The plasma membrane-localized receptor kinase SERK1 is present in a complex with the tomato immune receptor Mi-1.2.
Abstract
Somatic embryogenesis receptor kinases (SERKs) are transmembrane receptors involved in plant immunity. Tomato (Solanum lycopersicum) carries three SERK members. One of these, SlSERK1, is required for Mi-1.2-mediated resistance to potato aphids (Macrosiphum euphorbiae). Mi-1.2 encodes a coiled-coil nucleotide-binding leucine-rich repeat protein that in addition to potato aphids confers resistance to two additional phloem-feeding insects and to root-knot nematodes (Meloidogyne spp.). How SlSERK1 participates in Mi-1.2-mediated resistance is unknown, and no Mi-1.2 cognate pest effectors have been identified. Here, we study the mechanistic involvement of SlSERK1 in Mi-1.2-mediated resistance. We show that potato aphid saliva and protein extracts induce the Mi-1.2 defense marker gene SlWRKY72b, indicating that both saliva and extracts contain a Mi-1.2 recognized effector. Resistant tomato cultivar Motelle (Mi-1.2/Mi-1.2) plants overexpressing SlSERK1 were found to display enhanced resistance to potato aphids. Confocal microscopy revealed that Mi-1.2 localizes at three distinct subcellular compartments: the plasma membrane, cytoplasm, and nucleus. Coimmunoprecipitation experiments in these tomato plants and in Nicotiana benthamiana transiently expressing Mi-1.2 and SlSERK1 showed that Mi-1.2 and SlSERK1 colocalize only in a microsomal complex. Interestingly, bimolecular fluorescence complementation analysis showed that the interaction of Mi-1.2 and SlSERK1 at the plasma membrane distinctively changes in the presence of potato aphid saliva, suggesting a model in which a constitutive complex at the plasma membrane participates in defense signaling upon effector binding.
Plants are exposed to an environment rich in pathogenic microbes and pests. To protect themselves from these intruders, plants utilize physical and chemical barriers as well as innate immunity. Plant innate immunity relies on two major forms of active defense responses. The first form relies on recognition of conserved molecular patterns, defining a class of microbes, also known as microbe-associated molecular patterns (MAMPs), by cell surface-localized transmembrane pattern recognition receptors (PRRs; Boller and Felix, 2009). PRRs include receptor-like kinases and receptor-like proteins. MAMP recognition by a PRR triggers pattern-triggered immunity (PTI; Jones and Dangl, 2006). To circumvent PTI, adapted pathogens have evolved a diverse array of virulence factors, often referred to as effectors. To counteract these pathogens, plants evolved receptors that recognize specific effectors to mount a second layer of defense called effector-triggered immunity (ETI; Jones and Dangl, 2006).
ETI is mediated by resistance (R) genes generally encoding cytosolic nucleotide-binding (NB) Leu-rich repeat (LRR) proteins (NLR; Jones and Dangl, 2006). Based on their N-terminal domain, NLRs are typically divided into two groups. The NLRs carrying a toll-IL-1 receptor domain are placed in the TNL class, while the others, often carrying a coiled-coil (CC) domain, are collectively called CNLs. ETI triggers a highly effective defense response that includes rapid transcriptional reprogramming, production of pathogenesis-related proteins, reactive oxygen species and reactive nitrogen species, phytoalexins, and additional antimicrobial compounds (Jones and Dangl, 2006). Frequently, ETI is associated with the hypersensitive response (HR) a form of programmed cell death that limits the spread of biotrophic pathogens. Recognition of effectors by R proteins can be direct or indirect. There are only a few examples of direct interaction between effectors and R proteins (Dangl et al., 2013). The emerging model suggests that in most cases R proteins indirectly recognize effectors by detecting the modifications by the effectors to plant targets guarded by, or integrated in, R proteins (Takken and Goverse, 2012; Cesari et al., 2014; Kroj et al., 2016).
The tomato (Solanum lycopersicum) Mi-1.2 gene encodes a CNL protein that confers resistance to three species of root-knot nematodes (RKNs; Meloidogyne arenaria, Meloidogyne incognita, and Meloidogyne javanica), and three genera of phloem-feeding insects, namely, potato aphids (Macrosiphum euphorbiae), whiteflies (Bemisia tabaci), and psyllids (Bactericera cockerelli; Milligan et al., 1998; Nombela et al., 2003; Casteel et al., 2006). Using biochemical and genetic approaches, aspects of Mi-1.2 activation have been characterized. The Mi-1.2 protein has been shown to bind nucleotides and to possess ATPase activity (Tameling et al., 2002). Specific mutations in the Mi-1.2 gene confer autoactive phenotypes, indicating that Mi-1.2 is autoinhibited and under negative regulation (Hwang and Williamson, 2003). Transcomplementation studies and domain swapping experiments between Mi-1.2 and a nonfunctional Mi-1.1 homolog revealed that Mi-1.2 activation is a multistep process (Hwang et al., 2000). Whereas the LRR domain is thought to be involved in pest recognition and signal transduction, the extended N terminus, containing both a CC and a SD (Solanaceae domain), regulates Mi-1.2’s ability to induce cell death (Mucyn et al., 2006; Tameling and Takken, 2008; Gutierrez et al., 2010; Lukasik-Shreepaathy et al., 2012). Since Mi-1.2 cognate effectors have not been identified, most of these studies have been performed with autoactive and loss-of-function proteins.
Genetic components of Mi-1.2-mediated RKN and aphid resistance have been identified using virus-induced gene silencing (VIGS). Most of the genes identified to date encode generic factors involved in NLR R gene-mediated defenses, such as Hsp90, Sgt1, and members of the mitogen-activated protein kinase cascade (Li et al., 2006; Bhattarai et al., 2007). Besides these genes, WRKY transcription factors (TFs) WRKY70, WRKY72a, and WRKY72b were found to be required for Mi-1.2-mediated aphid and RKN resistance (Bhattarai et al., 2010; Atamian et al., 2012). These TFs are induced faster, and to higher levels, in resistant than in susceptible tomato plants following RKN infection or aphid infestation (Bhattarai et al., 2010; Atamian et al., 2012). A suppressor screen for autoactive Mi (Mi-DS4)-mediated cell death identified Somatic Embryogenesis Receptor Kinase1 (SERK1) to be required for Mi-1.2-mediated resistance (Mantelin et al., 2011). In SERK1-silenced Nicotiana benthamiana plants, Mi-DS4 cell death was abolished. SERK1 encodes a receptor-like kinase featuring an extracellular LRR, a transmembrane domain, and a cytoplasmic kinase domain. Silencing SERK1 (SlSERK1) in Mi-1.2 tomato plants revealed a role for SlSERK1 in Mi-1.2-mediated resistance to potato aphids, but surprisingly not in RKN resistance. This suggests a distinct role for SlSERK1 in the Mi-1.2-mediated recognition process of aphids and nematodes (Mantelin et al., 2011). Although interactions of SERK family members with plasma membrane-localized cell surface immune receptors have been characterized, no information exists about the interaction between SERKs and cytoplasmic localized immune receptors (Mantelin et al., 2011). Similarly, it is not known whether SERK1 and Mi-1.2 physically interact and if so where and whether such an interaction is conditional or requires effector recognition.
To characterize the role of SlSERK1 for Mi-1.2 function, we developed an overexpressing hemagglutinin (HA)-tagged SlSERK1 (SERK1-HA) fusion protein construct and used it for transient expression in N. benthamiana and to generate transgenic tomato cultivar (cv) Motelle plants. In these SlSERK1-overexpressing plants, which exhibit enhanced aphid resistance, we studied the interaction of SERK1 and Mi-1.2. Potato aphid saliva and aphid protein extracts were found to activate Mi-1.2-mediated responses, allowing us to investigate the localization and interaction pattern of SERK1 and Mi-1.2 before and after immune activation.
RESULTS
Functional Characterization of SERK1-Tagged Constructs
Before using SlSERK1-tagged constructs in various assays, we first evaluated the functionality of the SlSERK1 constructs labeled with different tags. Since SERK1 is required for cell death induced by autoactive Mi (Mantelin et al., 2011), we assessed whether the tagged SlSERK1 constructs could complement the SERK1 requirement in N. benthamiana in which NbSERK1 was silenced using Tobacco rattle virus (TRV)-based VIGS. We tested two C-terminal tagged SlSERK1 constructs, one with a single HA tag (P35S:SlSERK1-HA) and another with a tandem CFP-HA tag (P35S:SlSERK1-CFP-HA).
