Repetitive short-pulse illumination produces both superoxide and singlet oxygen within the thylakoid membranes, leading to inactivation of photosystem I.
Abstract
Photosystem I (PSI) photoinhibition suppresses plant photosynthesis and growth. However, the mechanism underlying PSI photoinhibition has not been fully clarified. In this study, in order to investigate the mechanism of PSI photoinhibition in higher plants, we applied repetitive short-pulse (rSP) illumination, which causes PSI-specific photoinhibition in chloroplasts isolated from spinach leaves. We found that rSP treatment caused PSI photoinhibition, but not PSII photoinhibition in isolated chloroplasts in the presence of O2. However, chloroplastic superoxide dismutase and ascorbate peroxidase activities failed to protect PSI from its photoinhibition. Importantly, PSI photoinhibition was largely alleviated in the presence of methyl viologen, which stimulates the production of reactive oxygen species (ROS) at the stromal region by accepting electrons from PSI, even under the conditions where CuZn-superoxide dismutase and ascorbate peroxidase activities were inactivated by KCN. These results suggest that the ROS production site, but not the ROS production rate, is critical for PSI photoinhibition. Furthermore, we found that not only superoxide (O2−) but also singlet oxygen (1O2) is involved in PSI photoinhibition induced by rSP treatment. From these results, we suggest that PSI photoinhibition is caused by both O2− and 1O2 produced within the thylakoid membranes when electron carriers in PSI become highly reduced. Here, we show, to our knowledge, new insight into the PSI photoinhibition in higher plants.
Higher plants need light to drive photosynthesis. However, excess light causes photoinhibition in chloroplasts (Melis, 1999). Photosystem (PS) II is very sensitive to environmental stress, such as high-light intensities or drought conditions, and such stress conditions can cause a decrease in D1 protein of the PSII reaction center (Aro et al., 1993). This phenomenon is called photoinhibition of PSII, and its occurrence greatly reduces plant productivity (Melis, 1999; Külheim et al., 2002). In previous studies, photoinhibition in PSII has been shown to be caused by singlet oxygen (1O2), which is produced through a charge recombination reaction between P680+ and the reduced secondary electron acceptor (QA−) in PSII. Next, 1O2 is thought to stimulate the degradation of D1 protein (Asada and Takahashi, 1987; Krieger-Liszkay, 2005; Hideg et al., 2007; Gill and Tuteja, 2010; Vass, 2011). In addition to 1O2, recent studies have revealed that superoxide (O2–) is also produced by PSII, which also causes photoinhibition (Bondarava et al., 2010; Zulfugarov et al., 2014). In contrast to the theory that oxidative degradation of the D1 protein causes photoinhibition in PSII, several studies now suggest that reactive oxygen species (ROS) suppress de novo D1 protein synthesis through the oxidative inactivation of the thioredoxin-regulated elongation factor G. The latter plays an important role in protein translation of the D1 protein (Kojima et al., 2007; Nishiyama et al., 2011).
In addition to PSII, PSI can also experience photoinhibition, and photoinhibition in PSI is similarly caused by ROS (Sonoike and Terashima, 1994; Terashima et al., 1994; Sonoike et al., 1995; Sonoike, 1995, 1996; Sejima et al., 2014). In PSI, the risk of ROS production increases when the photosynthetic electron transport chain is in a highly reduced state (Sonoike and Terashima, 1994; Allahverdiyeva et al., 2005; Oelze et al., 2012; Grieco et al., 2012). In fact, PSI photoinhibition occurs when the PSI electron carriers become reduced, impairing net carbon assimilation, and hence plant growth (Allahverdiyeva et al., 2005; Munekage et al., 2008; DalCorso et al., 2008; Suorsa et al., 2012; Grieco et al., 2012; Kono et al., 2014). An important difference between photoinhibition in PSI and PSII is that PSI recovers very slowly, whereas photoinhibited PSII recovers rapidly (t1/2 is about 60 min; Melis, 1999; Sonoike, 2011). Therefore, PSI photoinhibition has more severe consequences than PSII photoinhibition in higher plants.
Many studies have confirmed the occurrence of PSI photoinhibition, although the underlying molecular mechanisms have not yet been clarified. For example, the production site of ROS that induce PSI photoinhibition has not been identified. Furthermore, it is difficult to analyze PSI photoinhibition, since PSI is highly resistant to photoinhibition compared with PSII (Terashima et al., 1994). In previous studies, specific materials and experimental conditions (such as chilling sensitive plants and cold temperatures) were used to induce PSI photoinhibition in vivo (Terashima et al., 1994; Sonoike et al., 1995). These experimental limitations complicate the elucidation of the molecular mechanisms underlying PSI photoinhibition, as well as the mechanisms that protect PSI from photoinhibition.
Recently, our research group successfully established a method for specifically inducing PSI photoinhibition in the leaves of higher plants, by repetitively illuminating leaves with short-pulse light under dark conditions at room temperature (Sejima et al., 2014; Zivcak et al., 2015a, 2015b). This method was named “repetitive short-pulse” (rSP) treatment. PSI photoinhibition induced by the rSP treatment requires O2, and rSP treatment decreases the total content of P700 chlorophyll. Based on these results, we proposed that rSP treatment stimulates the production of ROS in PSI, and that ROS decreases P700 chlorophyll levels. Furthermore, we also observed a decrease in P700 chlorophyll when we used a light intensity similar to that of sunlight (2,000 μE m−2 s−1) in rSP treatment (Sejima et al., 2014). This result suggested that sun flecks stimulate PSI photoinhibition under natural field conditions. Therefore, the elucidation of PSI photoinhibition induced by rSP treatment would improve our understanding of the mechanism underlying photoinhibition in PSI under natural field conditions.
In this study, we focused on the mechanism underlying O2-dependent PSI photoinhibition induced by rSP treatment, and we investigated the relationship between the ROS production at PSI and PSI photoinhibition. We found that ROS produced within the thylakoid membranes promoted the degradation of P700 chlorophyll during rSP treatment and induced PSI photoinhibition. Importantly, we found that 1O2 is produced in PSI, and 1O2 also stimulates the PSI photoinhibition in addition to O2− although many studies are exclusively focused on O2− in PSI photoinhibition. On the basis of these results, we discuss the relationship between the ROS production mechanism and PSI photoinhibition.
