Nod factors induce massive reprogramming of gene expression in the root epidermis, including the CRE1 cytokinin pathway which leads to both positive and negative regulation of nodulation.
Abstract
Nod factors (NFs) are lipochitooligosaccharidic signal molecules produced by rhizobia, which play a key role in the rhizobium-legume symbiotic interaction. In this study, we analyzed the gene expression reprogramming induced by purified NF (4 and 24 h of treatment) in the root epidermis of the model legume Medicago truncatula. Tissue-specific transcriptome analysis was achieved by laser-capture microdissection coupled to high-depth RNA sequencing. The expression of 17,191 genes was detected in the epidermis, among which 1,070 were found to be regulated by NF addition, including previously characterized NF-induced marker genes. Many genes exhibited strong levels of transcriptional activation, sometimes only transiently at 4 h, indicating highly dynamic regulation. Expression reprogramming affected a variety of cellular processes, including perception, signaling, regulation of gene expression, as well as cell wall, cytoskeleton, transport, metabolism, and defense, with numerous NF-induced genes never identified before. Strikingly, early epidermal activation of cytokinin (CK) pathways was indicated, based on the induction of CK metabolic and signaling genes, including the CRE1 receptor essential to promote nodulation. These transcriptional activations were independently validated using promoter:β-glucuronidase fusions with the MtCRE1 CK receptor gene and a CK response reporter (TWO COMPONENT SIGNALING SENSOR NEW). A CK pretreatment reduced the NF induction of the EARLY NODULIN11 (ENOD11) symbiotic marker, while a CK-degrading enzyme (CYTOKININ OXIDASE/DEHYDROGENASE3) ectopically expressed in the root epidermis led to increased NF induction of ENOD11 and nodulation. Therefore, CK may play both positive and negative roles in M. truncatula nodulation.
The first step of nitrogen-fixing symbiosis consists of the mutual recognition of plants and bacteria by an exchange of diffusible signals during the so-called preinfection stage. This step enables later bacterial infection of root tissues, which, for legumes, generally takes place via plant tubular structures that originate in root hairs (RHs), the infection threads. Concomitantly, cell divisions are activated in the root to initiate the formation of specific organs, the nodules, in which bacteria released from infection threads differentiate in bacteroids and fix atmospheric nitrogen for the plant’s benefit (for review, see Desbrosses and Stougaard, 2011; Oldroyd et al., 2011; Popp and Ott, 2011).
In most documented rhizobium-legume symbioses, key signals for triggering the infection and nodulation processes in specific host plants are bacterial lipochitooligosaccharides (LCOs) known as Nod factors (NFs), structurally related to signals involved in the more ancient plant-arbuscular mycorrhizal fungi symbiosis and transduced via a common set of genes termed the common symbiotic signaling pathway (Oldroyd, 2013). Members of the LYSM receptor-like kinase (RLK) family, namely NOD FACTOR RECEPTOR1 (NFR1) and NFR5 in Lotus japonicus and NOD FACTOR PERCEPTION (NFP) and LYSM RECEPTOR KINASE3 (LYK3) in Medicago truncatula, play essential roles in the perception of NFs (Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003; Arrighi et al., 2006). Rhizobium spp. infection is strictly dependent on LYK3 and NFP, in contrast to the NF-induced responses (Mitra et al., 2004; Smit et al., 2007), for which only NFP is necessary. These LYSM receptors likely act in complexes with the symbiosis Leu-rich repeat receptor kinase (LRR RK) SYMRK in L. japonicus (Antolín-Llovera et al., 2014), orthologous to DOES NOT MAKE INFECTIONS2 (DMI2) in M. truncatula, as well as proteins associated with particular membrane microdomains, the symbiotic remorins (Lefebvre et al., 2010) and FLOTILLIN-LIKE2 (FLOT2) and FLOT4 (Haney et al., 2011). SYMRK and DMI2 interact with proteins shown to be important for nodulation, which are, respectively, a mitogen-activated protein kinase kinase (MAPKK) called SYMRK-INTERACTING2 (Chen et al., 2012) and a 3-HYDROXY-3-METHYLGLUTARYL COENZYME A REDUCTASE1 (MtHMGR1; Kevei et al., 2007). MtHMGR1 is thought to generate a secondary signal involved in triggering calcium oscillations within and around the nucleus, a key step of LCO signaling (Venkateshwaran et al., 2015), which involves cation channels (Ané et al., 2004; Charpentier et al., 2008; Venkateshwaran et al., 2012), a calcium pump (Capoen et al., 2011), and nucleoporins (Kanamori et al., 2006; Saito et al., 2007; Groth et al., 2010). Calcium oscillations are decoded by a calcium- and calmodulin-dependent Ser/Thr protein kinase (CCamK, also known as DMI3 in M. truncatula), which interacts with and phosphorylates CYCLOPS (called INTERACTING PROTEIN OF DMI3 in M. truncatula), a DNA-binding transcriptional activator (Singh et al., 2014).