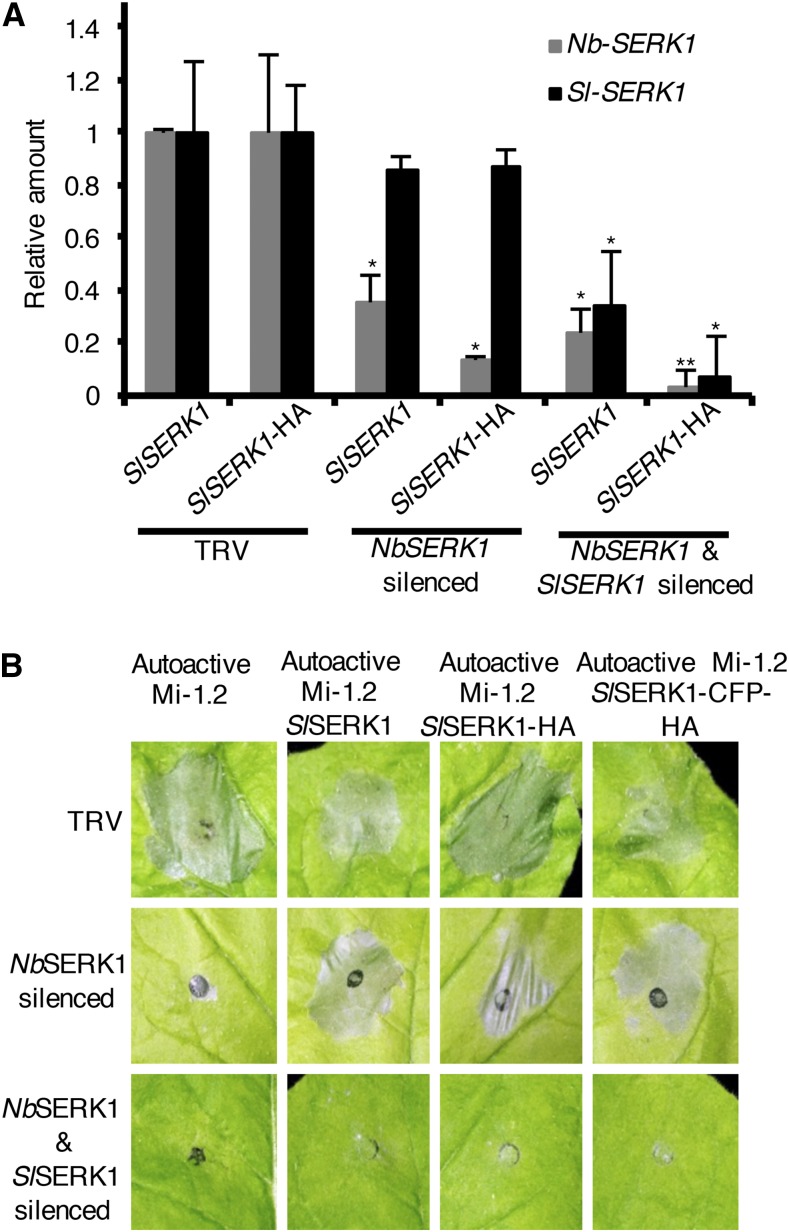
A TRV-NbSERK1 construct was used that could silence the endogenous NbSERK1 but not the transiently expressed SlSERK1 (Fig. 1A; Supplemental Fig. S1). As control for effective silencing, we included a TRV-SERK1 construct that is derived from NbSERK1 and able to silence both N. benthamiana and tomato SERK1 (Fig. 1A; Mantelin et al., 2011). As expected, agroinfiltration of autoactive Mi-1.2 or coagroinfiltration of autoactive Mi-1.2 with P35S:SlSERK1 in nonsilenced plants resulted in a HR (Fig. 1B). Similarly, coinfiltration of autoactive Mi-1.2 and P35S:SlSERK1-HA or P35S:SlSERK1-CFP-HA resulted in HR, demonstrating that these tagged constructs do not interfere with Mi-induced cell death (Fig. 1B). As expected, silencing NbSERK1 attenuated autoactive Mi-1.2-induced cell death (Fig. 1B). In contrast, coagroinfiltration of autoactive Mi-1.2 and P35S:SlSERK1-HA or P35S:SlSERK1-CFP-HA in the NbSERK1-silenced leaves restored cell death, demonstrating that the tagged SERK1 constructs can complement NbSERK1 function (Fig. 1B).
Figure 1.
SlSERK1-tagged constructs retain SERK1 function. A, Specificity of TRV-based VIGS of SlSERK1 and/or NbSERK1 was measured by RT-qPCR in N. benthamiana leaves infiltrated with Agrobacterium tumefaciens carrying various SlSERK constructs. Transcripts were normalized to N. benthamiana Ubiquitin (NbUbi) and compared to empty vector (TRV) control. Values are average ± se (n = 3). P values were generated by ANOVA using Dunnett’s test for multiple comparisons to empty vector control (*P < 0.05 and **P < 0.01). B, SlSERK1-tagged constructs rescue autoactive Mi-1.2-triggered HR in TRV-silenced N. benthamiana. N. benthamiana leaves silenced for NbSERK1 or cosilenced for NbSERK1 and SlSERK1 coagroinfiltrated with autoactive Mi-1.2 and SlSERK1, autoactive Mi-1.2 and SlSERK1-HA, autoactive Mi-1.2 and SlSERK1-CFP-HA, or autoactive Mi-1.2 alone. At 4 d after agroinfiltration, a representative leaf was photographed. This experiment was performed four times.
Cosilencing of NbSERK1 and SlSERK1 attenuated autoactive Mi-1.2-induced cell death irrespective of the SlSERK1 construct coagroinfiltrated, confirming the requirement of SERK1 for autoactive Mi-1.2-induced cell death (Fig. 1B). Taken together, these data show that SlSERK1-HA or SlSERK1-CFP-HA tagged constructs maintain SERK1 function and, therefore, SlSERK1 tagged constructs can be used in functional analyses.
Characterization of Tomato Transgenic Lines Overexpressing SlSERK1-HA
Transgenic tomato cv Motelle (Mi-1.2/Mi1.2) plants were generated harboring the P35S:SlSERK1-HA construct. Using PCR, eight independent transgenic T1 plants were identified that carried a single copy of the transgene (data not shown). Derived T2 progeny were evaluated for the presence of the transgene using PCR and/or immunoblot analysis. In T2 and T3 of three of these transgenic lines (lines 1, 10, and 27), SlSERK1-HA protein levels were evaluated using immunoblot analysis with an HA antibody. The lines differ in their levels of SlSERK1, with the highest levels detected in line 1 (Supplemental Fig. S2A). Therefore, line 1 was used for all subsequent studies. Transgenic SlSERK1-HA-overexpressing plants did not exhibit any apparent phenotype and were indistinguishable from the wild-type cv Motelle plants (Supplemental Fig. S2B).
Transgenic Motelle Tomato Plants Overexpressing SlSERK1 Exhibit Enhanced Resistance to Aphids
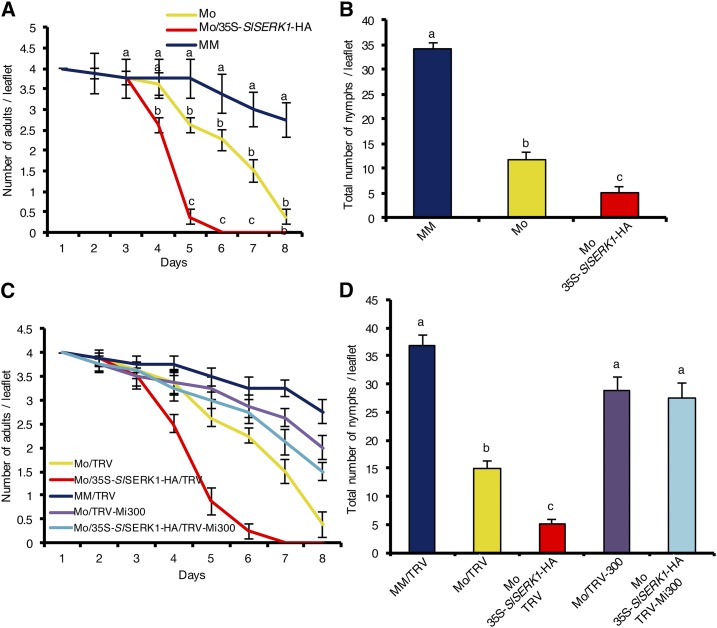
To assess the resistance phenotype of SERK1-overexpressing plants, we used them in aphid performance bioassays. To allow detection of subtle changes in aphid defense, plants were infested with age-synchronized 1-d-old adult potato aphids, and aphid survival and reproduction were monitored on a daily basis. Wild-type cv Motelle carrying the Mi-1 gene and near isogenic susceptible cv Moneymaker were used as control. Interestingly, aphid survival was reduced on SERK1-overexpressing Motelle compared to wild-type Motelle plants (Fig. 2A). Most aphids were dead by day 5 on SlSERK1-overexpressing Motelle, while a similar proportion of aphids were dead on day 8 on wild-type Motelle plants. This difference indicates that overexpression of SERK1 enhanced aphid resistance. On day 8, most aphids were still alive on the susceptible Moneymaker plants (Fig. 2A). Aphids reproduce parthenogenetically by laying viviparous nymphs. In keeping with the reduced survival rates described above, aphid reproduction as measured by the total number of newborn nymphs was significantly lower on SERK1-overexpressing Motelle compared to wild-type Motelle plants (Fig. 2B). As expected, the highest number of aphid progeny was observed on the susceptible cv Moneymaker.
Figure 2.
Tomato cv Motelle (Mo) plants overexpressing SlSERK1-HA (Mo/35S-SlSERK1-HA) are more resistant to potato aphids than wild-type Motelle. Aphid survival (A) and fecundity (B) on tomato cv Motelle 35S-SlSERK1-HA, the wild-type cv Motelle, and near isogenic susceptible cv Moneymaker (MM). Aphid survival (C) and fecundity (D) on Motelle 35S-SlSERK1-HA and Motelle silenced for Mi-1.2 (TRV-Mi300) or empty vector control (TRV). Four age-synchronized 1-d-old adult aphids were caged on a single leaflet of 7-week-old plants. Two leaflets per plants were infested and four plants per genotype were used (n = 8). Aphid survival and fecundity were monitored on a daily basis until all aphids were dead on Motelle. Error bars indicate ±se. Experiments were performed three times with similar results. Data from a single experiment are presented. Different letters denote a significant difference at P < 0.05 (ANOVA).