RESULTS
rSP Treatment Specifically Induces PSI Photoinhibition in Isolated Chloroplasts
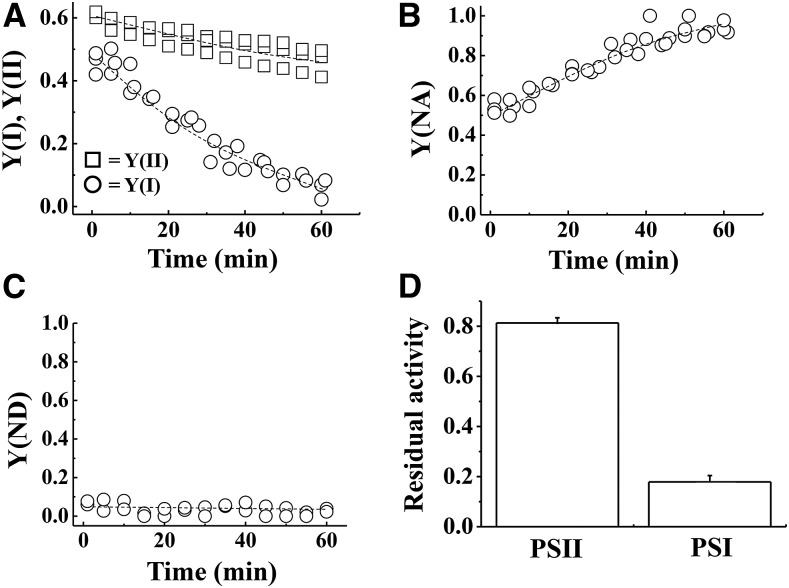
First, we applied rSP treatment to isolated chloroplasts, in order to elucidate the mechanism of PSI photoinhibition, using the method of Sejima et al. (2014) (Fig. 1). In the rSP treatment, the light intensity of the short pulse was set to 20,000 μE m−2 s−1, the pulse duration was set at 300 ms, and the illumination frequency was every 10 s under dark conditions. Figure 1A shows the time-course for the change in quantum yields in PSII [Y(II)] and PSI [Y(I)] throughout rSP treatment. Y(I) considerably decreased during rSP treatment in the absence of electron acceptors in the reaction mixture. Y(ND) and Y(NA) represent the extent of photosynthetic electron transport limitation in PSI at the donor and acceptor sides, respectively (Klughammer and Schreiber, 1994). Y(NA) increased throughout rSP treatment (Fig. 1B). In contrast, Y(ND) was not induced during the rSP treatment (Fig. 1C). These results indicate that rSP treatment suppresses the photosynthetic electron transport reaction at the acceptor side of PSI in isolated chloroplasts. On the other hand, Y(II) was less affected during rSP treatment, compared to Y(I) (Fig. 1A). These results are consistent with previous results obtained from intact leaves (Sejima et al., 2014; Zivcak et al., 2015a, 2015b).
Figure 1.
A to C, Time-course analysis of photosynthetic parameters [A, Y(I) and Y(II); B, Y(NA); C, Y(ND)] in isolated chloroplasts. The reaction mixture contained 30 μg ml−1 isolated chloroplasts. Isolated chloroplasts were illuminated every 10 s with a short-pulse (300 ms, 20,000 μE m−2 s−1) and for 1 h without AL illumination at 25°C. Experiments were repeated three times. D, After rSP treatment, the reaction mixture was kept in the dark for 30 min, and Fv/Fm and Pm were measured. Data were normalized to Fv/Fm and Pm before rSP treatment, and are represented as the residual activities of PSII and PSI after rSP treatment. Data are expressed as mean ± se of three independent experiments.
We estimated the residual activity of PSII and PSI after rSP treatment, based on the change in the maximum quantum yield of PSII (Fv/Fm) and the total amount of P700 chlorophyll (Pm) before and after rSP treatment (Fig. 1D). The rSP treatment decreased PSII activity to 80%, and PSI activity to 18%. We furthermore analyzed the effect of rSP treatment on PSII, PSI, and the whole-chain photosynthetic electron transport activities. PSII activity was estimated from DMBQ-dependent O2 evolution rates, and no difference was observed between the control and rSP treatment samples (Supplemental Fig. S1A). In contrast, PSI and the whole-chain photosynthetic electron activities, estimated from the MV-dependent O2 absorption rates, were considerably lower in the rSP treatment sample, compared to the control (Supplemental Fig. S1, B and C). These results indicate that rSP treatment induces photoinhibition that is specific to PSI in isolated chloroplasts, which is similar to the results seen in intact leaves (Sejima et al., 2014; Zivcak et al., 2015a, 2015b).
Furthermore, we performed western-blot analyses to confirm whether PSI photoinhibition induced by rSP treatment was accompanied by PSI protein degradation. We found that the amount of PSI core protein PsaA did not decrease after rSP treatment, even though the P700 chlorophyll content was largely decreased (Supplemental Fig. S2). This result indicates that rSP treatment does not stimulate protein degradation in PSI. Likewise, the PSII core protein PsbB did not decrease after rSP treatment (Supplemental Fig. S2). Therefore, rSP treatment-dependent PSI photoinhibition does not involve reductions of PSII and PSI proteins.
Effects of O2 and Methyl Viologen on PSI Photoinhibition in Isolated Chloroplasts
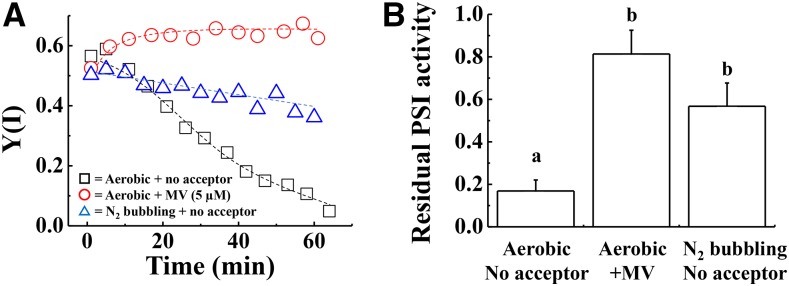
We analyzed the effect of rSP treatment under anaerobic conditions, in order to reveal whether PSI photoinhibition requires O2 in isolated chloroplasts in a similar manner to intact leaves. Nitrogen gas was bubbled through the reaction mixture to remove the diluted O2, and to create anaerobic conditions. The decrease in Y(I) during rSP treatment was alleviated under anaerobic conditions, compared to aerobic conditions (Fig. 2A). Furthermore, the residual activity of PSI after rSP treatment was significantly higher under anaerobic conditions, compared to aerobic conditions (Fig. 2B). Overall, these results indicate that the presence of O2 stimulates PSI photoinhibition.
Figure 2.
A, Time-course analysis of Y(I) in isolated chloroplasts under different experimental conditions. The reaction mixture contained 30 μg ml−1 isolated chloroplasts, and the reaction mixture was maintained at 25°C. Black squares indicate Y(I) in the absence of electron acceptors under aerobic conditions. Red circles indicate Y(I) in the presence of MV (5 μm) under aerobic conditions. Blue triangles indicate Y(I) in the absence of electron acceptors under anaerobic conditions. Experiments were repeated at least three times, and representative data are shown. B, After rSP treatment, the reaction mixture was kept in the dark for 30 min, and the Pm was measured. Data were normalized to Pm before rSP treatment, and are represented as the residual activity of PSI after rSP treatment. Data are expressed as mean ± se of three independent experiments. Different letters above the columns indicate a significant difference between the treatments (Tukey-Kramer HSD test, P < 0.05).
Next, we analyzed the effects of methyl viologen (MV) on the rSP treatment (Fig. 2A). MV accepts electrons from PSI, and the reduced MV subsequently donates electrons to O2 to produce the superoxide radical (O2−) under aerobic conditions (Babbs et al., 1989). In the presence of MV, there was hardly any decrease in Y(I) throughout rSP treatment (Fig. 2A). The residual activity of PSI was significantly higher than when MV was not added (Fig. 2B). In previous studies, PSI photoinhibition was suppressed by limiting photosynthetic electron flow from PSII (Sonoike, 1996; Sejima et al., 2014). That is, accumulation of the oxidized P700 suppresses rSP-dependent PSI photoinhibition (Sejima et al., 2014). Because MV is able to efficiently accept electrons from PSI, this means that the limiting step of the linear electron transport reaction moves from the acceptor side of PSI to plastoquinone (PQ), which is oxidized by cytochrome b6f turnover in the presence of MV (Tikhonov, 2013). MV is thought to have two effects on the oxidation of donor side of P700 in PSI: first, the stimulation of ΔpH formation to suppress the oxidation of reduced PQ (PQH2) by cytochrome b6f complex; and second, a stimulation of electron flow from P700 to O2 to oxidize the photosynthetic electron transport chain.
