Small reductions in leaf turgor can trigger foliar ABA biosynthesis in angiosperms, influencing stomatal responses to vapor pressure deficit.
Abstract
Stomatal responses to changes in vapor pressure deficit (VPD) constitute the predominant form of daytime gas-exchange regulation in plants. Stomatal closure in response to increased VPD is driven by the rapid up-regulation of foliar abscisic acid (ABA) biosynthesis and ABA levels in angiosperms; however, very little is known about the physiological trigger for this increase in ABA biosynthesis at increased VPD. Using a novel method of modifying leaf cell turgor by the application of external pressures, we test whether changes in turgor pressure can trigger increases in foliar ABA levels over 20 min, a period of time most relevant to the stomatal response to VPD. We found in angiosperm species that the biosynthesis of ABA was triggered by reductions in leaf turgor, and in two species tested, that a higher sensitivity of ABA synthesis to leaf turgor corresponded with a higher stomatal sensitivity to VPD. In contrast, representative species from nonflowering plant lineages did not show a rapid turgor-triggered increase in foliar ABA levels, which is consistent with previous studies demonstrating passive stomatal responses to changes in VPD in these lineages. Our method provides a new tool for characterizing the response of stomata to water availability.
The plant hormone abscisic acid (ABA) mediates a range of physiological processes in plants, from seed dormancy (Finkelstein et al., 2002) through to resource allocation (Sharp and LeNoble, 2002), yet arguably the most critical role for this hormone is that high levels close stomata during desiccation (Mittelheuser and Van Steveninck, 1969). The identification of ABA biosynthesis and signaling mutants, all of which have severely dysfunctional stomatal behavior (Tal and Nevo, 1973; Koornneef et al., 1982, 1984; Mustilli et al., 2002), has firmly established the central role of this hormone in the control of gas exchange in angiosperms (Nilson and Assmann, 2007). A major avenue of research since the discovery of ABA as an active hormone in plants has been the pursuit of endogenous triggers that stimulate natural increases in this critical hormone (Wright and Hiron, 1969).
Some of the earliest studies investigating the triggers for ABA biosynthesis observed a distinct leaf water potential (Ψl) threshold for the major, desiccation-stimulated, increases in foliar ABA level (Zabadal, 1974; Beardsell and Cohen, 1975). Leaves subjected to desiccation or osmotic stress were shown to reach this threshold Ψl close to bulk leaf turgor loss point (Ψtlp, when average leaf turgor equals 0; Pierce and Raschke, 1980, 1981; Davies et al., 1981; Creelman and Zeevaart, 1985). The link between Ψtlp and ABA biosynthesis appears consistent with respect to variation in Ψtlp among and within species (Pierce and Raschke, 1980). In all cases a significant augmentation of ABA levels occurred when leaves were dehydrated to a Ψl beyond Ψtlp for at least an hour (Ackerson and Radin, 1983). Given that ABA is critical for the effective closure of seed plant stomata during drought (Iuchi et al., 2001; Wilkinson and Davies, 2002), and stomatal closure during drought stress largely coincides with Ψtlp (Brodribb and Holbrook, 2003; Brodribb et al., 2003), it is reasonable to assume that turgor loss in leaf cells provides the endogenous signal for increasing ABA biosynthesis at Ψtlp leading to stomatal closure during water stress. Intriguingly, a number of early studies investigating the triggers for ABA accumulation in excised leaves or drought-stressed plants also observed up to, or greater than, a 20% increase in ABA levels before Ψtlp was reached (Pierce and Raschke, 1980; Henson, 1982; Creelman and Mullet, 1991; Dingkuhn et al., 1991). Very few studies have commented on the trigger for this more subtle increase in ABA level that occurs in dehydrated leaves at positive turgor pressures, yet these increases may be very important for the regulation of stomatal aperture in well-watered plants.
Studies observing stomatal apertures in epidermis removed from the leaf indicate that stomatal closure can occur at levels of ABA that are much lower than those measured in intact leaves during drought stress, suggesting that stomata may require comparatively small increases in ABA level to account for the majority of stomatal closure (Trejo et al., 1993). In support of this, the dynamic responses of stomata throughout the day to changes in humidity, or more precisely the vapor pressure deficit between the leaf and the atmosphere (VPD), appear to be driven by subtle yet functionally relevant changes in foliar ABA level in angiosperms (McAdam and Brodribb, 2015). In angiosperms, a transition to high VPD does not typically result in Ψl dropping below Ψtlp, yet ABA biosynthesis is rapidly activated at increased VPD, resulting in enhanced foliar ABA levels (Bauerle et al., 2004; McAdam and Brodribb, 2015; McAdam et al., 2016). Elevated foliar ABA levels observed at high VPD in angiosperms drive stomatal closure while the apparently slow rates of ABA catabolism also have a strong influence on hysteresis in the recovery of stomatal conductance in these species during reversible transitions in VPD (McAdam and Brodribb, 2015). Genetic evidence also supports this involvement of ABA in stomatal responses to VPD, with observations of dysfunctional VPD responses in mutants with impaired ABA synthesis or signaling (Xie et al., 2006; Bauer et al., 2013; Merilo et al., 2015; McAdam et al., 2016) and increased sensitivity in plants that constitutively overproduce ABA (Thompson et al., 2007). However, in wild-type plants it is unknown whether the rapid up-regulation of ABA biosynthesis at high VPD is caused by subtle reductions in leaf turgor due to increased transpiration (Buckley, 2016), or whether plants have an as yet unidentified direct sensor of atmospheric humidity or VPD that can rapidly provide a signal for up-regulating ABA biosynthesis.
In this study we specifically tested whether leaf turgor provides a quantitative regulatory signal for ABA biosynthesis over a time frame that is relevant to the stomatal response to VPD. We used precise physicochemical quantification of ABA levels to detect changes in foliar ABA when the turgor of leaves was reduced for a short period of time independent of VPD, by either the application of external pressure or the floating of leaves on osmotic solutions. We made these observations in representative species that spanned the phylogenetic diversity of vascular land plants, examining the variation in ABA biosynthesis in plants with ABA-sensitive stomata (seed plants) and ABA-insensitive stomata (ferns and lycophytes; Brodribb and McAdam, 2011; McAdam et al., 2016). Within our angiosperm sample we included species with a wide range in Ψtlp, including two herbaceous species and two woody species with native ranges from the Mediterranean and Southern Hemisphere cool-temperate rainforest. We also quantified hysteresis in the stomatal response to VPD among our sample of species to determine the relative importance of ABA as a regulator of stomatal conductance in response to VPD.
