehring C, Feijo JA (2015) Nitric oxide: a multitasked signaling gas in plants. Mol Plant 8: 506–520 [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY (2014) Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat Commun 5: 3129. [DOI] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM (2010) FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174: 332–341 [DOI] [PubMed] [Google Scholar]
- Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414: 53–61 [DOI] [PubMed] [Google Scholar]
- Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol Plant 9: 323–337 [DOI] [PubMed] [Google Scholar]
- Fan X, Hou J, Chen X, Chaudhry F, Staiger CJ, Ren H (2004) Identification and characterization of a Ca2+-dependent actin filament-severing protein from lily pollen. Plant Physiol 136: 3979–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favery B, Ryan E, Foreman J, Linstead P, Boudonck K, Steer M, Shaw P, Dolan L (2001) KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev 15: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119: 355–364 [Google Scholar]
- Francoz E, Ranocha P, Nguyen-Kim H, Jamet E, Burlat V, Dunand C (2015) Roles of cell wall peroxidases in plant development. Phytochemistry 112: 15–21 [DOI] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. (1998) Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 332: 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Kristensen A, Cuin TA, Schulze WX, Persson J, Thuesen KH, Ytting CK, Oehlenschlæger CB, Mahmood K, Sondergaard TE, et al. (2014) Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J 80: 951–964 [DOI] [PubMed] [Google Scholar]
- Galway ME, Eng RC, Schiefelbein JW, Wasteneys GO (2011) Root hair-specific disruption of cellulose and xyloglucan in AtCSLD3 mutants, and factors affecting the post-rupture resumption of mutant root hair growth. Planta 233: 985–999 [DOI] [PubMed] [Google Scholar]
- Gao QF, Fei CF, Dong JY, Gu LL, Wang YF (2014) Arabidopsis CNGC18 is a Ca2+-permeable channel. Mol Plant 7: 739–743 [DOI] [PubMed] [Google Scholar]
- Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF (2016) Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 113: 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Wang J, Gajdanowicz P, Gonzalez W, Hills A, Donald N, Riedelsberger J, Amtmann A, Dreyer I, Blatt MR (2010) A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J Biol Chem 285: 29286–29294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19: 623–630 [DOI] [PubMed] [Google Scholar]
- Gjetting KS, Ytting CK, Schulz A, Fuglsang AT (2012) Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J Exp Bot 63: 3207–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Nielsen E (2013) Targeting and regulation of cell wall synthesis during tip growth in plants. J Integr Plant Biol 55: 835–846 [DOI] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z (2005) A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 169: 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Vernoud V, Fu Y, Yang Z (2003) ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot 54: 93–101 [DOI] [PubMed] [Google Scholar]
- Guo K, Kong WW, Yang ZM (2009) Carbon monoxide promotes root hair development in tomato. Plant Cell Environ 32: 1033–1045 [DOI] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z (2008) The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol Plant 134: 499–507 [DOI] [PubMed] [Google Scholar]
- Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP (2009) Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem 284: 31532–31540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES (2015) Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350: 438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Gray WM, Sussman MR (2015) Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr Opin Plant Biol 28: 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Saito M, Matsuoka H, Iida K, Iida H (2004) Functional analysis of a rice putative voltage-dependent Ca2+ channel, OsTPC1, expressed in yeast cells lacking its homologous gene CCH1. Plant Cell Physiol 45: 496–500 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Marten I (2011) TPC1-SV channels gain shape. Mol Plant 4: 428–441 [DOI] [PubMed] [Google Scholar]
- Hernández-Barrera A, Velarde-Buendía A, Zepeda I, Sanchez F, Quinto C, Sánchez-Lopez R, Cheung AY, Wu HM, Cardenas L (2015) Hyper, a hydrogen peroxide sensor, indicates the sensitivity of the Arabidopsis root elongation zone to aluminum treatment. Sensors (Basel) 15: 855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero J, Fernández-Pérez F, Yebra T, Novo-Uzal E, Pomar F, Pedreño MÁ, Cuello J, Guéra A, Esteban-Carrasco A, Zapata JM (2013) Bioinformatic and functional characterization of the basic peroxidase 72 from Arabidopsis thaliana involved in lignin biosynthesis. Planta 237: 1599–1612 [DOI] [PubMed] [Google Scholar]
