Maize caffeoyl CoA O-methyltransferase and hydroxycinnamoyltransferase proteins, which are key enzymes in lignin biosynthesis, form a complex with NLR Rp1 protein to regulate the hypersensitive defense response.
Abstract
Disease resistance (R) genes encode nucleotide binding Leu-rich-repeat (NLR) proteins that confer resistance to specific pathogens. Upon pathogen recognition they trigger a defense response that usually includes a so-called hypersensitive response (HR), a rapid localized cell death at the site of pathogen infection. Intragenic recombination between two maize (Zea mays) NLRs, Rp1-D and Rp1-dp2, resulted in the formation of a hybrid NLR, Rp1-D21, which confers an autoactive HR in the absence of pathogen infection. From a previous quantitative trait loci and genome-wide association study, we identified genes encoding two key enzymes in lignin biosynthesis, hydroxycinnamoyltransferase (HCT) and caffeoyl CoA O-methyltransferase (CCoAOMT), adjacent to the nucleotide polymorphisms that were highly associated with variation in the severity of Rp1-D21-induced HR. We have previously shown that the two maize HCT homologs suppress the HR conferred by Rp1-D21 in a heterologous system, very likely through physical interaction. Here, we show, similarly, that CCoAOMT2 suppresses the HR induced by either the full-length or by the N-terminal coiled-coil domain of Rp1-D21 also likely via physical interaction and that the metabolic activity of CCoAOMT2 is unlikely to be necessary for its role in suppressing HR. We also demonstrate that CCoAOMT2, HCTs, and Rp1 proteins can form in the same complexes. A model is derived to explain the roles of CCoAOMT and HCT in Rp1-mediated defense resistance.
To protect against pathogen infection, plants have evolved multiple sophisticated defense mechanisms. One of the major mechanisms is mediated by resistance (R) genes, which directly or indirectly recognize corresponding pathogen-derived molecules called effectors (Bent and Mackey, 2007). The interaction between R genes and their cognate effectors usually triggers a number of defense responses, including activation of the hypersensitive response (HR), a rapid localized cell death at the pathogen infection site, generation of reactive oxygen species, induction of defense-related gene expression, production of secondary metabolites and antimicrobial compounds, and the lignification of cell walls (Morel and Dangl, 1997; Heath, 2000; Mur et al., 2008). The HR is thought to confine the pathogen, preventing it spreading from the infected site and often activates secondary metabolism and generates lignin or lignin-like phenolic compounds (Lange et al., 1995; Levine et al., 1996; Etalo et al., 2013).
In vascular plants, lignin is one of the most important products of the phenylpropanoid metabolic pathway. It contributes about 30% of the organic carbon in the biosphere, second after cellulose in abundance (Boerjan et al., 2003). Lignin provides mechanical strength to vascular tissues and protects plants from biotic stresses, including pathogen attack (Lewis and Yamamoto, 1990; Li et al., 2010). Lignin is mainly composed of three subunits: p-hydroxyphenyl lignin (H monolignol), guaiacyl lignin (G monolignol), and syringyl lignin (S monolignol). The biosynthesis of the monolignols starts with Phe, which is converted to cinnamic acid catalyzed by Phe ammonia-lyase (Supplemental Fig. S1). Cinnamic acid can be further converted to p-coumaroyl CoA, the precursor of lignin (Boerjan et al., 2003). p-Coumaroyl CoA is either converted to produce H monolignol catalyzed by cinnamoyl-CoA reductase and cinnamyl-alcohol dehydrogenase or G and S monolignols catalyzed by different enzymes including hydroxycinnamoyltransferase (HCT) and caffeoyl CoA O-methyltransferase (CCoAOMT). HCT catalyzes the reactions by converting coumaroyl CoA to coumaroyl shikimate/quinate and also caffeoyl shikimate/quinate to caffeoyl CoA (Hoffmann et al., 2003). CCoAOMT further catalyzes the reaction by methylating caffeoyl CoA to feruloyl CoA. Together with caffeic/5-hydroxyferulic acid O-methyltransferase, it is responsible for the methylation of the monolignol precursors (Ye et al., 1994; Zhong et al., 1998). Lignin is a tridimensional phenolic heteropolymer mainly derived from the oxidative polymerization of H, S, and G monolignols with H2O2 (Boerjan et al., 2003). Many important molecules associated with plant defense are produced by phenylpropanoid pathway. These include lignin itself as well as many phytoalexins, phytoanticipins, and the hormone salicylic acid (SA; Dixon et al., 2002). Not surprisingly, many genes encoding enzymes in the phenylpropanoid pathway have been implicated in the plant defense response, including Phe ammonia-lyase, cinnamoyl-CoA reductase, cinnamyl-alcohol dehydrogenase, caffeic/5-hydroxyferulic acid O-methyltransferase, HCT, and CCoAOMT (Kawasaki et al., 2006; Bhuiyan et al., 2009; Gallego-Giraldo et al., 2011b; Zhao and Dixon, 2014).
Most R genes are of the so-called NB-LRR (or NLR) class of proteins, which carry an NB-ARC (nucleotide binding, APAF-1, certain R proteins, and CED-4) domain and a Leu-rich-repeat (LRR) domain (Ellis et al., 2000; Dangl and Jones, 2001). Based on their N-terminal secondary structure, plant NLRs can be further divided into two major classes: One class, which is found in both monocots and dicots, carries a putative coiled-coil (CC) domain (CC-NB-LRR; CNL hereafter), and the other is dicot specific, which carries a Toll-IL 1 receptor (TIR) domain. The maize (Zea mays) R gene Rp1-D confers resistance to specific races of Puccinia sorghi, the causal agent of common rust, and encodes a typical CNL (Hu et al., 1996). The locus containing Rp1-D, Rp1, is complex and many haplotypes have been characterized (Smith et al., 2004). In the Rp1-D haplotype, nine NLR paralogs were identified, Rp1-dp1 to Rp1-dp8, with unknown function, and also Rp1-D itself (Sun et al., 2001). The high nucleotide sequence similarity among different Rp1 paralogs leads to occasional misalignments between homologous chromosomes during meiosis and consequent unequal crossing-over and intragenic recombination. Rp1-D21 is a chimeric gene derived from intragenic recombination between Rp1-D and Rp1-dp2 (Sun et al., 2001; Smith et al., 2010). Rp1-D21 causes an autoactive HR lesion phenotype in the absence of pathogen infection. Several other hallmarks of the pathogen-induced defense response are also associated with Rp1-D21-mediated HR phenotype in maize, including high accumulation of H2O2 and increased expression of defense-related genes (Chintamanani et al., 2010). The Rp1-D21 HR lesion phenotype is influenced by light, temperature, and genetic background (Chintamanani et al., 2010; Negeri et al., 2013).
We used Rp1-D21 as a tool to investigate the genetic basis of the control of HR by crossing a line carrying it into several mapping populations to identify loci, pathways, and genes associated with modulation of the HR strength (Chintamanani et al., 2010; Chaikam et al., 2011; Olukolu et al., 2013, 2014). One of the populations used, called the nested association mapping (NAM) population, is an extremely large and powerful mapping population that allows the mapping of quantitative trait loci (QTL) with high precision (McMullen et al., 2009). Using the NAM population (Olukolu et al., 2014), we identified single nucleotide polymorphism (SNP) markers highly associated with variation in the HR phenotype at 44 loci. Because the markers were mapped with extremely high resolution, we were able to tentatively identify candidate genes at the associated loci, which might underlie variation in the HR phenotype. Two genes encoding homologs of enzymes in the lignin biosynthesis pathway, HCT and CCoAOMT, were identified among these candidate genes. We furthermore showed that expression of both of these genes was highly induced by the defense response mediated by Rp1-D21 and that the products of two HCT candidate genes suppressed Rp1-D21-induced HR, very likely through physical interaction (Wang et al., 2015c).
In this study, we validate the role of CCoAOMT, the enzyme catalyzing the step immediately downstream of the HCT-catalyzed step in the lignin biosynthesis pathway, in modulating Rp1-D21-induced HR. We show that coexpression of CCoAOMT with Rp1-D21 in Nicotiana benthamiana suppresses Rp1-D21-induced HR and that the metabolic activity of CCoAOMT is unlikely to be necessary for its role in suppressing HR. Furthermore, we show that CCoAOMT physically associates with HCT and Rp1 proteins in a coimmunoprecipitation (Co-IP) assay, suggesting that CCoAOMT, HCT, and Rp1 proteins might form complexes in vivo. We discuss the roles of CCoAOMT and HCT in modulating the defense response and derive models for NLR protein function.
RESULTS
CCoAOMT2 Suppress Rp1-D21-Induced HR in N. benthamiana
From previous genome-wide associate study (GWAS) analysis in maize, we identified 44 SNPs associated with variation in Rp1-D21-induced HR (Olukolu et al., 2014) using the NAM population (McMullen et al., 2009). Furthermore, we demonstrated that two maize proteins homologous to HCT, termed HCT1806 and HCT4918, which were encoded by genes on chromosome 1, adjacent to the most highly associated SNP identified in the study, suppress Rp1-D21-induced HR via physical interaction (Wang et al., 2015c). An associated SNP on chromosome 9 is within a gene encoding a CCoAOMT homolog (GRMZM2G099363), the enzyme that catalyzes the step downstream of the HCT-catalyzed step in the lignin biosynthesis pathway (Supplemental Fig. S1).