We developed SlSERK1-overexpressing transgenic tomato only in the resistant cv Motelle background. To test whether overexpression of SlSERK1 alone is sufficient to enhance resistance against aphids, we silenced Mi-1.2 (TRV-Mi300) in Motelle and in the transgenic SlSERK1-overexpressing lines. These plants were subsequently used in aphid bioassays. Aphid survival (Fig. 2C) and fecundity (Fig. 2D) on both wild-type and transgenic Motelle tomatoes silenced for Mi-1.2 (TRV-Mi300; Supplemental Fig. S3) were similar to those on the susceptible cv Moneymaker. Hence, the increased aphid resistance observed following SlSERK1 overexpression is Mi-1.2 dependent, functionally linking these proteins.
Potato Aphid Saliva Induced Mi-1.2-Mediated Defense Response
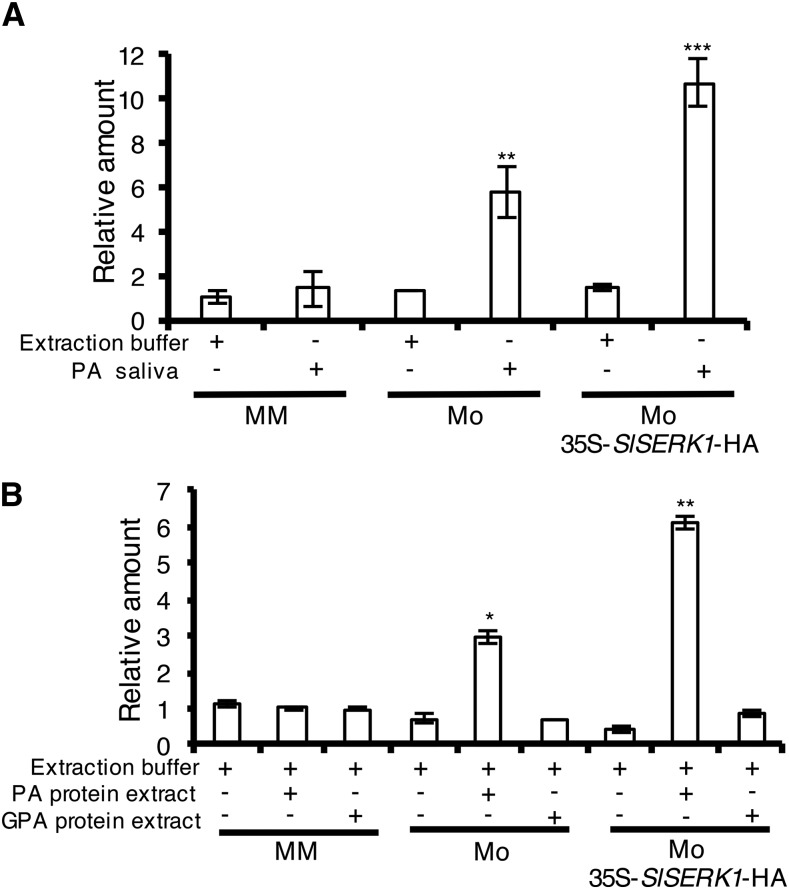
A functional interaction between SlSERK1 and Mi-1.2 might rely on a physical interaction between these proteins. In addition, formation of such a complex could rely on the presence of an effector triggering immune signaling. However, no Mi-1.2 recognized effector has been identified from insect or nematode pests. We speculated that aphid saliva might contain at least one Mi-1.2 effector and therefore tested whether aphid saliva, collected from feeding pouches in vitro, was able to induce Mi-1.2-mediated resistance responses. To evaluate induction of Mi-1.2-mediated responses, the expression of SlWRKY72b was monitored by reverse transcription quantitative PCR (qPCR) in resistant wild-type and SlSERK1-overexpressing cv Motelle plants and in susceptible cv Moneymaker after infiltration with aphid saliva. SlWRKY72b is a marker for Mi-1.2-mediated resistance signaling and is rapidly induced following potato aphid feeding (Bhattarai et al., 2010; Mantelin et al., 2011). Compared to the buffer control, infiltration of potato aphid saliva significantly induced expression of SlWRKY72b in tomato cv Motelle but not in cv Moneymaker (Fig. 3A). Interestingly, SlWRKY72b induction was higher in SlSERK1-overexpressing Motelle than in wild-type Motelle plants. Similar SlWRKY72b expression patterns were obtained whether gene expression was normalized to Actin (Fig. 3A) or Ubiquitin (Supplemental Fig. S4A). These results indicate the presence of effector(s) in the aphid saliva with the ability to trigger Mi-1.2 signaling and subsequent SlWRKY72b expression.
Figure 3.
SlWRKY72b shows Mi-1.2-specific expression following treatment with potato aphid saliva or total protein extract. Leaflets of tomato cv Motelle (Mo) overexpressing SlSERK1-HA, the wild-type cv Motelle, and near isogenic susceptible cv Moneymaker (MM) were infiltrated with potato aphid (PA) saliva collected in vitro in water (A) or PA or GPA protein extracts (B). At 6 h after infiltration, RNA was extracted from leaves and transcript levels were evaluated using RT-qPCR normalized against tomato Actin (SlActin) and calibrated to MM infiltrated with PA saliva or protein extract. Values are average ± se (n = 3). P values were generated by ANOVA using Dunnett’s test for multiple comparisons to MM infiltrated with PA saliva (*P < 0.05, **P < 0.01, and ***P < 0.001). Experiments were performed three times with similar results.
Potato Aphid Protein Extract Induced Mi-1.2-Mediated Defense Response
Collection of aphid saliva is laborious and requires handling of large number of aphids. The observation that in vitro collected aphid saliva is able to trigger Mi-1.2 defense responses suggests that no plant signal is required for the production of the effector. We therefore hypothesized that the effector might be constitutively produced and thus tested whether total aphid protein extracts could also be used to trigger Mi-1.2-mediated defense responses. Crude protein extracts from mixed developmental stages of potato aphids were isolated and used to infiltrate leaflets of SlSERK1-overexpressing Motelle lines, wild-type resistant cv Motelle, and susceptible cv Moneymaker. As with saliva, expression of SlWRKY72b was significantly induced only in leaves of SlSERK1-overexpressing lines and wild-type cv Motelle, but not in cv Moneymaker. As observed for the plants treated with the aphid saliva, SlWRKY72b was induced at higher levels in the SlSERK1-overexpressing line than in the wild-type cv Motelle plants that were infiltrated with aphid protein extracts (Fig. 3B). Similar SlWRKY72b expression patterns were obtained whether gene expression was normalized to Actin (Fig. 3B) or Ubiquitin (Supplemental Fig. S4B).
To test the specificity of the Mi-1.2-induced transcriptional response to the potato aphid extracts, we also prepared total protein extracts from green peach aphids (GPA; Myzus persicae) to which Mi-1.2 does not confer resistance (Goggin et al., 2001). Infiltration of the GPA protein extracts into leaflets of wild-type and SlSERK1-overexpressing Motelle, as well as in Moneymaker, did not induce SlWRKY72b expression in any of the genotypes (Fig. 3B; Supplemental Fig. S4B). These results show the specificity of the SlWRKY72b expression and demonstrate that potato aphid protein extracts can be used to specifically trigger a Mi-1.2-specific response.
SlSERK1 and Mi-1.2 Are Present in One Protein Complex in the Microsomal Fractions
To assess a potential SlSERK1 Mi-1.2 interaction in planta, the proteins were transiently coexpressed in N. benthamiana. Since SERK1 is plasma membrane localized, we predicted that the interaction between SlSERK1 and Mi-1.2 could occur at the plasma membrane. Therefore, an ultracentrifugation step was included to enrich for SlSERK-containing microsomal fractions. Before proceeding with coimmunoprecipitation experiments, we tested the specificity of the reported polyclonal anti-Mi antibody (van Ooijen et al., 2008) and its potential cross-reactivity with N. benthamiana Mi homologs in microsomal enriched fractions. Therefore, immunoblot analysis was performed following transient expression of either Mi-1.2-TAP and SlSERK1-HA or GFP and SlSERK1-HA. A band of 169 kD corresponding to the predicted Mr of Mi-1.2-TAP was detected in N. benthamiana leaves expressing Mi-1.2 but not in control leaves expressing GFP (Input, lower panel, Supplemental Fig. S5). This result confirms that the Mi polyclonal antibody specifically recognizes the tomato Mi-1.2 protein and either does not detect N. benthamiana homologs or that accumulation of such homologs is below its detection level. The presence of the Mi-1.2 protein in the microsomal fractions is interesting, as the resistant protein is not predicted to have transmembrane domains or membrane anchors, and suggests that Mi-1.2 is tethered to a membrane via another protein, possibly SERK1.