RESULTS
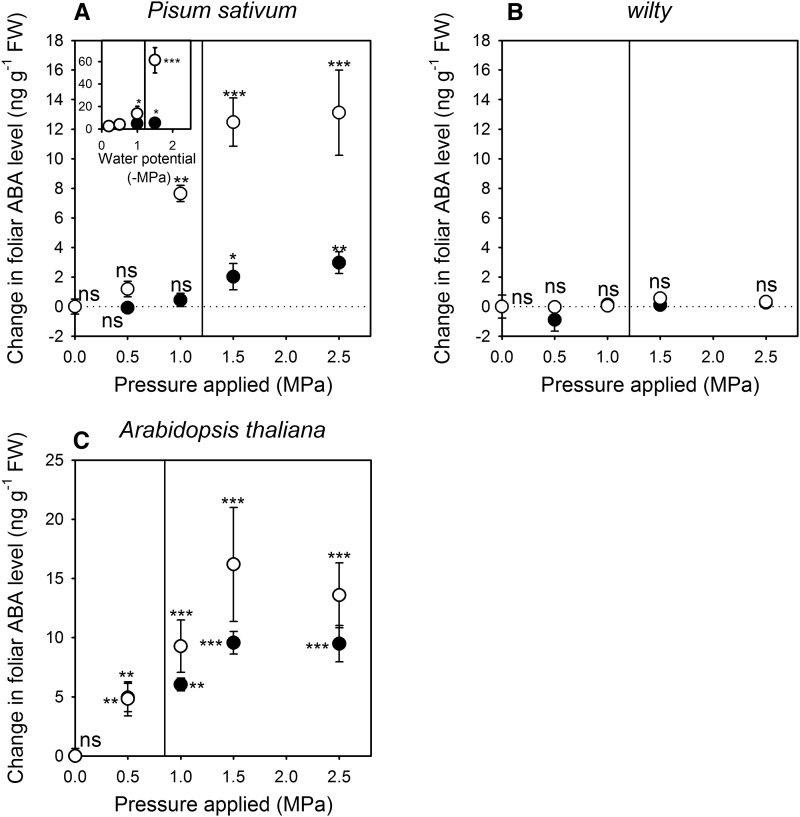
Lowering the turgor of excised leaves of wild-type angiosperm species, by either applying external pressure or floating exposed mesophyll on osmotic solutions, was found to induce a rapid (20 min) and significant increase in the level of ABA (Figs. 1 and 2; Supplemental Fig. S1). The magnitude of the increase in foliar ABA level in angiosperm species appeared to be dependent on the turgor pressure of the leaf, the ability of leaves to synthesize ABA, and, to a lesser extent, the time of exposure to this change in turgor pressure (Figs. 1 and 2; Supplemental Fig. S1). Major differences in the external pressure required to trigger a significant rise in foliar ABA levels were observed between species, with Arabidopsis (Arabidopsis thaliana) showing an increase in foliar ABA level after exposure of leaves to an external pressure of only 0.5 MPa, whereas the other herbaceous angiosperm, Pisum sativum, had a significant increase in foliar ABA level only when leaves were exposed to an external pressure of 1 MPa (Fig. 1). The difference between the trigger for ABA increase was more pronounced in the woody angiosperm species, with foliar ABA levels significantly increasing on exposure to between 0.5 and 1 MPa of external pressure in the cool-temperate rainforest tree Nothofagus cunninghamii, indicating much higher Ψl sensitivity of ABA synthesis than the Mediterranean tree species Olea europaea, which required 1.5 MPa of external pressure to trigger increases in foliar ABA levels (Fig. 2). These differences in the turgor pressure trigger for increases in foliar ABA level were correlated with differences in Ψtlp; however, in all species foliar ABA levels significantly increased while leaf turgor was +0.5 MPa (Supplemental Fig. S1). A similar pressure trigger for an increase in ABA level was observed when leaves were floated on osmotic solutions; however, this method resulted in a more substantial increase in foliar ABA levels than that observed in leaves exposed to external pressures (Fig. 1). The wilty ABA biosynthetic mutant of P. sativum, unlike wild-type plants, had no significant increase in foliar ABA level following exposure to external pressure (Fig. 1). This classic ABA biosynthetic mutant carries a lesion in the short-chain dehydrogenase/reductase responsible for converting xanthoxin to abscisic aldehyde (McAdam et al., 2015).
Figure 1.
The mean change in foliar ABA level (n = 3, ±95% confidence interval) in two herbaceous angiosperm species (P. sativum wild type [A] and wilty [B]; Arabidopsis [C]) after excised leaves were exposed to external pressures for 20 min (black circles) or 60 min (white circles). Vertical lines indicate Ψtlp (Supplemental Fig. S3). The insert in A depicts the mean change in foliar ABA level (n = 4, ±95% confidence interval) in leaves of P. sativum with the abaxial epidermis removed after floating on aqueous solutions of PEG 4000 mixed to particular water potentials for 20 min (black circles) or 60 min (white circles). Stars denote a significant change in foliar ABA level (n.s., not significant; * P < 0.05; ** P < 0.01; *** P < 0.001). Foliar ABA levels presented in terms of dry weight are shown in Supplemental Figure S4.
