Abstract
ROS, calcium, and electric signals mediate rapid systemic signaling in plants.
The systemic response of plants to pathogen infection (systemic acquired resistance [SAR]), or wounding has been extensively studied with a network of numerous compounds and signals implicated (for review, see Dempsey and Klessig, 2012; Shah and Zeier, 2013). In recent years a new type of systemic response, termed systemic acquired acclimation (SAA), has emerged as an important acclimation response of plants to abiotic stresses (e.g. Karpiński et al., 1999; Szechyńska-Hebda et al., 2010; Suzuki et al., 2013). This response is characterized by a rapid spread of the systemic signal(s) that can reach the systemic tissue within minutes from the application of abiotic stress to a local tissue. A number of different signaling mechanisms were implicated in this response, including the reactive oxygen species (ROS) wave (Miller et al., 2009), the calcium (Ca2+) wave (Choi et al., 2014), and electric signals (Szechyńska-Hebda et al., 2010). In this review we will focus on recent findings regarding each of these signals, as well as their integration, and attempt to propose a model for the propagation of rapid systemic signals during SAA and SAR. Due to space limitations, we will not address many other important aspects of ROS signaling that have been covered by a number of recent excellent reviews (for review, see Foyer and Noctor, 2013; Vaahtera et al., 2014; Considine et al., 2015; Dietz, 2015; Mignolet-Spruyt et al., 2016).
THE ROS WAVE
The ROS wave is an autopropagating wave of ROS production mediated via RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD) activation in each cell along its systemic path (Fig. 1). It was initially discovered by Miller et al. (2009), and was extensively reviewed by Mittler et al. (2011), Gilroy et al. (2014), and Mittler and Blumwald (2015). It can reach rates of up to 8.4 cm/min and is directly linked to the calcium wave (for review, see Gilroy et al., 2014) and possibly to electric signals (Suzuki et al., 2013; Fig. 2). It is required for SAA, but by itself it likely does not convey specificity to the systemic response of plants to different abiotic stresses (Suzuki et al., 2013; for review, see Mittler et al., 2011). The ROS wave is currently thought to be integrated with additional metabolic/signaling pathways and to enable rapid SAA responses and acclimation of plants, improving their overall fitness (Suzuki et al., 2013; for review, see Mittler et al., 2011; Mittler and Blumwald, 2015). Activation of the ROS wave by a local heat stress was shown, for example, to enhance the acclimation of systemic tissues to heat stress, and to be regulated by temporal and special interactions with ABA signaling (Suzuki et al., 2013; for review, see Mittler and Blumwald, 2015). In addition, local application of high light resulted in the activation of a ROS wave that enabled systemic tissues to withstand light stress, and was accompanied by the accumulation of photorespiratory amino acids, including Gly and Ser, in nonstressed systemic tissues (Suzuki et al., 2013).
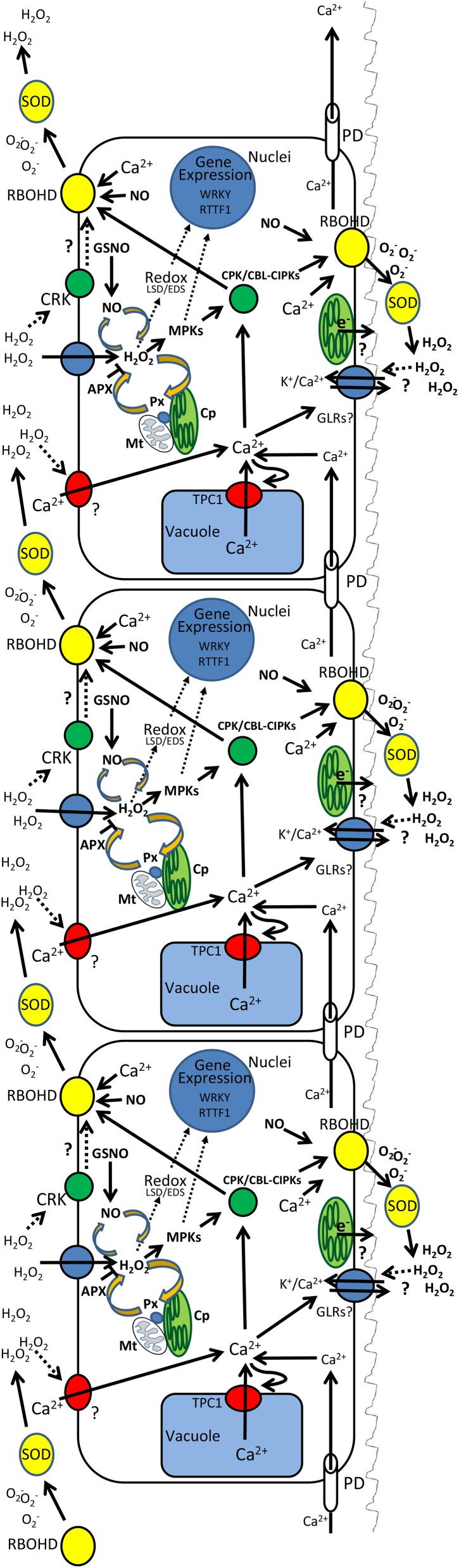
Figure 1.
Integration of the ROS, Ca2+, and electric waves in and between cells via the function of RBOH proteins and superoxide dismutases (SODs; yellow), Ca2+-dependent protein kinases (CPK/CBL-CIPKs; green), calcium channels such as TPC1 and H2O2-activated plasma membrane calcium channels (red), and GLRs and/or plasma membrane H2O2 channels (blue). Activation of RBOHD is shown to be mediated by CPK/CBL-CIPKs, Cys-rich receptor kinases (CRKs), or directly by Ca2+. Activation of Ca2+ channels is shown to be mediated by H2O2 or by calcium via calcium-induced calcium release. Activation of GLRs is proposed to be mediated by H2O2 and/or calcium levels via calcium-induced calcium release. The level of ROS in cells is proposed to be regulated by NO-ROS and NO-RBOH interactions, retrograde signaling, and ROS removal/production in the chloroplast (Cp), mitochondria (Mt), and peroxisomes (Px). The regulation of gene expression in the nuclei is shown to be mediated via redox/ROS changes, LESION SIMULATING DISEASE1 (LSD), ENHANCED DISEASE SUSCEPTIBILITY1 (EDS), MITOGEN-ACTIVATED PROTEIN KINASEs (MPKs), WRKY, and RRTF1. The electric signal is depicted as a sinus-like wave that travels along the plasma membrane and through the plasmodesmata (PD).