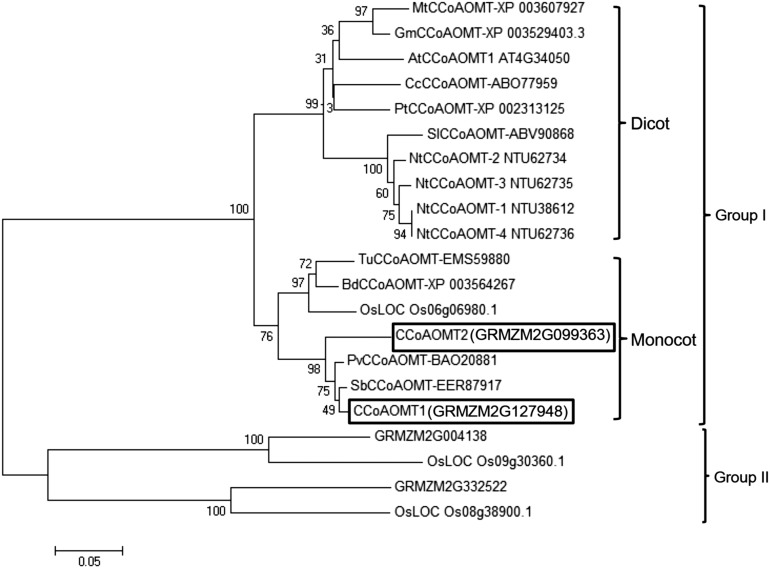
Using Arabidopsis (Arabidopsis thaliana) AtCCoAOMT1 as a query to search the maize genome database, we found that there are two closely CCoAOMT homologs in maize, GRMZM2G127948 and GRMZM2G099363, named CCoAOMT1 and CCoAOMT2 (Vélez-Bermúdez et al., 2015), respectively (Fig. 1), which are 93.1% similar at the amino acid level (Supplemental Fig. S2). CCoAOMT2 is the candidate gene on chromosome 9 associated with Rp1-D21-induced HR. Phylogenetic analysis showed that these two CCoAOMT homologs are closely related to other monocot CCoAOMTs, which together form a monocot-specific branch of the CCoAOMTs phylogenetic tree (Fig. 1).
Figure 1.
Phylogenetic analysis of CCoAOMTs from diverse plant species. The two maize CCoAOMTs (CCoAOMT1 and CCoAOMT2) are indicated in black boxes. At, Arabidopsis; Bd, Brachypodium distachyon; Cc, Coffea canephora; Gm, Glycine max; Mt, Medicago truncatula; Nt, Nicotiana tabacum; Os, Oryza sativa; Pt, Populus trichocarpa; Pv, Panicum virgatum; Sb, Sorghum bicolor; Sl, Solanum lycopersicum; Tu, Triticum urartu. All gene names starting with GRMZM are derived from maize.
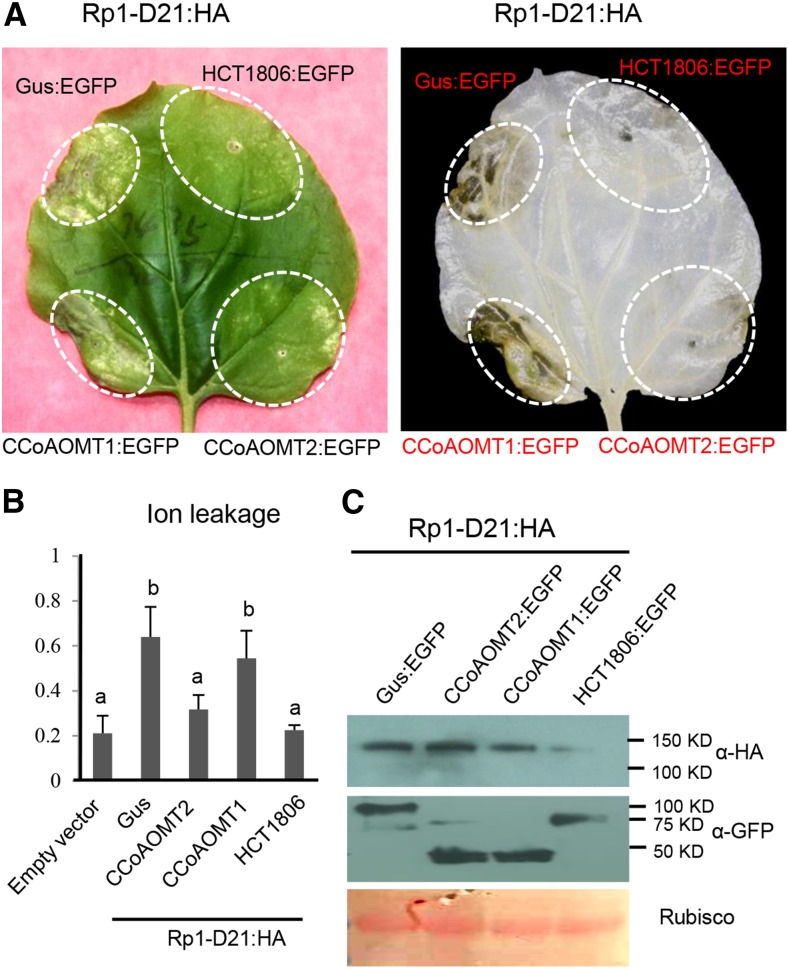
To explore whether CCoAOMT might be associated with variation in the severity of Rp1-D21-induced HR, we employed Agrobacterium tumefaciens-mediated transient expression in N. benthamiana. This system has been widely used for the functional analysis of HR mediated by NLRs from both monocots and dicots (Leister et al., 2005; van Ooijen et al., 2008; Slootweg et al., 2010; Tameling et al., 2010; Bai et al., 2012; Qi et al., 2012). We have recently shown that this is an appropriate system for the functional analysis of HR modulated by Rp1-D21 and its regulators (Wang and Balint-Kurti, 2015; Wang et al., 2015c, 2015d). When Rp1-D21:HA (Rp1-D21 fused with a 3× hemagglutinin tag at the C terminus) was transiently coexpressed with GUS:EGFP (a GUS gene fused with enhanced GFP at the C terminus), an obvious HR phenotype was observed at 3 d post infiltration, similar to our previous observation (Wang and Balint-Kurti, 2015; Wang et al., 2015c, 2015d). Also as previously observed, no HR was observed when HCT1806:EGFP and Rp1-D21:HA were transiently coexpressed (Fig. 2A; Wang et al., 2015c). Transient coexpression of CCoAOMT2:EGFP but not CCoAOMT1:EGFP with Rp1-D21:HA suppressed Rp1-D21-induced HR (Fig. 2A). Consistent with our visual observations, coexpression of CCoAOMT2:EGFP or HCT1806:EGFP with Rp1-D21:HA significantly reduced ion leakage levels compared to coexpression of the GUS:EGFP control or CCoAOMT1:EGFP with Rp1-D21:HA (Fig. 2B). Western-blot results showed that coexpression of CCoAOMT2 did not change the expression level of Rp1-D21, compared to coexpression of GUS:EGFP or CCoAOMT1:EGFP. All the EGFP-tagged proteins were expressed at substantial and broadly comparable levels (Fig. 2C).
Figure 2.
Investigating the function of maize CCoAOMT homologs in Rp1-D21-induced HR. A, The CCoAOMT homologs were transiently coexpressed with Rp1-D21 into N. benthamiana. The representative leaf was photographed at 3 d after inoculation (left), and the same leaf was cleared by ethanol (right). B, Ion leakage conductivity (average ± se, n > 5) was measured at 60 h after coexpression of GUS, HCT1806, or CCoAOMT homologs with Rp1-D21. Significant differences (P < 0.05; t test) between samples are indicated by different letters (a and b). C, Total protein was extracted from agroinfiltrated leaves at 30 hpi. Anti-HA was used to detect the expression of Rp1-D21, and anti-GFP was used to detect the expression of GUS, HCT1806, or CCoAOMT homologs. Equal loading of protein samples was shown by Ponceau S staining.
CCoAOMT2 Suppresses Rp1-D21-Induced HR More Than That Induced by Other Autoactive NLRs
To determine whether CCoAOMT2 could also suppress HR induced by other NLR proteins, we coexpressed it with Arabidopsis RPM1(D505V) and barley (Hordeum vulgare) MLA10(D502V), two autoactive CNL proteins that confer an HR phenotype when transiently expressed in N. benthamiana (Gao et al., 2011; Bai et al., 2012). Similar to our findings with HCT1806, we found that CCoAOMT2 and CCoAOMT1 did not have obvious roles in suppressing either RPM1(D505V)- or MLA10(D502V)-induced HR (Supplemental Fig. S3).