- Hooijmaijers C, Rhee JY, Kwak KJ, Chung GC, Horie T, Katsuhara M, Kang H (2012) Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J Plant Res 125: 147–153 [DOI] [PubMed] [Google Scholar]
- Horikawa K, Yamada Y, Matsuda T, Kobayashi K, Hashimoto M, Matsu-ura T, Miyawaki A, Michikawa T, Mikoshiba K, Nagai T (2010) Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat Methods 7: 729–732 [DOI] [PubMed] [Google Scholar]
- Hou C, Tian W, Kleist T, He K, Garcia V, Bai F, Hao Y, Luan S, Li L (2014) DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res 24: 632–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Qu X, Zhang R (2015) Plant villins: versatile actin regulatory proteins. J Integr Plant Biol 57: 40–49 [DOI] [PubMed] [Google Scholar]
- Huh SM, Noh EK, Kim HG, Jeon BW, Bae K, Hu HC, Kwak JM, Park OK (2010) Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol 51: 1499–1514 [DOI] [PubMed] [Google Scholar]
- Iwano M, Igarashi M, Tarutani Y, Kaothien-Nakayama P, Nakayama H, Moriyama H, Yakabe R, Entani T, Shimosato-Asano H, Ueki M, et al. (2014) A pollen coat-inducible autoinhibited Ca2+-ATPase expressed in stigmatic papilla cells is required for compatible pollination in the Brassicaceae. Plant Cell 26: 636–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang G, Dolan L (2011) Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1and PpRSL2 in Physcomitrella patens. New Phytol 192: 319–327 [DOI] [PubMed] [Google Scholar]
- Jin J, Hewezi T, Baum TJ (2011) Arabidopsis peroxidase AtPRX53 influences cell elongation and susceptibility to Heterodera schachtii. Plant Signal Behav 6: 1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkönen A, Kuchitsu K (2015) Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112: 22–32 [DOI] [PubMed] [Google Scholar]
- Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, et al. (2014) Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26: 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath NF, Waadt R, Brugman R, Schroeder JI, Grossmann G, Schumacher K, Krebs M (2015) Live cell imaging with R-GECO1 sheds light on flg22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Mol Plant 8: 1188–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K (2012) Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 1823: 398–405 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J (2009) The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad KR, Wudick MM, Feijó JA (2011) Calcium regulation of tip growth: new genes for old mechanisms. Curr Opin Plant Biol 14: 721–730 [DOI] [PubMed] [Google Scholar]
- Kunieda T, Shimada T, Kondo M, Nishimura M, Nishitani K, Hara-Nishimura I (2013) Spatiotemporal secretion of PEROXIDASE36 is required for seed coat mucilage extrusion in Arabidopsis. Plant Cell 25: 1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H (2013) Plant mechanosensing and Ca2+ transport. Trends Plant Sci 18: 227–233 [DOI] [PubMed] [Google Scholar]
- Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, Hamada H, Yamanaka T, Iida K, Nakagawa Y, Saji H, et al. (2012) Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwasniewski M, Daszkowska-Golec A, Janiak A, Chwialkowska K, Nowakowska U, Sablok G, Szarejko I (2016) Transcriptome analysis reveals the role of the root hairs as environmental sensors to maintain plant functions under water-deficiency conditions. J Exp Bot 67: 1079–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T, Sparks JA, Nakashima J, Allen SN, Tang Y, Blancaflor EB (2015) Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am J Bot 102: 21–35 [DOI] [PubMed] [Google Scholar]
- Lamport DT, Várnai P (2013) Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol 197: 58–64 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T (2014) Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 78: 94–106 [DOI] [PubMed] [Google Scholar]
- Lee RDW, Cho HT (2013) Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front Plant Sci 4: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Rubio MC, Alassimone J, Geldner N (2013) A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412 [DOI] [PubMed] [Google Scholar]
- Li X, Borsics T, Harrington HM, Christopher DA (2005) Arabidopsis AtCNGC10 rescues potassium channel mutants of E. coli, yeast and Arabidopsis and is regulated by calcium/calmodulin and cyclic GMP in E. coli. Funct Plant Biol 32: 643–653 [DOI] [PubMed] [Google Scholar]
- Liao HZ, Zhu MM, Cui HH, Du XY, Tang Y, Chen LQ, Ye D, Zhang XQ (2016) The MARIS plays important roles in Arabidopsis pollen tube and root hair growth. J Integr Plant Biol http://dx.doi.org/10.1111/jipb.12484 [DOI] [PubMed] [Google Scholar]