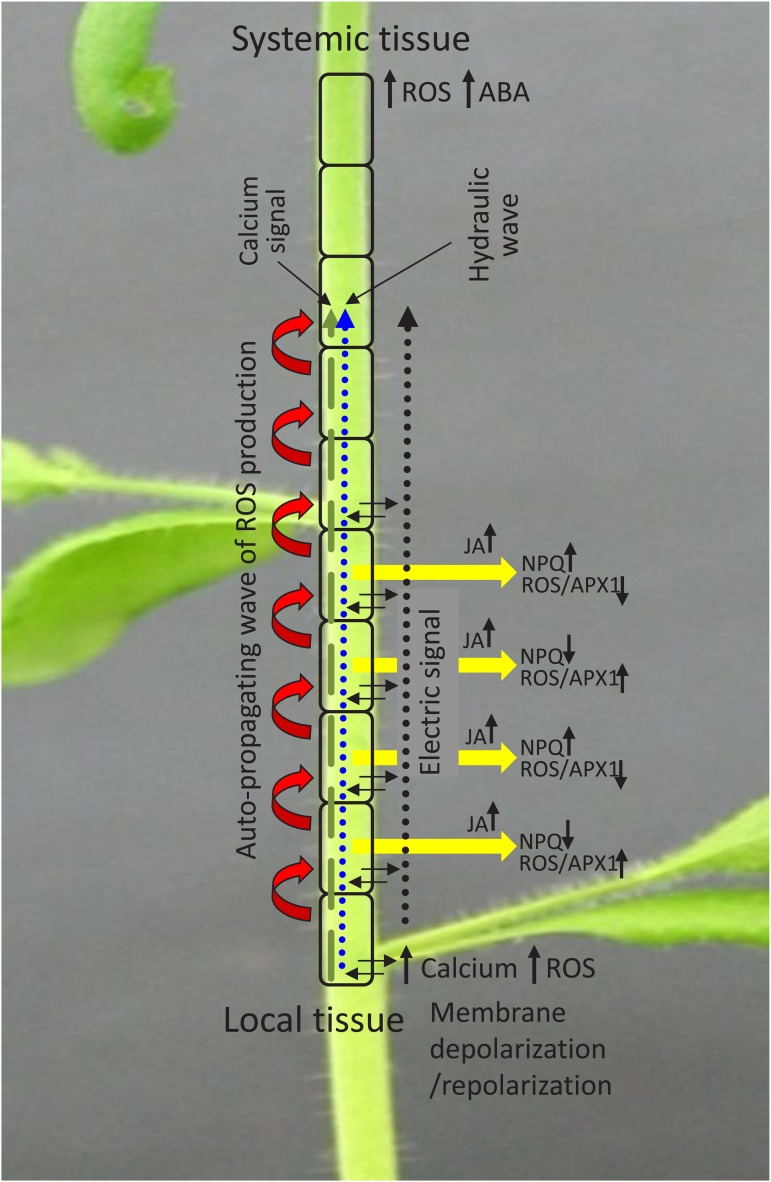
Figure 2.
Copropagation of the ROS, calcium, hydraulic, and electric waves during rapid systemic signaling. The ROS wave is shown as a series of red arrows, the calcium wave is shown as a dashed green arrow, the hydraulic wave is shown as a dotted blue arrow, and the electric wave is shown as a dotted black arrow. Different sections along the path of the signal (yellow arrows) are also shown to have alternating levels of NPQ and ROS/APX1 levels, and JA is shown to accumulate in cells along the systemic path. The local tissue is shown to have alterations in ROS, calcium, and membrane depolarization potential, and the systemic tissue is shown to have accumulation of ROS and abscisic acid (ABA). Black arrows indicate accumulation or suppression in the level of a particular chemical or transcript/protein, and dashed, dotted, and wide red arrows indicate direction of the signal.
LESSONS FROM SAR AND A POSSIBLE ROLE FOR NITRIC OXIDE
The integration of different signaling pathways with ROS-dependent systemic responses has been extensively studied during pathogen-induced SAR, and research focusing on phloem-mobile SAR signals have identified several biologically active molecules, including methyl salicylate, a glycerol-3-P (G3P) derivative, a lipid-transfer protein (DIR1), azelaic acid (AzA), dehydroabietinal, jasmonic acid (JA), and pipecolic acid (Návarová et al., 2012; for review, see Dempsey and Klessig, 2012; Shah and Zeier, 2013). Among these, methyl salicylate, AzA, dehydroabietinal, and G3P were shown to induce SAR when applied to local tissues (Kachroo and Robin, 2013), and JA was shown to rapidly accumulate throughout the path of the systemic signal (Glauser et al., 2009). Recent studies demonstrated that the synthesis of these molecules could be regulated by ROS. Therefore, Arabidopsis (Arabidopsis thaliana) plants deficient in RBOHD or RBOHF showed lower accumulation of AzA and G3P, and exogenous application of G3P was able to rescue SAR in these plants (for review, see Wang et al., 2014). These findings could suggest that the ROS wave is also activated during SAR.
Some of the most recent findings in SAR that may shed more light on how the ROS wave is mediated during SAA come from recent studies that focused on the interdependence of ROS signaling on nitric oxide (NO). The noa1/nia1 double mutant, deficient in NO accumulation, showed, for example, a fully compromised SAR accompanied by lower ROS accumulation in systemic tissues, that could be rescued by exogenous application of H2O2 (for review, see Wang et al., 2014). In addition, RBOHD was shown to be up-regulated through an NO-dependent process elicited by oligogalacturonides in response to pathogen attack (Rasul et al., 2012). Although these findings indicated that ROS could act downstream to NO in the SAR pathway, Arabidopsis deficient in RBOHD or RBOHF were unable to accumulate NO in response to pathogen attack (for review, see Wang et al., 2014; Wendehenne et al., 2014), suggesting that NO and ROS could operate in a feedback loop (Fig. 1). NO can react with reduced glutathione, by an S-nitrosylation reaction, to form S-nitrosoglutathione (GSNO; for review, see Considine et al., 2015; Del Río, 2015), and GSNO could function as a pool of NO ready to be used in ROS-NO interactions during SAR/SAA systemic signaling (Fig. 1). ROS, NO, and GSNO function as master-switches regulating various processes, including the SAR pathway and stomatal responses (for review, see Wang et al., 2014; Mittler and Blumwald, 2015; Considine et al., 2015). NO-dependent redox-based posttranslational modifications, such as addition of a glutathione to a protein Cys thiol in a glutathionylation reaction or a NO moiety to form a S-nitrosothiol, were shown to act as regulators of different processes (for review, see Zaffagnini et al., 2012; Considine et al., 2015), and these could also function during SAR or SAA to amplify or dampen the signal. In contrast to the up-regulation of RBOHD through an NO-dependent process enhanced by oligogalacturonides (Rasul et al., 2012), S-nitrosylation of RBOHD at Cys-890 was found to inhibit its enzymatic activity by impeding FAD binding (Yun et al., 2011), indicating a possible dual role for NO in the regulation of RBOHD. NO-mediated S-nitrosylation was also shown to inhibit ROS scavenging enzymes such as catalase and ascorbate peroxidase (Ortega-Galisteo et al., 2012; de Pinto et al., 2013), suggesting that NO could also promote an increase in H2O2 by reducing its decay (Suzuki et al., 2013). Moreover, glutaredoxin S12 in the chloroplast and Gly decarboxylase in the mitochondria were shown to be targets of glutathionylation, and these posttranslational modifications might also contribute to the regulation of ROS levels in cells (Palmieri et al., 2010; for review, see Zaffagnini et al., 2012). Although the involvement of NO in SAA has not been thoroughly studied, the regulation of RBOHD via NO signaling could implicate NO as a key player in modulating the ROS wave (Fig. 1). Furthermore, peroxisomes, mitochondria, and chloroplasts, known as sources of ROS, were also shown to generate NO that might regulate signal transduction involved in various biological processes (for review, see Del Río, 2015).