Effects of the Protonophore Nigericin on PSI Photoinhibition
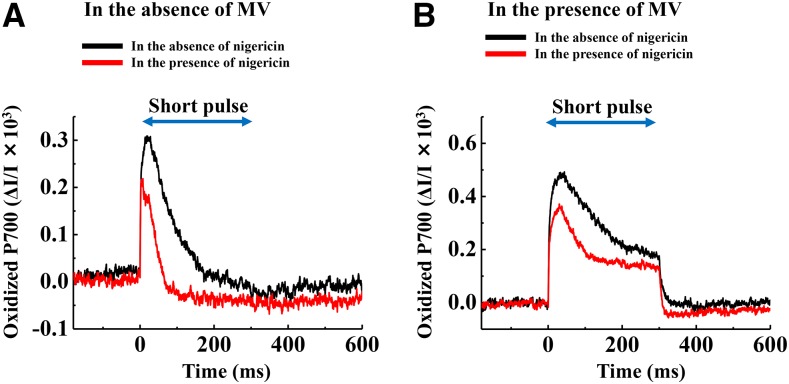
To examine whether MV forms a ΔpH to suppress PSI photoinhibition during rSP treatment, we applied a protonophore, nigericin, to isolated chloroplasts during rSP treatment. In the absence of MV, the addition of nigericin partially accelerated a decline in Y(I) throughout rSP treatment, compared to in the absence of nigericin (Supplemental Fig. S3). This indicated that a ΔpH was generated during rSP treatment and that electron flow from the donor side of PSI to the acceptor side was limited. This would suppress the reduction of O2 and the production of ROS in PSI. Indeed, the kinetics of oxidized P700 chlorophyll during short-pulse illumination differed considerably in the absence and presence of nigericin (Fig. 3A). In the presence of nigericin, oxidized P700 chlorophyll was more rapidly reduced during a short-pulse illumination, compared to when nigericin was not added. This result indicates that a ΔpH value was induced during the short-pulse illumination, and this would contribute to the suppression of photosynthetic electron flow from PSII to oxidized P700 chlorophyll. On the other hand, in the presence of MV, the addition of nigericin to the reaction mixture did not lead to a decrease in Y(I) (Supplemental Fig. S3). This result showed that the reduction in PSI photoinhibition by MV could not be due to the stimulation of ΔpH formation. The kinetics of oxidized P700 chlorophyll during a short-pulse illumination in the presence of MV showed that the oxidized state in P700 chlorophyll was maintained during short-pulse illumination, regardless of the addition of nigericin (Fig. 3B). This result indicated that MV effectively accepts electrons from PSI, and keeps P700 chlorophyll oxidized during a short-pulse illumination.
Figure 3.
Kinetics of oxidized P700 induced by a short-pulse in the absence of MV (A) and in the presence of MV (B). The data were obtained after rSP treatment was applied for 5 min. The black line shows the condition in the absence of nigericin. The red line shows the condition in the presence of nigericin. Experiments were performed at least three times. Short-pulse was illuminated every 10 s, and average data over three short-pulse illuminations are shown. The reaction mixture contained 30 μg ml−1 isolated chloroplasts, and the reaction mixture was maintained at 25°C.
Chloroplastic Superoxide Dismutase and Ascorbate Peroxidase Cannot Protect PSI from Photoinhibition Induced by rSP Treatment
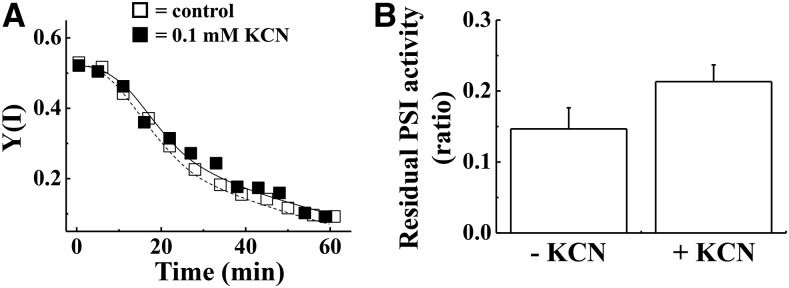
Higher plants have superoxide dismutase (SOD) and ascorbate peroxidase (APX) in their chloroplasts, and they help to detoxify ROS (Miyake and Asada, 1992; Asada, 2000). First, we analyzed the effects of rSP treatment on chloroplastic SOD and APX to assess whether chloroplastic SOD and APX activities are maintained in isolated chloroplasts throughout rSP treatment. A comparison of chloroplastic SOD and APX activities before and after rSP treatment showed that their activities did not change (Supplemental Fig. S4). This result indicated that ROS detoxification activities in isolated chloroplasts were maintained throughout rSP treatment. Furthermore, this result indicated that rSP treatment does not stimulate oxidative stress at the chloroplast stroma, because chloroplast APX activities are sensitive to ROS (Miyake and Asada, 1996; Mano et al., 2001). Thus, PSI photoinhibition induced by rSP treatment is probably independent of chloroplastic SOD and APX activities. To confirm this possibility, we added KCN to the reaction mixture to inhibit chloroplastic CuZn-SOD and APX activities, and applied rSP treatment. In spinach chloroplasts, the almost all SOD activities depend on CuZn-SOD (Asada et al., 1973, 1974). The addition of KCN did not affect the decrease in Y(I) during rSP treatment (Fig. 4A). Furthermore, the residual activity of PSI after rSP treatment did not show significant difference between in the absence or the presence of KCN (Fig. 4B). These results suggest that chloroplastic CuZn-SOD and APX do not protect PSI from photoinhibition induced by rSP treatment.
Figure 4.
Effect of KCN on PSI photoinhibition induced by rSP treatment. The reaction mixture contained 30 μg ml−1 isolated chloroplasts, and the reaction mixture was maintained at 25°C. A, Time-course analysis of Y(I) in isolated chloroplasts in the absence (white squares) and presence of KCN (0.1 mm; black squares). Experiments were repeated at least three times, and representative data are shown. B, After rSP treatment, the reaction mixture was kept in the dark for 30 min, and the Pm was measured. Data were normalized to the Pm before rSP treatment, and the residual activity of PSI after rSP treatment was calculated. Data are expressed as mean ± se of three independent experiments. Student’s t test revealed that there is no significant difference in the residual activities in between the absence and the presence of KCN (P > 0.1).
Surprisingly, we found that, even though KCN was present, MV suppressed PSI photoinhibition, when the production of O2− was enhanced during rSP treatment (Supplemental Fig. S5). From these facts, PSI photoinhibition would not be related to the production rate of ROS, and the scavenging of ROS outside the thylakoid membranes. Next, we studied the molecular species of ROS that is produced during rSP treatment.