Mutation in the Conserved Amino Acid Required for CCoAOMT Activity Does Not Affect Its Suppression of Rp1-D21-Induced HR
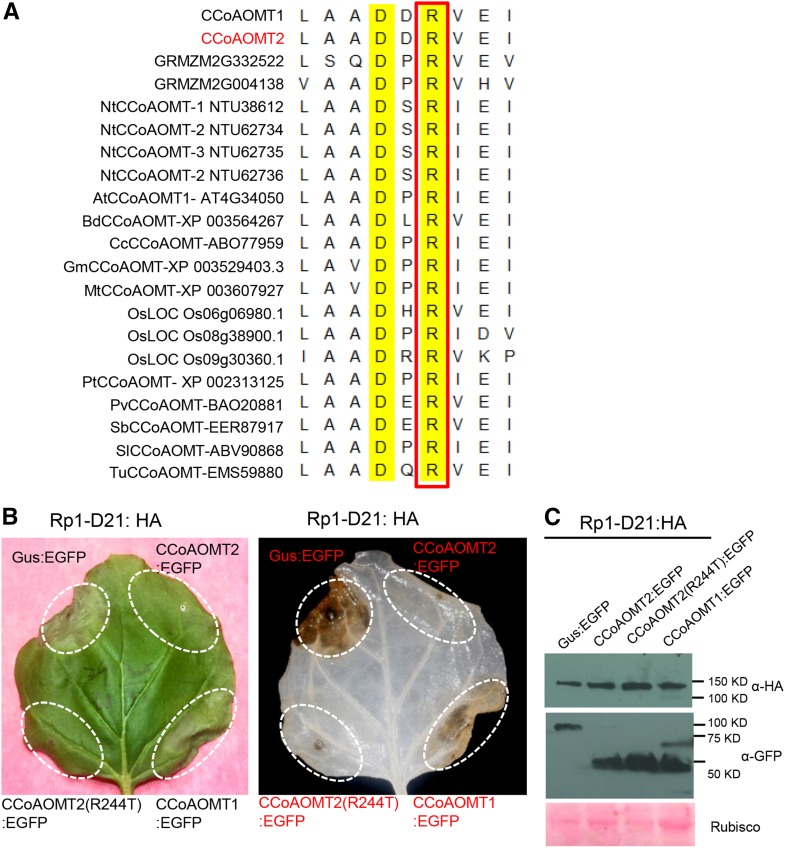
In tobacco CCoAOMT, the substitution of Arg with Thr at position 220 (R220T) results in the total loss of its enzymatic activity (Hoffmann et al., 2001). We found that R220 is conserved among different plant CCoAOMTs (Fig. 3A). To investigate whether the enzymatic activity of maize CCoAOMT2 is important for suppressing Rp1-D21-induced HR, we generated the corresponding mutation in the maize CCoAOMT2: CCoAOMT2(R244T). When coexpressed with Rp1-D21, it still suppressed Rp1-D21-induced HR (Fig. 3B). Based on these data, we speculate that the enzymatic activity of CCoAOMT2 is not required for its function in regulating Rp1-D21-induced HR.
Figure 3.
Mutation in the conserved residues required for CCoAOMT activity did not affect the suppressive role of CCoAOMT2 on Rp1-D21-induced HR. A, Multiple sequence alignment of plant CCoAOMTs. The conserved Arg (R) residue is boxed. B, The HR phenotypes resulting from transient coexpression of CCoAOMT2 and its mutant derivative with Rp1-D21. C, Total protein was extracted from agroinfiltrated leaves at 30 hpi. Anti-HA was used to detect the expression of Rp1-D21, and anti-GFP was used to detect the expression of CCoAOMT homologs or GUS. Equal loading of protein samples was shown by Ponceau S staining.
CCoAOMT2 Physically Interacts with Rp1
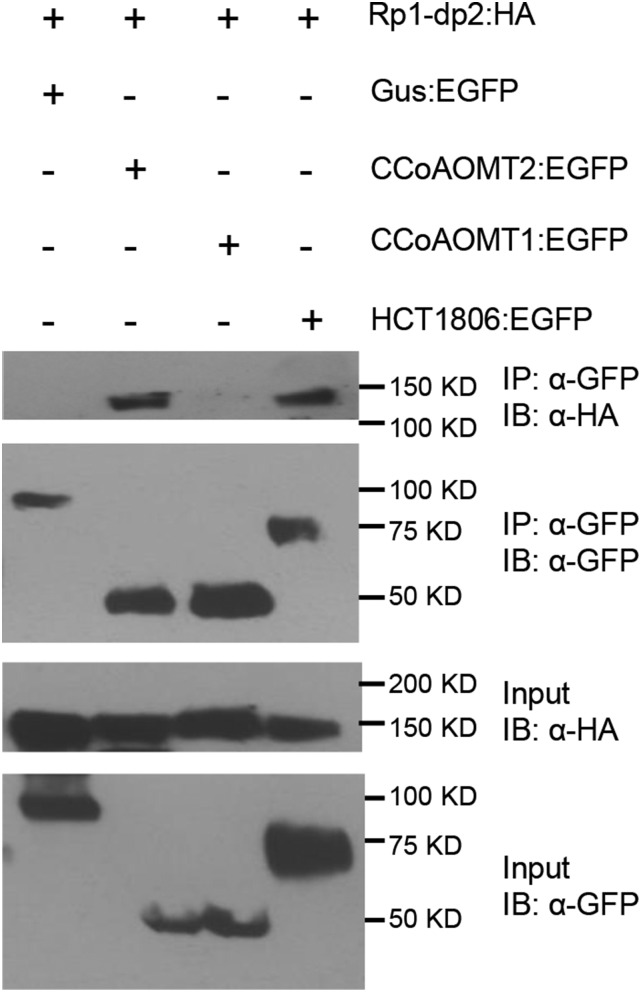
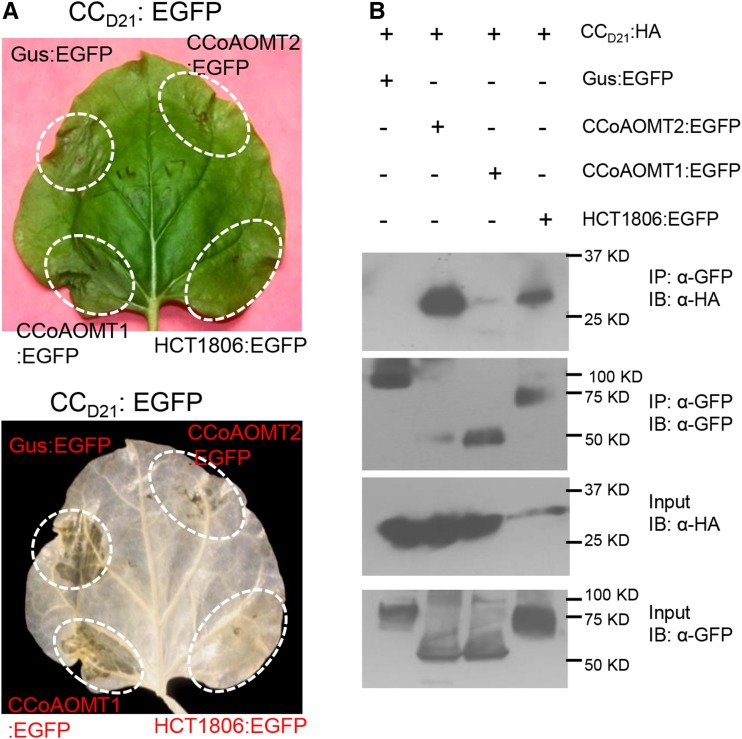
Since CCoAOMT2 suppressed Rp1-D21-induced HR, we wanted to test whether there was a physical association between CCoAOMT2 and Rp1 proteins. We have found that, when fused with HA tag, Rp1-dp2 has higher expression level than Rp1-D21 and Rp1-D (Wang et al., 2015d). Thus, we chose Rp1-dp2:HA to test the interaction between CCoAOMT and Rp1 proteins. We performed a Co-IP assay by coexpression of Rp1-dp2:HA and CCoAOMT:EGFP and found that CCoAOMT2 coimmunoprecipitated with Rp1-dp2 (Fig. 4). However, we did not observe coimmunoprecipitation between CCoAOMT1 and Rp1-dp2 (Fig. 4). Consistent with our previous results, HCT1806 also interacted with Rp1-dp2 (Fig. 4; Wang et al., 2015c).
Figure 4.
Investigating the interactions between Rp1-dp2 and CCoAOMT by Co-IP assay. EGFP- and 3×HA-tagged constructs were transiently coexpressed in N. benthamiana and samples were collected at 30 hpi for Co-IP assay. Protein extracts were immunoprecipitated (IP) by anti-GFP (α-GFP) microbeads and detected (immunblotted [IB]) by anti-GFP and anti-HA (α-HA) antibodies.