THE ROS WAVE, RETROGRADE SIGNALING, AND TRANSCRIPTIONAL REGULATION
Recent studies uncovered a possible integration between a hub of regulatory genes involved in programmed cell death, retrograde signaling, and hormone regulation, and ROS-dependent systemic responses to high light stress. Thus, genes such as LESION SIMULATING DISEASE1, ENHANCED DISEASE SUSCEPTIBILITY1, PHYTOALEXIN DEFICIENT4, ETHYLENE INSENSITIVE2, and MITOGEN-ACTIVATED PROTEIN KINASE 4 were shown to play important roles in the simultaneous regulation of SAA via ROS- and salicylic acid-dependent pathways (Mühlenbock et al., 2008; Szechyńska-Hebda et al., 2010; Wituszyńska et al., 2013; Gawroński et al., 2014; for review, see Karpiński et al., 2013; Mignolet-Spruyt et al., 2016). In addition, PHOTOSYSTEM II SUBUNIT S-dependent local and systemic wave-like regulation of nonphotochemical quenching (NPQ) and chlorophyll fluorescence decay time, important dissipation and quenching mechanisms of light absorbed in excess, were also proposed to be dependent on ROS and salicylic acid signaling during SAA (Szechyńska-Hebda et al., 2010; Gawroński et al., 2013, 2014; Ciszak et al., 2015; Fig. 2). Although a possible cross talk between ROS and retrograde signaling involving some of the genes indicated above has been proposed, and could explain how the systemic signal is integrated with redox and photosynthesis/respiration control, further studies are required to uncover how these pathways and the RBOHD-dependent ROS wave are coordinated during SAA and possibly SAR (for review, see Mignolet-Spruyt et al., 2016). For example, it is not clear if retrograde signaling functions downstream to rapid systemic signaling, or whether it is directly involved in attenuating or amplifying the signal.
A recent study demonstrated that REDOX RESPONSIVE TRANSCRIPTION FACTOR1 (RRTF1) might function as a key regulator of systemic ROS-dependent responses during high light stress (Matsuo et al., 2015). RRTF1-dependent systemic signaling was shown to be regulated by WRKY transcription factors (Matsuo et al., 2015), whose expression is enhanced during RBOHD-dependent SAA (Miller et al., 2009), but its rate of systemic response is slower than that of the ROS wave. RRTF1 could therefore play a role in the regulation of gene expression downstream to the ROS wave during SAA (Fig. 1).
Taken together, SAA might be regulated by a complex network linking NO, retrograde signaling, and RBOH-dependent ROS signaling that could function to amplify or attenuate the systemic signal (Fig. 1). Further studies are of course required to determine the mode of integration of NO and ROS signals in SAA that could be a key aspect of systemic signaling during abiotic stress.
THE CA2+ WAVE
Along with ROS, changes in cytosolic Ca2+ have also been linked to rapid, systemic signaling activity throughout the plant. Calcium is a ubiquitous cellular regulator involved in a wide range of physiological processes and responses to both biotic and abiotic stresses (for review, see Dodd et al., 2010; Steinhorst and Kudla, 2014). Direct Ca2+ movement through xylem has been proposed as one component of this long-range Ca2+-dependent response network (Tang et al., 2007). However, in addition to this apoplastic route for a movement of Ca2+ signals, there is now also evidence for the systemic spread of a cytosolic Ca2+ increase. This Ca2+ “wave” appears to couple local sensing of stimuli such as wounding or salt stress to plant-wide adaptive responses (Figs. 1 and 2).
Thus, local salt stress has been shown to trigger a cytosolic Ca2+ increase that propagates from sites of local perception in both root and shoot in Arabidopsis (Choi et al., 2014; Xiong et al., 2014). This Ca2+ elevation spreads through the cortex and endodermal cell layers in the root (Choi et al., 2014), but appears to spread more broadly across the leaves (Xiong et al., 2014). When directly challenged with NaCl, all root cell types can respond, implying specialized molecular mechanisms related to transmission in the cortical and endodermal cell layers. Similarly, mechanical wounding and insect damage trigger electrical signals (Mousavi et al., 2013; Salvador-Recatalà et al., 2014) that are likely strongly coupled to a Ca2+ wave system (Kiep et al., 2015) that propagates from wounded to unwounded leaves, with phloem providing at least one of the tissues used for transmission of the mobile signal (Salvador-Recatalà et al., 2014). Indeed, electrical signaling in the phloem has been closely linked to wound responses (for review, see Hedrich et al., 2016). Clustering of Ca2+ channels at the sieve plates and plasmodesmal connections to companion cells has also been proposed as a key player in sustaining systemic propagation of Ca2+ increases linked to these phloem responses (Furch et al., 2009; also see discussion in van Bel et al., 2014).
CA2+ CHANNELS AND THE CA2+ WAVE
Although identifying the channels involved in the processes described above at the molecular level remains a major challenge, recent insights into the role of the vacuolar channel TWO PORE CHANNEL1 (TPC1) and Glu Receptor-Like channels (GLRs) have begun to shed some light on potential mechanisms (Fig. 1). Thus, propagation of NaCl- and wound-induced Ca2+ waves are dependent on the slow vacuolar ion channel encoded by TPC1 (Choi et al., 2014; Kiep et al., 2015). Mutation in the vacuolar Ca2+-sensing domain of this channel (Beyhl et al., 2009; Dadacz-Narloch et al., 2011; Guo et al., 2016) also leads to up-regulation of jasmonate production, a hormone intimately linked to systemic wound responses (e.g. Bonaventure et al., 2007), reinforcing the idea that the regulation of this channel might be linked to these kinds of systemic response systems. The lack of a TPC1 mutant Ca2+ response phenotype to a range of biotic and abiotic stresses when monitored at the whole-plant level (Ranf et al., 2008) is consistent with a role for this channel as a systemic element of Ca2+ responses. Thus, the spread of Ca2+ increase is suppressed in the tpc1-2 knockout in response to both local NaCl and wound treatments, whereas local/direct Ca2+ elevations are either slightly delayed (NaCl; Choi et al., 2014) or even extended (wounding; Kiep et al., 2015).
TPC1 also serves as an example of the complex set of potential interactions we can expect to contribute to and/or accompany these systemic propagation events. Thus, in addition to its vacuolar Ca2+ sensor, TPC1 contains two classic Ca2+-binding EF-hand domains on its cytosolic face (although only one may be functional in Ca2+ activation; Schulze et al., 2011; Guo et al., 2016; Kintzer and Stroud, 2016), and both vacuolar and cytosolic Ca2+ levels are thought to modulate channel activity through effects on the channel’s voltage-sensitive gating mechanism (Guo et al., 2016; for review, see Hedrich and Marten, 2011). In addition, roles for a host of other regulatory activities likely impose control on the channel ranging from phosphorylation (e.g. Kintzer and Stroud, 2016; see also discussion in Hedrich and Marten, 2011) and ROS (Pottosin et al., 2009), to polyunsaturated fatty acids (Gutla et al., 2012), 14-3-3 proteins (Latz et al., 2007), and an array of ionic interactions that modulate channel gating (for review, see Hedrich and Marten, 2011). The combination of these multiple regulatory mechanisms may help in repressing channel activity until a cytosolic signal increase switches it to respond, providing a way to rapidly amplify the initial triggering signal. However, whether any of these other regulators plays an important role in modulating the Ca2+ wave has yet to be characterized. In addition, although TPC1 is permeable to a range of cations including Ca2+ (e.g. Ward and Schroeder, 1994; for review, see Hedrich and Marten, 2011; Peiter, 2011), whether it directly releases vacuolar Ca2+ or acts indirectly by modulating other vacuolar activities remains to be defined.