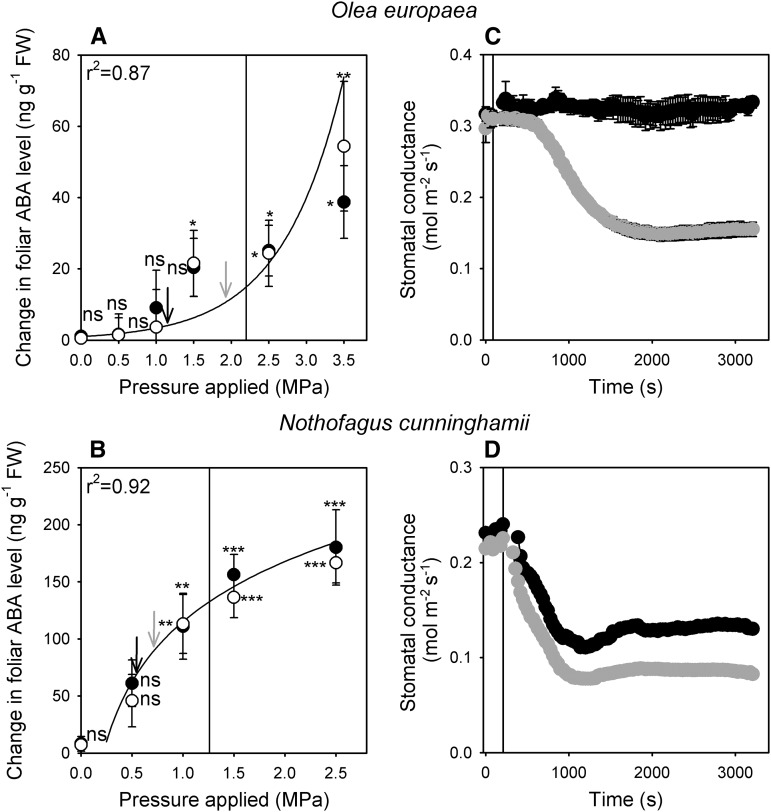
Figure 2.
A and B, The mean change in foliar ABA level (n = 3, ±95% confidence interval) in two woody angiosperm species (O. europaea and N. cunninghamii) after excised leaves were exposed to external pressures for 20 min (black circles) or 60 min (white circles). Vertical lines indicate Ψtlp (Supplemental Fig. S3). Stars denote a significant change in foliar ABA level (n.s., not significant; * P < 0.05; ** P < 0.01; *** P < 0.001). C and D, The mean response of stomatal conductance (n = 3, ±se) to a step change in VPD (depicted by vertical lines) from 0.7 kPa to 1.5 kPa (black circles) or 0.7 kPa to 2.2 kPa (gray circles). Arrows above the regressions in A and B correspond to incipient Ψl measured in branches immediately prior to stomatal closure when exposed to a step change in VPD from 0.7 kPa to 1.5 kPa (black arrows) or 0.7 kPa to 2.2 kPa (gray arrows). Foliar ABA levels presented in terms of dry weight are shown in Supplemental Figure S4.
The pronounced differences in threshold turgor pressure required for the synthesis of foliar ABA in the woody angiosperm species was associated with differences in the sensitivity of stomata to VPD (Fig. 2). Stomatal conductance of the Mediterranean native O. europaea did not respond to a step increase in VPD from 0.7 kPa to 1.5 kPa. This step change in VPD caused Ψl to drop to −1.18 MPa, which would be insufficient to trigger an increase in foliar ABA level after either 20 or 60 min based on the relationship in Figure 2A. However, when leaves of O. europaea were exposed to a severe step increase in VPD from 0.7 kPa to 2.2 kPa, stomata responded by closing over a period of 20 min (Fig. 2). During this more severe step increase in VPD, leaves of O. europaea experienced a minimum Ψl as low as −1.85 MPa, which, based on the relationship between turgor pressure and ABA biosynthesis, would be sufficient to elicit a significant increase in foliar ABA levels (Fig. 2A). In contrast to O. europaea, the stomata of the cool-temperate rainforest tree N. cunninghamii responded to both a mild doubling in VPD from 0.7 kPa to 1.5 kPa as well as the more severe transition from 0.7 kPa to 2.2 kPa, to a greater degree (Fig. 2). For both of these transitions, leaves of N. cunninghamii experienced a Ψl that was low enough to trigger a significant increase in foliar ABA levels (based on the observations from increases in foliar ABA levels after external pressurization experiments; Fig. 2B).
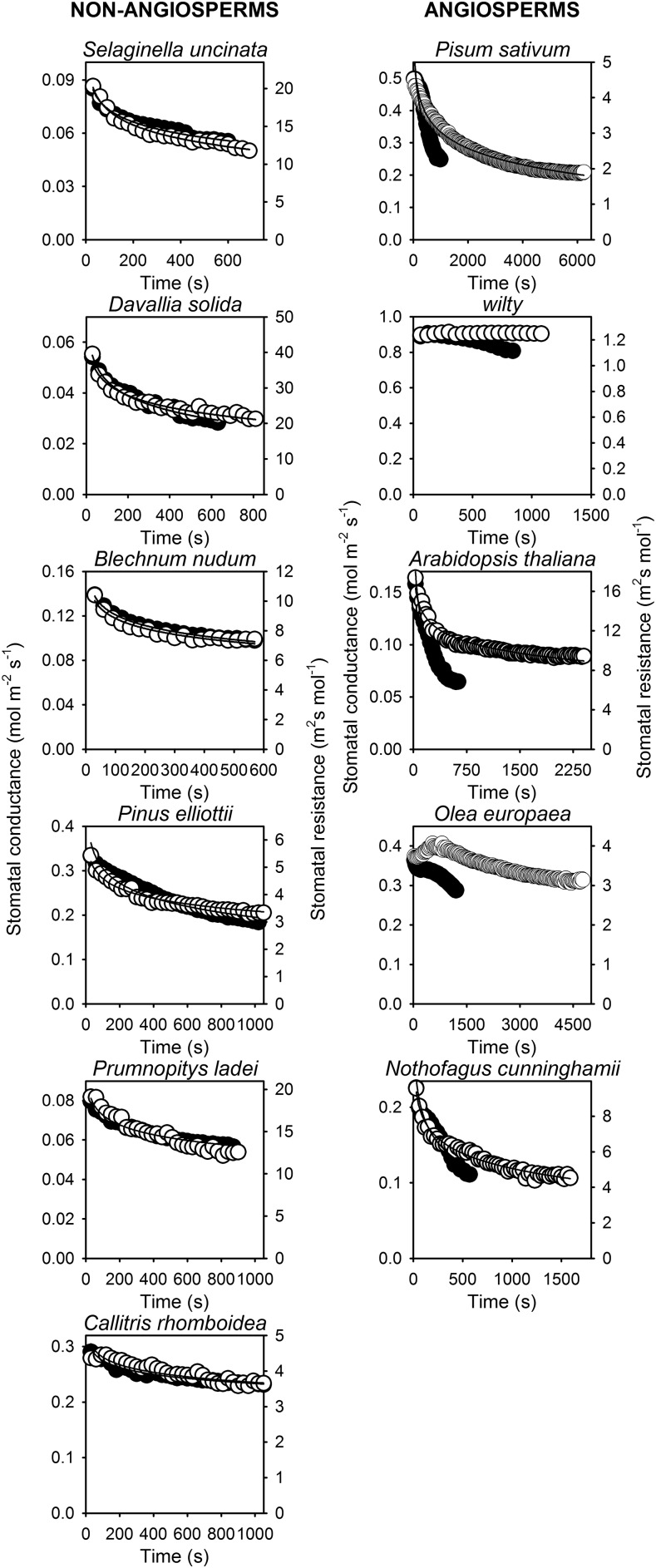