CCoAOMT2 Suppress CCD21-Induced HR through Physical Association
Rp1-D21 contains three major domains: an N-terminal CC domain that we termed CCD21, a middle NB-ARC domain, and a C-terminal LRR domain. We have shown that CCD21, but not the NB-ARC or LRR domain, conferred autoactive HR when fused with EGFP (Wang et al., 2015d). We have also shown that HCT1806/4918 interact with CCD21 to suppress CCD21:EGFP-induced HR (Wang et al., 2015c). To determine whether CCoAOMT can inhibit CCD21-induced HR, we coexpressed CCD21:EGFP and CCoAOMT in N. benthamiana and found that CCoAOMT2, but not CCoAOMT1, suppressed CCD21-induced HR, which was similar to the coexpression of CCD21:EGFP and HCT1806 (Fig. 5A). Consistent with the phenotypic data, we found that CCoAOMT2 and HCT1806, physically associated with CCD21 as detected by Co-IP assays (Fig. 5). However, CCoAOMT1 had no or very weak interaction with CCD21 (Fig. 5).
Figure 5.
Investigating the function of CCoAOMT in CCD21-induced HR. A, CCoAOMT2 but not CCoAOMT1 suppressed CCD21-induced HR in N. benthamiana. CCD21:EGFP was transiently coexpressed with EGFP-tagged CCoAOMT1, CCoAOMT2, HCT1806, or GUS, and a representative HR picture was taken at 2 d after inoculation. B, Investigating the interactions between CCD21 and CCoAOMT and HCT1806 by Co-IP assay. EGFP- and 3×HA-tagged constructs were transiently coexpressed in N. benthamiana, and samples were collected at 30 hpi for Co-IP assay. Protein extracts were immunoprecipitated (IP) by anti-GFP (α-GFP) microbeads and detected (immunblotted [IB]) by anti-GFP and anti-HA (α-HA) antibodies.
CCoAOMT2 Physically Associates with HCT1806/4918
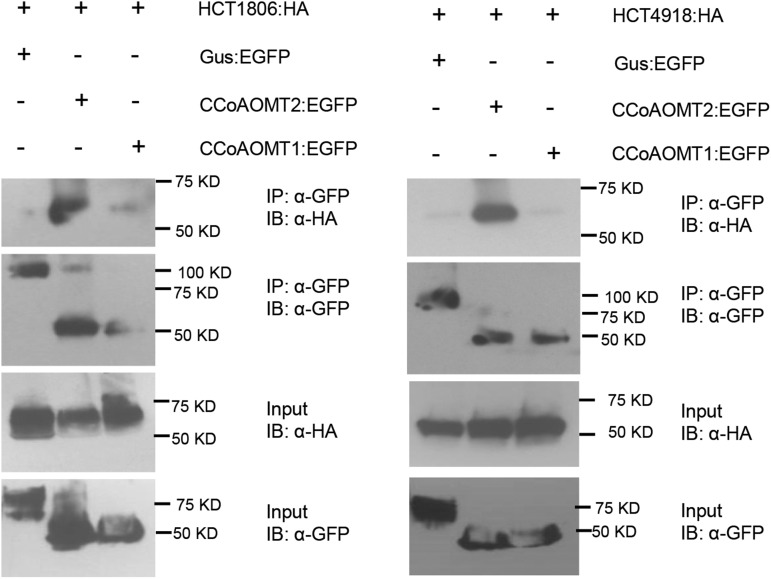
Since CCoAOMT2 and HCT1806/4918 suppressed CCD21-induced HR via physical interaction (Fig. 5; Wang et al., 2015c), we tested whether CCoAOMT could physically interact with both HCTs. We performed Co-IP experiments by coexpressing EGFP-tagged CCoAOMT and HA-tagged HCT and found CCoAOMT2 interacted strongly with both HCT1806 and HCT4918 (Fig. 6). However, CCoAOMT1 had no or very weak interaction with both HCTs (Fig. 6).
Figure 6.
Investigating the interactions between HCT1806/4918 and CCoAOMTs by Co-IP assay. EGFP- and 3×HA-tagged constructs were transiently coexpressed in N. benthamiana, and samples were collected at 30 hpi for Co-IP assay. Protein extracts were immunoprecipitated (IP) by anti-GFP (α-GFP) microbeads and detected (immunblotted [IB]) by anti-GFP and anti-HA (α-HA) antibodies.
CCoAOMT2, HCT1806/4918, and Rp1 Proteins Form Complexes
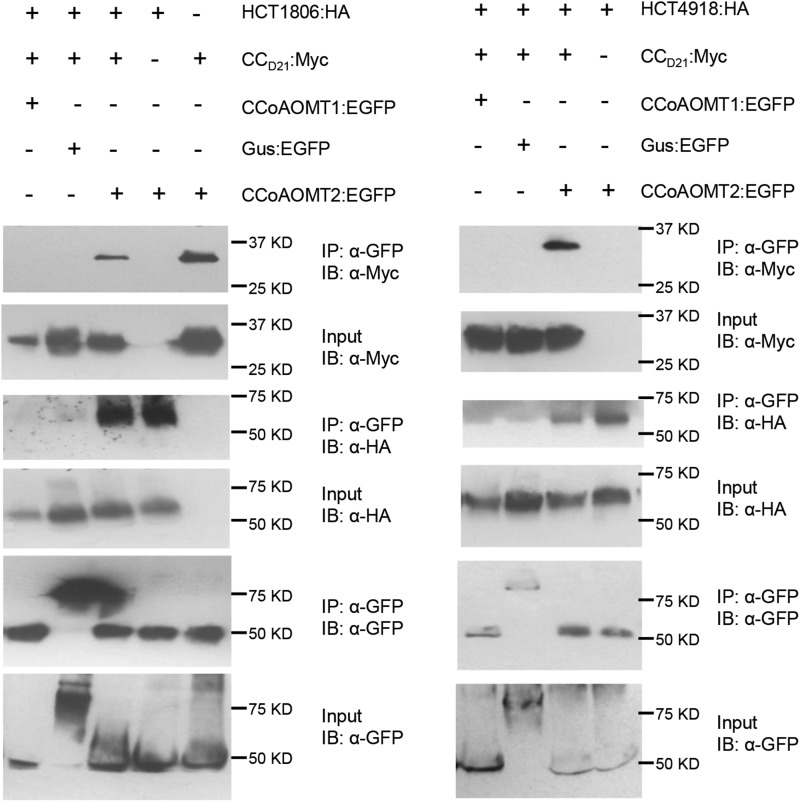
To test whether CCoAOMT2, HCT1806/4918, and CCD21 could form complexes incorporating all three proteins, we performed Co-IP experiments by coexpression of EGFP-tagged CCoAOMT, HA-tagged HCT, and Myc-tagged CCD21 in N. benthamiana. We found that CCoAOMT2, but not CCoAOMT1 and GUS control, interacted strongly with both HCT1806/4918 and CCD21 in the same complex(es) (Fig. 7).
Figure 7.
Investigating the interactions between CCD21, HCT and CCoAOMT by Co-IP assay. EGFP-, Myc-, and 3×HA-tagged constructs were transiently coexpressed in N. benthamiana, and samples were collected at 30 hpi for Co-IP assay. Protein extracts were immunoprecipitated (IP) by anti-GFP (α-GFP) microbeads and detected (immunblotted [IB]) by α-GFP, anti-Myc (α-Myc), and anti-HA (α-HA) antibodies.
Alternate Alleles of CCoAOMT2
In our previous study using the NAM population (Olukolu et al., 2014), we identified a QTL allelic series at the CCoAOMT2 locus with different alleles from the 26 NAM parents conferring different strengths of effect on HR. Among these, the QTL alleles from the lines NC350 and M37W had among the strongest suppressive effects compared to the B73 allele, while the effect of the B97 allele was similar to the effect of the B73 allele. We cloned and sequenced the CCoAOMT2 alleles from these three lines. The predicted amino acid sequences for the four CCoAOMT2 alleles are 94 to 98% similar with all the difference occurring in a small patch (Patch 1 in Supplemental Fig. S2) close to the N terminus.
The HR-suppressive activities of the B73 and NC350 CCoAOMT2 alleles were compared using the N. benthamiana transient expression system. They suppressed Rp1-D21-induced HR indistinguishably on a visual level (Supplemental Fig. S4A). However, the NC350 CCoAOMT2 allele had a significantly higher suppressive effect on ion leakage (Supplemental Fig. S4B). All the HA- and EGFP-tagged proteins were expressed at substantial and broadly comparable levels (Supplemental Fig. S4C).
DISCUSSION
From previous GWAS analysis, we identified two SNP loci that were highly associated with variation in the severity of HR induced by Rp1-D21 (Olukolu et al., 2014) and were adjacent to or within genes encoding enzymes, HCT and CCoAOMT, involved in lignin biosynthesis pathway. We have previously shown that two HCT homologs that are encoded by genes adjacent to the HR-associated SNP on chromosome 1 (HCT1806 and HCT4918) suppress Rp1-D21-induced HR through physical interaction, while other HCT homologs, encoded by genes distant from any associated SNPs, confer no or weak suppression (Wang et al., 2015c). Here, we provide strong evidence that CCoAOMT2, encoded by a gene encompassing an HR-associated SNP on chromosome 9, also suppresses Rp1-D21-induced HR through physical interaction. Importantly, CCoAOMT1, encoded by a gene on chromosome 6 distant from any HR-associated SNP, does not have obvious suppressive effect, even though it has very high similarity with CCoAOMT2. Consistent with the data, we have shown that the transcript levels of HCT1806, HCT4918, and CCoAOMT2, but not CCoAOMT1, are increased in the maize lines containing Rp1-D21 mutant compared to its isogenic wild-type lines (Wang et al., 2015c).