Both O2− and 1O2 Contribute to PSI Photoinhibition Induced by rSP Treatment
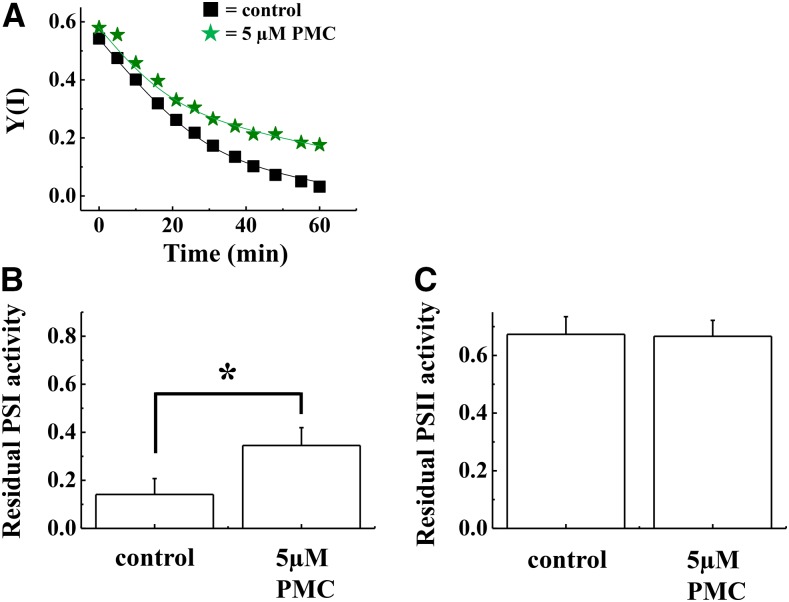
PSI is a major production site of ROS, and O2− has been generally recognized as a main ROS to be produced at PSI (Takahashi and Asada, 1982). However, recent reports provide evidence that PSI also produces 1O2, which is related to PSI photoinhibition (Cazzaniga et al., 2012; Rutherford et al., 2012). Furthermore, we showed that inactivation of APX and CuZn-SOD does not affect PSI photoinhibition (Fig. 4). This might indicate that PSI photoinhibition was induced by 1O2 but not O2− during rSP treatment. To examine the possibility whether 1O2 relates to PSI photoinhibition induced by rSP treatment, we added a soluble α-tocopherol analog, 2, 2, 5, 7, 8-pentamethyl-6-chromanol (PMC), which is an amphipathic 1O2 scavenger, to the reaction mixture, and conducted rSP treatment (Fryer, 1992; Grams and Inglett, 1972; Munné-Bosch, 2005). The addition of PMC did not affect the early decrease in Y(I) during rSP treatment (Fig. 5A). In contrast, PMC alleviated the late decrease in Y(I), compared to that seen in the control (Fig. 5A). The residual activity of PSI after rSP treatment for 1 h revealed that the addition of PMC significantly alleviated the inactivation of PSI, compared to control (Fig. 5B). In contrast, the residual activity of PSII was not different in the absence and the presence of PMC (Fig. 5C).
Figure 5.
Effect of PMC on PSI photoinhibition induced by rSP treatment. The reaction mixture contains 30 μg ml−1 isolated chloroplasts, and the reaction mixture was maintained at 25°C. A, Time-course analysis of Y(I) in isolated chloroplasts in the absence (black squares) and presence of PMC (5 μm; green stars). Experiments were repeated at least three times, and representative data are shown. B and C, After rSP treatment, the reaction mixture was kept in the dark for 30 min, and the Pm and Fv/Fm values were measured. Data were normalized to the Pm and Fv/Fm values before rSP treatment, and the residual activity of PSI (B) and PSII (C) after rSP treatment were calculated. Data are expressed as mean ± se of three independent experiments.
To examine whether PMC acts as an electron acceptor like MV, we analyzed the kinetics of oxidized P700 chlorophyll during the short-pulse illumination. We found that PMC did not affect the kinetics of oxidized P700 chlorophyll during a short-pulse illumination (Supplemental Fig. S6). Furthermore, we compared the O2 absorption rate and Y(II) in the absence and presence of PMC using an O2-electrode and chlorophyll fluorescence. Under continuous actinic light (AL) illumination (500 μE m−2 s−1), PMC did not affect O2 absorption rate and Y(II) in isolated chloroplasts (Supplemental Table S1). From these results, we revealed that PMC does not act as an electron acceptor. Furthermore, doubling the PMC concentration from 5 μm to 10 μm did not affect the residual activity of PSI after rSP treatment for 1 h [PSI residual activity = 0.35 ± 0.06 (n = 3)]. This result indicated that 5 μm PMC is enough to protect PSI from photoinhibition induced by 1O2.
DISCUSSION
In this study, we aimed to reveal the mechanisms by which rSP treatment induces photoinhibition specific to PSI by using isolated chloroplasts. We observed that rSP treatment induces PSI-specific photoinhibition in isolated chloroplasts when O2 was present, in a similar manner to leaves (Sejima et al., 2014). In addition, rSP treatment caused a limitation of the photosynthetic electron transport reaction at the acceptor side in PSI (Fig. 1; Supplemental Fig. S1). These results suggest that rSP treatment critically affected chloroplasts, but other organelles were not involved in PSI photoinhibition.
We propose that PSI photoinhibition is triggered by ROS produced within the thylakoid membranes. Although several components of ROS production have been proposed in previous reports, the ROS production mechanism in PSI has not been clarified (Misra and Fridovich, 1971; Miyake et al., 1998; Asada, 2000; Voss et al., 2011; Kozuleva and Ivanov, 2010). ROS are known to trigger PSI photoinhibition and to degrade its components because PSI photoinhibition requires O2 (Allahverdiyeva et al., 2005; Sonoike and Terashima, 1994; Sonoike, 1996). However, the production site of the ROS that trigger PSI photoinhibition has been not clarified. In our study, we observed that the addition of MV to isolated chloroplasts greatly alleviates PSI photoinhibition induced by rSP treatment (Fig. 2). This result is consistent with a previous report on thylakoid membranes (Sonoike, 1996). A relationship between the suppression of PSI photoinhibition and oxidation of P700 in PSI would clarify the molecular mechanism of MV to alleviate PSI photoinhibition. We have thought that an increase in P700+ decreases the ratio of the ground state P700 and excited P700 against the total P700 amount, of which both can donate electrons to O2 to produce O2− in the thylakoid membranes. However, even in the presence of KCN or nigericin, the presence of MV suppressed rSP-treatment-dependent PSI photoinhibition (Supplemental Figs. S3 and S5). These situations should stimulate the production of ROS in the chloroplast thylakoid membranes and stroma. Based on these results, the production rate of ROS, and the quantity of ROS should not relate to the PSI photoinhibition in chloroplasts. Therefore, we suggest that the production site of ROS, rather than the quantity of ROS, is important for causing PSI photoinhibition.
MV accepts electrons from PSI and stimulates the production of O2− through their radical form (MV; Babbs et al., 1989). Then, where is ROS produced by MV⋅? According to Kozuleva et al. (2014), the midpoint redox potential (Em) of O2/O2− in the lipid phase is a lower value (−500 mV/−600 mV vs. normal hydrogen electrode), compared to the Em value of O2/O2− in the aqueous phase. Since MV accepts electrons from electron carriers in PSI [FX, FA, or FB; their Em values are higher than the Em value of O2/O2− in the lipid phase (Kozuleva et al., 2014)], MV would have a higher Em value than the Em value of O2/O2− in the lipid phase (Michaelis and Hill, 1933; Stombaugh et al., 1976). Based on the latter and thermodynamics, MV· effectively reduces O2 to produce O2− at the stromal region in chloroplasts, although the reduction of O2 within the thylakoid membranes might be insufficient. Furthermore, we used 5 μm MV in the rSP treatment. Under these conditions, MV reacts with FA/FB at the stromal side in PSI (Sonoike and Terashima, 1994). Based on this report, MV would stimulate the production of ROS in the chloroplast stroma, and we suggest ROS produced in the stromal region does not contribute to PSI photoinhibition. This suggestion is also supported by the result that chloroplastic CuZn-SOD and APX activities were independent of PSI photoinhibition (Fig. 4; Supplemental Figs. S4 and S5).