The significant increases in foliar ABA level in angiosperm species caused by reductions in turgor were associated with a significant hysteresis in the response of stomata to a 20-min reversible transition in VPD from 0.7 kPa to 1.5 kPa and returning to 0.7 kPa (Fig. 3). In all angiosperm species, except O. europaea (for which stomata did not respond to this mild transition in VPD, likely because the ABA levels did not increase; Fig. 2A), the dynamics of stomatal opening upon returning to 0.7 kPa from 1.5 kPa were significantly slower than the rates of stomatal closure when leaves were exposed to 1.5 kPa from 0.7 kPa (Fig. 3). Significant hysteresis was observed in the recovery of gs when O. europaea leaves were exposed to a 20-min reversible transition during the more severe VPD transition (0.7 kPa to 2.2 kPa and returning back to 0.7 kPa; Fig. 3). In contrast to angiosperm species, the stomata of all conifer, fern, and lycophyte species responded to the mild, reversible transition in VPD without hysteresis, or difference in the dynamics of stomatal response, on returning to 0.7 kPa from 1.5 kPa (Fig. 3).
Figure 3.
The response of both stomatal conductance (black) to a step increase in VPD from 0.7 kPa to 1.5 kPa and stomatal resistance (white) to the reversed step change in VPD from 1.5 kPa to 0.7 kPa. Data were collected from the same leaf through a reversible sequence in VPD from 0.7 kPa to 1.5 kPa and returning to 0.7 kPa; in O. europaea a step change of 0.7 kPa to 2.2 kPa and returning to 0.7 kPa was used. Time zero for each trace marks the time of a change in VPD.
Lowering the turgor of conifer, fern, and lycophyte leaves by applying external pressure for either 20 min or 1 h did not stimulate an increase in the level of foliar ABA (Fig. 4; Supplemental Fig. S2). This absence of an increase in foliar ABA level after applying external pressure to the leaves of nonangiosperm species was not because these species were exposed to pressures that were significantly lower than Ψtlp (Fig. 4). Ψtlp in the nonangiosperm species spanned the same range of Ψtlp as the angiosperm species (Supplementary Fig. S3).
Figure 4.
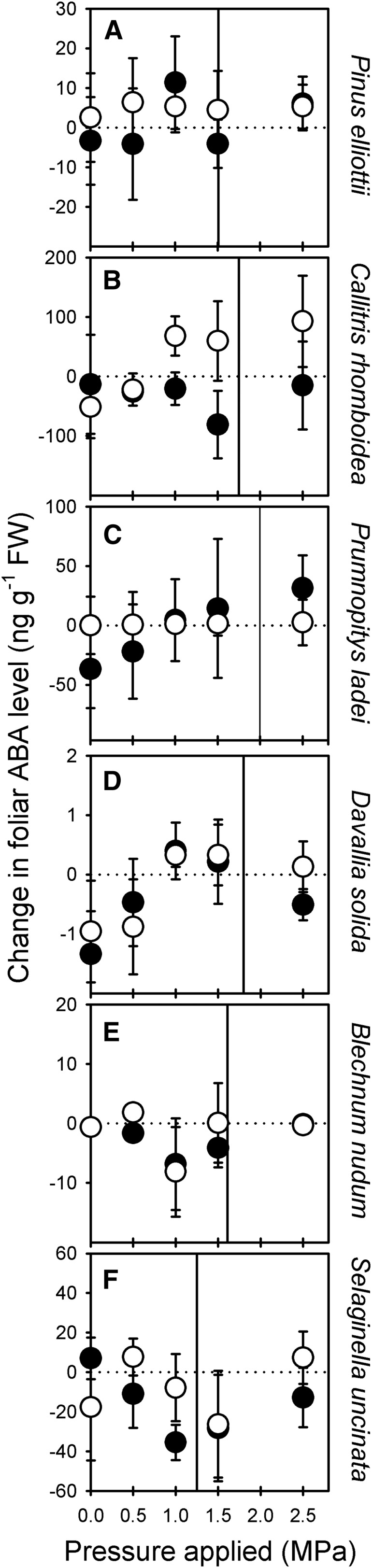
The mean change in foliar ABA level (n = 3, ±95% confidence interval) in three conifer species (A–C), two fern species (D and E), and a lycophyte (F) after excised leaves were exposed to external pressures for 20 min (black circles) or 60 min (white circles). Vertical lines indicate Ψtlp (Supplemental Fig. S1). In all species, changes in foliar ABA level were not significant. Foliar ABA levels presented in terms of dry weight are shown in Supplemental Figure S4.