The CCoAOMT2 allele from NC350 suppresses Rp1-D21-induced HR more strongly than the B73 allele in the N. benthamiana transient expression system (Supplemental Fig. S4), which is consistent with the differential allelic effects we detected previously (Olukolu et al., 2014). The alleles are 94 to 98% similar at the amino acid level with the only polymorphisms occurring close to the N terminus (Patch 1 in Supplemental Fig. S2). Therefore, this region would appear to be responsible for the differential effects on HR.
Many genes in the phenylpropanoid pathway, including HCT and CCoAOMT, have been implicated in the plant defense response. Knocking down the expression of HCT in both Arabidopsis and alfalfa (Medicago sativa) leads to a dwarf phenotype, with constitutive activation of defense responses, including increased PR gene expression and elevated SA levels (Hoffmann et al., 2004; Li et al., 2010; Gallego-Giraldo et al., 2011a, 2011b). HCT-down-regulated alfalfa also enhances disease resistance to the fungus Colletotrichum trifolii, the causal agent of alfalfa anthracnose (Gallego-Giraldo et al., 2011b). In parsley (Petroselinum crispum) suspension cells, the enzymatic activity of CCoAOMT rapidly increased after fungal elicitor treatment (Pakusch et al., 1989). Down-regulation of CCoAOMT in N. benthamiana results in higher susceptibility to Pseudomonas syringae pv tabaci (Senthil-Kumar and Mysore, 2010), and silencing of CCoAOMT in wheat (Triticum aestivum) compromises the defense against powdery mildew (Bhuiyan et al., 2009). An Arabidopsis CCoAOMT mutant line displayed increased disease symptoms (Senthil-Kumar et al., 2010). CCoAOMT was also identified as a candidate gene in resistance to maize southern leaf blight from the NAM-GWAS analysis (Kump et al., 2011; Bian et al., 2014), suggesting a possible association between disease resistance and HR in this case.
In maize, many genes involved in phenylpropanoid pathway were upregulated in a near-isogenic line resistance line after being infected by Fusarium graminearum (Ye et al., 2013). In addition, the levels of SA and three phenols (caffeic acid, coumaric acid, and ferulic acid) are higher in a line resistant to F. graminearum compared to their levels in a near-isogenic line with lower resistance (Ye et al., 2013). Pantoea stewartii ssp. Stewartii (Pnss) is a bacterial pathogen that causes Stewart’s wilt leaf blight in maize. WtsE is a functional type III effector secreted from Pnss. Delivering WtsE into the cells of maize seedling leaves by an Escherichia coli system induces disease-like cell death symptoms in leaves of susceptible maize seedlings and elicits numerous plant responses, including the induced expression of genes in the phenylpropanoid pathway and enhanced accumulation of coumaroyl tyramine (Ham et al., 2006, 2009; Asselin et al., 2015). Interestingly, HCT1806, HCT4918, and the two CCoAOMT homologs are also induced in leaves expressing WtsE (Asselin et al., 2015). Consistent with these results, we found that the presence of Rp1-D21 induced expression of nearly all genes in lignin biosynthesis pathway, the hyperaccumulation of some metabolites (p-coumaroyl quinate and p-coumaroyl shikimate) catalyzed by HCT, and increased lignin levels (Wang et al., 2015c). These data indicate that the phenylpropanoid/lignin biosynthesis pathway plays pivotal roles in Rp1-D21-mediated HR and plant disease resistance.
HCT belongs to the BAHD family of plant acyl-CoA-dependent acyltransferases, which possess a conserved HxxxD motif (Burhenne et al., 2003; Ma et al., 2005; D’Auria, 2006). The residue His (H) in the HxxxD motif is important for the enzymatic activity of HCT, and mutation of this amino acid greatly reduces its catalytic activity (Lallemand et al., 2012; Walker et al., 2013). We have shown that mutations at this conserved H residue in HCT1806/4918 did not reduce their ability to suppress Rp1-D21-induced HR (Wang et al., 2015c). In CCoAOMT, the conserved amino acid Arg is also important for its enzymatic activity (Hoffmann et al., 2001). A mutation in the maize CCoAOMT2(R244T) also has no obvious effect on the ability of CCoAOMT2 to inhibit Rp1-D21-induced HR when the genes are transiently coexpressed in N. benthamiana (Fig. 3). These data suggest that the catalytic activity of neither HCT1806/4918 nor CCoAOMT is required for regulating the function of Rp1-D21.
The activity of NLR resistance genes must be finely controlled. On the one hand, activation of the defense pathway needs to be rapid in order for it to be effective against pathogen infection. On the other, inappropriate activation can result in severe stunting and loss of fitness (Negeri et al., 2013). The precise control of NLR activation has been investigated in several systems. While the precise details vary in each case, there are several general features; NLR proteins are held in an inactive state by a delicate combination of self-inhibitory intra- and intermolecular interactions, including homomer formation and interactions with other NLRs or other cofactors, including so-called “guardees” (see below). In a wild-type situation, specific pathogen-derived effectors disturb this balance and activate the NLR, leading to HR and the defense response. After activation, it is crucial that the HR area is contained in a small region, sufficient to confer resistance to pathogen but not large enough to cause significant damage to the host. Autophagy is involved in restricting the spread of HR (Liu et al., 2005). We have shown that levels of mRNAs encoding HCT1806/4918 and CCoAOMT2 are up-regulated in lines carrying Rp1-D21 compared to isogenic wild-type lines (Wang et al., 2015c), presumably by the HR. After HR activation, the presumably increased levels of both HCTs and CCoAOMT2 would be expected to produce substantial reservoirs of these proteins, which may serve to inhibit further Rp1 activation and to contain the spread of the HR.
Effector molecules are secreted by the pathogen to facilitate infection and pathogenesis. Effectors often interact with and disrupt the function of specific plant proteins involved in the defense response. Some NLRs detect effectors by direct physical binding, while others detect them indirectly via other host cofactors targeted by pathogens (Van der Biezen and Jones, 1998; DeYoung and Innes, 2006). In the later case, the NLR resistance protein “guards” the virulence target (the guardee) of the pathogen and a defense response is activated when the structure of the guardee is disrupted by the effector detected by the NLR. An elaboration of this guard model is the decoy model (van der Hoorn and Kamoun, 2008), under which the guardee loses its original biochemical function and is employed by the plant solely as a means to monitor the presence of the pathogen, in other words as a bait.
Many likely guardee proteins that modulate the innate immunity mediated by NLRs through physical interaction have been identified, and we have recently summarized these interactions (Wang et al., 2015c). RIN4 is an important regulator of plant basal immunity in Arabidopsis and is targeted by at least four different bacterial effectors (Chung et al., 2014). In contrast, the same NLR can also interact with different host cofactors targeted by different pathogen effectors. In Arabidopsis, the NLR ZAR1 is required for the recognition of the HopZ1a effector from P. syringae (Lewis et al., 2010). ZAR1 is activated upon acetylation of the pseudokinase ZED1, by HopZ1a (Lewis et al., 2013). Recently, it was reported that ZAR1 is also important for the recognition of the effector AvrAC from Xanthomonas campestris pv campestris (Wang et al., 2015a). ZAR1 interacts with another host protein RKS1, a pseudokinase closely related to ZED1. However, neither ZAR1 nor RKS1, but the receptor-like cytoplasmic kinase PBL2, is the substrate of AvrAC. AvrAC can uridylylate PBL2, and the uridylylated PBL2 interacts with RKS1. Thus, ZAR1 is indirectly activated by AvrAC-uridylalated PBL2, with RKS1 as an intermediary protein (Wang et al., 2015a). In both cases, ZED1 and PBL2 are thought to be the decoys of ZAR1 for recognition of their cognate pathogen effectors since their absence does not significantly alter the virulence effects of HopZ1a and AvrAC, respectively.
We have previously identified HCT1806/4918 as negative regulators of Rp1-D21, likely via direct interaction with Rp1-D21 (Wang et al., 2015c). In this study, we provide evidence that CCoAOMT, the enzyme downstream of HCT in the lignin biosynthesis pathway, is also a negative regulator of Rp1-D21. CCoAOMT2 interacts with both HCT1806 and HCT4918, suggesting that they might work as a complex in regulating the activity of Rp1 proteins. We also show that CCoAOMT2 interacts with both full-length Rp1-dp2 and with CCD21 and that CCoAOMT2, HCT1806/4918, and CCD21 can form the same complex comprising all three proteins (Figs. 4–7), further suggesting that CCoAOMT2 and HCT1806/4918 can modulate the activity of Rp1 through physical interaction.
