How do plants manage to grow under adverse conditions? The current zeitgeist of plant sciences proposes that kinetic components of transient changes in internal free calcium ion concentrations ([Ca2+]i) in response to environmental challenges determine many downstream cellular processes in plants. However, recently, Minguet-Parramona et al. (2016) concluded that oscillations of [Ca2+]i are a by-product of the transport activities in guard cells and that the frequency optimum associated with maximal closure rates is subject to the balance in these activities. This provokes the question as to whether any other kind of [Ca2+]i variation observed in plant cells is primarily responsible for a specific physiological response, or is rather a by-product of transport processes initiated to reset cellular disturbances caused by environmental stimuli. Indeed, there are many reasons why calcium is an effective ion with biological activity, not least because the activities of many proteins involved in signal transmission depend on it (Edel and Kudla, 2015). However, the question as to how changes in the free concentration of calcium can determine many different activities in plant cells with such remarkable specificity has not yet been satisfactorily answered experimentally, although it has often been asked (Blatt, 2000; Sanders et al., 2002).
THE “CALCIUM SIGNATURE HYPOTHESIS”
One plausible answer, adopted from nonplant research fields (Berridge, 1997) and put forward in plant sciences as a stimulating hypothesis (McAinsh et al., 1997), is the “calcium signature hypothesis.” It postulates that if each stimulus causes a unique set of transient variations or oscillations in [Ca2+]i, then the information necessary to produce specificity in the physiological response may be encoded in the kinetics of the transient (Sanders et al., 2002). This exciting explanation makes calcium ions distinct from other molecular messengers, e.g. phytohormones. It proposes that, while Ca2+ ions per se do not have the specificity of a typical signaling compound that bind to a receptor in order to transmit a specific information, the variation of [Ca2+]i is a signaling process. In other words, information is transmitted by a specific kinetic component in the “Ca2+ signature” that precisely activates the relevant Ca2+ receptor protein, which thereby works like a “molecular antenna.”
This signature hypothesis has stimulated diverse fields of research. Indeed, signatures known from other active but otherwise unspecific signal intermediates, such as H2O2, pH, and nitric oxide (NO), have come to be of interest (Mittler et al., 2011; Bose et al., 2014). Doubts about the signature concept have been expressed only sporadically.
To convey the signature concept to a broader audience, radio broadcasting has been introduced as a metaphor to explain calcium signaling in plant cells (Hofmann, 2013). However, circumspection is needed in this case for the reasons outlined below.
In analog broadcasting the acoustic audio input signal (pitch and volume) is modulated onto an invariant electromagnetic “carrier” wave for signal transmission and is decoded by the radio to bring voice and music to our ears. In plants, however, no invariant kinetic components have yet been found in Ca2+ signatures, which are modulated to encode information such as stimulus type or stimulus intensity. Hence, the broadcasting metaphor and the signature hypothesis both lack an invariant kinetic carrier component.
Often different stimuli come in parallel (e.g. heat and drought, drought and salt) and may trigger multiple calcium signatures in parallel. This leads to heterodyning of [Ca2+]i kinetics and requires signal filtering by calcium-dependent molecular antennas to select specific information and to orchestrate an appropriate response. Many such molecular antennas have been found, and others are the focus of current research (Edel and Kudla, 2015). Nevertheless, their individual filter functions in terms of responsiveness to specific calcium kinetics remain elusive.
Hypotheses are rewarding for any scientific discussion. They are excellent when they provoke research activity. This is the case for the calcium signature hypothesis (McAinsh et al., 1997). However, hypotheses have a limited lifetime during which they are challenged. They are abandoned sooner or later, if they do not yield knowledge. The signature hypothesis has come of age and almost 20 years after its inauguration is still alive. Albeit, to our knowledge, a plant signal transduction cascade, beginning with a stimulus, its receptors, through all intermediate cellular transmitters, down to the physiological response, has never been completely described with a specific kinetic calcium signature being a sufficient and necessary intermediate.
The requirement for sufficiency and necessity as preconditions for a “second messenger” was demanded more than 30 years ago. Three rules must be fulfilled before calcium can be called a second messenger (Jaffe, 1980):
-
(1)
“A measurement of [...] cytoplasmic calcium during activation” (p. 87).
-
(2)
“Evidence that direct artificial induction of [...] free calcium will activate (the response)” (p. 87). This means that a specific [Ca2+]i signature is sufficient to cause the corresponding physiological response.