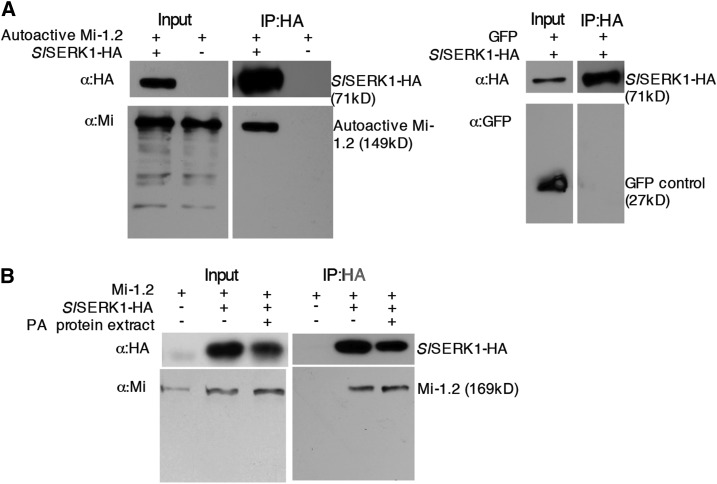
To test whether SlSERK1 associates with Mi-1.2 in planta, we coexpressed autoactive Mi-1.2 with SlSERK1-HA in N. benthamiana leaves. SlSERK1-HA was pulled down using anti-HA trap beads from microsomal enriched fractions, and the immunoprecipitates were subjected to immunoblot analysis with antibodies recognizing Mi and HA. A band of 149 kD corresponding to the predicted molecular weight of autoactive Mi-1.2 was detected in N. benthamiana leaves expressing this Mi-1.2 variant (Fig. 4A). In control leaves expressing only autoactive Mi-1.2, no Mi-1.2 was pulled down following SERK1-HA immunoprecipitation (Fig. 4A). To confirm that the SlSERK1 association with autoactive Mi-1.2 is specific, we also coexpressed SlSERK1-HA with GFP alone and performed immunoprecipitation and immunoblot analysis with antibodies recognizing GFP. Although GFP was expressed, no GFP was detected in the immunoprecipitates (Fig. 4A). Taken together, these results indicate that SlSERK1 specifically interacts with the transiently expressed autoactive Mi-1.2 variant.
Figure 4.
Mi-1.2 coimmunoprecipitates with SlSERK1 in N. benthamiana microsomal fractions. N. benthamiana microsomal enriched proteins were extracted from leaves transiently coexpressing SlSERK1-HA and autoactive TAP-Mi-1.2 or GFP (A) or wild-type Mi-1.2 expressed either alone or in combination with SlSERK-HA (B). Potato aphid (PA) total protein extracts or extraction buffer control was infiltrated 15 min before sample harvest. Total proteins (input) were subjected to immunoprecipitation with anti-HA affinity beads, and associated proteins were detected using immunoblot analyses. This experiment was performed three times with similar results.
Having identified an interaction between SlSERK1 and activated Mi-1.2, we set out to evaluate the dynamics of this association during immune activation. Therefore, we coexpressed wild-type Mi-1.2 and SlSERK1-HA following treatment with aphid protein extracts. Forty-eight hours after agroinfiltration, leaves were infiltrated with either aphid protein extracts or with buffer and within 15 min processed for isolation of microsomal fractions. Immunoprecipitation and immunoblot analyses were performed, and as observed before, no Mi-1.2 was detected in microsomal fractions in the absence of SlSERK1-HA (Fig. 4B). However, a band of 169 kD, corresponding to the predicted Mr of Mi-1.2, was detected in N. benthamiana leaves expressing SlSERK1-HA irrespective of potato aphid protein extract treatment (Fig. 4B). This result indicates that in planta, Mi-1.2 forms a constitutive complex with SERK1, irrespective of the proposed activation state of the NLR protein or effector presence.
Mi-1.2 Does Not Associate with SlSERK3A or SlSERK3B
Besides SlSERK1, tomato has two additional SERK members: SlSERK3A and SlSERK3B (Mantelin et al., 2011; Peng and Kaloshian, 2014). To test the specificity of the Mi-1.2 and SERK1 interaction, we tested whether Mi-1.2 can also associate with SlSERK3A and/or SlSERK3B. We coagroinfiltrated Mi-1.2 with either HA-tagged SlSERK3A (SlSERK3A-HA) or SlSERK3B (SlSERK3B-HA) and processed the microsomal fractions for immunoprecipitation using anti-HA trap beads. The presence of Mi-1 in the precipitates was detected using immunoblot analyses and the Mi antibody. Although the SERK proteins were successfully immunoprecipitated, Mi-1.2 was not detected in either SlSERK3A (Supplemental Fig. S6A) or SlSERK3B (Supplemental Fig. S6B) immunoprecipitates. The Arabidopsis (Arabidopsis thaliana) ortholog of SlSERK3A and SlSERK3B, BAK1, forms a complex with partner receptors only after MAMP perception (Sun et al., 2013; Böhm et al., 2014; Halter et al., 2014). Therefore, we evaluated whether SlSERK3A and SlSERK3B could participate in Mi-1.2 signaling following effector recognition. Leaves coexpressing Mi-1.2 and either SlSERK3A or SlSERK3B were infiltrated with potato aphid protein extracts or buffer and processed for isolation of the microsomal fraction, immunoprecipitation, and immunoblot analyses. Mi-1.2 was not detected in either SlSERK3A (Supplemental Fig. S6A) or SlSERK3B (Supplemental Fig. S6B) immunoprecipitates following potato aphid extract treatment. These data reveal specificity of the interaction between Mi-1.2 and SlSERK1 as no interaction with the other SERK members was found irrespective of the presence of the aphid effector and proposed activation state of Mi-1.2.
Native Mi-1.2 and SlSERK1-HA Associate at the Tomato Plasma Membrane
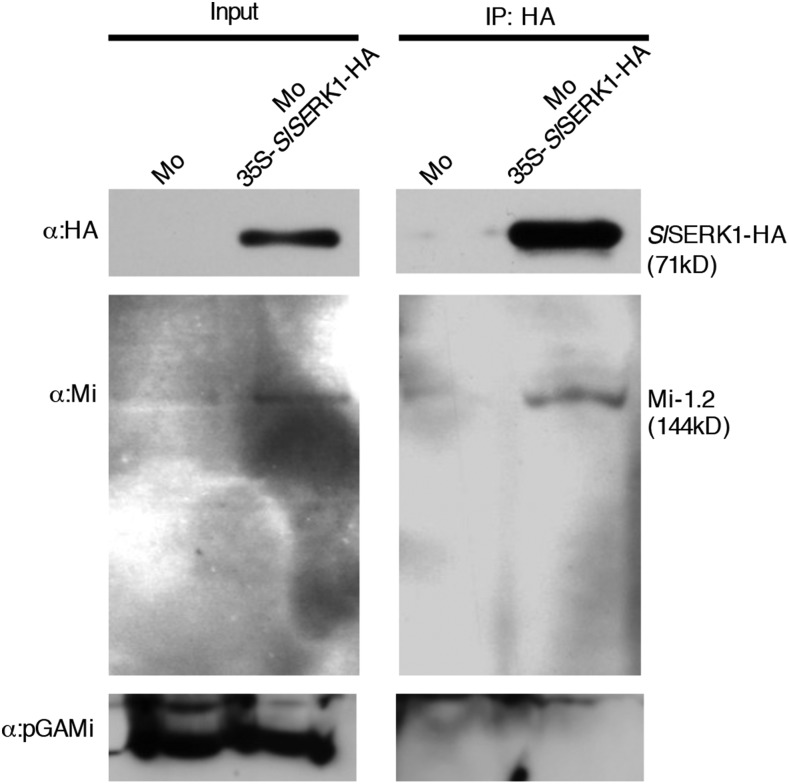
To assess whether Mi-1.2 and SlSERK1 also associate in their endogenous plant, we explored native Mi-1.2 associations with SlSERK1 in the transgenic SlSERK1-HA overexpressing tomato cv Motelle. Since immunoprecipitation experiments in N. benthamiana indicated that Mi-1.2 constitutively associates with SlSERK1, tomato leaves were not treated with aphid protein extracts. Microsomal fractionations isolated from wild-type and transgenic SlSERK1-HA-overexpressing Motelle leaves were used for immunoprecipitation experiments using HA-trap beads. The immunoprecipitates were subjected to immunoblot analysis using the Mi antibody. As shown in the left panel of Figure 5, native Mi-1.2 was detected in the microsomal fractions of both lines showing that at least part of the Mi-1.2 protein pool constitutively interacts with a membrane structure. Like in N. benthamiana (Fig. 4B), more Mi-1.2 is present in the microsomal fractions following SlSERK1 overexpression, suggesting that only part of the Mi-1.2 pool is bound to SERK1 when expressed at its endogenous level. Following immunoprecipitation, Mi-1.2 was only isolated from the transgenic SlSERK1-HA-overexpressing Motelle plants (Fig. 5), indicating that native Mi-1.2 is present in a complex with SlSERK1-HA.
Figure 5.
Mi-1.2 coimmunoprecipitates with SlSERK1 in tomato microsomal fractions. Total protein (input) extracted from tomato cv Motelle (Mo) leaves overexpressing 35S-SlSERK1-HA or wild-type Motelle were subjected to immunoprecipitation with anti-HA affinity beads, and Mi-1.2 was detected using immunoblot analyses. AntipGAMi antibody was used as negative control for microsomal fractions. This experiment was repeated once with similar results.