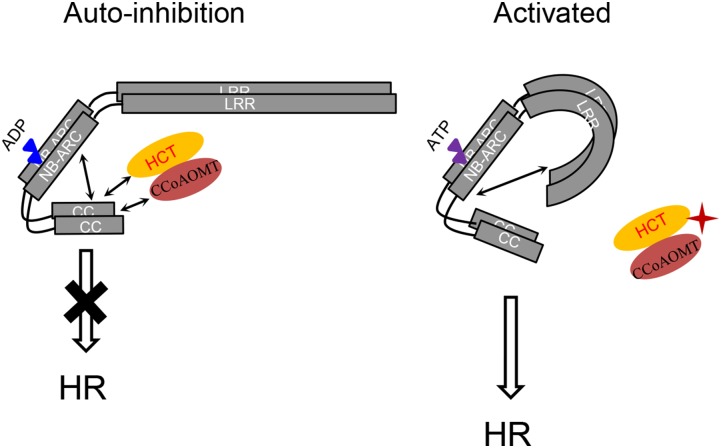
Rp1-D confers resistance to maize common rust caused by P. sorghi (Hu et al., 1996). However, the effector that elicits the response associated with Rp1-D has not been identified. We previously derived models to elucidate the self-activation/repression of Rp1 proteins (Wang et al., 2015c, 2015d). In a suppressed state, Rp1 proteins are held in an inactive state through the intramolecular interaction of different domains and the interaction between different Rp1 proteins (Wang et al., 2015d). The data presented here support an elaboration of this model (Fig. 8) in which the suppressed state is maintained through interaction with other molecules, including CCoAOMT2 and HCT1806/HCT4918 (Wang et al., 2015c). Since CCoAOMT2, HCT1806/HCT4918, and the lignin pathway are important for the defense response, it is reasonable to speculate that CCoAOMT2 or HCT1806/HCT4918 might be effector targets and might be guarded by Rp1 proteins. In both cases, other very similar homologous genes are expressed that do not have any apparent effect on Rp1-D21-induced HR, so it is possible that HCT1806/4918 or CCoAOMT2 function primarily as decoys, while the other homologous genes provide the catalytic functions. In a wild type situation, modification of CCoAOMT2 or HCT1806/4918 homologs by a rust effector may be sufficient to trigger the switch between the inactive and active states of the Rp1 proteins (Fig. 8). Without knowledge of the specific pathogen effectors involved, we do not know whether CCoAOMT2 or HCT1806/4918 is the direct target of effectors. By analogy to the ZAR1-AvrAC example stated above, we hypothesize that one might be the direct target of pathogen effector while the other is the adapter protein of Rp1.
Figure 8.
Model for the suppression and activation of Rp1 proteins, adapted from our previous study (Wang et al., 2015c). Rp1 proteins form homo- and heteromers. The inhibited state of Rp1 proteins (left) is maintained through autoinhibitory intramolecular interaction between the CC and NB-ARC domains and also through interactions between CCoAOMT/HCT complex and the CC domain. In the activated state (right), pathogen effectors modify CCoAOMT/HCT complex, disrupting the interaction between Rp1 proteins and CCoAOMT/HCT. This in turn disrupts the autoinhibitory intramolecular interactions; the interaction between the LRR and NB-ARC domains is strengthened and the CC/NB-ARC interaction is weakened, leading to activation and HR. Blue and pink triangles indicate ADP and ATP, respectively. Arrows indicate intra- and intermolecular interactions. The putative pathogen effector is labeled in red star.
A metabolon is defined as an assembly of cooperating enzymes existing as a complex within the cell often associated with a structural complex. The complex is held together through noncovalent interactions and enables the efficient channeling of a metabolite between two or more active sites, so-called “metabolic channeling” in which processing of metabolic intermediates occurs more quickly than would be expected in a homogeneous aqueous environment. The enzymes of the Calvin and tricarboxylic acid cycles are among the best characterized metabolons (Winkel, 2004; Jørgensen et al., 2005). Metabolic channeling has been demonstrated to occur in the plant phenylpropanoid pathway, and the enzymes of the phenylpropanoid pathway are thought to form such a metabolon, held in place by close associations with membrane-integral cytochrome P450 enzymes (Winkel, 2004; Jørgensen et al., 2005; Lee et al., 2012). Of particular interest here is the suggestion that HCT (also known as CST for hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase) and CCoAOMT directly interact in this metabolon, as would be expected of two enzymes catalyzing adjacent steps in the pathway (Winkel, 2004; Jørgensen et al., 2005). In this article, we provide direct evidence for such an interaction. As discussed above, the phenylpropanoid pathway is of central importance to plant defense. It is therefore not surprising that pathogen effectors may target it and that, in turn, NLR resistance proteins might guard specific components.
In summary, homologs of both CCoAOMT and HCT (CCoAOMT2 and HCT1806/HCT4918, respectively), which catalyze sequential steps in the lignin biosynthesis pathway, were previously identified as candidate genes for modulating the severity of HR induced by Rp1-D21. With this study we have now validated the roles of both as cofactors of Rp1 proteins, and we provide evidence that CCoAOMT2 forms complex(es) with HCT1806/HCT4918 and Rp1 to regulate the activity of Rp1 proteins. Though the protein interaction data were obtained from transient expression in N. benthamiana, we assume there are similar interactions in maize. We hope in the future to verify these results in maize, though these experiments are much more technically challenging. These data form the basis for the continuing investigation of the association between Rp1 and other NLR proteins with these and other proteins of the phenylpropanoid pathway metabolon.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Wild-type Nicotiana benthamiana plants were grown at 23°C with a cycle of 16 h light/8 h dark. Maize (Zea mays) line B73 harboring Rp1-D21 was used for isolating the cDNA of CCoAOMT1 and CCoAOMT2 homologous genes. The maize seedlings were grown in constant 22°C with 12 h day/12 h dark.
Plasmid Construction
Rp1-D21, Rp1-dp2, and the CC domain of Rp1-D21 (CCD21) were constructed into pGWB14 (with a 3×HA epitope tag in the C terminus) as reported in our previous study (Wang et al., 2015d). GUS:EGFP, CCD21:EGFP, HCT1806:EGFP, HCT4918:HA, and HCT1806:HA were generated previously (Wang et al., 2015c, 2015d). CCD21:Myc was constructed by cloning CCD21 into pGWB617 (with a Myc epitope tag in the C terminus). CCoAOMT1 and CCoAOMT2 genes were amplified from cDNA by primers (CCoAOMT-B1, GGGGACAAGTTTGTACAAAAAAGCAGGCTCCATGGCCACCACGGCGACC; CCoAOMT-B2, GGGGACCACTTTGTACAAGAAAGCTGGGTGCTTGACGCGGCGGCAGAG) and cloned into pDONR207 vector by BP reactions. After sequencing, they were transferred into pSITEII-N1-EGFP vector (Martin et al., 2009) by LR reactions.
Site-Directed Mutagenesis
Overlapping extension PCR primer (GTCGCCGACGGGGAGCTGGCAGATCTCGACGGTGTCGTCGGC) was designed for generating the site-directed mutation: CCoAOMT2(R244T). The site-directed mutation was cloned into pDONR207 vector by BP reactions and verified by sequencing. Then it was moved to Gateway vector pSITEII-N1-EGFP by LR reactions.
Sequence Alignment and Phylogenetic Analysis
CCoAOMT amino acids sequences from different plant species were aligned by ClustalW (www.ebi.ac.uk) and edited by BioEdit software. The phylogenetic tree was constructed with the MEGA 6.0 software (Tamura et al., 2013). The specific algorithms used for tree building was neighbor-joining according to previous studies (Wang et al., 2011, 2015b).
Agrobacterium tumefaciens-Mediated Transient Expression
A. tumefaciens strain GV3101 (pMP90) transformed with binary vector constructs was grown at 28°C overnight in 10 mL of L-broth medium supplemented with appropriate antibiotics. The detailed procedures were performed according to our previous studies (Wang et al., 2015c, 2015d). Unless otherwise indicated, all the experiments were repeated three times with substantially similar results.
Ion Leakage Measurement
Ion leakage was measured according to previous studies (Wang and Balint-Kurti, 2015; Wang et al., 2015c). Briefly, at least five leaf discs (1.2 cm diameter) were collected from different plants and put in 4 mL of sterile water in a 15-mL polypropylene tube. The samples were shaken at room temperature for 3 h, and the conductivity (C1) was measured by a conductivity meter (model 4403; Markson Science). Samples were then boiled for 15 min, and the total conductivity (C2) was measured again. The ion leakage was calculated as C1/C2 ratio.