- Lin CY, Huang LY, Chi WC, Huang TL, Kakimoto T, Tsai CR, Huang HJ (2015) Pathways involved in vanadate-induced root hair formation in Arabidopsis. Physiol Plant 153: 137–148 [DOI] [PubMed] [Google Scholar]
- Lin WD, Liao YY, Yang TJ, Pan CY, Buckhout TJ, Schmidt W (2011) Coexpression-based clustering of Arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiol 155: 1383–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MC, Lamattina L (2012) Nitric oxide is essential for vesicle formation and trafficking in Arabidopsis root hair growth. J Exp Bot 63: 4875–4885 [DOI] [PubMed] [Google Scholar]
- López-Bucio JS, Dubrovsky JG, Raya-González J, Ugartechea-Chirino Y, López-Bucio J, de Luna-Valdez LA, Ramos-Vega M, León P, Guevara-García AA (2014) Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. J Exp Bot 65: 169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson N, Takeda S, Shang Z, Dark A, Mortimer JC, Brownlee C, Dolan L, Davies JM (2008) NADPH oxidase involvement in cellular integrity. Planta 227: 1415–1418 [DOI] [PubMed] [Google Scholar]
- Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, Orman-Ligeza B, Van Isterdael G, Beeckman T, Draye X, Casero P, Del Pozo JC (2014) The emerging role of reactive oxygen species signalling during lateral root development. Plant Physiol 165: 1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Rejano EM, Camacho-Cristóbal JJ, Herrera-Rodríguez MB, Rexach J, Navarro-Gochicoa MT, González-Fontes A (2011) Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol Plant 142: 170–178 [DOI] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijó JA (2009) The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol 53: 1609–1622 [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Mishina NM, Markvicheva KN, Bilan DS, Matlashov ME, Shirmanova MV, Liebl D, Schultz C, Lukyanov S, Belousov VV (2013) Visualization of intracellular hydrogen peroxide with HyPer, a genetically encoded fluorescent probe. Methods Enzymol 526: 45–59 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104: 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S (2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011) Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J 65: 309–318 [DOI] [PubMed] [Google Scholar]
- Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak SS, Kim DH, Nam J, Bahk J, et al. (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA 100: 358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009) The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol 184: 885–897 [DOI] [PubMed] [Google Scholar]
- Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281: 38697–38704 [DOI] [PubMed] [Google Scholar]
- Nakano M, Iida K, Nyunoya H, Iida H (2011) Determination of structural regions important for Ca2+ uptake activity in Arabidopsis MCA1 and MCA2 expressed in yeast. Plant Cell Physiol 52: 1915–1930 [DOI] [PubMed] [Google Scholar]
- Nakano M, Samejima R, Iida H (2014) Mechanosensitive channel candidate MCA2 is involved in touch-induced root responses in Arabidopsis. Front Plant Sci 5: 421. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nestler J, Liu S, Wen TJ, Paschold A, Marcon C, Tang HM, Li D, Li L, Meeley RB, Sakai H, et al. (2014) Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J 79: 729–740 [DOI] [PubMed] [Google Scholar]
- Niedre M, Patterson MS, Wilson BC (2002) Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem Photobiol 75: 382–391 [DOI] [PubMed] [Google Scholar]
- Nissen KS, Willats WG, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci 15: 310–316 [DOI] [PubMed] [Google Scholar]
- Niu Y, Chai R, Liu L, Jin G, Liu M, Tang C, Zhang Y (2014) Magnesium availability regulates the development of root hairs in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ 37: 2795–2813 [DOI] [PubMed] [Google Scholar]
- Niu YF, Jin GL, Chai RS, Wang H, Zhang YS (2011) Responses of root hair development to elevated CO2. Plant Signal Behav 6: 1414–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AM, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Hashimoto H, Kuwabara N, Akashi S, Hayashi K, Kojima C, Wong HL, Kawasaki T, Shimamoto K, Sato M, et al. (2010) Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J Biol Chem 285: 1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GE. (2001) Dissecting symbiosis: developments in Nod factor signal transduction. Ann Bot (Lond) 87: 709–718 [Google Scholar]
- Oldroyd GE, Dixon R (2014) Biotechnological solutions to the nitrogen problem. Curr Opin Biotechnol 26: 19–24 [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Kangasjärvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K (2002) The IRE gene encodes a protein kinase homologue and modulates root hair growth in Arabidopsis. Plant J 30: 289–299 [DOI] [PubMed] [Google Scholar]
- Palin R, Geitmann A (2012) The role of pectin in plant morphogenesis. Biosystems 109: 397–402 [DOI] [PubMed] [Google Scholar]