Mi-1.2 Is Present in Different Subcellular Compartments and Colocalized with SlSERK1 on the Plasma Membrane
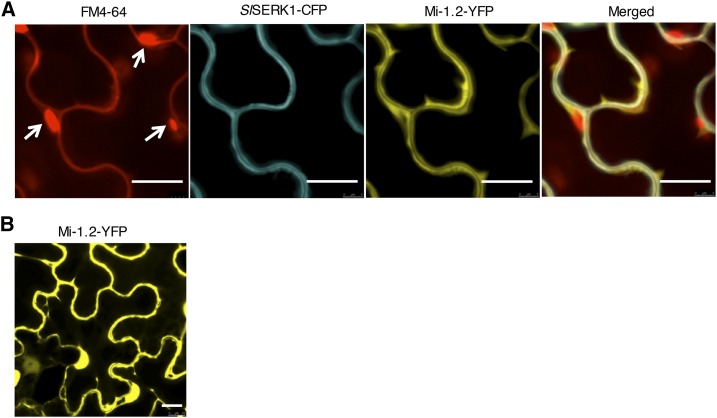
To determine the subcellular localization of Mi-1.2 and reveal where it interacts with SlSERK1, we transiently coexpressed a C-terminal tagged SlSERK1 fused with cyan fluorescent protein (CFP; SlSERK1-CFP) and Mi-1.2 fused to yellow fluorescent protein (YFP; Mi-1.2-YFP) in N. benthamiana (Supplemental Fig. S7). To label the plasma membrane, the styryl dye FM4-64 was applied immediately before processing the samples for confocal microscopy (Betz et al., 1996; Bolte et al., 2004). As expected, SlSERK1-CFP was detected solely at the plasma membrane (Fig. 6A). Like SlSERK1, Mi-1.2 was present at the plasma membrane and in the overlay, the yellow and cyan fluorescence overlap. In addition, the yellow fluorescence emitted by Mi-1.2-YFP was observed in the cytoplasm (Fig. 6) and, surprisingly, in the nucleus (Fig. 6B). These data indicate that Mi-1.2 is present in three distinct subcellular compartments of which one overlaps with that of SlSERK1.
Figure 6.
Mi-1.2 is present in different cellular compartments and colocalized with SlSERK1 on the plasma membrane. A, Colocalization of SlSERK1 and Mi-1.2 on the plasma membrane. White arrows point to autofluorescence of chloroplasts. B, Localization of Mi-1.2 in the cytoplasm and the nucleus. Mi-1.2-YFP-HA and SlSERK1-CFP-HA were transiently coexpressed in N. benthamiana leaves using A. tumefaciens. Fluorescence was monitored using confocal microscopy 48 h after agroinfiltration. Leaves were infiltrated with FM4-64 20 min before observation. Bar = 20 µm.
The Presence of Aphid Effector(s) Brings Mi-1.2 and SlSERK1 into Close Proximity
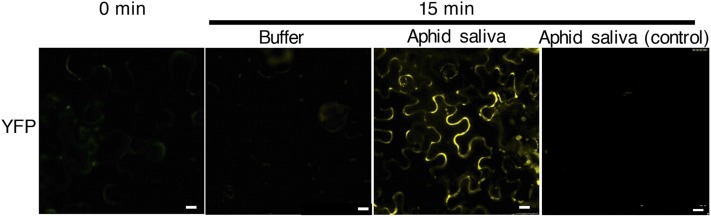
The immunoprecipitation experiments and confocal studies together indicated that SlSERK1 and Mi-1.2 are constitutively present in one protein complex localized at the plasma membrane. To further assess this Mi-1.2 and SlSERK1 association, we utilized the bimolecular fluorescence complementation (BiFC) assay. We coexpressed SlSERK1, fused with C-terminal residues of YFP (SlSERK1-cYFP), and Mi-1.2, fused to the N-terminal residues of YFP (Mi-1.2-nYFP; Supplemental Fig. S8), and studied their interaction using confocal microscopy. Surprisingly, no fluorescent signal was detected following coexpression of these constructs, indicating that SlSERK1 and Mi-1.2 are not in sufficient proximity to allow fluorescence complementation (Fig. 7). We tested whether the aphid effector could change the conformation of the Mi-1.2-SlSERK1 complex and, hence, the physical distance between the fluorophore halves fused to the immune regulators. Leaves coexpressing the SlSERK1-cYFP Mi-1.2-nYFP proteins were infiltrated with potato aphid saliva and monitored using fluorescence microscopy. Notably, within 15 min following infiltration, YFP fluorescence was observed in saliva-infiltrated regions but not in sectors infiltrated with the buffer control. The reconstituted YFP fluorescence suggests a conformation change in the Mi-1.2-SlSERK1 complex allowing the YFP protein halves to interact (Fig. 7). This reconstituted YFP fluorescence was mainly localized at the plasma membrane, although a few strong puncta of unknown identity were observed in different subcellular compartments (Fig. 7). Together, these data indicate the presence of a Mi-1.2 - SlSERK1 complex at the plasma membrane and suggests rapid conformational changes following Mi-1.2 activation by the aphid effector.
Figure 7.
SlSERK1 associates with Mi-1.2 after infiltration with potato aphid saliva. BiFC analysis of N. benthamiana leaves coexpressing Mi-1.2-nYFP and SlSERK1-cYFP 0 min or 15 min after infiltration with potato aphid saliva or buffer. Aphid saliva control is a nontransformed leaf infiltrated with aphid saliva. Leaf samples were harvested 48 h after agroinfiltration for confocal microscopy. Bar = 20 µm.
DISCUSSION
Previous studies revealed that members of the SERK family interact with membrane-localized cell surface immune receptors and are required for their function (Liebrand et al., 2014; Zipfel, 2014). Earlier, we reported that SlSERK1 is also required for the function of an NLR immune receptor, the presumed cytoplasmic localized tomato Mi-1.2 protein (Mantelin et al., 2011). Here, we show that Mi-1.2 is indeed localized at the cytoplasm, but can also be found in the nucleus and interacts with SlSERK1 at the plasma membrane in a protein complex whose properties changes upon treatment with potato aphid saliva.
Certain C-terminal tagged SERK3/BAK1 fusion proteins can still form ligand-induced complexes with PRRs but are partially compromised in their ability to support PTI signaling (Ntoukakis et al., 2011). In our experiments, C-terminal tagging of SlSERK1 did not affect its ability to complement NbSERK1 in Mi-1.2-mediated cell death in N. benthamiana, thereby justifying the use of C-terminal tagged SlSERK1 fusion proteins for both protein interaction and immunoprecipitation assays, as well as in evaluating Mi-1.2-mediated resistance to aphids.
Mi-1.2-mediated resistance to aphids is not absolute, allowing some aphids to survive and reproduce on Mi-1.2-containing tomato plants. Interestingly, overexpression of SlSERK1 in tomato resulted in an enhanced Mi-1.2-mediated immune response resulting in reduced aphid survival and fecundity and enhanced SlWRKY72b expression. This enhanced resistance suggests that SlSERK1 is a limiting factor in the immune response. This phenotype cannot be attributed solely to SlSERK1 abundance, as no enhanced aphid resistance was observed in Mi-1.2-silenced plants overexpressing SlSERK1, showing a reliance on Mi-1.2. Hence, increased availability of SlSERK1 may boost the formation of a SlSERK1-Mi-1.2 immune complex. Indeed, more Mi-1.2 protein was detected at microsomal fractions in both N. benthamiana and tomato plants overexpressing SlSERK1 (Figs. 4 and 5). These results suggest enhancement of existing resistances in crops could be achieved by overexpressing key immune regulators.
To date, three insect-derived avirulence effectors have been identified from the Dipteran Hessian fly Mayetiola destructor (Aggarwal et al., 2014; Zhao et al., 2015). However, their cognate R genes have not yet been cloned and the mechanism of recognition of these effectors is not known (Harris et al., 2015; Stuart, 2015). No aphid effector recognized by an R protein, including Mi-1.2, has yet been identified (Kaloshian and Walling, 2016). Although aphid saliva (De Vos and Jander, 2009) and aphid total protein (Prince et al., 2014) have been used to induce plant immune responses, these sources have not been described as a source for isolating aphid or other insect avirulence effectors. Here, we show that a Mi-1.2-specific effector is present in in vitro collected saliva and in aphid total protein extracts. Its recognition is specific as Mi-1.2-dependent up-regulation of SlWRKY72b only occurred in response to treatment with saliva or protein extracts from Mi-1.2 avirulent potato aphids and not following treatment with green peach aphid extracts. Having an effector source allowed us to mimic synchronous infestation and enrich for leaf cells undergoing a simultaneous immune response, making the study of Mi-1.2 complex dynamics possible.
Immunoprecipitation experiments combined with confocal microscopy indicated that SlSERK1 and Mi-1.2 are present in a protein complex at the plasma membrane. This interaction is intriguing as SERK family members typically do not associate with cytosolic immune receptors, but rather with cell surface-localized immune receptors such as the Arabidopsis FLAGELLIN SENSING2 (FLS2) and EF-TU RECEPTOR (EFR) that are both membrane-bound proteins (Chinchilla et al., 2007; Roux et al., 2011). FLS2 and EFR are the best-characterized Arabidopsis PRRs and perceive bacterial flagellin and elongation factor TU, respectively. Both receptors require SERK3 for function, but neither FLS2 nor EFR constitutively interacts with SERK members; complex formation happens only following ligand binding (Chinchilla et al., 2007; Schulze et al., 2010; Roux et al., 2011; Schwessinger et al., 2011). Our immunoprecipitation experiments show that SlSERK1 and Mi-1.2 localize constitutively in the same protein complex, although their relative orientation changes upon exposure to aphid saliva. Our experiments were performed with a SERK1 overexpression construct or transgenic line. It remains to be investigated whether Mi-1.2 interacts specifically and constitutively with a SERK1 construct expressed by its native promoter.