A POSSIBLE ROLE FOR GLU RECEPTOR-LIKE CHANNELS IN THE CALCIUM WAVE
In addition to TPC1, the plant GLRs have also emerged as key players in systemic signaling mechanisms. The GLRs are a 20-member family (in Arabidopsis) of Ca2+-permeable channels (Vincill et al., 2012, 2013; for review, see Dodd et al., 2010) that appear intimately linked to rapid long-range electrical signaling. Thus, knockouts in GLR3.2, GLR3.3, and GLR3.6 attenuate wound-induced systemic signaling (Mousavi et al., 2013; Salvador-Recatalà et al., 2014; Salvador-Recatalà, 2016). Conversely, GLR3.5 has been reported to play a role in limiting the spread of the wound signal (Salvador-Recatalà, 2016), raising the intriguing possibility that the same family of channels acts to both facilitate signal transmission to some organs and to define boundaries to where the systemic signal can move. Propagation of both the wound electrical and Ca2+ signals does show distinct patterning, with preferential coupling between subsets of leaves (Kiep et al., 2015). Such patterning of connectivity likely partly reflects anatomical linkages between organs, but it is capable of being rerouted if necessary, for example, in response to removal of a target leaf, indicating a more dynamic element in its regulation. Similarly, the spread of the Ca2+ increase in the aerial parts of the plant induced by local treatment of roots with NaCl shows distinct leaf-to-leaf differences in the kinetics of propagation (Xiong et al., 2014). Whether such patterns reflect differences in the pathways of communication within the plant or response capacities between different organs represents an important question to be more fully addressed.
Further, although the glr3.3 glr3.6 double mutant blocks electrical signaling from wounded to unwounded leaves, it does not silence wound-triggered fast action potentials (APs) in the phloem (Hedrich et al., 2016), reinforcing the idea of the likely multifaceted nature of the systemic signaling system. The GLRs have extracellular ligand binding sites and are gated by a range of amino acids (e.g. Stephens et al., 2008; Vincill et al., 2012). However, whether such amino acid sensitivity plays an important role in their action in propagation of systemic signals remains to be defined. It is also important to note that the potential role for these channels in processes such as developmental regulation (Singh et al., 2016) and specific hormone signaling (Kong et al., 2015) may be superimposed on their role(s) in systemic signal propagation, and so defining functions in long-range signaling will require careful evaluation of any mutant phenotypes.
HYDRAULIC WAVES, MECHANO-SENSORS, AND THE CALCIUM WAVE
The observation of vascular movement of Ca2+ and other systemic signals has also suggested another propagation mechanism whereby a hydraulic signal moving through the vasculature could represent the mobile signal (e.g. Farmer et al., 2014). In this model, a wave of altered pressure would trigger mechano-sensitive elements that in turn act to trigger an initial Ca2+ influx to cells bordering xylem vessels or phloem sieve tubes (Fig. 3). This influx would then prime the cell for further amplification and propagation. Although Ca2+ increases are strongly linked to mechanical signaling in plants (for review, see Toyota and Gilroy, 2013), and an array of likely plant mechano-sensitive elements such as OSCA1 (Yuan et al., 2014), receptor-like kinases (Shih et al., 2014), and the MSL, MCA, and TPK channels (for review, see Hamilton et al., 2015) have been identified, the possible roles of such mechanical sensors and channels in rapid systemic signaling have yet to be fully explored.
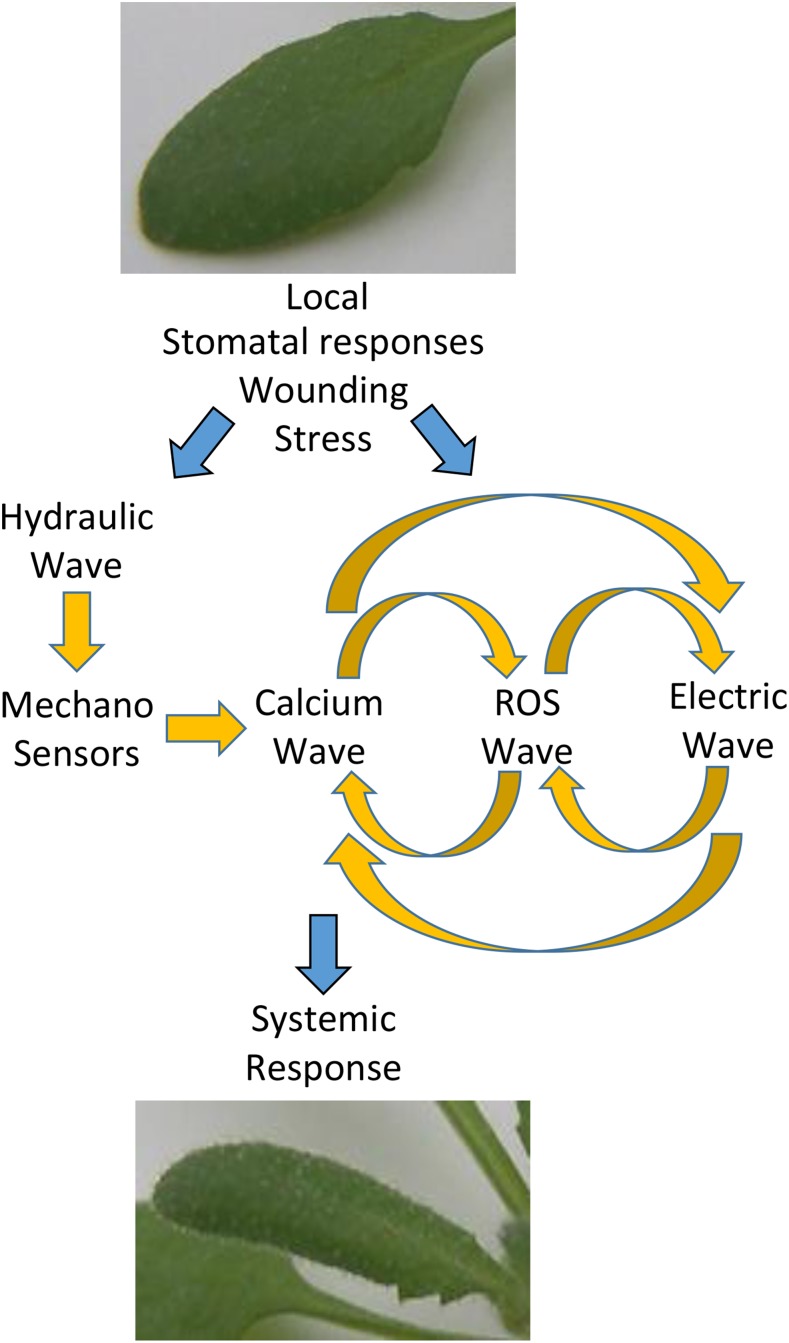
Figure 3.