Protein Analysis
Three leaf discs (1.2 cm diameter) were collected from different single plants at 30 h post inoculation (hpi) for protein expression analysis. The samples were ground with prechilled plastic pestles in liquid nitrogen, and total protein was extracted in 160 µL extraction buffer (20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 10 mm DTT, 1 mm EDTA, pH 8.0, 0.1% SDS, 1% Triton X-100, 40 µM MG132, and 1× plant protein protease inhibitor mixture [Sigma-Aldrich]). For the Co-IP assay, EGFP-, Myc-, or 3×HA-tagged constructs were transiently coexpressed in N. benthamiana. Samples were collected at 30 hpi, and proteins were extracted by grinding 0.8 g of leaf tissues in 2.4 mL extraction buffer (50 mm HEPES, pH 7.5, 50 mm NaCl, 4 mm DTT, 10 mm EDTA, pH 8.0, 0.5% Triton X-100, and 1× plant protein protease inhibitor mixture [Sigma-Aldrich]). The detailed procedures for protein analysis were conducted according to our previous study (Wang et al., 2015c, 2015d). HA detection was performed using a 1:350 dilution of anti-HA-HRP (horseradish peroxidase) (catalog no. 12013819001; Roche). Myc detection was performed using a 1:8,000 dilution of primary mouse monoclonal anti-Myc (catalog no. 05-724; Millipore), followed by hybridization with a 1:15,000 dilution of anti-mouse-HRP second antibody (catalog no. A4426; Sigma-Aldrich). GFP detection was performed using a 1:8,000 dilution of primary mouse monoclonal anti-GFP (catalog no. ab1218; Abcam), followed by hybridization with a 1:15,000 dilution of anti-mouse-HRP second antibody. The HRP signal was detected by ECL substrate kit (Supersignal West femto chemiluminescent substrate; Thermo Scientific).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: nucleotide sequences: Rp1-D21 (KF951062), Rp1-dp2 (KF951063), CCoAOMT2-NC350 (KX110046), CCoAOMT2-M37W (KX110057), and CCoAOMT2-B97 (KX110056); protein sequences: Rp1-D21 (AIW65617) and Rp1-dp2 (AIW65618); and CCoAOMT2-NC350 (AND78162), CCoAOMT2-M37W (AND80699), CCoAOMT2-B97 (AND80698).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The phenylpropanoid pathway, adapted from previous studies (Hoffmann et al., 2004; Li et al., 2010; Wang et al., 2015c).
Supplemental Figure S2. Sequence alignment of CCoAOMT.
Supplemental Figure S3. CCoAOMT2 did not suppress MLA10(D502V)- and RPM1(D505V)-induced HR in N. benthamiana.
Supplemental Figure S4. Investigating the function of CCoAOMT2 from different maize lines in Rp1-D21-induced HR.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Dangl for providing strains of RPM1(D505V) and Dr. Qian-Hua Shen for providing MLA10(D502V) plasmid. We are grateful to Dr. Shannon Sermons for help with obtaining reagents, Dr. Qin Yang for providing the PCR product used as template for cloning CCoAOMT2 from B73, and Drs. Guri Johal, Tiffany Jamann, Yijian He, Bode Olukolu, and Jim Holland for helpful discussions. The Genomic Sciences Laboratory at NC State University provided DNA sequencing services. Controlled environment facilities were provided by the NCSU Phytotron.
Glossary
- HR
hypersensitive response
- CCoAOMT
caffeoyl CoA O-methyltransferase
- HCT
hydroxycinnamoyltransferase
- SA
salicylic acid
- NAM
nested association mapping
- QTL
quantitative trait loci
- SNP
single nucleotide polymorphism
- Co-IP
coimmunoprecipitation
- GWAS
genome-wide associate study
- hpi
hours postinoculation
Footnotes
This work was supported by National Science Foundation (grant nos. 0822495 and 1444503), the U.S. Department of Agriculture-Agricultural Research Service, and Qilu Scholarship from Shandong University of China (grant no.11200085963025 to G.-F.W.).
References
- Asselin JA, Lin J, Perez-Quintero AL, Gentzel I, Majerczak D, Opiyo SO, Zhao W, Paek SM, Kim MG, Coplin DL, Blakeslee JJ, Mackey D (2015) Perturbation of maize phenylpropanoid metabolism by an AvrE family type III effector from Pantoea stewartii. Plant Physiol 167: 1117–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Liu J, Chang C, Zhang L, Maekawa T, Wang Q, Xiao W, Liu Y, Chai J, Takken FL, Schulze-Lefert P, Shen QH (2012) Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog 8: e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Mackey D (2007) Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol 45: 399–436 [DOI] [PubMed] [Google Scholar]
- Bhuiyan NH, Selvaraj G, Wei Y, King J (2009) Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J Exp Bot 60: 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Y, Yang Q, Balint-Kurti PJ, Wisser RJ, Holland JB (2014) Limits on the reproducibility of marker associations with southern leaf blight resistance in the maize nested association mapping population. BMC Genomics 15: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Burhenne K, Kristensen BK, Rasmussen SK (2003) A new class of N-hydroxycinnamoyltransferases. Purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3.1.64). J Biol Chem 278: 13919–13927 [DOI] [PubMed] [Google Scholar]
- Chaikam V, Negeri A, Dhawan R, Puchaka B, Ji J, Chintamanani S, Gachomo EW, Zillmer A, Doran T, Weil C, Balint-Kurti P, Johal G (2011) Use of mutant-assisted gene identification and characterization (MAGIC) to identify novel genetic loci that modify the maize hypersensitive response. Theor Appl Genet 123: 985–997 [DOI] [PubMed] [Google Scholar]
- Chintamanani S, Hulbert SH, Johal GS, Balint-Kurti PJ (2010) Identification of a maize locus that modulates the hypersensitive defense response, using mutant-assisted gene identification and characterization. Genetics 184: 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E-H, El-Kasmi F, He Y, Loehr A, Dangl JL (2014) A plant phosphoswitch platform repeatedly targeted by type III effector proteins regulates the output of both tiers of plant immune receptors. Cell Host Microbe 16: 484–494 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JD (2001) Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 [DOI] [PubMed] [Google Scholar]
- D’Auria JC. (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9: 331–340 [DOI] [PubMed] [Google Scholar]
- DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7: 1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence-a genomics perspective. Mol Plant Pathol 3: 371–390 [DOI] [PubMed] [Google Scholar]
- Ellis J, Dodds P, Pryor T (2000) Structure, function and evolution of plant disease resistance genes. Curr Opin Plant Biol 3: 278–284 [DOI] [PubMed] [Google Scholar]
- Etalo DW, Stulemeijer IJ, van Esse HP, de Vos RC, Bouwmeester HJ, Joosten MH (2013) System-wide hypersensitive response-associated transcriptome and metabolome reprogramming in tomato. Plant Physiol 162: 1599–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Giraldo L, Escamilla-Trevino L, Jackson LA, Dixon RA (2011a) Salicylic acid mediates the reduced growth of lignin down-regulated plants. Proc Natl Acad Sci USA 108: 20814–20819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Giraldo L, Jikumaru Y, Kamiya Y, Tang Y, Dixon RA (2011b) Selective lignin downregulation leads to constitutive defense response expression in alfalfa (Medicago sativa L.). New Phytol 190: 627–639 [DOI] [PubMed] [Google Scholar]
- Gao Z, Chung EH, Eitas TK, Dangl JL (2011) Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc Natl Acad Sci USA 108: 7619–7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham JH, Majerczak DR, Arroyo-Rodriguez AS, Mackey DM, Coplin DL (2006) WtsE, an AvrE-family effector protein from Pantoea stewartii subsp. stewartii, causes disease-associated cell death in corn and requires a chaperone protein for stability. Mol Plant Microbe Interact 19: 1092–1102 [DOI] [PubMed] [Google Scholar]
- Ham JH, Majerczak DR, Nomura K, Mecey C, Uribe F, He SY, Mackey D, Coplin DL (2009) Multiple activities of the plant pathogen type III effector proteins WtsE and AvrE require WxxxE motifs. Mol Plant Microbe Interact 22: 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath MC. (2000) Hypersensitive response-related death. Plant Mol Biol 44: 321–334 [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M (2004) Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell 16: 1446–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L, Maury S, Bergdoll M, Thion L, Erard M, Legrand M (2001) Identification of the enzymatic active site of tobacco caffeoyl-coenzyme A O-methyltransferase by site-directed mutagenesis. J Biol Chem 276: 36831–36838 [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M (2003) Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem 278: 95–103 [DOI] [PubMed] [Google Scholar]
- Hu G, Richter TE, Hulbert SH, Pryor T (1996) Disease lesion mimicry caused by mutations in the rust resistance gene rp1. Plant Cell 8: 1367–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005) Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8: 280–291 [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, Umemura K, Umezawa T, Shimamoto K (2006) Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci USA 103: 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kump KL, Bradbury PJ, Wisser RJ, Buckler ES, Belcher AR, Oropeza-Rosas MA, Zwonitzer JC, Kresovich S, McMullen MD, Ware D, Balint-Kurti PJ, Holland JB (2011) Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat Genet 43: 163–168 [DOI] [PubMed] [Google Scholar]