The order of the signaling events following effector perception is currently unclear. Overexpression of SlSERK1 potentiates Mi-1.2 signaling, while silencing abrogates its function. These data are consistent with a model in which Mi-1.2 guards SlSERK1 or a yet unidentified tomato PRR that requires SlSERK1 for its function. A similar model for a CNL guarding a PRR has been proposed for the CNL Resistance to RESISTANCE TO PSEUDOMONAS SYRINGAE PV TOMATO2 (RPS2), which was found in a constitutive complex with the PRR FLS2 (Qi et al., 2011). However, a functional relationship between RPS2 and FLS2 has not been demonstrated. Mi-1.2 confers resistance to a variety of distinct pathogens that might carry unrelated effectors, suggesting the need for many distinct PRRs. But to date, no PRR for a conserved aphid-associated molecular pattern has been identified (Chaudhary et al., 2014; Prince et al., 2014; Kaloshian and Walling, 2016). If many perturbations of the SlSERK1 receptor complex can trigger Mi-1.2 signaling, then it is rather surprising that Mi-1.2 is not required for immune responses triggered by known PRRs that rely on SlSERK1.
An alternative scenario is that effector recognition is mediated by Mi-1.2 and that the signal is transduced to SlSERK1 to feed into the PTI pathway. The lack of HR following aphid feeding on Mi-1.2 tomato is in line with a PTI-type defense response (Martinez de Ilarduya et al., 2003). Arguing against this model is that Mi-1.2-mediated HR can be observed following infestation with avirulent nematodes or upon transient expression in heterologous systems. A third option is that the interaction with SlSERK1 merely serves to position Mi-1.2 at the proper subcellular localization to perceive the effector or its action. In this scenario, SlSERK1 acts as a scaffold and following its overexpression more Mi-1.2 is present at the proper location to intercept effector action. As proposed by the switch model (Takken et al., 2006), the conformation of Mi-1.2 changes following effector perception, apparently resulting in a reorientation of the fluorophore half resulting in fluorescence complementation in our assays (Fig. 7). Earlier, we had shown that SERK1 is not required for Mi-1.2-mediated resistance to RKN (Mantelin et al., 2011). Therefore, it is likely that the RKN effector or its action is perceived by Mi-1.2 in a different subcellular location. It is not known whether SERK1 is required for Mi-1.2-mediated whitefly or psyllid resistance and whether the effectors from these two insect species are perceived in a similar manner as that of the aphid or that of the nematode.
The tripartite localization of the protein in the cell, plasma membrane, cytoplasm, and nucleus makes it challenging to speculate about potential downstream signaling substrates of activated Mi-1.2. One cytoplasmic candidate is a downstream NLR, NRC1 (NB-LRR Required for Hypersensitive-Response-Associated Cell Death1). NRC1 has been shown to be required for autoactive Mi-1.2 cell death in N. benthamiana (Gabriëls et al., 2007; Sueldo et al., 2015). N. benthamiana encodes at least four NRC1-like proteins, and silencing specific NRC-like proteins did not compromise the HR-inducing activity of an autoactive Mi-1.2 variant, suggesting that there might be functional redundancy among these NRC-like genes (Wu et al., 2016).
Mi-1.2 does not have a canonical nuclear localization signal (Milligan et al., 1998; Vos et al., 1998). However, nuclear localization for Mi-1.2 is forecasted by the NucPred protein subcellular localization prediction program (NucPred score 0.71; Brameier et al., 2007; Caplan et al., 2008). Since the estimated size of the YFP-tagged Mi-1.2 construct (M-1.2-YFP-HA) is about 160 kD, far larger than the 40-kD nuclear exclusion size limit, the movement of Mi-1.2 into the nucleus must be enabled by a yet unidentified protein (DeYoung and Innes, 2006). The discovery that Mi-1.2 is localized to both the cytoplasm and nucleus adds a new member to a growing list of NLR proteins that are constitutively present in these two subcellular compartments (Caplan et al., 2008; Liu and Coaker, 2008; Chang et al., 2013). It is unclear whether the dynamic of Mi-1.2 localization in these cellular compartments changes after pest recognition. Similarly, the functional importance of Mi-1.2 nuclear localization for immune signaling remains to be investigated. Nevertheless, it is intriguing to speculate that Mi-1.2 directly interacts with TFs WRKY72a or WRKY72b to regulate immune responses or might act directly on the plant DNA like Rx and I-2, two solanaceous C proteins recently found to interact with DNA following their activation (Fenyk et al., 2015, 2016).
The presence of Mi-1.2 at the plasma membrane, cytoplasm, and the nucleus and the altered interaction of Mi-1.2 with SlSERK1 after aphid saliva treatment suggest that the aphid effector might be recognized at the plasma membrane but that activation of defense signaling could occur at a different subcellular compartment. Further experimentation is required to shed light on the dynamics of SlSERK1 and Mi-1.2 interactions following aphid perception and to identify additional components of this dynamic immune complex.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum) plants of near-isogenic cv Motelle (Mi-1/Mi-1) and Moneymaker (mi-1/mi-1), 35S-SlSERK1-HA transgenic Motelle, and Nicotiana benthamiana were grown in CA mix II (University of California) or sand. Plants were maintained in plant growth rooms at 22 to 24°C with 16-h-light/8-h-dark photoperiod under a light intensity of 700 μmol m−2 s−1, supplemented with a slow release fertilizer Osmocote (The Scotts Company) and fertilized biweekly with MiracleGro (Stern’s MiracleGro). VIGS-treated plants were maintained at 19°C for 5 weeks until used in aphid bioassays (Mantelin et al., 2011).
Constructs
The coding sequences of SlSERK1 without a stop codon in pENTR221 (Mantelin et al., 2011), wild-type Mi-1.2 construct pG54 (Mi-1.2-TAP), and the autoactive Mi-1.2 variant pFP2221 (TAP-Nt2-NB-ARC-LRR; Lukasik-Shreepaathy et al., 2012) used in this work have been described previously.
To develop transgenic tomato overexpressing a SlSERK1-HA, the binary clone pBIN61-35S-SlSERK1-HA (P35S:SlSERK1-HA) was constructed. The HA tag was fused to the C terminus of SlSERK1 by PCR and ligated into 5′XbaI and 3′BamHI sites of the pBIN61 binary vector. Primers used for cloning can be found in Supplemental Table S1.
Split YFP-tagged proteins, SlSERK1-cYFP and Mi-1.2-nYFP, were generated for BiFC analysis. pENTR221-SlSERK1 was recombined into pSAT5(A)-DEST-cEYFP175-end-N1 (pE3132) to produce SlSERK1-cYFP. The pG54 (Mi-1.2) clone was recombined into pSAT4(A)- DEST-nEYFP1-174-N1 (pE3134) to produce Mi-1.2-nYFP.
For localization studies, pENTR221-SlSERK1 was recombined into pEarleyGate101 generating a C-terminal CFP-HA-tag fusion, while pG54 (Mi-1.2) was recombined into pEarleyGate102 using a one-tube recombination protocol generating a C-terminal YFP-His-tag fusion construct. Both constructs were cloned behind the 35S promoter.
BiFC expression cassettes from these satellite plasmids were transferred into the I-SceI or I-CeuI sites of the pPZP-RCS1 binary vector (Goderis et al., 2002). All resulting constructs were sequence verified and transformed into Agrobacterium tumefaciens strain GV3101 (Koncz and Schell, 1986).
Generating Transgenic Tomato
The pBIN61-35S-SlSERK1-HA construct was used to transform tomato cv Motelle. Transgenic tomato lines were generated by the UC Riverside Plant Transformation Research Center using standard A. tumefaciens-mediated gene transfer procedures. Independent transformed plant pools were kept separate for selection of independent transgenic lines based on their kanamycin resistance and presence of the transgene. Plants of T2 and T3 generation were chosen for experiments. To assay transgene expression, immunoblot analysis was performed on crude leaf protein extracts using a monoclonal anti-HA-HRP (Santa Cruz) at a 1:2,000 dilution.
VIGS Constructs and A. tumefaciens-Mediated Virus Infection
VIGS was performed by infiltration with A. tumefaciens strain GV3101 containing the bipartite TRV, pTRV1, and pTRV2 vectors (Liu et al., 2002). Two- to three-week-old tomato or four-week-old N. benthamiana plantlets were agroinfiltrated. Bacteria were prepared as described previously (Li et al., 2006). Equal volumes of A. tumefaciens (OD600 = 1) with pTRV1 and suspensions containing pTRV2-derived constructs or pTRV2 empty vector were mixed before infiltration using a needleless syringe. The TRV-SERK1 construct that can silence both NbSERK1 and SlSERK1 was used as previously described (Mantelin et al., 2011). The NbSERK1-specific VIGS construct spans nucleotides 1399 to 1545 (Supplemental Fig. S1). The tomato TRV-Mi300 VIGS construct was used as previously published (Bhattarai et al., 2007).
Transient Expression in N. benthamiana
A. tumefaciens containing constructs were grown overnight in LB medium supplemented with appropriate antibiotics. Cultures were resuspended in 10 mm MgCl2, 10 mm MES, and 150 μm acetosyringone to a final OD600 = 0.2 to 0.5. After 3 h induction, cultures were infiltrated into 3- to 4-week-old N. benthamiana leaves using a needleless syringe.
Hypersensitive Response Assays in N. benthamiana
Three to four weeks after TRV infection, N. benthamiana leaves were spot infiltrated with A. tumefaciens carrying different constructs. Plants were kept under low light exposure, and HR symptoms were recorded 3 to 4 d after spot infiltration. Constructs were assayed at least three times with a minimum of three plants per construct and two leaves per plant.