-
(3)
“Evidence that prevention of the natural rise (of [Ca2+]i) will block activation” (p. 87). In relation to a specific [Ca2+]i signature, it is therefore expected to be necessary and any different signature will fail to cause the response.
For specific rapid processes in animal neurons, muscles, and eggs, these rules apply, and in some cases [Ca2+]i modulations have been found to determine cellular responses (Berridge, 1997). Plants, however, are different, and the frequently invoked parallelism between animal and plant signal transduction does not always hold up to close scrutiny. Many studies lack one of the above rules. Consequently, the resulting data can at most serve as a vague indication for a role of [Ca2+]i.
“SINGLE-FILE” VERSUS “NETWORK” SIGNALING
The signature hypothesis is based on a “single-file signaling” view. It proposes that the kinetic transient is the information cargo of [Ca2+]i that encodes specificity, which is subsequently decoded by calcium-dependent molecular antennas and converted into the correct physiological response. Such a way of signaling is important for rapid signal cascades in cells like neurons and muscles, where split-second responses to environmental stimuli are critical. Rapid responses can also be observed in plants. Mimosa or carnivorous plants are frequently quoted in this context. However, these examples are the exception rather than the rule. The rule is that plants respond more sedately as most environmental stimuli relevant to plants appear in a time frame of minutes to hours. Consequently, a resource-consuming response only makes sense after having integrated an environmental factor “F” for a while so that information about it has accumulated to a level of reliability. Plants minimize this integration period by sensing the rate of change dF/dt rather than the amplitude F of the primary environmental signal. Indeed, [Ca2+]i is an indicator for this kind of optimized dF/dt sensing (Plieth et al., 1999). However, [Ca2+]i transients indicating dF/dt sensing do not allow the conclusion that these transients are indispensable for adaptation to the environmental factor F.
On reflection it seems more likely that complex, interwoven mechanisms provide the plant with an increased chance for survival and reproduction rather than single-file signaling. There are three layers of complexity:
-
(1)
External, primary stimuli typically have simultaneous effects on diverse cellular actuating variables, and [Ca2+]i is simply one of them. There is a multitude of other variables determining the molecular environment in each cell, for example, the membrane potential, the cellular energy level [ATP], the dissolved oxygen concentration [O2], the cellular redox state, antioxidative capacity [GSH], reactive oxygen species [ROS], [NO], or pH.
-
(2)
The activity of any protein involved in signal transmission depends simultaneously on several such cellular variables.
-
(3)
Cellular actuating variables are interdependent (e.g. [Ca2+]i depends on pHi and ROS depend on [O2]). Their interdependence is hard to delineate and may be sequential.
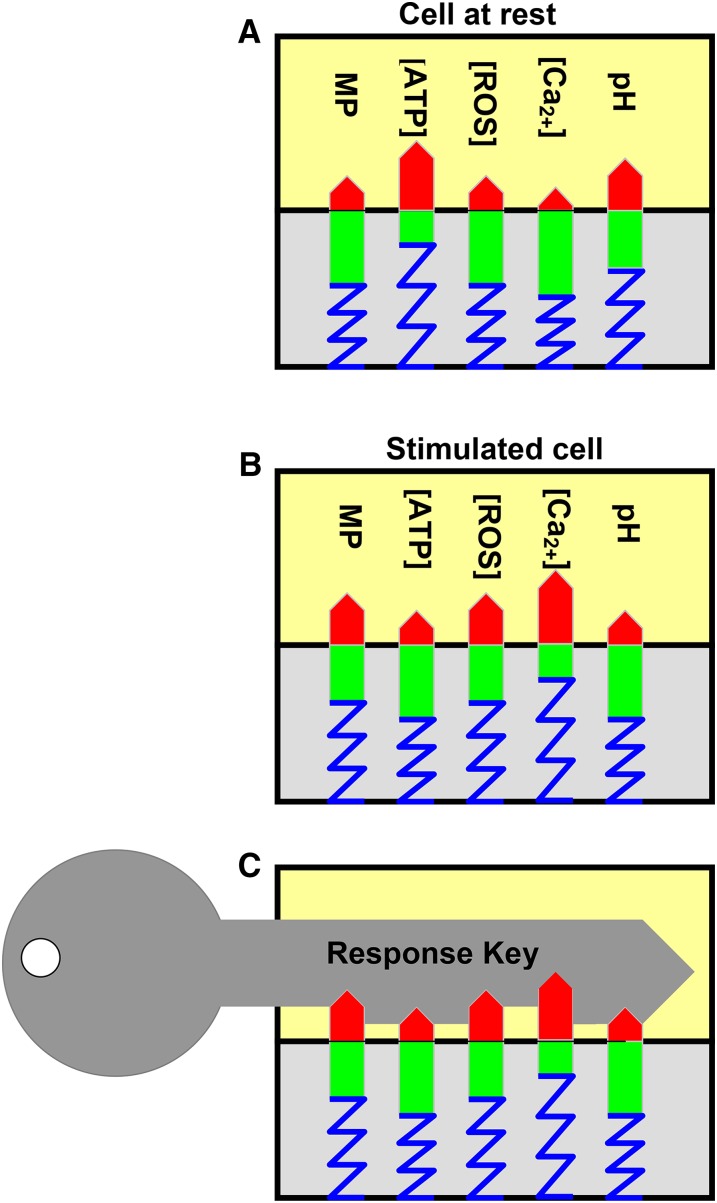