DISCUSSION
A Leaf Turgor Mechanism for Increases in ABA Levels during VPD Transitions in Angiosperms
In angiosperms it is widely recognized that ABA levels increase as leaf turgor pressure approaches zero during desiccation or drought stress (Pierce and Raschke, 1980; Henson, 1982). Here, using a new technique to characterize the relationship between Ψl and ABA levels, we show that foliar ABA levels in angiosperms significantly increase in leaves well before (more than 0.5 MPa) the complete loss of bulk leaf turgor (Supplemental Fig. S1). Reductions in leaf turgor produced by external pressure or osmotic solutions led to enhanced ABA levels over a very short period of time (20 min). These increases in ABA level are similar to the increases in ABA levels observed during VPD transitions (McAdam and Brodribb, 2015). This observation is consistent with the subtle but continuous increases in foliar ABA levels observed in desiccation experiments prior to Ψtlp (Pierce and Raschke, 1980; Henson, 1982; Creelman and Mullet, 1991; Dingkuhn et al., 1991), and we propose that these increases are due to a reduction in leaf turgor in the same manner as the major increases in foliar ABA levels during desiccation are due to a loss of leaf turgor (Pierce and Raschke, 1980; Davies et al., 1981; Creelman and Zeevaart, 1985). While our method of applying external pressure results in both a decrease in leaf turgor and cell solute potential, we can attribute the increase in foliar ABA levels solely to a decrease in cell turgor, as variation in cell osmotic potential in the absence of changes to cell turgor does not affect ABA levels (Creelman and Zeevaart, 1985).
Our results suggest that changes in leaf turgor during a VPD transition trigger the observed rapid ABA biosynthesis reported during these transitions (Bauerle et al., 2004; McAdam and Brodribb, 2015) and that this provides the signal for stomatal responses to VPD. Our results, as well as measurements that show ABA levels in the epidermis increase slower than those in the leaf following changes in VPD (McAdam and Brodribb, 2015), challenge the hypothesis that guard cell ABA synthesis solely drives stomatal responses VPD (Bauer et al., 2013).
The key finding from our study, that foliar ABA levels in angiosperms can significantly increase following reductions in leaf turgor over a very short period of time, contrasts with earlier studies that suggested a period of at least an hour following a loss of turgor is required before significant increases in foliar ABA level could be detected (Ackerson and Radin, 1983). This difference is likely due to the vastly improved resolution provided by modern methods of liquid chromatography and physicochemical identification of ABA, methods that now are able to detect the subtle yet functionally significant increases in foliar ABA levels that we observe here.
Given that the key rate-limiting carotenoid-cleavage step in ABA biosynthesis, mediated by NCED (9-cis-carotenoid deoxygenase) genes (Qin and Zeevaart, 1999; Thompson et al., 2000), is the only step in the ABA biosynthetic pathway to be up-regulated over the 20-min time frame of a stomatal response to increased VPD (McAdam et al., 2016), it is likely that turgor-activated transcription factors for these genes operate in angiosperms. In support of this hypothesis, a number of genes have been identified that have the capacity to perceive and signal changes in leaf turgor, ranging from those that can directly perceive reductions in cell turgor through to those that can sense changes in tension on the cell membrane (Christmann et al., 2013). Of high importance among these genes are the reports that some may be involved in regulating ABA biosynthesis during severe osmotic stress (Wohlbach et al., 2008; Wang et al., 2011). Whether these turgor-sensing genes are responsible for regulating NCED expression and thus rapid ABA biosynthesis either during a VPD transition or over a time frame relevant to the stomatal response to VPD is yet to be tested.
Our data suggest that ABA synthesis is triggered before turgor loss and at a Ψl expected to occur in well-watered plants exposed to moderate changes in VPD. Further work is required to determine both in which leaf tissues ABA is being synthesized, although there is strong molecular evidence to suggest that the source of foliar ABA levels is in the vasculature, specifically the phloem companion cells (Kuromori et al., 2014), and whether the trigger for synthesis is falling turgor, plasmolysis, or cytorrhysis in these particular cells.
Differences in the Threshold for ABA Biosynthesis Strongly Influences Stomatal Responses to VPD in Angiosperms
Just as differences in Ψtlp can determine species-specific differences in the threshold for the major increases in ABA levels observed during desiccation (Pierce and Raschke, 1980; Davies et al., 1981), here we report striking differences in the threshold for which significant rapid increases in ABA levels occurred in our angiosperm sample. Of particular note was the major difference we observed in the imposed external pressures required to trigger rapid ABA biosynthesis in the two woody angiosperm species from contrasting native ecological ranges. While increases in foliar ABA levels in the temperate rainforest tree N. cunninghamii appeared to be very sensitive to Ψl manipulation by applied external pressures, the Mediterranean tree O. europaea required greater external pressure, up to 1 MPa more than N. cunninghamii, before significant increases in foliar ABA level were observed. This difference in the threshold for rapid ABA biosynthesis in response to external pressures was mirrored by striking differences in the sensitivity of stomata to changes in VPD, with O. europaea stomata being insensitive to mild changes in VPD that resulted in an insufficient reduction in Ψl to trigger the rapid biosynthesis of ABA. Our study may thus provide a very effective method for assessing the potential variation in gas-exchange regulation between species in natural settings. A key question that arises from our study is: just as osmotic adjustment can change Ψtlp, and therefore the threshold for major ABA biosynthesis during water stress (Pierce and Raschke, 1980), can an individual alter the turgor threshold for rapid foliar ABA biosynthesis and therefore the sensitivity of stomata to changes in VPD? It has been shown that leaves grown under different conditions can have quite different sensitivities to changes in VPD, although this has largely been attributed to differences in leaf hydraulic parameters (Appleby and Davies, 1983). Further research into the possibility that this difference could be due to altered thresholds for rapid ABA biosynthesis is required. In addition, stomatal responses to VPD in angiosperms generally begin immediately following the transition in VPD (Fig. 3), suggesting that an increase in ABA level sufficient to activate stomatal closure can occur over extremely short periods of time. Further work is required to investigate the speed with which ABA biosynthesis is activated following a reduction in turgor.