- Lallemand LA, Zubieta C, Lee SG, Wang Y, Acajjaoui S, Timmins J, McSweeney S, Jez JM, McCarthy JG, McCarthy AA (2012) A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol 160: 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Lapierre C, Sandermann H Jr (1995) Elicitor-induced spruce stress lignin (Structural similarity to early developmental lignins). Plant Physiol 108: 1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Escamilla-Treviño L, Dixon RA, Voit EO (2012) Functional analysis of metabolic channeling and regulation in lignin biosynthesis: a computational approach. PLOS Comput Biol 8: e1002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister RT, Dahlbeck D, Day B, Li Y, Chesnokova O, Staskawicz BJ (2005) Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana. Plant Cell 17: 1268–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C (1996) Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol 6: 427–437 [DOI] [PubMed] [Google Scholar]
- Lewis JD, Lee AH, Hassan JA, Wan J, Hurley B, Jhingree JR, Wang PW, Lo T, Youn JY, Guttman DS, Desveaux D (2013) The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the Pseudomonas syringae type III effector HopZ1a. Proc Natl Acad Sci USA 110: 18722–18727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Wu R, Guttman DS, Desveaux D (2010) Allele-specific virulence attenuation of the Pseudomonas syringae HopZ1a type III effector via the Arabidopsis ZAR1 resistance protein. PLoS Genet 6: e1000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41: 455–496 [DOI] [PubMed] [Google Scholar]
- Li X, Bonawitz ND, Weng JK, Chapple C (2010) The growth reduction associated with repressed lignin biosynthesis in Arabidopsis thaliana is independent of flavonoids. Plant Cell 22: 1620–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121: 567–577 [DOI] [PubMed] [Google Scholar]
- Ma X, Koepke J, Panjikar S, Fritzsch G, Stöckigt J (2005) Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. J Biol Chem 280: 13576–13583 [DOI] [PubMed] [Google Scholar]
- Martin K, Kopperud K, Chakrabarty R, Banerjee R, Brooks R, Goodin MM (2009) Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J 59: 150–162 [DOI] [PubMed] [Google Scholar]
- McMullen MD, Kresovich S, Villeda HS, Bradbury P, Li H, Sun Q, Flint-Garcia S, Thornsberry J, Acharya C, Bottoms C, et al. (2009) Genetic properties of the maize nested association mapping population. Science 325: 737–740 [DOI] [PubMed] [Google Scholar]
- Morel JB, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death Differ 4: 671–683 [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E (2008) The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 59: 501–520 [DOI] [PubMed] [Google Scholar]
- Negeri A, Wang G-F, Benavente L, Kibiti CM, Chaikam V, Johal G, Balint-Kurti P (2013) Characterization of temperature and light effects on the defense response phenotypes associated with the maize Rp1-D21 autoactive resistance gene. BMC Plant Biol 13: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukolu BA, Negeri A, Dhawan R, Venkata BP, Sharma P, Garg A, Gachomo E, Marla S, Chu K, Hasan A, et al. (2013) A connected set of genes associated with programmed cell death implicated in controlling the hypersensitive response in maize. Genetics 193: 609–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukolu BA, Wang GF, Vontimitta V, Venkata BP, Marla S, Ji J, Gachomo E, Chu K, Negeri A, Benson J, et al. (2014) A genome-wide association study of the maize hypersensitive defense response identifies genes that cluster in related pathways. PLoS Genet 10: e1004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakusch AE, Kneusel RE, Matern U (1989) S-adenosyl-L-methionine:trans-caffeoyl-coenzyme A 3-O-methyltransferase from elicitor-treated parsley cell suspension cultures. Arch Biochem Biophys 271: 488–494 [DOI] [PubMed] [Google Scholar]
- Qi D, DeYoung BJ, Innes RW (2012) Structure-function analysis of the coiled-coil and leucine-rich repeat domains of the RPS5 disease resistance protein. Plant Physiol 158: 1819–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil-Kumar M, Hema R, Suryachandra TR, Ramegowda HV, Gopalakrishna R, Rama N, Udayakumar M, Mysore KS (2010) Functional characterization of three water deficit stress-induced genes in tobacco and Arabidopsis: an approach based on gene down regulation. Plant Physiol Biochem 48: 35–44 [DOI] [PubMed] [Google Scholar]
- Senthil-Kumar M, Mysore KS (2010) Assessing functional role of three water deficit stress-induced genes in nonhost disease resistance using virus-induced gene silencing in Nicotiana benthamiana. Plant Signal Behav 5: 586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slootweg E, Roosien J, Spiridon LN, Petrescu AJ, Tameling W, Joosten M, Pomp R, van Schaik C, Dees R, Borst JW, et al. (2010) Nucleocytoplasmic distribution is required for activation of resistance by the potato NB-LRR receptor Rx1 and is balanced by its functional domains. Plant Cell 22: 4195–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Pryor AJ, Hulbert SH (2004) Allelic and haplotypic diversity at the rp1 rust resistance locus of maize. Genetics 167: 1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Steinau M, Trick HN, Hulbert SH (2010) Recombinant Rp1 genes confer necrotic or nonspecific resistance phenotypes. Mol Genet Genomics 283: 591–602 [DOI] [PubMed] [Google Scholar]
- Sun Q, Collins NC, Ayliffe M, Smith SM, Drake J, Pryor T, Hulbert SH (2001) Recombination between paralogues at the Rp1 rust resistance locus in maize. Genetics 158: 423–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tameling WI, Nooijen C, Ludwig N, Boter M, Slootweg E, Goverse A, Shirasu K, Joosten MH (2010) RanGAP2 mediates nucleocytoplasmic partitioning of the NB-LRR immune receptor Rx in the Solanaceae, thereby dictating Rx function. Plant Cell 22: 4176–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Biezen EA, Jones JD (1998) Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci 23: 454–456 [DOI] [PubMed] [Google Scholar]
- van der Hoorn RAL, Kamoun S (2008) From Guard to Decoy: a new model for perception of plant pathogen effectors. Plant Cell 20: 2009–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooijen G, Mayr G, Albrecht M, Cornelissen BJ, Takken FL (2008) Transcomplementation, but not physical association of the CC-NB-ARC and LRR domains of tomato R protein Mi-1.2 is altered by mutations in the ARC2 subdomain. Mol Plant 1: 401–410 [DOI] [PubMed] [Google Scholar]
- Vélez-Bermúdez IC, Salazar-Henao JE, Fornalé S, López-Vidriero I, Franco-Zorrilla JM, Grotewold E, Gray J, Solano R, Schmidt W, Pagés M, Riera M, Caparros-Ruiz D (2015) A MYB/ZML complex regulates wound-induced lignin genes in maize. Plant Cell 27: 3245–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AM, Hayes RP, Youn B, Vermerris W, Sattler SE, Kang C (2013) Elucidation of the structure and reaction mechanism of sorghum hydroxycinnamoyltransferase and its structural relationship to other coenzyme a-dependent transferases and synthases. Plant Physiol 162: 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Roux B, Feng F, Guy E, Li L, Li N, Zhang X, Lautier M, Jardinaud MF, Chabannes M, et al. (2015a) The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe 18: 285–295 [DOI] [PubMed] [Google Scholar]
- Wang GF, Balint-Kurti PJ (2015) Cytoplasmic and nuclear localizations are important for the hypersensitive response conferred by maize autoactive Rp1-D21 protein. Mol Plant Microbe Interact 28: 1023–1031 [DOI] [PubMed] [Google Scholar]
- Wang GF, Fan R, Wang X, Wang D, Zhang X (2015b) TaRAR1 and TaSGT1 associate with TaHsp90 to function in bread wheat (Triticum aestivum L.) seedling growth and stripe rust resistance. Plant Mol Biol 87: 577–589 [DOI] [PubMed] [Google Scholar]
- Wang GF, He Y, Strauch R, Olukolu BA, Nielsen D, Li X, Balint-Kurti PJ (2015c) Maize homologs of hydroxycinnamoyltransferase, a key enzyme in lignin biosynthesis, bind the nucleotide binding leucine-rich repeat Rp1 proteins to modulate the defense response. Plant Physiol 169: 2230–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GF, Ji J, El-Kasmi F, Dangl JL, Johal G, Balint-Kurti PJ (2015d) Molecular and functional analyses of a maize autoactive NB-LRR protein identify precise structural requirements for activity. PLoS Pathog 11: e1004674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GF, Wei X, Fan R, Zhou H, Wang X, Yu C, Dong L, Dong Z, Wang X, Kang Z, et al. (2011) Molecular analysis of common wheat genes encoding three types of cytosolic heat shock protein 90 (Hsp90): functional involvement of cytosolic Hsp90s in the control of wheat seedling growth and disease resistance. New Phytol 191: 418–431 [DOI] [PubMed] [Google Scholar]
- Winkel BS. (2004) Metabolic channeling in plants. Annu Rev Plant Biol 55: 85–107 [DOI] [PubMed] [Google Scholar]
- Ye J, Guo Y, Zhang D, Zhang N, Wang C, Xu M (2013) Cytological and molecular characterization of quantitative trait locus qRfg1, which confers resistance to gibberella stalk rot in maize. Mol Plant Microbe Interact 26: 1417–1428 [DOI] [PubMed] [Google Scholar]
- Ye ZH, Kneusel RE, Matern U, Varner JE (1994) An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell 6: 1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Dixon RA (2014) Altering the cell wall and its impact on plant disease: from forage to bioenergy. Annu Rev Phytopathol 52: 69–91 [DOI] [PubMed] [Google Scholar]
- Zhong R, Iii WH, Negrel J, Ye ZH (1998) Dual methylation pathways in lignin biosynthesis. Plant Cell 10: 2033–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.