Taken together, the specificity needed to activate a signal-transmitting protein is given by the amplitude pattern of diverse cellular variables that are addressed by the primary stimulus. Any signal transmitter can thus be regarded as a key (Fig. 1), and its molecular environment as a lock with a coding continuously changing in response to incoming external stimuli. The key is activated the moment it fits the lock’s code, thereby unlocking a correct physiological response, from the repertoire, the plant species has “learned” during its evolution. This “lock-and-key” metaphor (Fig. 1) can be regarded as a possible alternative to the radio broadcasting metaphor. Lock-and-key mechanisms make calcium just one of many interdependent cellular determinants. They are not new (Sato et al., 2010) and have already been anticipated as “cellular signaling networks” for plant sciences (Blatt, 2000; Trewavas, 2011), but, due to their complexity, have not received the recognition they deserve. Here, mathematical modeling will be helpful in the future to understand signaling networks in more detail.
Figure 1.
A cylinder lock as alternative key metaphor for cellular stimulus-response coupling. A, An environmental stimulus has effects on diverse cellular actuating variables, such as [Ca2+], [H2O2], pH, [ATP], and membrane potential. Each variable is reflected here by a red bar. Together they form an amplitude pattern. B, An environmental stimulus affects some cellular actuating variables, and a new amplitude pattern (cellular state) is established. C, The new pattern is decoded by a specific key (i.e. a cellular receptor protein with specific sensitivity to the corresponding cellular actuating variables). The key that fits unlocks a gate in the signaling network and provides access to the correct physiological response that the plant species has learned during its evolution.
“Network signaling” provides several advantages, such as tolerance, robustness, redundancy, resilience, and flexibility. Tolerance is the bandwidth for each environmental factor. Variations within the bandwidth do not trigger resource-consuming responses. Robustness means that environmental factors exceeding the tolerance bandwidth are reliably transmitted. Redundancy means that if part of the network fails, then an alternative pathway will ensure the correct physiological response (Roelfsema and Hedrich, 2010; Sato et al., 2010). Tolerance, robustness, and redundancy make the network resilient, and resilience is the ability of a network to stay operating even under adverse conditions. Graded responses according to the severity of the environmental stimulus represent flexibilities.
PLANT RESEARCH AHEAD: CORRECT QUESTIONS AND TRUTHFUL ANSWERS
In science, the usual way to ask nature a question is by experimentation. Good experiments should be designed in such a way that nature has a chance to give reasonable answers. Reasonable answers help to reveal interdependencies, and interdependencies are needed for proper mathematical modeling. Consequently, there are four levels of plant research ahead:
-
(1)
“Physiologically meaningful” experimentation: It is important to apply experimental conditions that mimic natural situations and avoid conditions that the plant, during its evolution, has not learned to deal with. Meaningful experimentation will produce reliable data that reflect a response the plant is able to activate from its repertoire and does not force reactions that are prone to erroneous interpretation.
-
2)
Exploring the lock: Given the multitude of cellular variables driving cellular events and signal transmission, we need to develop tools with which interdependence and cause-effect relationships can be investigated. This implies precise kinetic recording of multiple parameters (such as [Ca2+]i, pH, redox state, movements, and growth), and correlation analysis to see whether [Ca2+]i changes precede or follow the cellular process under study (Coelho and Malhó, 2006). We need to know if and how [Ca2+]i interacts with other detectable cellular signals. This in turn implies the development of novel cellular indicators that allow simultaneous recording of diverse parameters.