Aphid Colony and Bioassays
A Mi-1-avirulent potato aphid colony was maintained on tomato cv UC82B in a pesticide-free glasshouse. Age-synchronized 1-d-old adult aphids were developed as described by Bhattarai et al. (2010). Six- to eight-week-old tomato plants were infested with four apterous 1-d-old adults in a cage on a single leaflet, and aphid survival and fecundity were monitored daily. Four leaflets were caged per plant and four to six plants were used per genotype.
Aphid Saliva Collection and Protein Extraction
Saliva was collected from mixed developmental stages of the aphid in water in feeding chambers as described by Chaudhary et al. (2014). Aphid soluble proteins were extracted from mixed developmental stages by homogenizing the tissues in liquid N2 using a mortar and pestle. An extraction buffer (50 mm Tris-HCl, pH 7.5, and 150 mm NaCl) was added to the aphid powder at a ratio of 1 mL of buffer to 1 g of aphids and frozen overnight (Yang et al., 2008). After centrifugation at 13,000g for 30 min, at 4°C, the supernatant was used for assays after adjusting the protein concentration to 1 mg/mL as determined with a Bradford assay using bovine serum albumin as a standard.
RNA Extraction and RT-qPCR
RNA was extracted using TRIzol (Invitrogen) and treated with DNase I (New England Biolabs). Five micrograms of RNA was reverse-transcribed using SuperScript III reverse transcriptase (Invitrogen) and oligo(dT) primer. For qPCR, transcripts were amplified from 1 μL of a 5× diluted cDNA, except for SlWRKY72b, where undiluted cDNA template was used in a 15-μL reaction using gene-specific primers (Supplemental Table S2) and iQ SYBR Green Supermix (Bio-Rad). PCRs were performed using three biological replicates. The PCR amplification consisted of 3 min at 94°C, 40 cycles of 30 s at 94°C, 30 s at 58°C, and 1 min at 72°C, 15 min at 72°C, followed by the generation of a dissociation curve. The generated threshold cycle (CT) was used to calculate the transcript abundance relative to N. benthamiana Ubiquitin (NbUbi; Jin et al., 2002), tomato Actin (SlActin; Kim et al., 2009), or Ubiquitin (SlUbi3; Bhattarai et al., 2010) genes, as described previously (Ginzinger, 2002). DNase-treated RNA was used as negative control.
Protein Isolation and Microsomal Membrane Protein Preparation
Five grams of leaves were chopped with a hand-held blade for 2 min in 20 mL of ice-cold buffer (50 mm MOPS, 5 mm EDTA, 0.33 m Suc, 1× plant protease cocktail [Sigma-Aldrich], 20 mm DTT, 0.04 g PVPP, and 1 mm PMSF, adjusted to pH 6.8 with KOH) and were then ground with a cold mortar and pestle in a 4°C room. The plant debris was filtered out using two layers of Miracloth (Calbiochem), and the remaining solution was cleared by centrifugation at 10,000g for 20 min at 4°C. The supernatant was centrifuged at 50,000g in an SW 28 rotor (Beckman) for 1 h at 4°C. Proteins from the microsomal pellet were extracted in 700 μL of extraction buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% glycerol, 10 mm DTT, 10 mm EDTA, 1 mm NaF, 1 mm Na2MoO40.2H2O, 1% [v/v] P9599 protease inhibitor cocktail [Sigma-Aldrich], and 1% [v/v] Nonidet P-40) and incubated for 3 h on an orbital shaker at 4°C. The insoluble debris was removed from the microsomal extracts by centrifugation at 10,000g for 15 min at 4°C. Protein concentration was quantified using a Bradford assay.
Coimmunoprecipitation and Immunoblot Analyses
Microsomal proteins were adjusted to a concentration of 1 mg mL−1, and immunoprecipitation was performed using 1 mL proteins and 20 μL anti-HA affinity matrix (Roche) for 3 h at 4°C. Beads were washed three times with TBS containing 0.5% (v/v) Triton X-100 and samples eluted in 5% Gly (pH 3.0).
Samples were electrophoresed on 8% SDS-acrylamide gels and electroblotted onto nitrocellulose membranes (Bio-Rad). Blocked membranes were incubated overnight with primary antibody (anti-Mi [1:3,500; van Ooijen et al., 2008], anti-HA [Santa Cruz; 1:2,000], anti-GFP [Santa Cruz, 1:2,000], and antipGAMi [Santa Cruz; 1:500]) and washed in PBST (PBS with 0.1% [w/v] Tween 20). Except for the anti-HA antibody, blots were incubated with an anti-rabbit HRP-conjugated secondary antibody (Santa Cruz; 1:3,000). Signals were visualized using chemiluminescent substrate (Thermo Scientific) before exposure to x-ray film.
Microscopy
For colocalization, A. tumefaciens GV3101 strains containing the pEARLEYGATE101-SlSERK1-CFP or pEARLEY102-Mi-1-YFP constructs were coinfiltrated into 3-week-old N. benthamiana leaves as described above. Fluorescence was visualized in epidermal cell layers after 2 to 3 d of infiltration, using a Leica SP5 confocal microscope. Microscopy was performed with 458- and 514-nm filters to excite the CFP and YFP, respectively, and images were collected through band emission filters at 460 to 500 and 520 to 550 nm, respectively.
For BiFC analysis, A. tumefaciens GV3101 strains containing the BiFC constructs were coinfiltrated into 4-week-old N. benthamiana leaves, and YFP fluorescence was visualized as described previously, except confocal microscopy was performed at 0 and 15 min after infiltration with potato aphid saliva.
Supplemental Data
The following supplemental materials are available
Supplemental Figure S1. Alignment of the TRV-NbSERK1 construct with the NbSERK1 and SlSERK1 sequences.
Supplemental Figure S2. Transgenic Motelle (Mo; Mi-1.2/Mi1.2) tomato overexpressing SlSERK1-HA.
Supplemental Figure S3. Expression of Mi-1.2 transcripts in Mi-1.2-silenced tomato plants cv Motelle (Mo; Mi-1.2/Mi-1.2) or cv Motelle overexpressing SlSERK1 (Mo/SERK1-HA) and controls.
Supplemental Figure S4. SlWRKY72b expression in tomato following treatment with potato aphid saliva or total protein extract normalized to Ubiquitin.
Supplemental Figure S5. Mi-1.2 coimmunoprecipitates with SlSERK1 in N. benthamiana microsomal fractions.
Supplemental Figure S6. Mi-1.2 did not coimmunoprecipitate with SlSERK3A or SlSERK3B in N. benthamiana microsomal fractions.
Supplemental Figure S7. Immunoblot showing accumulation of Mi-1.2 and SlSERK1 tagged constructs used in colocalization in N. benthamiana.
Supplemental Figure S8. Immunoblot showing expression of Mi-1.2 and SlSERK1 constructs used in BiFC analysis in N. benthamiana.
Supplemental Table S1. List of primers used in cloning.
Supplemental Table S2. List of primers used in qPCR.
Supplementary Material
Acknowledgments
We thank Beibei Li for technical help and Dr. Gitta Coaker for sharing protocols.
Glossary
- MAMP
microbe-associated molecular pattern
- PRR
pattern recognition receptor
- PTI
pattern-triggered immunity
- ETI
effector-triggered immunity
- HR
hypersensitive response
- RKN
root-knot nematode
- VIGS
virus-induced gene silencing
- TF
transcription factor
- BiFC
bimolecular fluorescence complementation
Footnotes
Articles can be viewed without a subscription.
This work was funded by a grant from University of California Riverside Experimental Station to I.K.