- Palmgren MG. (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52: 817–845 [DOI] [PubMed] [Google Scholar]
- Pang CY, Wang H, Pang Y, Xu C, Jiao Y, Qin YM, Western TL, Yu SX, Zhu YX (2010) Comparative proteomics indicates that biosynthesis of pectic precursors is important for cotton fiber and Arabidopsis root hair elongation. Mol Cell Proteomics 9: 2019–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Szumlanski AL, Gu F, Guo F, Nielsen E (2011) A role for CSLD3 during cell-wall synthesis in apical plasma membranes of tip-growing root-hair cells. Nat Cell Biol 13: 973–980 [DOI] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9: 534–540 [DOI] [PubMed] [Google Scholar]
- Passardi F, Tognolli M, De Meyer M, Penel C, Dunand C (2006) Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta 223: 965–974 [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS (2010) Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol 5: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira J, Herrera MT, Zarra I, Revilla G (2011) The overexpression of AtPrx37, an apoplastic peroxidase, reduces growth in Arabidopsis. Physiol Plant 141: 177–187 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Kong Y, York WS, O’Neill MA (2012) A galacturonic acid-containing xyloglucan is involved in Arabidopsis root hair tip growth. Plant Cell 24: 4511–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK (1994) Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. Plant Cell 6: 1815–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina C, Pinto F, Feijó JA, Becker JD (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol 138: 744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires ND, Yi K, Breuninger H, Catarino B, Menand B, Dolan L (2013) Recruitment and remodeling of an ancient gene regulatory network during land plant evolution. Proc Natl Acad Sci USA 110: 9571–9576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Vollbehr S (2012) Calcium promotes activity and confers heat stability on plant peroxidases. Plant Signal Behav 7: 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust H, Honkanen S, Jones VA, Morieri G, Prescott H, Kelly S, Ishizaki K, Kohchi T, Dolan L (2016) RSL class I genes controlled the development of epidermal structures in the common ancestor of land plants. Curr Biol 26: 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, Mackinlay J, Loake GJ, Mundy J, Morris PC (2008) Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol 148: 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al. (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Richards SL, Laohavisit A, Mortimer JC, Shabala L, Swarbreck SM, Shabala S, Davies JM (2014) Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J 77: 136–145 [DOI] [PubMed] [Google Scholar]
- Ringli C. (2010) The hydroxyproline-rich glycoprotein domain of the Arabidopsis LRX1 requires Tyr for function but not for insolubilization in the cell wall. Plant J 63: 662–669 [DOI] [PubMed] [Google Scholar]
- Röckel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53: 133–143 [DOI] [PubMed] [Google Scholar]
- Rounds CM, Bezanilla M (2013) Growth mechanisms in tip-growing plant cells. Annu Rev Plant Biol 64: 243–265 [DOI] [PubMed] [Google Scholar]
- Roy SJ, Gilliham M, Berger B, Essah PA, Cheffings C, Miller AJ, Davenport RJ, Liu LH, Skynner MJ, Davies JM, et al. (2008) Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ 31: 861–871 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiøtt M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214: 821–828 [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ (2008) Confocal imaging of glutathione redox potential in living plant cells. J Microsc 231: 299–316 [DOI] [PubMed] [Google Scholar]
- Shigeto J, Kiyonaga Y, Fujita K, Kondo R, Tsutsumi Y (2013) Putative cationic cell-wall-bound peroxidase homologues in Arabidopsis, AtPrx2, AtPrx25, and AtPrx71, are involved in lignification. J Agric Food Chem 61: 3781–3788 [DOI] [PubMed] [Google Scholar]
- Shih HW, DePew CL, Miller ND, Monshausen GB (2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol 25: 3119–3125 [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Sondergaard TE, Schulz A, Palmgren MG (2004) Energization of transport processes in plants: roles of the plasma membrane H+-ATPase. Plant Physiol 136: 2475–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Martinoia E, Lee J, Kim D, Kim DY, Vogt E, Shim D, Choi KS, Hwang I, Lee Y (2004) A novel family of Cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol 135: 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SJ, Choi WG, Chanoca A, Gilroy S (2011) In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu Rev Plant Biol 62: 273–297 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kimura S, Kaya H, Iizuka A, Wong HL, Shimamoto K, Kuchitsu K (2012) Reactive oxygen species production and activation mechanism of the rice NADPH oxidase OsRbohB. J Biochem 152: 37–43 [DOI] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531: 245–248 [DOI] [PubMed] [Google Scholar]