Integration of the different waves that mediate rapid systemic signaling during SAA. Local stimuli are shown to trigger the ROS/calcium/electric wave, as well as a hydraulic wave that in turn triggers the calcium wave via mechano-sensors. The calcium and ROS waves are shown to be linked (possibly via RBOH and TPC1/CPK function), the ROS and electric waves are shown to be linked (possibly through RBOHD and GLR function), and the calcium and electric waves are shown to be linked (possibly via channels such as GLRs or similar). The different waves are shown to mediate the propagation of each other and to trigger a response in the systemic tissue.
ROS, RBOHS, AND THE CALCIUM WAVE
One further element of proposed models of the molecular machinery behind Ca2+ wave propagation comes from the finding of a parallel role for ROS and the RBOHs in long-range signal transmission (as outlined above). These observations suggest a mechanism for propagation of a cellular Ca2+ increase where apoplastic ROS produced by NADPH oxidase activation could trigger channels allowing Ca2+ influx at the plasma membrane. Indeed, ROS-activated Ca2+ channels and transporters in the plasma membrane have been identified at the electrophysiological (e.g. Allen et al., 2000; Foreman et al., 2003; Demidchik et al., 2007) and molecular levels (e.g. the Ca2+-permeable Stelar K+ Outward Rectifier or Annexin1; Garcia-Mata et al., 2010; Richards et al., 2014; Fig. 1), although to date the relevant channels related to systemic signaling have not been identified.
Once such an initial ‘priming’ Ca2+ influx has occurred, the resulting cytosolic Ca2+ increase could then be amplified, e.g. through a vacuolar Ca2+ release system, potentially involving TPC1, and a feed-forward loop of RBOH activation triggering further cycles of Ca2+ influx and propagation to adjacent cells (Fig. 1). Indeed, RBOHs are known to be modulated by Ca2+-dependent signaling both directly through their EF-hand motifs (Takeda et al., 2008; Kimura et al., 2012) and through posttranslational modulation, such as Ca2+-dependent protein kinase activity (e.g. Kimura et al., 2012; Drerup et al., 2013; Dubiella et al., 2013; Li et al., 2014; Monaghan et al., 2014). In such a model where ROS and Ca2+ interact to propagate a cell-to-cell signal, whether Ca2+ or ROS lies upstream of the other becomes somewhat of a moot point as the response involves cycles of Ca2+-triggered ROS production that are themselves intimately linked to ROS-dependent Ca2+ fluxes (Fig. 1).
Such coupling to ROS-dependent events could even provide a mechanism for termination of the cellular amplification loop, leading to the wave-like progression of the Ca2+ changes from cell to cell. For example, after the initial extracellular ROS production has elicited rapid ion fluxes to the cytosol and the triggering of responses in adjacent cells, a slower entry of extracellular ROS to the cell (e.g. through aquaporins; Hooijmaijers et al., 2012) could lead to inactivation of important amplification components (such as TPC1; Pottosin et al., 2009) and so termination of the signal in that cell. The prior propagation to adjacent cells would then lead to a wave-like rise-and-fall cell-to-cell progression of the Ca2+ change. Further regulatory elements that could contribute to this propagating increase and decrease in Ca2+ are also hinted at in the literature. For example, cyclic nucleotides have been reported to attenuate ROS-related Ca2+ influx in the root in vivo (Ordoñez et al., 2014). Given the known relationships between cyclic nucleotides/cyclic nucleotide-gated channels, Ca2+, stress response, and NO (for review, see Jeandroz et al., 2013) and the above discussion of potential relationships between ROS, NO, and long-range signaling, continued dissection of the interactions between these elements may be a very fruitful avenue to help define the machinery behind systemic signal propagation and response.
THE ELECTRIC WAVE
Electrical signals in plants are divided into: APs (Dziubińska et al., 2001), evoked by nondamaging stimuli (e.g. cold, mechanical stimuli) and spread rapidly (ranging in various plants from a few millimeters to several centimeters per second); variation potentials (VPs), induced by injury (e.g. heat, wounding), transmitted through the xylem, and regulated by hydraulic pressure (for review, see Fromm and Lautner, 2007); and system potentials that depend on the apoplastic ions pool and self-propagate (Zimmermann et al., 2009).
Over short distances (from cell to cell) electrical signals can propagate along the cell membrane likely via plasmodesmata (Fig. 1). Electrical coupling between adjacent cells via plasmodesmata has been shown in species like Elodea, Avena, and Arabidopsis (Lew, 1994). However, very little is known about the properties of plasmodesmata that would regulate electrical signals transduction. Another possibility is that the generation of a local current on one cell membrane could lead to depolarization of the cell membrane of an adjacent cell without direct connection (Ping et al., 1990). In both cases resistance would be too high for electrical signals to travel over distances larger than few neighboring cells. For signal transmission throughout the whole plant, the vascular bundles are more suitable.
Sieve tubes create a low-resistance pathway due to their plate pores and plasma membrane continuity. In addition, Ca2+-permeable channels that are located in the plasma membrane of the sieve tubes are associated with propagation of electrical potentials induced by biotic and abiotic stresses (for review, see van Bel et al., 2014). As noted above, xylem may also be involved in systemic signal propagation, for example, in VP generation. Following wounding the hydrostatic pressure in xylem vessels changes, leading to turgor changes in the adjacent parenchyma cells that cause membrane potential changes potentially via mechano-sensors (Stahlberg et al., 2006; for review, see Farmer et al., 2014; van Bel et al., 2014). Transmission of the signal between mesophyll and phloem is thought to be mediated by bundle-sheath cells (Szechyńska-Hebda et al., 2010; Fromm et al., 2013). Such electrical signal propagation along the vascular tissue in the leaves of Helianthus annuus was recently shown by spatio-temporal surface recording (Zhao et al., 2014). Nevertheless, the identity of the specific ion channels mediating various types of long-distance electrical signals is currently unknown. Recently it was reported that GLR genes encoding putative cation channels could mediate wound-stimulated electrical signals (Mousavi et al., 2013; Fig. 1).
SYSTEMIC EFFECT OF ELECTRICAL SIGNALS ON PLANT PHYSIOLOGY
To date, numerous studies have shown a systemic effect of electrical signals on various physiological processes in higher plants. The most apparent examples are fast leaf movements in Mimosa pudica and Venus flytrap (Dionea muscipula) upon mechanical stimulation. These movements depend on generation of APs (Volkov et al., 2010). To shut the Dionea trap, two APs elicited by touch are needed, and recently it was discovered that five APs are required for digestive glands to be activated, which suggests that Venus flytrap plant is able to count (Böhm et al., 2016). An additional effect of mechanically triggered APs in Dionea is photosynthesis inhibition via regulation of the dark reaction and CO2 assimilation (Pavlovic et al., 2011). Heat stimulation of a Mimosa leaf was shown to trigger VPs that cause a transient decrease of quantum yield of photosystem II and CO2 net uptake (Lautner et al., 2014). Similar effects of VPs on photosynthesis were found in other species such as Populus trichocarpa and soybean (Glycine max; Gallé et al., 2013). In addition, studies of plant defense against herbivory or pathogen attack indicated that electrical signaling plays an important role in the initiation of systemic reactions such as the activation of various genes (for review, see Davies and Stankovic, 2006; Howe and Jander, 2008), including JA biosynthesis (Mousavi et al., 2013), and sieve tube occlusion (Furch et al., 2010). Recently it was demonstrated that bundle-sheath cells, Ca2+-permeable channels, chloroplast ROS, photosynthetic electron transport, and NPQ play an important role in long-distance electrical signaling in plants (Figs. 1 and 2; Szechyńska-Hebda et al., 2010). Electrical signaling was also shown to generate a wave-like systemic change in ROS, APX expression, and NPQ, and systemic waves-like changes in ROS and APX gene expression were found to be present in a contrast phase to the waves of NPQ changes (Fig. 2; Szechyńska-Hebda et al., 2010).