Evolution of a Rapid, Turgor-Triggered Regulation of ABA Biosynthesis
Measurements of foliar ABA levels in nonflowering plants revealed an absence of rapid ABA biosynthesis when leaves were exposed to external pressures. This contrasts strongly with the significant increase in foliar ABA levels observed in representative wild-type angiosperm species. While some controversy remains as to whether the stomata of basal vascular land plants respond to ABA (Brodribb and McAdam, 2011; Ruszala et al., 2011), there is an unchallenged compendium of literature based on leaf gas exchange showing highly predictable and ABA-independent responses of fern and lycophyte guard cells to changes in leaf water status (Lösch, 1977, 1979; Lösch and Tenhunen, 1981; Brodribb and McAdam, 2011; McAdam and Brodribb, 2012, 2013; Husby et al., 2014; McAdam and Brodribb, 2014, 2015; Martins et al., 2016). Our data support this literature in showing that the biosynthesis of ABA in ferns and lycophytes does not occur over a time frame that is relevant to the stomatal response to VPD.
Even among seed plants with ABA-dependent stomatal regulation there is evidence of variation in the role of ABA in regulating daytime stomatal aperture. Recent data suggest that species of conifers and ferns are unable to rapidly increase foliar ABA levels during a VPD transition, which is unlike angiosperm species (McAdam and Brodribb, 2015). There are three possibilities, which are not mutually exclusive, that may explain the absence of a rapid foliar ABA biosynthesis in species of nonflowering plants: (1) the turgor-sensing mechanisms that rapidly up-regulate rate-limiting NCED expression evolved in the earliest angiosperms and are absent in basal lineages; (2) ABA biosynthesis is slower in the basal lineages of land plants; or (3) the trigger for ABA biosynthesis, particularly in ferns and lycophytes, is closer to the point of leaf death (McAdam and Brodribb, 2013). The first two of these hypotheses remain to be tested; however, there is evidence that ABA biosynthesis may be slower in more basal lineages of land plants, with recent bioinformatics analysis revealing that the genomes of the lycophyte Selaginella moellendorffii and conifer Picea abies lack genes encoding the ABA-specific biosynthetic enzymes responsible for at least one or both of the last two steps in the biosynthetic pathway following carotenoid cleavage (Hanada et al., 2011; McAdam et al., 2015). In angiosperms, which have enzymes to specifically catalyze these final two steps in the biosynthetic pathway, conversion of the cleavage product xanthoxin to ABA takes place very quickly (Milborrow, 2001).
CONCLUSION
Our method has revealed that subtle reductions in leaf turgor can trigger the biosynthesis of ABA over a time frame relevant to the stomatal response to VPD, as has been recently hypothesized (Buckley, 2016). Furthermore, we show that the relationship between Ψl and foliar ABA level can be characterized in different species using positive pressure to directly modify Ψl. This relationship between Ψl and foliar ABA levels provides a novel means of assessing the stomatal sensitivity to VPD in angiosperms. Stark differences in the threshold trigger for ABA biosynthesis observed between our two woody angiosperm species corresponded to pronounced differences in the stomatal sensitivity to VPD between these species. Further work is required under both controlled conditions and field settings to investigate whether differences in the stomatal sensitivity to VPD both within and between species might be due to differences in the threshold leaf turgor required for activating ABA biosynthesis. Whether differences in the rate or trigger of ABA catabolism, which also has a major influence over ABA levels (Okamoto et al., 2009), similarly influences the stomatal response to VPD is also yet to be tested.
MATERIALS AND METHODS
Plant Material
Potted individuals from a wide phylogenetic and ecological range of vascular land plants were specifically selected for this study. These included the two herbaceous angiosperm species Arabidopsis (Arabidopsis thaliana ‘Wassilewskija’; Brassicaceae) and Pisum sativum (‘Argenteum’ and the ABA-biosynthetic mutant ‘wilty’; Fabaceae); two woody angiosperm species from differing native ecological ranges, the cultivated Mediterranean tree Olea europaea ‘Kalamata’ (Oleaceae) and the cool-temperate rainforest tree Nothofagus cunninghamii (Nothofagaceae); three conifer species, including Pinus elliottii (Pinaceae), Callitris rhomboidea (Cupressaceae), and Prumnopitys ladei (Podocarpaceae); two ferns, including the epiphyte Davallia solida (Davalliaceae) and terrestrial fern Blechnum nudum (Blechnaceae); and the lycophyte Selaginella uncinata (Selaginellaceae). Prior to experimentation all plants, except Arabidopsis, were grown under controlled glasshouse conditions and natural light supplemented and extended to a 16-h photoperiod by sodium vapor lamps, which ensured a minimum 300 µmol quanta m−2 s−1 at the pot surface and 23°C/16°C day/night temperatures. Arabidopsis individuals were grown in a growth cabinet under a 10-h photoperiod provided by cool-white fluorescent tubes supplying 100 µmol quanta m−2 s−1 at the pot surface and 22°C/16°C day/night temperatures. All plants received daily watering and weekly applications of liquid nutrients.
Changing Turgor by Controlled Pressurization
Branches or compound leaves were excised from individuals, and a sample leaf adjacent to the manipulated leaves was taken for foliar ABA quantification to act as a control. Branches or leaves were then wrapped in damp paper towel to prevent dehydration by transpiration and enclosed in a Scholander pressure chamber with the excised end emerging from the chamber. A positive pressure provided by compressed air was applied to the sample to a prescribed MPa (0.5 MPa, 1 MPa, 1.5 MPa, and 2.5 MPa [and 3.5 MPa for O. europaea]) for both 20 and 60 min. Increases and decreases in pressure were made gradually (less than 0.1 bar s−1). Control samples were also included where leaves were enclosed in the chamber without pressurization. Pressure applied to leaves enclosed entirely (without protruding petiole) within a pressure chamber does not cause an increase in ABA level (Ackerson and Radin, 1983). Following pressurization, a sample was again taken for foliar ABA quantification. A microscope was used to monitor the excised end of the branch or leaf during the experiment to ensure that tissue in the chamber did not desiccate to a Ψl below the pressure applied.
Changing Turgor by Osmotic Solution
Using leaves of the Argenteum mutant of P. sativum, which has an epidermis isolated from the mesophyll (Hoch et al., 1980) and is thus easy to remove without damaging the mesophyll or veins, the abaxial epidermis was removed and the lamina was floated on aqueous solutions of polyethylene glycol (PEG; average Mr 4000 g mol−1) prepared to water potentials of −0.2 MPa, −0.5 MPa, −1 MPa, or −1.5 MPa based on the relationship between water potential and molarity of PEG 4000 determined by Steuter et al. (1981). After a period of 20 and 60 min, leaves were removed from the aqueous solutions, the surface PEG solution was removed with damp paper towel, and samples were immediately taken for ABA quantification.