-
3)
Investigating key functions: There is a need to investigate functions and actions of proteins involved in signal transmission (such as CDPKs, CBLs, and calmodulins, as well as GTPases, SNAREs, etc.) in vivo and on a single-cell level. The development of recombinant indicators providing optical output measurands for specific cellular variables has shown during the past two decades that both multiple-parameter recordings on the cellular level and the quantification of specific protein activities are achievable. Advancement of these genetic engineering and cellular imaging technologies should be pushed to reevaluate older data sets.
-
4)
Modeling and yielding knowledge: Systems biology provides software platforms and other tools for modeling interdependent cellular processes. When a mathematical model is able to describe and to predict physiological responses to environmental challenges (i.e. “plant behavior”), then the underlying theory can be regarded as knowledge and the cellular processes can be regarded as understood. Consequently, these tools (e.g. Wang et al., 2012) need to be at the forefront of experimentation so that network signaling is transformed from just a vogue term into an established research subfield with quantifiable outputs within plant sciences.
THE CHANGING ZEITGEIST
Ernst Mayr (1982) stated: “Written histories, like science itself, are constantly in need of revision. Erroneous interpretations of an earlier author eventually become myths, accepted without question and carried forward from generation to generation. […] The main reason, however, why histories are in constant need of revision is that at any given time they merely reflect the present state of understanding; they depend on how the author interpreted the current zeitgeist of biology and on his own conceptual framework and background” (p. 1).
The zeitgeist is changing, and we are closer to understanding molecular mechanisms down to the atomic level. There are many molecular antennas responding to calcium [Ca2+]i (Edel and Kudla, 2015). Whether their activities during a transient cellular [Ca2+]i increase are switched by the transient’s kinetics still needs to be demonstrated by novel experimental approaches. However, there are many other equally important molecular antennas (Simon and Dresselhaus, 2015) that, by contrast, respond to cellular actuating variables other than [Ca2+]i. Each may form a key (Fig. 1) that activates a physiological response that is appropriate to the prevailing environmental challenge.
Acknowledgments
I thank Lee Shaw and Hartmut Kaiser for helpful comments.
Footnotes
Articles can be viewed without a subscription.
References
- Berridge MJ. (1997) The AM and FM of calcium signalling. Nature 386: 759–760 [DOI] [PubMed] [Google Scholar]
- Blatt MR. (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65: 1241–1257 [DOI] [PubMed] [Google Scholar]
- Coelho PC, Malhó R (2006) Correlative analysis of [Ca2+]C and apical secretion during pollen tube growth and reorientation. Plant Signal Behav 1: 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edel KH, Kudla J (2015) Increasing complexity and versatility: how the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium 57: 231–246 [DOI] [PubMed] [Google Scholar]
- Hofmann NR. (2013) Calmodulin methylation: another layer of regulation in calcium signaling. Plant Cell 25: 4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF. (1980) Calcium explosions as triggers of development. Ann N Y Acad Sci 339: 86–101 [DOI] [PubMed] [Google Scholar]
- Mayr E. (1982) The Growth of Biological Thought. Harvard University Press, Cambridge, MA [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM (1997) Calcium ions as secondary messengers in guard cell signal transduction. Physiol Plant 100: 16–29 [Google Scholar]
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2016) An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol 170: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Plieth C, Hansen U-P, Knight H, Knight MR (1999) Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J 18: 491–497 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2010) Making sense out of Ca(2+) signals: their role in regulating stomatal movements. Plant Cell Environ 33: 305–321 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Tsuda K, Wang L, Coller J, Watanabe Y, Glazebrook J, Katagiri F (2010) Network modeling reveals prevalent negative regulatory relationships between signaling sectors in Arabidopsis immune signaling. PLoS Pathog 6: e1001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Dresselhaus T (2015) Peptides take centre stage in plant signaling. Preface. J Exp Bot 66: 5135–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. (2011) Plant cell calcium, past and future. In Luang S, ed, Coding and Decoding of Calcium Signals in Plants. Springer, Heidelberg, pp 1–7 [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR (2012) Systems dynamic modeling of a guard cell Cl- channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]