In addition to the above considerations, we also suggest the possibility that MV-dependent oxidation of the electron transport chain alleviated PSI photoinhibition. A recent study showed that P700 in PSI is oxidized under electron sink-limitation conditions in the cyanobacteria Synechococcus elongatus PCC 7942 (Shaku et al., 2015). Shaku et al. (2015) demonstrated that an oxidized high-potential chain, including cytochrome f and plastocyanin-scavenged O2−, was produced in thylakoid membranes. This scavenging rate was comparable to the electron flow rate in linear electron transport (Tanaka et al., 1978; Takahashi et al., 1980). We observed that MV successfully oxidized the P700 during a short-pulse illumination (Fig. 3). Therefore, the stimulated oxidation of photosynthetic electron transport chain by MV could scavenge O2−, even though the production of ROS was stimulated (Supplemental Fig. S5).
In leaves, PSI photoinhibition induced by rSP treatment is suppressed under continuous AL illumination, compared to dark conditions (Sejima et al., 2014). This is because constant AL illumination stimulates the oxidation of PSI by activating the electron sink in photosynthesis (like the Calvin cycle and photorespiration) and the formation of ΔpH across the thylakoid membrane (Zaks et al., 2012). Indeed, we observed that ΔpH and electron donation from PSI to MV protect PSI photoinhibition in isolated chloroplasts (Fig. 3). In the study by Sejima et al. (2014), PSI photoinhibition was suppressed, in accordance with the induction of Y(ND) under AL illumination. Based on this result, in leaves, the formation of a large ΔpH across the thylakoid membranes is more critical for the protection of PSI photoinhibition, compared to the consumption of electrons in the electron sink in photosynthesis. Therefore, these results and those by Sejima et al. (2014) show that rSP treatment under dark conditions promotes the reduction of electron carriers in PSI without activating the electron sink and forming a large ΔpH, which suppresses the photosynthetic electron transport reaction from PSII to PSI in vivo. Accordingly, PSI photoinhibition would be triggered by the production of ROS within the thylakoid membranes in vivo, in the same way as in isolated chloroplasts.
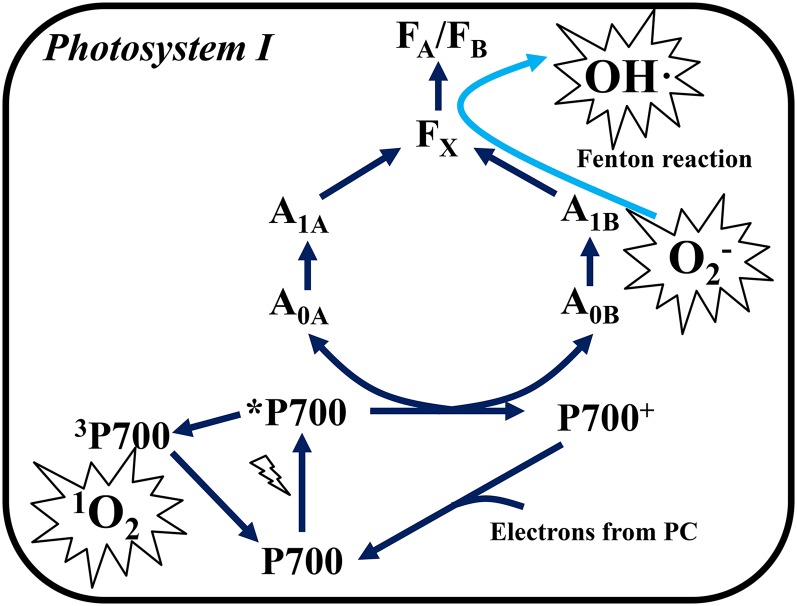
We propose that PSI photoinhibition proceeds in two different ways by the different kinds of ROS produced within the thylakoid membrane (Fig. 6). First, the production of O2− and OH· at a secondary electron acceptor in PSI induces PSI photoinhibition (Takahashi and Asada 1988; Sonoike et al., 1995). PSI has four electron acceptors (A0 as a primary acceptor, and A1, FX, and FA/FB as secondary acceptors). It has been suggested that the ROS production site in PSI is within the thylakoid membranes and that the secondary acceptor, A1, is a major contributor to the Mehler reaction in vivo (Kozuleva and Ivanov, 2010; Kozuleva et al., 2014). Two types of A1, which have different Em values (A1a, −671 mV; A1b, −844 mV), exist in PSI, and A1a and A1b are coupled with PsaA and PsaB, respectively (Rutherford et al., 2012). The degradation of PsaB is more promoted during PSI photoinhibition, compared to the degradation of PsaA (Sonoike, 1996; Sonoike et al., 1997). Therefore, A1b, which has a lower Em value than A1a, might be a primary ROS production site in PSI, and O2− produced at A1b could trigger PSI photoinhibition (Sonoike and Terashima, 1994). We suggest that ROS production and the oxidative attacks by ROS in PSI occur within the thylakoid membranes. Therefore, chloroplastic SOD and APX cannot protect PSI from its photoinhibition (Fig. 4; Supplemental Fig. S4). In fact, Takahashi and Asada (1988) reported that O2 reduction detected by O2−-dependent cytochrome c reduction was stimulated in thylakoid membranes that were disintegrated by detergent. This means that the O2 reduction site does not exist on the thylakoid membrane surface. Furthermore, they revealed that high concentrations of NH4Cl, which induced the protonation of O2−, stimulated the production of H2O2 in thylakoid membranes, and simultaneously NH4Cl suppressed the O2−-dependent cytochrome c reduction (Takahashi and Asada, 1988). From these observations, they concluded that O2− was produced in the aprotic region of the thylakoid membranes, that is, interior regions of the thylakoid membrane. Their conclusion is consistent with our observations.
Figure 6.
The scheme of ROS production and photoinhibition in PSI. When electron carriers are highly reduced in PSI, the production of O2− is stimulated at the A1 site (Kozuleva and Ivanov, 2010; Kozuleva et al., 2014). O2− either directly attacks the peripheral component, or is converted to OH· via the Fenton reaction between the iron-sulfur center in FX, FA, or FB in the diffusing process (Inoue et al., 1986; Sonoike et al., 1995; Takahashi and Asada, 1988). The limitation of electron transfer from P700 to downstream electron acceptors would suppress the charge separation between P700 and chlorophyll A0 (Shuvalov et al., 1986). Furthermore, the production of the P700 triplet-state (3P700) is initiated (Rutherford et al., 2012; Shuvalov et al., 1986). 3P700 reacts with O2 to produce singlet O2 (1O2). 1O2 attacks the peripheral component in PSI, and precedes PSI photoinhibition (Cazzaniga et al., 2012). These reactions proceed within the thylakoid membranes. Therefore, a decrease in P700 chlorophyll occurs, but protein degradation does not accompany PSI photoinhibition. Blue arrows indicate the energy transfer pathway in PSI, and the light-blue arrow indicates the diffusion of O2− in the thylakoid membranes (Takahashi and Asada, 1988).