CYCLOPS transactivates the expression of the NODULE INCEPTION (NIN) gene, encoding a transcriptional regulator that plays distinct roles in different Medicago spp. root tissues (Vernié et al., 2015). NIN is required to initiate nodule formation in the cortex (Schauser et al., 1999) and to transcriptionally activate NF-YA1 (Soyano et al., 2013; Laloum et al., 2014), a transcription factor (TF) involved in various nodulation steps (Combier et al., 2006; Soyano et al., 2013; Laporte et al., 2014; Xiao et al., 2014). In the epidermis, NIN is necessary for the onset of rhizobium infection (Xie et al., 2012; Fournier et al., 2015) but restricts the expression of EARLY NODULIN11 (ENOD11), a marker of the preinfection and infection steps (Marsh et al., 2007; Vernié et al., 2015). ENOD11 is up-regulated in response to NF by ETHYLENE RESPONSE FACTOR REQUIRED FOR NODULATION1 (ERN1; Andriankaja et al., 2007; Middleton et al., 2007) as well as NUCLEAR TRANSCRIPTION FACTOR Y (MtNF-YA1) and MtNF-YA2 (Laloum et al., 2014). It was shown earlier that two other transcriptional regulators, NODULATION SIGNALING PATHWAY1 (NSP1) and NSP2, are essential for nodulation (Kaló et al., 2005; Smit et al., 2005) and form a complex binding the promoter of NIN, ERN1, and MtENOD11 (Hirsch et al., 2009). Thus, there is a requirement of apparently overlapping transcriptional regulators during NF signaling (preinfection stage), rhizobium infection, and nodule initiation that can be mobilized using different protein complexes or promoter regions (Cerri et al., 2012).
The NF signaling and nodulation pathways are strongly interconnected with hormonal cues and also trigger rapid and dynamic modifications in reactive oxygen species (ROS; Cárdenas et al., 2008), probably impacting the regulation of symbiotic genes (Ramu et al., 2002; Andrio et al., 2013). Early responses to NF, notably calcium oscillations, and consequently nodulation, are negatively regulated by ethylene, jasmonic acid, and abscisic acid (Penmetsa and Cook, 1997; Oldroyd et al., 2001; Suzuki et al., 2004; Sun et al., 2006; Ding et al., 2008). By contrast, auxins and cytokinins (CKs) play positive roles in nodule initiation and development. Rhizobium spp. inoculation or LCO application modulates auxin fluxes in the root, with local auxin accumulation at the site of cortical cell divisions leading to nodule initiation (Mathesius et al., 1998; Ng et al., 2015). Auxin also is involved in controlling the progression of rhizobium infections (Breakspear et al., 2014; Laplaze et al., 2015). The positive role of CK in nodule initiation was demonstrated by the phenotype of mutants affecting the CK receptors MtCHK1/CRE1 and LjLHK1. Loss-of-function alleles are defective in nodule formation (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Plet et al., 2011), while the spontaneous nodule formation2 (snf2) mutant carrying a gain-of-function mutation in LHK1 produces nodules in the absence of rhizobia (Tirichine et al., 2007). An autoactive form of CCamK/DMI3 (snf1 mutant) requires LHK1 to produce spontaneous nodules, while snf2 does not require CCamK/DMI3 to induce spontaneous nodules, indicating that CK/LHK1 acts downstream from CCamK (Tirichine et al., 2007). The CK biosynthesis genes ISOPENTENYL TRANSFERASE3 (IPT3) in L. japonicus and LONELY GUY1 (LOG1) and LOG2 in M. truncatula are up-regulated during nodulation via CRE1/LHK1, while decreasing MtLOG1 or LjIPT3 expression by RNA interference leads to decreased nodulation (Chen et al., 2014; Mortier et al., 2014). The CK-CRE1/LHK1 pathway controls polar auxin transport (Plet et al., 2011; Suzaki et al., 2012) and the Rhizobium spp. induction of specific flavonoids that act as polar auxin transport inhibitors, able to rescue nodulation defects of the cre1 mutant (Ng et al., 2015). Exogenous CK treatments induce NIN and NF-YA1 in L. japonicus (Heckmann et al., 2011) and NIN, NSP2, and ERN1 in M. truncatula (Plet et al., 2011), while many M. truncatula genes require MtCRE1 for a full induction by NF (van Zeijl et al., 2015), consistent with a role of CRE1 and CK in NF signaling.