- Tian GW, Chen MH, Zaltsman A, Citovsky V (2006) Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol 294: 83–91 [DOI] [PubMed] [Google Scholar]
- Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H (2016) Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol (in press)10.1104/pp.15.01237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14: 219–228 [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A, Frietsch S, Myers CT, Poulsen LR, Malhó R, Harper JF (2013) Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 8: e55277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart W, Chin K, Ung H, Moeder W, Yoshioka K (2011) The cyclic nucleotide-gated channels AtCNGC11 and 12 are involved in multiple Ca²⁺-dependent physiological responses and act in a synergistic manner. J Exp Bot 62: 3671–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslu VV, Grossmann G (2016) The biosensor toolbox for plant developmental biology. Curr Opin Plant Biol 29: 138–147 [DOI] [PubMed] [Google Scholar]
- Velasquez SM, Klein-Vein J, Estevez JM (2016) Auxin and cellular elongation. Plant Physiol 170: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez SM, Marzol E, Borassi C, Pol-Fachin L, Ricardi MM, Mangano S, Juarez SP, Salter JD, Dorosz JG, Marcus SE, et al. (2015) Low sugar is not always good: impact of specific O-glycan defects on tip growth in Arabidopsis. Plant Physiol 168: 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez SM, Ricardi MM, Dorosz JG, Fernandez PV, Nadra AD, Pol-Fachin L, Egelund J, Gille S, Harholt J, Ciancia M, et al. (2011) O-Glycosylated cell wall proteins are essential in root hair growth. Science 332: 1401–1403 [DOI] [PubMed] [Google Scholar]
- Wagner S, Nietzel T, Aller I, Costa A, Fricker MD, Meyer AJ, Schwarzländer M (2015) Analysis of plant mitochondrial function using fluorescent protein sensors. Methods Mol Biol 1305: 241–252 [DOI] [PubMed] [Google Scholar]
- Wang C, Li S, Ng S, Zhang B, Zhou Y, Whelan J, Wu P, Shou H (2014) Mutation in xyloglucan 6-xylosytransferase results in abnormal root hair development in Oryza sativa. J Exp Bot 65: 4149–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC (2016) A receptor heteromer mediates the male perception of female attractants in plants. Nature 531: 241–244 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH (2008) Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol 148: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Yu JN, Chen T, Zhang ZG, Hao YJ, Zhang JS, Chen SY (2005) Functional analysis of a putative Ca2+ channel gene TaTPC1 from wheat. J Exp Bot 56: 3051–3060 [DOI] [PubMed] [Google Scholar]
- Wolf S, Höfte H (2014) Growth control: a saga of cell walls, ROS, and peptide receptors. Plant Cell 26: 1848–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J (2009) Homogalacturonan methyl-esterification and plant development. Mol Plant 2: 851–860 [DOI] [PubMed] [Google Scholar]
- Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, et al. (2007) Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell 19: 4022–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzaczek M, Brosché M, Salojärvi J, Kangasjärvi S, Idänheimo N, Mersmann S, Robatzek S, Karpiński S, Karpińska B, Kangasjärvi J (2010) Transcriptional regulation of the CRK/DUF26 group of receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Shang Z, Wu J, Jiang X, Moschou PN, Sun W, Roubelakis-Angelakis KA, Zhang S (2010) Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J 63: 1042–1053 [DOI] [PubMed] [Google Scholar]
- Wudick MM, Feijó JA (2014) At the intersection: merging Ca2+ and ROS signaling pathways in pollen. Mol Plant 7: 1595–1597 [DOI] [PubMed] [Google Scholar]
- Xie HT, Wan ZY, Li S, Zhang Y (2014) Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 26: 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42: 264–267 [DOI] [PubMed] [Google Scholar]
- Yu X, Pasternak T, Eiblmeier M, Ditengou F, Kochersperger P, Sun J, Wang H, Rennenberg H, Teale W, Paponov I, et al. (2013) Plastid-localized glutathione reductase2-regulated glutathione redox status is essential for Arabidopsis root apical meristem maintenance. Plant Cell 25: 4451–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371 [DOI] [PubMed] [Google Scholar]
- Zabotina OA, Avci U, Cavalier D, Pattathil S, Chou YH, Eberhard S, Danhof L, Keegstra K, Hahn MG (2012) Mutations in multiple XXT genes of Arabidopsis reveal the complexity of xyloglucan biosynthesis. Plant Physiol 159: 1367–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]