Second, the production of 1O2 by charge recombination in PSI proceeds further PSI photoinhibition (Fig. 6). We revealed that PMC, which acts as a 1O2 scavenger, suppressed the decrease in Y(I) in isolated chloroplasts, and PMC significantly protected PSI activity after rSP treatment for 1 h, compared to the control (Fig. 5). In a similar way to P680 chlorophyll, the transient state of the excited P700 chlorophyll in PSI is de-excited to the triplet state (3P700) through charge recombination, and then the 3P700 reacts with O2 to produce 1O2 (Shuvalov et al., 1986; Rutherford et al., 2012). In a previous study, it has been reported that 1O2 is not produced in PSI under photoinhibitory conditions for PSII (Hideg and Vass, 1995). However, in that study, the decrease in PSI activity was not observed under photoinhibitory conditions although PSII activity was successfully impaired (Hideg and Vass, 1995). This might indicate that electron carriers in PSI did not become reduced to produce ROS in PSI under photoinhibitory conditions for PSII. In contrast, Cazzaniga et al. (2012) reported that 1O2 is produced in the PSI-LHCI complex isolated from Arabidopsis (Arabidopsis thaliana). Furthermore, they also reported that the Arabidopsis szl1 mutant, which contains less β-carotene than the wild-type, is susceptible to PSI photoinhibition under high-light and low-temperature conditions. This means that 1O2 produced in PSI caused PSI photoinhibition because β-carotene is a major 1O2 quencher (Telfer, 2014). Therefore, we suggest that 1O2 is actually produced in PSI, and 1O2 is involved in PSI photoinhibition in higher plants. In PSI photoinhibition, FA, FB, and FX were primarily destroyed and they lost electron transport activity (Inoue et al., 1986; Sonoike et al., 1995). Based on these reports, O2− produced at A1 would diffuse in the thylakoid membrane and react with the [Fe-S] cluster in FX, FA, or FB. Furthermore, OH· produced from O2− through the Fenton reaction would then inactivate their electron transfer activities (Takahashi and Asada, 1988; Sonoike et al., 1997). When the electron transport reaction in PSI is limited after the destruction of FX, FA, and FB, the charge separation of P700 chlorophyll would be suppressed, and then the charge recombination between P700+ and A0, or A1 could be further accelerated (Shuvalov et al., 1986; Kozuleva and Ivanov, 2010).
We observed that PSI core protein content did not decrease after PSI photoinhibition, even though P700 chlorophyll content and PSI activity largely decreased in isolated chloroplasts as well as in leaves (Supplemental Fig. S2). Previous studies also showed that the decrease in PSI activity and P700 chlorophyll content does not correlate with the decrease in PSI protein content after PSI photoinhibition (Sonoike et al.,1997; Tjus et al., 1998; Zhang and Scheller, 2004). Therefore, these results indicate that PSI protein degradation is not the first step of PSI photoinhibition. These results support the idea that ROS produced within the thylakoid membrane specifically target electron transport carriers in PSI, and hence precede PSI photoinhibition.
We have shown that rSP treatment stimulated specific PSI photoinhibition in vitro. Furthermore, the kinetics of Y(I) during rSP treatment were quite similar in the leaves and isolated chloroplasts (Fig. 1A; Sejima et al., 2014; Zivcak et al., 2015a, 2015b). To date, there has not been a common method to cause PSI photoinhibition in vivo and in vitro. This means that the rSP treatment improves PSI photoinhibition analysis efficiency. Because PSI photoinhibition is a severe phenomenon, reducing plant fitness, the identification of the protection mechanism against PSI photoinhibition would contribute to the improvement of plant fitness. We suggest that the rSP treatment should make it possible to uncover the protection mechanism against PSI photoinhibition through a forward or reverse genetic approach, such as the selection of mutagenized plants that have a higher susceptibility or tolerance to PSI photoinhibition.
MATERIALS AND METHODS
Isolation of Intact Chloroplasts from Spinach Leaves
Intact chloroplasts were isolated from spinach (Spinacia oleracea) leaves purchased from a local market and purified by a Percoll density gradient centrifugation, as described previously by Takagi et al (2012). The isolated chloroplasts were suspended in a reaction mixture of HEPES-KOH [50 mm 4-(2-hydroxyethyl) piperazine-1-ethansulfonic acid-potassium hydrate], pH 7.6; 0.33 m sorbitol; 10 mm NaCl (sodium chloride); 1 mm MgCl2 (magnesium chloride); 2 mm EDTA; and 0.5 mm KH2PO4 (monopotassium phosphate). The intactness of the purified chloroplasts was determined with the ferricyanide method (Heber and Santarius, 1970), and 85% to 95% of the intact chloroplasts were used in our experiment. The chloroplast chlorophyll content was determined as described previously by Arnon (1949).
Oxygen Exchange Analysis in Isolated Chloroplasts
A chloroplast suspension that had either been incubated in the dark for 1 h or subjected to repetitive short pulse (rSP) treatment for 1 h was placed in an oxygen electrode cuvette (DW 2/2; Hansatech, King’s Lyn, UK) equipped with actinic red light (AL; >640 nm). Temperature was maintained at 25°C and controlled by circulating temperature-controlled water through the water jacket. The PSII, PSI, and whole-chain activities were measured as described by Miyake and Okamura (2003). PSII activity in the chloroplasts was determined by the O2 evolution rate. To achieve this, 2, 6-dimethyl benzoquinone (500 μm) and nigericin (0.5 μm) were added to the chloroplast suspension, and illuminated with AL. PSI activity was determined by the O2 absorption rate. For this measurement, dichlorophenolindophenol (70 μm), nigericin (0.5 μm), KCN (0.1 mm), ascorbate (1 mm), methyl viologen (100 μm), and 3-(3,4‐dichlorophenyl)‐1,1‐dimethylurea (10 μm) were added to the chloroplast suspension, and illuminated with AL. Whole-chain activity of the photosynthetic electron transport reaction was determined by the O2 absorption rate. For this measurement, nigericin (0.5 μm), KCN (0.1 mm), and methyl viologen (100 μm) were also added to the chloroplast suspension, and illuminated with AL.
Enzyme Assay of Chloroplastic SOD and APX
Chloroplastic APX activities were determined in a reaction mixture containing isolated chloroplasts (60 μg Chl), 50 mm potassium phosphate (pH 7.0), 0.5 mm ascorbate, and 5 mm H2O2 (Miyake and Asada, 1992). APX activities were determined by measuring the H2O2-dependent oxidation of ascorbate. The oxidation of ascorbate was monitored by the decrease in A290 (the absorption coefficient was 2.8 mm−1 cm−1). Chloroplastic SOD activities were determined using the xanthine oxidase/cytochrome c method (McCord and Fridovich, 1969). Chloroplastic SOD activities were measured in a reaction mixture containing isolated chloroplasts (60 μg Chl), 300 mm potassium phosphate (pH 8.0), 60 μm cytochrome c, 0.3 mm xanthine, and 120 mU xanthine oxidase. The reduction of cytochrome c was measured at 550 nm.