Even though the different CK receptors were all shown to have positive roles in nodule initiation (Held et al., 2014; Boivin et al., 2016), CK also may play negative roles at later nodulation stages. Indeed, CKs have been shown in L. japonicus to mimic the activity of the shoot-derived signal that inhibits nodule formation during systemic autoregulation of nodulation (Sasaki et al., 2014), while CKs induce, via CRE1/LHK1 in M. truncatula and L. japonicus, the expression of CLAVATA3-LIKE (CLE) peptides promoting systemic autoregulation of nodulation (Mortier et al., 2012a; Soyano et al., 2014). In addition, the observations that, first, the expression of a CK-responsive reporter (TWO COMPONENT SIGNALING SENSOR [TCS]; Müller and Sheen, 2008),increases with time in the epidermis of rhizobium-infected L. japonicus roots and, second, that the lhk1-1 mutant is hyperinfected led to the suggestion that CK might be involved in locally restricting the number of infections in L. japonicus (Held et al., 2014).
Molecular mechanisms linking NF signaling in the root epidermis and the activation of cell divisions in the root cortex are still unclear. A central role for NIN in the coordination of epidermal and cortical responses was proposed recently, with NIN promoting MtCRE1 expression in a positive feedback loop (Vernié et al., 2015). The pMtCRE1:GUS construct is up-regulated a few hours after Sinorhizobium meliloti inoculation in cortical cells (Lohar et al., 2006), while expression of the primary CK-response gene MtRR4 (encoding a type-A response regulator or RRA; Heyl et al., 2013) is detected in pericycle and cortical cells (Plet et al., 2011; Vernié et al., 2015). This led to the conclusion that the primary sites of CK action are inner root tissues, and notably the cortex, consistent with the snf phenotypes induced by a gain-of-function LHK1 mutation. This was supported recently by the use of the TCS:GUS reporter, which was detected at early time points only in inner root tissues following NF addition or Rhizobium spp. inoculation and not in the epidermis (Held et al., 2014; van Zeijl et al., 2015).
Transcriptome analysis is a powerful way to investigate genes associated with signaling pathways. NF-induced gene expression reprogramming was analyzed recently in M. truncatula using whole roots or root segments (Czaja et al., 2012; Rose et al., 2012; van Zeijl et al., 2015) as well as isolated RHs (Breakspear et al., 2014). Two of these studies used Affymetrix microarrays representing approximately 70% of the genome (Czaja et al., 2012; Breakspear et al., 2014), while the two others used RNA sequencing (RNAseq), with sequencing reads mapped on the partial (Mt3.5) or the full (Mt4.0) M. truncatula genome sequence (Rose et al., 2012; van Zeijl et al., 2015). In addition, genes whose transcriptional activation by Rhizobium is NF dependent were identified by root RNAseq analysis (Larrainzar et al., 2015). Laser-capture microdissection (LCM) coupled to RNAseq enables a sensitive and genome-wide analysis of gene expression in specific tissues or organ regions, as shown for M. truncatula nodule zones (Roux et al., 2014). In this study, we used LCM-RNAseq to analyze early NF signaling in the root epidermis. Hundreds of genes were found to be up-regulated within a few hours and involved in a variety of cellular processes. Strikingly, an early epidermal activation of the CK pathway was evidenced based on the induction of CK metabolic and signaling genes and independently validated using both the recently improved CK-responsive reporter TWO COMPONENT SIGNALING SENSOR NEW (TCSn):GUS (Zürcher et al., 2013) and a pCRE1:GUS fusion. The functional relevance of this epidermal activation of CK signaling pathways was explored by testing the impact of CK on the NF induction of ENOD11 and the nodulation efficiency.