Stomatal Responses to VPD
In all species the degree of dynamic hysteresis in the recovery of gs during a reversible, mild transition in VPD was assessed using a portable infrared gas analyzer (Li-6400; LI-COR Biosciences). Well-watered potted plants were brought into the laboratory the night before the experiment to reduce perturbation prior to gas-exchange measurements. The environmental conditions in the cuvette of the gas analyzer were controlled for the duration of the experiment at an air temperature of 22°C, light intensity of 1000 µmol quanta m−2 s−1, and VPD regulated initially at 0.7 kPa using a portable dew-point generator (Li-610; LI-COR Biosciences). Leaf gas exchange and cuvette environmental conditions were logged every 30 s. Leaves were allowed to equilibrate to the conditions inside the cuvette until leaf gas exchange had reached a maximum and stabilized. Following stabilization, VPD in the cuvette was increased to 1.5 kPa and maintained for 20 min, after which it was returned to 0.7 kPa and maintained until gs had recovered and/or stabilized.
In the two woody angiosperm species O. europaea and N. cunninghamii, the response of stomatal conductance (gs) to single step changes in VPD, including a mild transition (0.7 kPa to 1.5 kPa) and a large transition (0.7 kPa to 2.2 kPa), was monitored over 60 min. Conditions in the cuvette of the gas analyzer were maintained as described above. To determine the change in Ψl that the leaves of these species experienced during these two transitions in VPD, shoots of each species were enclosed in a lighted conifer chamber (Li-6400-22L; LI-COR Biosciences) connected to the infrared gas analyzer while the rest of the plant remained under laboratory conditions. Conditions in the chamber were maintained as described above for the leaf cuvette. Branches were initially allowed to equilibrate to chamber conditions until gs had reached a maximum and stabilized, after which the step change in VPD was made. Approximately 5 min after this transition in VPD and before stomata had closed, the branch was removed from the chamber, excised, immediately wrapped in damp paper towel, and Ψl assessed using a Scholander pressure chamber and microscope to precisely measure the balance pressure.
Turgor Loss Point and Determining Leaf Turgor
To determine Ψtlp, pressure-volume curves were constructed from three leaves of each species (Tyree and Hammel, 1972). Leaves were cut underwater and allowed to hydrate to >−0.05 MPa, after which they were allowed to slowly dry on the bench while leaf weight and Ψl were periodically measured until Ψl stopped falling. Relative water content was plotted against Ψl for each leaf, and Ψtlp was determined as the point of inflection between the linear and nonlinear portions of the plot. Results were also plotted against average leaf turgor pressure, which was derived by subtracting leaf osmotic potential (quantified from pressure-volume curves as the intersection of the Ψl axis by a linear regression fitted through the data after turgor loss point) from the external pressure, or water potential of the osmotic solution, that the leaf was exposed to using the spreadsheet tool for pressure-volume curve analysis of Sack and Pasquet-Kok (2011).
Foliar ABA Quantification and Analysis
Foliar ABA level was extracted, purified, and physicochemically quantified by the high precision method of ultra-performance liquid chromatography tandem mass spectrometry with an added internal standard according to the method of McAdam (2015). When the results of foliar ABA levels were analyzed in terms of fresh weight and dry weight (based on calculating dry masses from the balance pressures applied and pressure-volume relationships), similar results were obtained (Supplemental Fig. S4).
Statistical Analysis
In all species to test whether changing leaf turgor (by either external pressurization or floating on osmotic solutions) significantly increased foliar ABA levels, one-way ANOVA followed by posthoc Tukey’s test was undertaken using R Statistical Software (version 3.2.2). For O. europaea and N. cunninghamii, exponential growth or rise curves, respectively, were fitted to the relationships between the change in foliar ABA level and external pressure applied using the curve-fitting functions of the Sigma Plot software. To compare the response of stomata with a reversible transition in VPD to observe any dynamic hysteresis, time courses of the response of stomatal conductance to an increase in VPD were overlaid with time courses during a decrease in VPD of the inverse measure, stomatal resistance.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The mean change in foliar ABA level plotted against leaf turgor pressure in the angiosperm species.
Supplemental Figure S2. The mean change in foliar ABA level plotted against leaf turgor pressure in the conifer, fern, and lycophyte species.
Supplemental Figure S3. Pressure-volume relationships for all species.
Supplemental Figure S4. The mean change in foliar ABA level in terms of dry weight.
Supplementary Material
Acknowledgments
We would like to thank Shelley Urquhart and Theodore Dimitriou for helping prepare samples for ABA analysis; David Nichols for chromatography; John Ross for ongoing counsel regarding sample preparation and analysis; Frances Sussmilch for providing critical comments; and Charlotte Ragus, Emma Greenwood, Julia Rhodes, Marcus Gregory, and Sophie McCoull for assistance during the PEG incubation experiments.
Glossary
- VPD
vapor pressure deficit
Footnotes
This work was supported by the Australian Research Council (grant nos. DE140100946 [S.A.M.M.] and DP140100666 [T.J.B.]).
Articles can be viewed without a subscription.