Although changes in light intensity or switching from light to dark conditions was recently found to induce systemic electrical signals that were influenced by cytosolic Ca2+ and were light wavelength-specific (Szechyńska-Hebda et al., 2010; for review, see Marten et al., 2010), questions about the molecular components involved in mediating and regulating these electrical waves during SAA remain at present unresolved (Fig. 1).
INTEGRATION OF THE WAVES
A number of different waves are proposed to be involved in rapid systemic signaling during SAA and SAR (Fig. 2), and there are now some indications of the molecular mechanisms whereby these waves could be interlinked and regulate each other (Fig. 3). The integration of the Ca2+ wave with the ROS wave could be mediated via the interaction of elements such as TPC1, Ca2+-regulated kinases (CPK/CBL-CIPKs), and RBOHD (Fig. 1). The recent identification of GLRs as potential mediators of systemic electric signals (Mousavi et al., 2013; Salvador-Recatalà et al., 2014; Salvador-Recatalà, 2016) and the findings that in the absence of RBOHD electric signaling is attenuated (Suzuki et al., 2013) could point to a link between ROS signaling and electric signaling (potentially mediated via GLRs and RBOHD; Fig. 1). GLRs could therefore be regulated by ROS-NO-RBOHD interactions and regulate Ca2+ or electric signals. In addition, hydraulic waves that could initiate at the local tissue due to wounding (for review, see Farmer et al., 2014), rapid changes in stomatal responses, and/or local stress perception (for review, see Mittler and Blumwald, 2015), could also be translated into, or integrated with, Ca2+ and ROS waves by triggering mechanical sensors that affect Ca2+ fluxes and/or ROS production (e.g. Monshausen et al., 2009).
A general model could therefore emerge integrating the different waves (Fig. 3). As shown in Figure 3, local stimuli could directly affect Ca2+ fluxes or trigger a hydraulic wave that would be converted into a Ca2+ signal via mechanical sensors. The Ca2+ wave would in turn be integrated with the ROS wave via the activation of RBOH proteins by direct Ca2+ binding or Ca2+-derived CPK/CBL-CIPK RBOH phosphorylation that would trigger enhanced ROS production (which in turn will further activate or inhibit Ca2+ channels such as TPC1 and/or plasma membrane channels). The ROS wave could be integrated with the electric wave via ROS-induced activation of GLRs or other ion channels that will depolarize membrane potential and regulate electric signals (these could of course further activate RBOHs via Ca2+ signaling). The Ca2+ wave could additionally be integrated with the electric wave via GLRs and TPC1 in a calcium-induced calcium release-like process. The three waves could therefore amplify and regulate each other and carry the systemic signal all the way to the systemic tissue (Fig. 3).
It should be emphasized that the different waves described here involve the transmission of an activated state between cells, and not necessarily the transmission of a particular systemic compound from one cell to the other. The ROS wave is mediated by the transmission of an RBOH activation state between cells, the Ca2+ wave by the transmission of a Ca2+ release activation state (such as through regulation of GLRs or TPC1), and the electric signal is similarly mediated by the transmission of an ion transport activation state (GLRs or equivalent). As described above, each of these processes likely involves a complex interplay of cellular regulators that fine-tune each other’s outcome, the activated state. Each cell along the path of the signal turns on therefore a particular set of proteins/enzymes in response to a signal that is transmitted to it by the cells preceding it, resulting in the production of a signal that is transferred to the cells proceeding it in the pathway (Fig. 1). The transmitted signal need not always be the same, and triggering one element of the wave system could feedback or initiate the others. Of course once a new activated state is established within the different cells along the pathway, each of these cells could be synthesizing a particular compound or hormone (e.g. JA or ABA; Glauser et al., 2009; Suzuki et al., 2013), or activating a particular genetic or metabolic program that would make them more resistant to abiotic stress (e.g. the accumulation of Gly and Ser; Suzuki et al., 2013). This autopropagating wave signal should therefore be viewed more like a line of domino pieces tripping each other until the “fall” state reaches the systemic tissue (as opposed to a particular compound that is transferred from cell to cell until it reaches the systemic tissue). It is possible that the integration of the different signals with respect to their timing and intensity conveys specificity to the signal, or that an additional, yet unknown compound or signal is responsible for triggering a stress-specific response in the systemic tissue (Suzuki et al., 2013; for review, see Mittler et al., 2011). Further studies are of course needed to answer this important question.
Glossary
- SAR
systemic acquired resistance
- SAA
systemic acquired acclimation
- NO
nitric oxide
- G3P
glycerol-3-P
- AzA
azelaic acid
- GSNO
S-nitrosoglutathione
- NPQ
nonphotochemical quenching
- AP
action potential
- VP
variation potential
Footnotes
This work was supported by funding from the National Science Foundation (IOS-1353886, IOS-0639964, IOS-0743954, IOS-1063287, IOS-0943128, IOS-11213800, and MCB-1329723) and NASA (NNX13AM50G and NNX14AT25G), the University of North Texas (College of Arts and Sciences), and Sophia University (Faculty of Science and Technology). S.K., M.B., and M.G. were supported by the Opus 4 (UMO-2012/07/B/NZ3/00228), Opus 6 (UMO-2013/11/B/NZ3/00973), and Maestro 6 (UMO-2014/14/A/NZ1/00218) projects financed by the Polish National Science Center, and also co-supported by the project (PBS3/A9/37/2015) financed by the Polish National Research and Development Center. The funders had no role in the design, data collection, analysis, decision to publish, or preparation of the article.
Articles can be viewed without a subscription.