RESULTS
LCM-RNAseq Analysis of the Root Epidermal Response to NF Treatment
To identify genes associated with early NF signaling in the epidermis, M. truncatula roots treated with 10−8 m NF (4 and 24 h) were compared with mock-treated roots. The root region responsive to NF was defined using the stable pENOD11:GUS M. truncatula L416 transgenic line (Journet et al., 2001), grown in parallel. Root segments (approximately 1 cm long) were collected for laser dissection of epidermal cells (Supplemental Fig. S1), and RNAseq analysis was carried out following a procedure described previously (Roux et al., 2014). About 247 million read pairs were unambiguously mapped on the full M. truncatula genome sequence Mt20120830 (accessible at the SYMbiMICS Web site https://iant.toulouse.inra.fr/symbimics/; download section), with an average of 61.9 million read pairs per condition (Supplemental Table S1). Using a threshold statistically defined following Rau et al. (2013), these data enabled the detection of 17,191 genes, which included MtEXP7, a gene specifically expressed in the epidermis (Murray et al., 2011). Complete results, including correspondence with Mt4.0 gene models and annotation data, are provided in Supplemental Table S2 or at the SYMbiMICS Web site, where epidermis and previous nodule RNAseq data can be easily queried using various requests (M. truncatula gene or Affymetrix oligonucleotide identifiers, keywords, and BLAST search).
A set of 1,070 differentially expressed genes was identified (adjusted P < 0.05 and fold change > 2), with 722 up-regulated and 348 down-regulated genes (670 and 310 corresponding genes in Mt4.0, respectively). To validate the experimental setup, we compared expression data obtained in this study versus recent transcriptome analyses of NF responses for a set of 19 NF-induced marker genes (Table I). Different conditions were used in these studies, with different NF concentrations, durations of NF treatment, and either all root tissues or isolated RH (see Fig. 1 legend). Data also were compared with those obtained with S. meliloti-inoculated RH in the hyperinfected sickle (skl) mutant (affected in the ETHYLENE INSENSITIVE2 gene; Penmetsa et al., 2008), used to maximize the plant symbiotic response (Breakspear et al., 2014). Pairwise comparisons are shown in Supplemental Figure S2, which also includes data from Sinorhizobium medicae-inoculated roots (expression groups G1, G2, and G3, predicted to be NF induced; Larrainzar et al., 2015). Table I shows that the LCM-RNAseq approach used in our study enabled the detection of strong differential expression for all 19 NF-induced marker genes, validating both the samples and the technical approach.
Table I. Detection of known NF-induced genes in this study and in recent transcriptomic analyses.
Values correspond to the regulation levels (fold change) following NF treatment for the indicated times or S. meliloti inoculation at 5 d post inoculation (RH-5dpi; skl mutant background), determined in this study versus previous studies: a, Rose et al. (2012); b, van Zeijl et al. (2015); c, Czaja et al. (2012); and d, Breakspear et al. (2014). For entries in parentheses, differences were not statistically significant (adjusted P > 0.05). ART, All root tissues from whole root systems (a) or 1- to 2-cm-long root segments (b and c); NA, not applicable (no corresponding gene in the M. truncatula genome sequence used); NF, RNAseq reads were detected only in the NF-treated samples.