References
- Ackerson RC, Radin JW (1983) Abscisic acid accumulation in cotton leaves in response to dehydration at high pressure. Plant Physiol 71: 432–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby RF, Davies WJ (1983) A possible evaporation site in the guard cell wall and the influence of leaf structure on the humidity response by stomata of woody plants. Oecologia 56: 30–40 [DOI] [PubMed] [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Whitlow TH, Setter TL, Vermeylen FM (2004) Abscisic acid synthesis in Acer rubrum L. leaves—a vapor-pressure-deficit-mediated response. J Am Soc Hortic Sci 129: 182–187 [Google Scholar]
- Beardsell MF, Cohen D (1975) Relationships between leaf water status, abscisic acid levels, and stomatal resistance in maize and sorghum. Plant Physiol 56: 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol 132: 2166–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26: 443–450 [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Buckley TN. (2016) Stomatal responses to humidity: Has the “black box” finally been opened? Plant Cell Environ 39: 482–484 [DOI] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1991) Abscisic acid accumulates at positive turgor potential in excised soybean seedling growing zones. Plant Physiol 95: 1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Zeevaart JAD (1985) Abscisic acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol 77: 25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Wilson JA, Sharp RE, Osonubi O (1981) Control of stomatal behaviour in water-stressed plants. In Jarvis PG, Mansfield TA, eds, Stomatal Physiology. Cambridge University Press, Cambridge, UK, pp 163–185 [Google Scholar]
- Dingkuhn M, Cruz RT, O’Toole JC, Turner NC, Doerffling K (1991) Responses of seven diverse rice cultivars to water deficits. III. Accumulation of abscisic acid and proline in relation to leaf water-potential and osmotic adjustment. Field Crops Res 27: 103–117 [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Hase T, Toyoda T, Shinozaki K, Okamoto M (2011) Origin and evolution of genes related to ABA metabolism and its signaling pathways. J Plant Res 124: 455–465 [DOI] [PubMed] [Google Scholar]
- Henson IE. (1982) Abscisic acid and water relations of rice (Oryza sativa L.): sequential responses to water stress in the leaf. Ann Bot (Lond) 50: 9–24 [Google Scholar]
- Hoch HC, Pratt C, Marx GA (1980) Subepidermal air spaces: basis for the phenotypic expression of the Argenteum mutant of Pisum. Am J Bot 67: 905–911 [Google Scholar]
- Husby CE, Delatorre-Herrera J, Oberbauer SF, Grau A, Novara L (2014) Stomatal conductance patterns of Equisetum giganteum stems in response to environmental factors in South America. Botany 92: 701–712 [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) heynh. Theor Appl Genet 61: 385–393 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61: 377–383 [Google Scholar]
- Kuromori T, Sugimoto E, Shinozaki K (2014) Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol 164: 1587–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösch R. (1977) Responses of stomata to environmental factors: experiments with isolated epidermal strips of Polypodium vulgare. I. Temperature and humidity. Oecologia 29: 85–97 [DOI] [PubMed] [Google Scholar]
- Lösch R. (1979) Responses of stomata to environmental factors: experiments with isolated epidermal strips of Polypodium vulgare. II. Leaf bulk water potential, air humidity and temperature. Oecologia 39: 229–238 [DOI] [PubMed] [Google Scholar]
- Lösch R, Tenhunen JD (1981) Stomatal responses to humidity - phenomenon and mechanism. In Jarvis PG, Mansfield TA, eds, Stomatal Physiology. Cambridge University Press, Cambridge, UK [Google Scholar]
- Martins SCV, McAdam SAM, Deans RM, DaMatta FM, Brodribb TJ (2016) Stomatal dynamics are limited by leaf hydraulics in ferns and conifers: results from simultaneous measurements of liquid and vapour fluxes in leaves. Plant Cell Environ 39: 694–705 [DOI] [PubMed] [Google Scholar]
- McAdam SAM. (2015) Physicochemical quantification of abscisic acid levels in plant tissues with an added internal standard by ultra-performance liquid chromatography. Bio Protoc 5: e1599 [Google Scholar]
- McAdam SAM, Brodribb TJ (2012) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2013) Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol 198: 429–441 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2014) Separating active and passive influences on stomatal control of transpiration. Plant Physiol 164: 1578–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2015) The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiol 167: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ (2016) Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ 39: 485–491 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ, Ross JJ (2015) Molecular characterization of a mutation affecting abscisic acid biosynthesis and consequently stomatal responses to humidity in an agriculturally important species. AoB Plants 7: plv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Jalakas P, Kollist H, Brosché M (2015) The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol Plant 8: 657–659 [DOI] [PubMed] [Google Scholar]
- Milborrow BV. (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52: 1145–1164 [PubMed] [Google Scholar]
- Mittelheuser CJ, Van Steveninck RFM (1969) Stomatal closure and inhibition of transpiration induced by (RS)-abscisic acid. Nature 221: 281–282 [Google Scholar]
- Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM (2007) The control of transpiration. Insights from Arabidopsis. Plant Physiol 143: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E (2009) High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol 149: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M, Raschke K (1980) Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 148: 174–182 [DOI] [PubMed] [Google Scholar]
- Pierce M, Raschke K (1981) Synthesis and metabolism of abscisic acid in detached leaves of Phaseolus vulgaris L. after loss and recovery of turgor. Planta 153: 156–165 [DOI] [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96: 15354–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Sack L, Pasquet-Kok J (2011) Leaf pressure-volume curve parameters. http://prometheuswiki.publish.csiro.au/tiki-citation.php?page=Leaf%20pressure-volume%20curve%20parameters#sthash.1KxZH2vQ.dpuf
- Sharp RE, LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53: 33–37 [PubMed] [Google Scholar]
- Steuter AA, Mozafar A, Goodin JR (1981) Water potential of aqueous polyethylene glycol. Plant Physiol 67: 64–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Nevo Y (1973) Abnormal stomatal behavior and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochem Genet 8: 291–300 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, et al. (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143: 1905–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR, Blake PS, Burbidge A, Taylor IB (2000) Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J 23: 363–374 [DOI] [PubMed] [Google Scholar]
- Trejo CL, Davies WJ, Ruiz L (1993) Sensitivity of stomata to abscisic acid (an effect of the mesophyll). Plant Physiol 102: 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Hammel HT (1972) The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J Exp Bot 23: 267–282 [Google Scholar]
- Wang Z-Y, Xiong L, Li W, Zhu J-K, Zhu J (2011) The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 23: 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25: 195–210 [DOI] [PubMed] [Google Scholar]
- Wohlbach DJ, Quirino BF, Sussman MR (2008) Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell 20: 1101–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright STC, Hiron RWP (1969) (+)-Abscisic acid, the growth inhibitor induced in detached wheat leaves by a period of wilting. Nature 224: 719–720 [Google Scholar]
- Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HMO, et al. (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr Biol 16: 882–887 [DOI] [PubMed] [Google Scholar]
- Zabadal TJ. (1974) A water potential threshold for the increase of abscisic acid in leaves. Plant Physiol 53: 125–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.