References
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Beyhl D, Hörtensteiner S, Martinoia E, Farmer EE, Fromm J, Marten I, Hedrich R (2009) The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J 58: 715–723 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Proebsting WM, Hörtensteiner S, Chételat A, Martinoia E, Farmer EE (2007) A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J 49: 889–898 [DOI] [PubMed] [Google Scholar]
- Böhm J, Scherzer S, Krol E, Kreuzer I, von Meyer K, Lorey C, Mueller TD, Shabala L, Monte I, Solano R, et al. (2016) The Venus flytrap Dionaea muscipula counts prey-induced action potentials to induce sodium uptake. Curr Biol 26: 286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciszak K, Kulasek M, Barczak A, Grzelak J, Maćkowski S, Karpiński S (2015) PsbS is required for systemic acquired acclimation and post-excess-light-stress optimization of chlorophyll fluorescence decay times in Arabidopsis. Plant Signal Behav 10: e982018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ, Sandalio LM, Foyer CH (2015) Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann Bot (Lond) 116: 469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadacz-Narloch B, Beyhl D, Larisch C, López-Sanjurjo EJ, Reski R, Kuchitsu K, Müller TD, Becker D, Schönknecht G, Hedrich R (2011) A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell 23: 2696–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E, Stankovic B (2006) Electrical signals, the cytoskeleton, and gene expression: a hypothesis on the coherence of the cellular responses to environmental insult. In Baluška F, Mancuso S, Volkmann D, eds, Communication in Plants. Springer, Berlin, pp 309–320 [Google Scholar]
- de Pinto MC, Locato V, Sgobba A, Romero-Puertas MdelC, Gadaleta C, Delledonne M, De Gara L (2013) S-Nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco Bright Yellow-2 cells. Plant Physiol 163: 1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río LA. (2015) ROS and RNS in plant physiology: an overview. J Exp Bot 66: 2827–2837 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Davies JM (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49: 377–386 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Klessig DF (2012) SOS - too many signals for systemic acquired resistance? Trends Plant Sci 17: 538–545 [DOI] [PubMed] [Google Scholar]
- Dietz KJ. (2015) Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J Exp Bot 66: 2401–2414 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Drerup MM, Schlücking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J (2013) The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant 6: 559–569 [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziubińska H, Trębacz K, Zawadzki T (2001) Transmission route for action potentials and variation potentials in Helianthus annuus L. J Plant Physiol 158: 1167–1172 [Google Scholar]
- Farmer EE, Gasperini D, Acosta IF (2014) The squeeze cell hypothesis for the activation of jasmonate synthesis in response to wounding. New Phytol 204: 282–288 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2013) Redox signaling in plants. Antioxid Redox Signal 18: 2087–2090 [DOI] [PubMed] [Google Scholar]
- Fromm J, Hajirezaei MR, Becker VK, Lautner S (2013) Electrical signaling along the phloem and its physiological responses in the maize leaf. Front Plant Sci 4: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm J, Lautner S (2007) Electrical signals and their physiological significance in plants. Plant Cell Environ 30: 249–257 [DOI] [PubMed] [Google Scholar]
- Furch AC, van Bel AJ, Fricker MD, Felle HH, Fuchs M, Hafke JB (2009) Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell 21: 2118–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furch AC, Zimmermann MR, Will T, Hafke JB, van Bel AJ (2010) Remote-controlled stop of phloem mass flow by biphasic occlusion in Cucurbita maxima. J Exp Bot 61: 3697–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallé A, Lautner S, Flexas J, Ribas-Carbo M, Hanson D, Roesgen J, Fromm J (2013) Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO(2). Plant Cell Environ 36: 542–552 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Wang J, Gajdanowicz P, Gonzalez W, Hills A, Donald N, Riedelsberger J, Amtmann A, Dreyer I, Blatt MR (2010) A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J Biol Chem 285: 29286–29294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawroński P, Górecka M, Bederska M, Rusaczonek A, Ślesak I, Kruk J, Karpiński S (2013) Isochorismate synthase 1 is required for thylakoid organization, optimal plastoquinone redox status, and state transitions in Arabidopsis thaliana. J Exp Bot 64: 3669–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawroński P, Witoń D, Vashutina K, Bederska M, Betliński B, Rusaczonek A, Karpiński S (2014) Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol Plant 7: 1151–1166 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19: 623–630 [DOI] [PubMed] [Google Scholar]
- Glauser G, Dubugnon L, Mousavi SA, Rudaz S, Wolfender JL, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284: 34506–34513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, Jiang Y (2016) Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 531: 196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutla PV, Boccaccio A, De Angeli A, Gambale F, Carpaneto A (2012) Modulation of plant TPC channels by polyunsaturated fatty acids. J Exp Bot 63: 6187–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ES, Schlegel AM, Haswell ES (2015) United in diversity: mechanosensitive ion channels in plants. Annu Rev Plant Biol 66: 113–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Marten I (2011) TPC1-SV channels gain shape. Mol Plant 4: 428–441 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Salvador-Recatalà V, Dreyer I (2016) Electrical wiring and long-distance plant communication. Trends Plant Sci 21: 376–387 [DOI] [PubMed] [Google Scholar]
- Hooijmaijers C, Rhee JY, Kwak KJ, Chung GC, Horie T, Katsuhara M, Kang H (2012) Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J Plant Res 125: 147–153 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Jeandroz S, Lamotte O, Astier J, Rasul S, Trapet P, Besson-Bard A, Bourque S, Nicolas-Francès V, Ma W, Berkowitz GA, et al. (2013) There’s more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiol 163: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Robin GP (2013) Systemic signaling during plant defense. Curr Opin Plant Biol 16: 527–533 [DOI] [PubMed] [Google Scholar]
- Karpiński S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Karpiński S, Szechyńska-Hebda M, Wituszyńska W, Burdiak P (2013) Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant Cell Environ 36: 736–744 [DOI] [PubMed] [Google Scholar]
- Kiep V, Vadassery J, Lattke J, Maaß JP, Boland W, Peiter E, Mithöfer A (2015) Systemic cytosolic Ca(2+) elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207: 996–1004 [DOI] [PubMed] [Google Scholar]
- Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K (2012) Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 1823: 398–405 [DOI] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM (2016) Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 531: 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Ju C, Parihar A, Kim S, Cho D, Kwak JM (2015) Arabidopsis glutamate receptor homolog3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination. Plant Physiol 167: 1630–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautner S, Stummer M, Matyssek R, Fromm J, Grams TE (2014) Involvement of respiratory processes in the transient knockout of net CO2 uptake in Mimosa pudica upon heat stimulation. Plant Cell Environ 37: 254–260 [DOI] [PubMed] [Google Scholar]
- Latz A, Becker D, Hekman M, Müller T, Beyhl D, Marten I, Eing C, Fischer A, Dunkel M, Bertl A, et al. (2007) TPK1, a Ca(2+)-regulated Arabidopsis vacuole two-pore K(+) channel is activated by 14-3-3 proteins. Plant J 52: 449–459 [DOI] [PubMed] [Google Scholar]
- Lew R. (1994) Regulation of electrical coupling between Arabidopsis root hairs. Planta 193: 67–73 [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. (2014) The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Marten I, Deeken R, Hedrich R, Roelfsema MR (2010) Light-induced modification of plant plasma membrane ion transport. Plant Biol (Stuttg) (Suppl 1) 12: 64–79 [DOI] [PubMed] [Google Scholar]