| Mt20120830 | MtV4.0 | Annotation | LCM-4h | LCM-24h | ART-1h (a) | ART-3h (b) | ART-6h (c) | ART-24h (c) | RH-24h (d) | RH-5dpi (d) | Gene Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mt0004_00313 | Medtr1g056530 | MtNF-YA1 | 3513.9 | NF | 294.7 | 1.9 | 4.5 | 53.7 | 557.7 | Laloum et al. (2014) | |
| Mt0017_10456 | Medtr3g415670 | MtENOD11 | 2560.1 | NF | NA | 1,915.0 | 8.1 | 29.6 | 166.0 | 759.6 | Journet et al. (2001) |
| Mt0039_00030 | Medtr1g090807 | MtRPG | 526.0 | NF | NA | 372.3 | 20.1 | 23.1 | 78.7 | 121.8 | Arrighi et al. (2008) |
| Mt0006_10188 | Medtr3g086320 | MtNPL | NF | NF | 1,456.7 | 2.6 | 3.2 | 36.5 | 217.2 | Xie et al. (2012) | |
| Mt0017_10454 | Medtr3g415650 | MtENOD12 | NF | (NF) | NA | 96.9 | 68.4 | 469.2 | Journet et al. (1994) | ||
| Mt0012_10641 | none | MtENOD40-1 | 339.2 | 10.2 | NA | NA | 21.8 | 127.2 | Fang and Hirsch (1998) | ||
| Mt0037_10123 | Medtr8g038210 | MtAnn1 | 319.3 | 31.6 | 97.5 | 306.3 | de Carvalho et al. (1998) | ||||
| Mt0010_00289 | Medtr5g083030 | MtPUB1 | 106.9 | 10.7 | 9.6 | 14.3 | 14.3 | 6.0 | 3.6 | 4.0 | Mbengue et al. (2010) |
| Mt0005_10038 | Medtr5g005290 | Mt NMN1 | 104.8 | NF | 1,090.5 | 18.7 | 12.0 | 36.8 | 40.3 | Libault et al. (2011) | |
| Mt0001_00292 | Medtr4g129010 | MtSPK1 | 52.3 | 13.2 | 228.2 | 10.5 | 4.8 | 64.9 | 68.5 | Andrio et al. (2013) | |
| Mt0001_00813 | Medtr4g116990 | MtNFH1 | 34.9 | NF | NA | 40.0 | Tian et al. (2013) | ||||
| Mt0010_01109 | Medtr5g099060 | MtNIN | 23.8 | 16.5 | 2.5 | 13.8 | 10.3 | 16.4 | 34.0 | 116.8 | Marsh et al. (2007) |
| Mt0033_10061 | Medtr6g027840 | MtVAPYRIN | 10.4 | 4.5 | NA | 81.4 | 8.0 | 8.1 | 7.4 | 9.6 | Murray et al. (2011) |
| Mt0011_00459 | Medtr7g085810 | MtERN1 | (0.97) | 21.1 | 2.3 | 4.0 | 4.4 | 3.8 | 2.5 | 9.2 | Middleton et al. (2007) |
| Mt0033_10028 | Medtr6g029180 | MtERN2 | (1.22) | 5.8 | 2.2 | 1.5 | 2.5 | Andriankaja et al. (2007) | |||
| Mt0028_00160 | Medtr8g020840 | MtNSP1 | 7.1 | 7.4 | 5.4 | 6.1 | 13.6 | Smit et al. (2005) | |||
| Mt0005_10559 | Medtr5g016320 | MtGH3 | 6.4 | (0.2) | 4.0 | 21.9 | 34.1 | Mathesius et al. (1998) | |||
| Mt0027_10212 | Medtr1g094960 | MtARF16a | 5.2 | 13.5 | 2.1 | 2.9 | 2.4 | 14.4 | Breakspear et al. (2014) | ||
| Mt0008_10953 | Medtr5g074860 | MtRIP1 | 3.2 | 2.9 | NA | 1.5 | 2.1 | Cook et al. (1995) |
Figure 1.