References
- Aggarwal R, Subramanyam S, Zhao C, Chen MS, Harris MO, Stuart JJ (2014) Avirulence effector discovery in a plant galling and plant parasitic arthropod, the Hessian fly (Mayetiola destructor). PLoS One 9: e100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamian HS, Eulgem T, Kaloshian I (2012) SlWRKY70 is required for Mi-1-mediated resistance to aphids and nematodes in tomato. Planta 235: 299–309 [DOI] [PubMed] [Google Scholar]
- Betz WJ, Mao F, Smith CB (1996) Imaging exocytosis and endocytosis. Curr Opin Neurobiol 6: 365–371 [DOI] [PubMed] [Google Scholar]
- Bhattarai KK, Atamian HS, Kaloshian I, Eulgem T (2010) WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J 63: 229–240 [DOI] [PubMed] [Google Scholar]
- Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I (2007) The MI-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol 144: 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm H, Albert I, Fan L, Reinhard A, Nürnberger T (2014) Immune receptor complexes at the plant cell surface. Curr Opin Plant Biol 20: 47–54 [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159–173 [DOI] [PubMed] [Google Scholar]
- Brameier M, Krings A, MacCallum RM (2007) NucPred--predicting nuclear localization of proteins. Bioinformatics 23: 1159–1160 [DOI] [PubMed] [Google Scholar]
- Caplan J, Padmanabhan M, Dinesh-Kumar SP (2008) Plant NB-LRR immune receptors: from recognition to transcriptional reprogramming. Cell Host Microbe 3: 126–135 [DOI] [PubMed] [Google Scholar]
- Casteel CL, Walling LL, Paine TD (2006) Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol Exp Appl 121: 67–72 [Google Scholar]
- Cesari S, Bernoux M, Moncuquet P, Kroj T, Dodds PN (2014) A novel conserved mechanism for plant NLR protein pairs: the “integrated decoy” hypothesis. Front Plant Sci 5: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Zhang L, Shen QH (2013) Partitioning, repressing and derepressing: dynamic regulations in MLA immune receptor triggered defense signaling. Front Plant Sci 4: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary R, Atamian HS, Shen Z, Briggs SP, Kaloshian I (2014) GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc Natl Acad Sci USA 111: 8919–8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science 341: 746–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Jander G (2009) Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ 32: 1548–1560 [DOI] [PubMed] [Google Scholar]
- DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7: 1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyk S, Dixon CH, Gittens WH, Townsend PD, Sharples GJ, Pålsson LO, Takken FL, Cann MJ (2016) The tomato nucleotide-binding leucine-rich repeat Immune receptor I-2 couples DNA-binding to nucleotide-binding domain nucleotide exchange. J Biol Chem 291: 1137–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyk S, Townsend PD, Dixon CH, Spies GB, de San Eustaquio Campillo A, Slootweg EJ, Westerhof LB, Gawehns FK, Knight MR, Sharples GJ, et al. (2015) The potato nucleotide-binding leucine-rich repeat (NLR) immune receptor Rx1 Is a pathogen-dependent DNA-deforming protein. J Biol Chem 290: 24945–24960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriëls SHEJ, Vossen JH, Ekengren SK, van Ooijen G, Abd-El-Haliem AM, van den Berg GCM, Rainey DY, Martin GB, Takken FLW, de Wit PJGM, Joosten MHAJ (2007) An NB-LRR protein required for HR signalling mediated by both extra- and intracellular resistance proteins. Plant J 50: 14–28 [DOI] [PubMed] [Google Scholar]
- Ginzinger DG. (2002) Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30: 503–512 [DOI] [PubMed] [Google Scholar]
- Goderis IJ, De Bolle MF, François IE, Wouters PF, Broekaert WF, Cammue BP (2002) A set of modular plant transformation vectors allowing flexible insertion of up to six expression units. Plant Mol Biol 50: 17–27 [DOI] [PubMed] [Google Scholar]
- Goggin FL, Williamson VM, Ullman DE (2001) Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene Mi. Environ Entomol 30: 101–106 [Google Scholar]
- Gutierrez JR, Balmuth AL, Ntoukakis V, Mucyn TS, Gimenez-Ibanez S, Jones AME, Rathjen JP (2010) Prf immune complexes of tomato are oligomeric and contain multiple Pto-like kinases that diversify effector recognition. Plant J 61: 507–518 [DOI] [PubMed] [Google Scholar]
- Halter T, Imkampe J, Mazzotta S, Wierzba M, Postel S, Bücherl C, Kiefer C, Stahl M, Chinchilla D, Wang X, et al. (2014) The leucine-rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr Biol 24: 134–143 [DOI] [PubMed] [Google Scholar]
- Harris MO, Friesen TL, Xu SS, Chen MS, Giron D, Stuart JJ (2015) Pivoting from Arabidopsis to wheat to understand how agricultural plants integrate responses to biotic stress. J Exp Bot 66: 513–531 [DOI] [PubMed] [Google Scholar]
- Hwang CF, Bhakta AV, Truesdell GM, Pudlo WM, Williamson VM (2000) Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell 12: 1319–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CF, Williamson VM (2003) Leucine-rich repeat-mediated intramolecular interactions in nematode recognition and cell death signaling by the tomato resistance protein Mi. Plant J 34: 585–593 [DOI] [PubMed] [Google Scholar]
- Jin H, Axtell MJ, Dahlbeck D, Ekwenna O, Zhang S, Staskawicz B, Baker B (2002) NPK1, an MEKK1-like mitogen-activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev Cell 3: 291–297 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kaloshian I, Walling LL (2016) Hemipteran and dipteran pests: Effectors and plant host immune regulators. J Integr Plant Biol 58: 350–361 [DOI] [PubMed] [Google Scholar]
- Kim JG, Li X, Roden JA, Taylor KW, Aakre CD, Su B, Lalonde S, Kirik A, Chen Y, Baranage G, et al. (2009) Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. Plant Cell 21: 1305–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of Tl-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Kroj T, Chanclud E, Michel-Romiti C, Grand X, Morel JB (2016) Integration of decoy domains derived from protein targets of pathogen effectors into plant immune receptors is widespread. New Phytol 210: 618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xie QG, Smith-Becker J, Navarre DA, Kaloshian I (2006) Mi-1-Mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol Plant Microbe Interact 19: 655–664 [DOI] [PubMed] [Google Scholar]
- Liebrand TW, van den Burg HA, Joosten MH (2014) Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci 19: 123–132 [DOI] [PubMed] [Google Scholar]
- Liu J, Coaker G (2008) Nuclear trafficking during plant innate immunity. Mol Plant 1: 411–422 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30: 415–429 [DOI] [PubMed] [Google Scholar]
- Lukasik-Shreepaathy E, Slootweg E, Richter H, Goverse A, Cornelissen BJ, Takken FL (2012) Dual regulatory roles of the extended N terminus for activation of the tomato MI-1.2 resistance protein. Mol Plant Microbe Interact 25: 1045–1057 [DOI] [PubMed] [Google Scholar]
- Mantelin S, Peng HC, Li B, Atamian HS, Takken FL, Kaloshian I (2011) The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J 67: 459–471 [DOI] [PubMed] [Google Scholar]
- Martinez de Ilarduya O, Xie Q, Kaloshian I (2003) Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol Plant Microbe Interact 16: 699–708 [DOI] [PubMed] [Google Scholar]
- Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10: 1307–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucyn TS, Clemente A, Andriotis VM, Balmuth AL, Oldroyd GE, Staskawicz BJ, Rathjen JP (2006) The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell 18: 2792–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela G, Williamson VM, Muñiz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Interact 16: 645–649 [DOI] [PubMed] [Google Scholar]
- Ntoukakis V, Schwessinger B, Segonzac C, Zipfel C (2011) Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell 23: 3871–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H-C, Kaloshian I (2014) The tomato leucine-rich repeat receptor-like kinases SlSERK3A and SlSERK3B have overlapping functions in bacterial and nematode innate immunity. PLoS One 9: e93302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DC, Drurey C, Zipfel C, Hogenhout SA (2014) The leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 and the cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol 164: 2207–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Tsuda K, Nguyen V, Wang X, Lin J, Murphy AS, Glazebrook J, Thordal-Christensen H, Katagiri F (2011) Physical association of Arabidopsis hypersensitive induced reaction proteins (HIRs) with the immune receptor RPS2. J Biol Chem 286: 31297–31307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C (2011) Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. (2015) Insect effectors and gene-for-gene interactions with host plants. Curr Opin Insect Sci 9: 56–61 [DOI] [PubMed] [Google Scholar]
- Sueldo DJ, Shimels M, Spiridon LN, Caldararu O, Petrescu AJ, Joosten MH, Tameling WI (2015) Random mutagenesis of the nucleotide-binding domain of NRC1 (NB-LRR Required for Hypersensitive Response-Associated Cell Death-1), a downstream signalling nucleotide-binding, leucine-rich repeat (NB-LRR) protein, identifies gain-of-function mutations in the nucleotide-binding pocket. New Phytol 208: 210–223 [DOI] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J (2013) Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628 [DOI] [PubMed] [Google Scholar]
- Takken FL, Albrecht M, Tameling WI (2006) Resistance proteins: molecular switches of plant defence. Curr Opin Plant Biol 9: 383–390 [DOI] [PubMed] [Google Scholar]
- Takken FL, Goverse A (2012) How to build a pathogen detector: structural basis of NB-LRR function. Curr Opin Plant Biol 15: 375–384 [DOI] [PubMed] [Google Scholar]
- Tameling WIL, Elzinga SDJ, Darmin PS, Vossen JH, Takken FLW, Haring MA, Cornelissen BJC (2002) The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell 14: 2929–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tameling WIL, Takken FLW (2008) Resistance proteins: scouts of the plant innate immune system. Eur J Plant Pathol 121: 243–255 [Google Scholar]
- van Ooijen G, Mayr G, Kasiem MM, Albrecht M, Cornelissen BJ, Takken FL (2008) Structure-function analysis of the NB-ARC domain of plant disease resistance proteins. J Exp Bot 59: 1383–1397 [DOI] [PubMed] [Google Scholar]
- Vos P, Simons G, Jesse T, Wijbrandi J, Heinen L, Hogers R, Frijters A, Groenendijk J, Diergaarde P, Reijans M, et al. (1998) The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat Biotechnol 16: 1365–1369 [DOI] [PubMed] [Google Scholar]
- Wu CH, Belhaj K, Bozkurt TO, Birk MS, Kamoun S (2016) Helper NLR proteins NRC2a/b and NRC3 but not NRC1 are required for Pto-mediated cell death and resistance in Nicotiana benthamiana. New Phytol 209: 1344–1352 [DOI] [PubMed] [Google Scholar]
- Yang X, Thannhauser TW, Burrows M, Cox-Foster D, Gildow FE, Gray SM (2008) Coupling genetics and proteomics to identify aphid proteins associated with vector-specific transmission of polerovirus (luteoviridae). J Virol 82: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Escalante LN, Chen H, Benatti TR, Qu J, Chellapilla S, Waterhouse RM, Wheeler D, Andersson MN, Bao R, et al. (2015) A massive expansion of effector genes underlies gall-formation in the wheat pest Mayetiola destructor. Curr Biol 25: 613–620 [DOI] [PubMed] [Google Scholar]
- Zipfel C. (2014) Plant pattern-recognition receptors. Trends Immunol 35: 345–351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.