Measurement of Chlorophyll Fluorescence and P700+
Chlorophyll fluorescence and P700+ were simultaneously measured with a Dual-PAM-100 measuring system (Heintz Walz, Effeltrich, Germany). The reaction mixture was maintained at 25°C by temperature control unit US-T (Heintz Walz). The chlorophyll fluorescence parameters were calculated according to Baker (2008): maximum quantum efficiency of PSII photochemistry, Fv/Fm = (Fm − Fo)/Fm; quantum yield of photochemical energy conversion in PSII, Y(II) = (Fm′ − Fs)/Fm′; Fo, minimum fluorescence yield; Fm, maximum fluorescence yield; and Fs, steady-state fluorescence yield. Measuring light (0.1 μE m−2 s−1) and saturated pulse (20,000 μE m−2 s−1, 300 ms) were applied to determine Fo and Fm. The oxidation-reduction state of P700+ was determined according to the methods of Klughammer and Schreiber (1994) as follows: quantum yield of photochemical energy in PSI, Y(I) = (Pm′ − P)/Pm; quantum yield of nonphotochemical quenching due to the acceptor side limitation, Y(NA) = (Pm − Pm′)/Pm; and quantum yield of nonphotochemical quenching due to the donor side limitation, Y(ND) = P/Pm. The maximum oxidation level of P700 chlorophyll (Pm) was obtained by a saturated pulse under far-red light, which reflected the maximum amount of photooxidized P700 chlorophyll. The parameter P reflects the steady-state oxidation level of P700+, and Pm′ was obtained by a saturated pulse at a steady state.
rSP Treatment
rSP treatment was applied to chloroplasts that had been isolated from spinach leaves, as described previously by Sejima et al. (2014). Isolated chloroplasts were illuminated with short pulses (20,000 μE m−2 s−1, 300 ms) every 10 s in the absence of AL for 1 h under the experimental conditions indicated in the figure legends. During rSP treatment, the reaction mixture containing isolated chloroplasts was maintained at 25°C by temperature control unit US-T (Heintz Walz). After rSP treatment, the photosynthetic parameters of PSII and PSI activity were determined as described in the figure legends.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
For the protein analysis, isolated chloroplasts suspended in the reaction mixture were collected by the centrifugation at 5000g for 1 min at 4°C. After the centrifugation, the isolated chloroplasts were resuspended in the protein-solubilizing solution [80 mm Tris-HCl (pH 6.8), 1 mm EDTA, 5% glycerol (v/v), 2% n-octyl-β-d-glycoside (w/v)]. The isolated chloroplasts were electrophoresed on 12.5% (w/v) sodium dodecyl sulfate-polyacrylamide gels containing 8 m urea, as described by Laemmli (1970).
Western Blotting
The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Billerica, MA), and then blocked with the commercially available Blocking One reagent (Nakalai Tesque, Kyoto, Japan) for 30 min at room temperature (25°C). The PVDF membrane was subsequently incubated with a specific peptide antibody for 1 h at room temperature (25°C). The PVDF membrane was washed three times with TBS Tween buffer [10 mm Tris-HCl (pH 7.4), 0.14 m NaCl, 0.1% (v/v) Tween 20] and incubated with ECL peroxidase-labeled secondary anti-rabbit antibody (GE Healthcare, Buckinghamshire, UK) for 1 h at room temperature (25°C). The PVDF membrane was washed three times with TBS TWEEN buffer. Proteins were detected with an alkaline phosphatase labeling kit. The protein content was quantified by ImageJ version 1.49 (National Institutes of Health, Bethesda, MD). The protein content after rSP treatment was expressed by relative value, compared to control sample.
Statistical Analysis
All data were expressed as mean ± se of at least three independent analyses. We used ANOVAs, Student’s t tests, and Tukey-Kramer HSD tests to detect differences. All statistical analyses were performed using Microsoft Excel 2010 (Microsoft, Redmond, WA) and JMP8 (SAS Institute, Tokyo, Japan).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The light response of PSII, PSI, and whole-chain photosynthetic electron transport activity in isolated chloroplasts before and after rSP treatment.
Supplemental Figure S2. The comparison of PSII and PSI core protein content in isolated chloroplast between before and after rSP treatment.
Supplemental Figure S3. The effect of nigericin on rSP treatment in the absence and presence of MV.
Supplemental Figure S4. The change in SOD and APX activities before and after rSP treatment.
Supplemental Figure S5. The effect of KCN on short-pulse treatment in the absence and presence of MV.
Supplemental Figure S6. The kinetics of oxidized P700 induced by a short-pulse in the absence of and in the presence of PMC.
Supplemental Table S1. Oxygen evolution rate and Y(II) in the absence and in the presence of PMC.
Supplementary Material
Acknowledgments
The authors thank assistant professor Dr. Kentaro Ifuku and Mr. Taishi Nishimura in Kyoto University for giving us fruitful advice about the experimental technique. We also thank Professor Dr. Isao Enami in Tokyo University of Science, Professor Dr. Kintake Sonoike in Waseda University, and Professor Dr. Toshiharu Shikanai in Kyoto University for giving us antibodies for western-blot analysis. Finally, the authors thank Editage (Cactus Communications Inc., http://www.editage.jp/) for editing our manuscript.
Glossary
- AL
actinic light
- MV
methyl viologen
- PMC
2, 2, 5, 7, 8-pentamethyl-6-chromanol
- PQ
plastoquinone
- PS
photosystem
- ROS
reactive oxygen species
- rSP
repetitive short pulse
Footnotes
Articles can be viewed without a subscription.
References
- Allahverdiyeva Y, Mamedov F, Mäenpää P, Vass I, Aro EM (2005) Modulation of photosynthetic electron transport in the absence of terminal electron acceptors: characterization of the rbcL deletion mutant of tobacco. Biochim Biophys Acta 1709: 69–83 [DOI] [PubMed] [Google Scholar]
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Asada K. (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In Kyle DJ, Osmond CB, Arntzen CJ, eds, Photoinhibition. Elsevier Science, Amsterdam, The Netherlands, pp 227–287 [Google Scholar]
- Asada K, Takahashi M, Nagate M (1974) Assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 38: 471–473 [Google Scholar]
- Asada K, Urano M, Takahashi M (1973) Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem 36: 257–266 [DOI] [PubMed] [Google Scholar]
- Babbs CF, Pham JA, Coolbaugh RC (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90: 1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Bondarava N, Gross CM, Mubarakshina M, Golecki JR, Johnson GN, Krieger-Liszkay A (2010) Putative function of cytochrome b559 as a plastoquinol oxidase. Physiol Plant 138: 463–473 [DOI] [PubMed] [Google Scholar]
- Cazzaniga S, Li Z, Niyogi KK, Bassi R, Dall’Osto L (2012) The Arabidopsis szl1 mutant reveals a critical role of β-carotene in photosystem I photoprotection. Plant Physiol 159: 1745–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132: 273–285 [DOI] [PubMed] [Google Scholar]
- Fryer MJ. (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15: 381–392 [Google Scholar]
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48: 909–930 [DOI] [PubMed] [Google Scholar]
- Grams G, Inglett GE (1972) Sensitized photooxidation of α-tocopherol and of 2, 2, 5, 7, 8-pentamethyl-6-chromanol in ethyl acetate. Lipids 7: 442–444 [Google Scholar]
- Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro EM (2012) Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol 160: 1896–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Santarius KA (1970) Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B 25: 718–728 [DOI] [PubMed] [Google Scholar]
- Hideg E, Kós PB, Vass I (2007) Photosystem II damage induced by chemically generated singlet oxygen in tobacco leaves. Physiol Plant 131: 33–40 [DOI] [PubMed] [Google Scholar]
- Hideg É, Vass I (1995) Singlet oxygen is not produced in photosystem I under photoinhibitory conditions. Photochem Photobiol 62: 949–952 [Google Scholar]
- Inoue K, Sakurai H, Hiyama T (1986) Photoinactivation sites of photosystem I in isolated chloroplasts. Plant Cell Physiol 27: 961–968 [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192: 261–268 [Google Scholar]