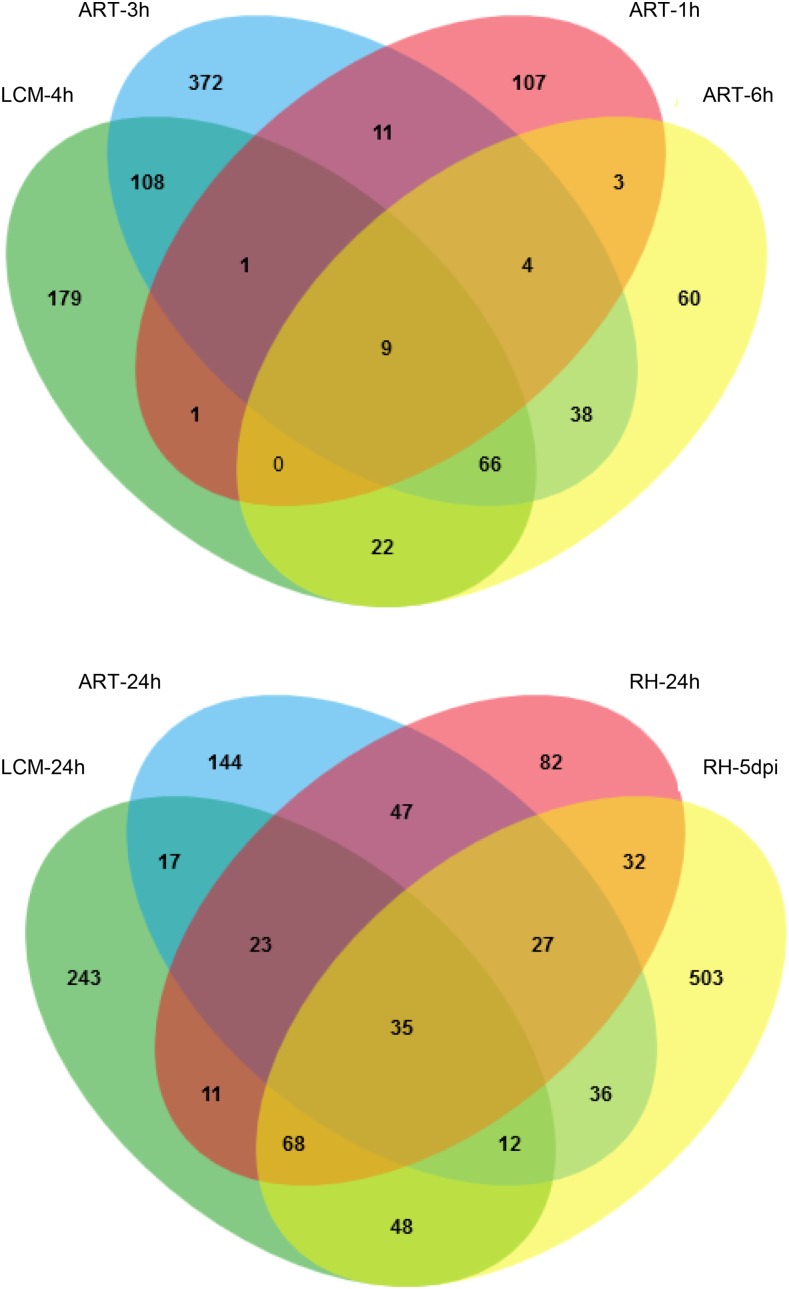
Multiple comparisons (Venn diagrams) of NF up-regulated M. truncatula genes detected in recent transcriptomics experiments. Top, 1- to 6-h NF treatments; bottom, 24-h NF treatment and S. meliloti-inoculated RHs 5 d post inoculation (RH-5dpi) in a hyperinfected skl mutant background. ART-1h, 10−8 m NF-treated (1 h) whole roots (Rose et al., 2012); ART-3h, 10−9 m NF-treated (3 h) root segments in the presence of 1 µm aminoethoxyvinylglycine (AVG), an inhibitor of ethylene biosynthesis (van Zeijl et al., 2015); ART-6h and ART-24h, 10−8 m NF-treated root segments (6 and 24 h; Czaja et al., 2012); LCM-4h/-24h, laser-dissected epidermal cells (this study) with 4 or 24 h of 10−8 m NF treatment, respectively; RH-24h, 10−6 m NF-treated RHs (24 h; Breakspear et al., 2014). MtV4.0 identifiers were used in all cases. Thresholds chosen to identify up-regulated genes were defined in the corresponding publications. Venn diagrams were generated with the tool provided by Bardou et al. (2014).