- Matsuo M, Johnson JM, Hieno A, Tokizawa M, Nomoto M, Tada Y, Godfrey R, Obokata J, Sherameti I, Yamamoto YY, et al. (2015) High REDOX RESPONSIVE TRANSCRIPTION FACTOR1 levels result in accumulation of reactive oxygen species in Arabidopsis thaliana shoots and roots. Mol Plant 8: 1253–1273 [DOI] [PubMed] [Google Scholar]
- Mignolet-Spruyt L, Xu E, Idänheimo N, Hoeberichts FA, Mühlenbock P, Brosché M, Van Breusegem F, Kangasjärvi J (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot http://dx.doi.org/10.1093/jxb/erw080 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R, Blumwald E (2015) The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27: 64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Monaghan J, Matschi S, Shorinola O, Rovenich H, Matei A, Segonzac C, Malinovsky FG, Rathjen JP, MacLean D, Romeis T, et al. (2014) The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 16: 605–615 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21: 2341–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Mühlenbock P, Szechyńska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, Parker JE, Karpinska B, Karpinski S (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20: 2339–2356; erratum [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenbock P, Szechyńska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, Parker JE, Karpinska B, Karpinski S (2008) Plant Cell 20: 3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Návarová H, Bernsdorff F, Döring AC, Zeier J (2012) Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24: 5123–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñez NM, Marondedze C, Thomas L, Pasqualini S, Shabala L, Shabala S, Gehring C (2014) Cyclic mononucleotides modulate potassium and calcium flux responses to H2O2 in Arabidopsis roots. FEBS Lett 588: 1008–1015 [DOI] [PubMed] [Google Scholar]
- Ortega-Galisteo AP, Rodríguez-Serrano M, Pazmiño DM, Gupta DK, Sandalio LM, Romero-Puertas MC (2012) S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: changes under abiotic stress. J Exp Bot 63: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri MC, Lindermayr C, Bauwe H, Steinhauser C, Durner J (2010) Regulation of plant glycine decarboxylase by S-nitrosylation and glutathionylation. Plant Physiol 152: 1514–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic A, Slováková L, Pandolfi C, Mancuso S (2011) On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J Exp Bot 62: 1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiter E. (2011) The plant vacuole: emitter and receiver of calcium signals. Cell Calcium 50: 120–128 [DOI] [PubMed] [Google Scholar]
- Ping Z, Mimura T, Tazawa M (1990) Jumping transmission of action potential between separately placed internodal cells of Chora corallina. Plant Cell Physiol 31: 299–302 [Google Scholar]
- Pottosin I, Wherrett T, Shabala S (2009) SV channels dominate the vacuolar Ca2+ release during intracellular signaling. FEBS Lett 583: 921–926 [DOI] [PubMed] [Google Scholar]
- Ranf S, Wünnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P (2008) Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 53: 287–299 [DOI] [PubMed] [Google Scholar]
- Rasul S, Dubreuil-Maurizi C, Lamotte O, Koen E, Poinssot B, Alcaraz G, Wendehenne D, Jeandroz S (2012) Nitric oxide production mediates oligogalacturonide-triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana. Plant Cell Environ 35: 1483–1499 [DOI] [PubMed] [Google Scholar]
- Richards SL, Laohavisit A, Mortimer JC, Shabala L, Swarbreck SM, Shabala S, Davies JM (2014) Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J 77: 136–145 [DOI] [PubMed] [Google Scholar]
- Salvador-Recatalà V. (2016) New roles for the GLUTAMATE RECEPTOR-LIKE 3.3, 3.5, and 3.6 genes as on/off switches of wound-induced systemic electrical signals. Plant Signal Behav 11: e1161879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Recatalà V, Tjallingii WF, Farmer EE (2014) Real-time, in vivo intracellular recordings of caterpillar-induced depolarization waves in sieve elements using aphid electrodes. New Phytol 203: 674–684 [DOI] [PubMed] [Google Scholar]
- Schulze C, Sticht H, Meyerhoff P, Dietrich P (2011) Differential contribution of EF-hands to the Ca²⁺-dependent activation in the plant two-pore channel TPC1. Plant J 68: 424–432 [DOI] [PubMed] [Google Scholar]
- Shah J, Zeier J (2013) Long-distance communication and signal amplification in systemic acquired resistance. Front Plant Sci 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Singh SK, Chien CT, Chang IF (2016) The Arabidopsis glutamate receptor-like gene GLR3.6 controls root development by repressing the Kip-related protein gene KRP4. J Exp Bot 67: 1853–1869 [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Cleland R, Van Volkenburgh E (2006) Slow wave potentials - a propagating electrical signal unique to higher plants. In Baluška F, Mancuso S, Volkmann D, eds, Communication in Plants. Springer, Berlin, pp 291–308 [Google Scholar]
- Steinhorst L, Kudla J (2014) Signaling in cells and organisms - calcium holds the line. Curr Opin Plant Biol 22: 14–21 [DOI] [PubMed] [Google Scholar]
- Stephens NR, Qi Z, Spalding EP (2008) Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3.3 and GLR3.4 genes. Plant Physiol 146: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Kruk J, Górecka M, Karpińska B, Karpiński S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Tang RH, Han S, Zheng H, Cook CW, Choi CS, Woerner TE, Jackson RB, Pei ZM (2007) Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science 315: 1423–1426 [DOI] [PubMed] [Google Scholar]
- Toyota M, Gilroy S (2013) Gravitropism and mechanical signaling in plants. Am J Bot 100: 111–125 [DOI] [PubMed] [Google Scholar]
- Vaahtera L, Brosché M, Wrzaczek M, Kangasjärvi J (2014) Specificity in ROS signaling and transcript signatures. Antioxid Redox Signal 21: 1422–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel AJ, Furch AC, Will T, Buxa SV, Musetti R, Hafke JB (2014) Spread the news: systemic dissemination and local impact of Ca²⁺ signals along the phloem pathway. J Exp Bot 65: 1761–1787 [DOI] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP (2012) Ca(2+) conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Clarin AE, Molenda JN, Spalding EP (2013) Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. Plant Cell 25: 1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Foster JC, Ashby TA, Walker RK, Johnson JA, Markin VS (2010) Mimosa pudica: electrical and mechanical stimulation of plant movements. Plant Cell Environ 33: 163–173 [DOI] [PubMed] [Google Scholar]
- Wang C, El-Shetehy M, Shine MB, Yu K, Navarre D, Wendehenne D, Kachroo A, Kachroo P (2014) Free radicals mediate systemic acquired resistance. Cell Reports 7: 348–355 [DOI] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Gao QM, Kachroo A, Kachroo P (2014) Free radical-mediated systemic immunity in plants. Curr Opin Plant Biol 20: 127–134 [DOI] [PubMed] [Google Scholar]
- Wituszyńska W, Ślesak I, Vanderauwera S, Szechyńska-Hebda M, Kornaś A, Van Der Kelen K, Mühlenbock P, Karpińska B, Maćkowski S, Van Breusegem F, et al. (2013) Lesion simulating disease1, enhanced disease susceptibility1, and phytoalexin deficient4 conditionally regulate cellular signaling homeostasis, photosynthesis, water use efficiency, and seed yield in Arabidopsis. Plant Physiol 161: 1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong TC, Ronzier E, Sanchez F, Corratgé-Faillie C, Mazars C, Thibaud JB (2014) Imaging long distance propagating calcium signals in intact plant leaves with the BRET-based GFP-aequorin reporter. Front Plant Sci 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, et al. (2011) S-Nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268 [DOI] [PubMed] [Google Scholar]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M, Bedhomme M, Lemaire SD, Trost P (2012) The emerging roles of protein glutathionylation in chloroplasts. Plant Sci 185-186: 86–96 [DOI] [PubMed] [Google Scholar]
- Zhao DJ, Wang ZY, Huang L, Jia YP, Leng JQ (2014) Spatio-temporal mapping of variation potentials in leaves of Helianthus annuus L. seedlings in situ using multi-electrode array. Sci Rep 4: 5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MR, Maischak H, Mithöfer A, Boland W, Felle HH (2009) System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol 149: 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]