- Kojima K, Oshita M, Nanjo Y, Kasai K, Tozawa Y, Hayashi H, Nishiyama Y (2007) Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Mol Microbiol 65: 936–947 [DOI] [PubMed] [Google Scholar]
- Kono M, Noguchi K, Terashima I (2014) Roles of the cyclic electron flow around PSI (CEF-PSI) and O₂-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol 55: 990–1004 [DOI] [PubMed] [Google Scholar]
- Kozuleva MA, Ivanov BN (2010) Evaluation of the participation of ferredoxin in oxygen reduction in the photosynthetic electron transport chain of isolated pea thylakoids. Photosynth Res 105: 51–61 [DOI] [PubMed] [Google Scholar]
- Kozuleva MA, Petrova AA, Mamedov MD, Semenov AY, Ivanov BN (2014) O2 reduction by photosystem I involves phylloquinone under steady-state illumination. FEBS Lett 588: 4364–4368 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A. (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56: 337–346 [DOI] [PubMed] [Google Scholar]
- Külheim C, Ågren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Mano J, Ohno C, Domae Y, Asada K (2001) Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochim Biophys Acta 1504: 275–287 [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055 [PubMed] [Google Scholar]
- Melis A. (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage? Trends Plant Sci 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Michaelis L, Hill ES (1933) The viologen indicators. J Gen Physiol 16: 859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra HP, Fridovich I (1971) The generation of superoxide radical during the autoxidation of ferredoxins. J Biol Chem 246: 6886–6890 [PubMed] [Google Scholar]
- Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33: 541–553 [Google Scholar]
- Miyake C, Asada K (1996) Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37: 423–430 [Google Scholar]
- Miyake C, Okamura M (2003) Cyclic electron flow within PSII protects PSII from its photoinhibition in thylakoid membranes from spinach chloroplasts. Plant Cell Physiol 44: 457–462 [DOI] [PubMed] [Google Scholar]
- Miyake C, Schreiber U, Hormann H, Sano S, Asada K (1998) The FAD-enzyme monodehydroascorbate radical reductase mediates photoproduction of superoxide radicals in spinach thylakoid membranes. Plant Cell Physiol 39: 821–829 [Google Scholar]
- Munekage YN, Genty B, Peltier G (2008) Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Physiol 49: 1688–1698 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S. (2005) The role of α-tocopherol in plant stress tolerance. J Plant Physiol 162: 743–748 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142: 35–46 [DOI] [PubMed] [Google Scholar]
- Oelze ML, Vogel MO, Alsharafa K, Kahmann U, Viehhauser A, Maurino VG, Dietz KJ (2012) Efficient acclimation of the chloroplast antioxidant defence of Arabidopsis thaliana leaves in response to a 10- or 100-fold light increment and the possible involvement of retrograde signals. J Exp Bot 63: 1297–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford AW, Osyczka A, Rappaport F (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: redox tuning to survive life in O2. FEBS Lett 586: 603–616 [DOI] [PubMed] [Google Scholar]
- Sejima T, Takagi D, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55: 1184–1193 [DOI] [PubMed] [Google Scholar]
- Shaku K, Shimakawa G, Hashiguchi M, Miyake C (2015) Reduction-induced suppression of electron flow (RISE) in the photosynthetic electron transport system of Synechococcus elongatus PCC 7942. Plant Cell Physiol http://dx.doi.org/10.1093/pcp/pcv198 [DOI] [PubMed] [Google Scholar]
- Shuvalov VA, Nuijs AM, van Gorkom HJ, Smit HWJ, Duysens LNM (1986) Picosecond absorbance changes upon selective excitation of the primary electron donor P-700 in photosystem I. Biochim Biophys Acta Bioenerg 850: 319–323 [Google Scholar]
- Sonoike K. (1996) Degradation of psaB gene product, the reaction center subunit of photosystem I, is caused during photoinhibition of photosystem I: possible involvement of active oxygen species. Plant Sci 115: 157–164 [Google Scholar]
- Sonoike K. (2011) Photoinhibition of photosystem I. Physiol Plant 142: 56–64 [DOI] [PubMed] [Google Scholar]
- Sonoike K. (1995) Selective photoinhibition of photosystem I in isolated thylakoid membranes from cucumber and spinach. Plant Cell Physiol 36: 825–830 [Google Scholar]
- Sonoike K, Kamo M, Hihara Y, Hiyama T, Enami I (1997) The mechanism of the degradation of psaB gene product, one of the photosynthetic reaction center subunits of photosystem I, upon photoinhibition. Photosynth Res 53: 55–63 [Google Scholar]
- Sonoike K, Terashima I (1994) Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta 194: 287–293 [Google Scholar]
- Sonoike K, Terashima I, Iwaki M, Itoh S (1995) Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett 362: 235–238 [DOI] [PubMed] [Google Scholar]
- Stombaugh NA, Sundquist JE, Burris RH, Orme-Johnson WH (1976) Oxidation-reduction properties of several low potential iron-sulfur proteins and of methylviologen. Biochemistry 15: 2633–2641 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi D, Yamamoto H, Amako K, Makino A, Sugimoto T, Miyake C (2012) O2 supports 3-phosphoglycerate-dependent O2 evolution in chloroplasts from spinach leaves. Soil Sci Plant Nutr 58: 462–468 [Google Scholar]
- Takahashi M, Asada K (1982) Dependence of oxygen affinity for Mehler reaction on photochemical activity of chloroplast thylakoids. Plant Cell Physiol 23: 1457–1461 [Google Scholar]
- Takahashi M, Asada K (1988) Superoxide production in aprotic interior of chloroplast thylakoids. Arch Biochem Biophys 267: 714–722 [DOI] [PubMed] [Google Scholar]
- Takahashi MA, Kono Y, Asada K (1980) Reduction of plastocyanin with O2- and superoxide dismutase-dependent oxidation of plastocyanin by H2O2. Plant Cell Physiol 21: 1431–1438 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Takahashi MA, Asada K (1978) Isolation of monomeric cytochrome f from Japanese radish and a mechanism of autoreduction. J Biol Chem 253: 7397–7403 [PubMed] [Google Scholar]
- Telfer A. (2014) Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of β-carotene. Plant Cell Physiol 55: 1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I, Funayama S, Sonoike K (1994) The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 193: 300–306 [Google Scholar]
- Tikhonov AN. (2013) pH-dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynth Res 116: 511–534 [DOI] [PubMed] [Google Scholar]
- Tjus SE, Møller BL, Scheller HV (1998) Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures. Plant Physiol 116: 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I. (2011) Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol Plant 142: 6–16 [DOI] [PubMed] [Google Scholar]
- Voss I, Goss T, Murozuka E, Altmann B, McLean KJ, Rigby SE, Munro AW, Scheibe R, Hase T, Hanke GT (2011) FdC1, a novel ferredoxin protein capable of alternative electron partitioning, increases in conditions of acceptor limitation at photosystem I. J Biol Chem 286: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR (2012) A kinetic model of rapidly reversible nonphotochemical quenching. Proc Natl Acad Sci USA 109: 15757–15762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Scheller HV (2004) Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol 45: 1595–1602 [DOI] [PubMed] [Google Scholar]
- Zivcak M, Brestic M, Kunderlikova K, Olsovska K, Allakhverdiev SI (2015a) Effect of photosystem I inactivation on chlorophyll a fluorescence induction in wheat leaves: does activity of photosystem I play any role in OJIP rise? J Photochem Photobiol B 152(Pt B): 318–324 [DOI] [PubMed] [Google Scholar]
- Zivcak M, Brestic M, Kunderlikova K, Sytar O, Allakhverdiev SI (2015b) Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth Res 162: 449–463 [DOI] [PubMed] [Google Scholar]
- Zulfugarov IS, Tovuu A, Eu YJ, Dogsom B, Poudyal RS, Nath K, Hall M, Banerjee M, Yoon UC, Moon YH, et al. (2014) Production of superoxide from Photosystem II in a rice (Oryza sativa L.) mutant lacking PsbS. BMC Plant Biol 14: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.