Sets of 413 and 499 genes (386 and 457 corresponding genes identified in Mt4.0, respectively) were scored as up-regulated in response to 4 or 24 h of NF treatment, respectively (for complete data, including comparison with other recently published transcriptome analyses, see Supplemental Table S3). Strikingly, a large number of them exhibited a high level of up-regulation (greater than 10-fold for 314 genes and greater than 50-fold for 143 genes). About half of the NF-induced genes at 4 or 24 h had not been identified in the above-mentioned transcriptomics studies (Fig. 1; 33.4% at 4 h when also taking into account S. meliloti-inoculated skl RH). Only 45% of the genes induced at 4 h were still up-regulated at 24 h (Supplemental Fig. S2), showing a relatively large proportion of transiently induced genes. We identified a set of genes found in all or most studies of the NF-regulated transcriptome, with in total 76 and 42 genes found in five and six conditions, respectively, and thus representing highly robust NF-induced marker genes (Supplemental Table S3).
The situation was somewhat different for NF down-regulated genes, with both a lower number of genes (247 and 139 at 4 and 24 h of NF treatment, respectively, with 225 and 98 corresponding genes in Mt4.0, respectively; Supplemental Table S4) and a higher proportion of genes not found in other studies (Supplemental Fig. S3). Only 14.7% (33 genes) of the NF down-regulated genes at 4 h were still scored down-regulated at 24 h, indicating a very high proportion of transient regulation, while only seven genes were found in common among the LCM, RH, and all root tissues 24-h down-regulated genes (Supplemental Table S4). Repressing gene expression, therefore, seems to be a less critical component for the preinfection than gene induction.
A Variety of Functional Classes Is Observed among NF Up-Regulated Genes
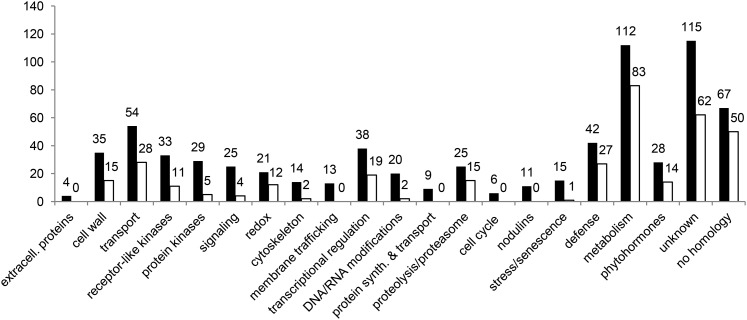
An enrichment analysis of Gene Ontology functional categories among the NF-regulated genes was performed (Supplemental Figs. S4 and S5), revealing a predominance of extracellular, cell wall, and cytoskeleton components, as well as protein kinases, membrane transport, and signaling components. A functional classification was independently performed, based upon the best BLAST hits in SWISSPROT and The Arabidopsis Information Resource databases (Fig. 2). Selected relevant genes in relation to the biology of rhizobial infections in RH and to the activation of signaling pathways are detailed below.
Figure 2.
Functional classification of M. truncatula NF-regulated genes in the root epidermis. Categories were manually defined, based upon best BLASTP hits using predicted encoded proteins against SWISSPROT and The Arabidopsis Information Resource databases. The number of genes is indicated for each category, with black and white bars corresponding to up- and down-regulated genes, respectively.
Genes Encoding Proteins Involved in Cell Dynamics
NF perception leads to morphological modifications of RH in the nodulation-competent zone of the root, with swelling followed by branching associated with a reorientation of RH growth (for review, see Gage, 2004). RH growth is governed by multiple factors: vesicle and organelle movement using myosin-based motors traveling along actin filaments, microtubules that are notably involved in nuclear positioning, and the buildup of a tip-focused calcium gradient, modulated by ROS and small GTPase activity (Gage, 2004). This study revealed 11 genes encoding proteins related to the actin and microtubule cytoskeleton that are strongly NF induced (Table II; Supplemental Table S3), such as the kinesin-4 microtubule-binding motor proteins, the microtubule-associated proteins MAP65 and MAP70, the TPX2 microtubule-targeting protein, and the tubulin- or actin-binding formin-like proteins. Four of these proteins have been reported previously to be phosphorylated 1 h after NF addition (Rose et al., 2012), indicating a combination of transcriptional and posttranscriptional regulation.
Table II. Transcriptomic and protein phosphorylation data for selected NF-induced genes involved in cell dynamics.
The eight right columns indicate detected NF-induced phosphorylation (P) sites and the regulation levels (fold change) following NF treatment for the indicated times or S. meliloti inoculation at 5 d post inoculation (RH-5dpi; skl mutant background) determined in this study versus previous studies: a, Rose et al. (2012); b, van Zeijl et al. (2015); c, Czaja et al. (2012); and d, Breakspear et al. (2014). For entries in parentheses, differences were not statistically significant (adjusted P > 0.05). ART, All root tissues from whole root systems (a) or 1- to 2-cm-long root segments (b and c); ND, not detected; NF, RNAseq reads detected only in the NF-treated samples.
| Mt20120830 | MtV4.0 | Functional Category | Annotation | P Sites (a) | LCM-4h | LCM-24h | ART-3h (b) | ART-6h (c) | ART-24h (c) | RH-24h (d) | RH-5 dpi (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mt0017_10221 | Medtr3g010330 | Cell wall | Cellulase3-like | 15.3 | 19.6 | 1.4 | 1.9 | 33.3 | |||
| Mt0006_00323 | Medtr3g082440 | Cell wall | KOR2 endoglucanase | 10.1 | 1.8 | 3.3 | 5.5 | 6.1 | 4.0 | ||
| Mt0008_01038 | Medtr0118s0070 | Cell wall | Expansin EXPB14-like | 740.3 | 152.5 | 177.1 | 259.6 | 118.4 | 93.5 | 795.4 | |
| Mt0004_11139 | Medtr1g073320 | Cell wall | Plasmodesmata-located protein3-like | 20.9 | 0.2 | ||||||
| Mt0015_10081 | Medtr8g006790 | Transport | Plasma membrane proton pump MtAHA1 | NF | NF | 268.8 | 11.6 | 149.6 | |||
| Mt0009_00137 | Medtr8g087710 | Transport | MtNip1 (NOD26) | 384.0 | 187.2 | 206.3 | 8.1 | 5.1 | 56.0 | 688.2 | |
| Mt0005_10852 | Medtr5g023240 | Transport | Lys-His transporter | S27 | 49.2 | 31.6 | 3.4 | 10.8 | 5.7 | 35.6 | 164.2 |
| Mt0040_00155 | Medtr3g112460 | Transport | Peptide transporter PTR1-like | 163.1 | 121.3 | 433.9 | 3.3 | 5.6 | |||
| Mt0023_10223 | Medtr2g011570 | Transport | Equilibrative nucleotide transporter | NF | NF | ||||||
| Mt0007_00517 | Medtr3g098930 | Transport | Sugar transporter SWEET13 | NF | ND | 652.5 | 23.2 | ||||
| Mt0006_10110 | Medtr3g087730 | Transport | Sulfate transporter | 1,335.9 | 337.0 | 105.4 | |||||
| Mt0009_00487 | Medtr8g095360 | Membrane trafficking | Patellin | 7.9 | 366.4 | 4.3 | 2.6 | 1.9 | 5.1 | 31.3 | |
| Mt0037_10126 | Medtr8g038220 | Membrane trafficking | MtAnn2 annexin | 447.6 | 0.2 | 149.5 | |||||
| Mt0057_00074 | Medtr1g105930 | Membrane trafficking | Inositol-1,3,4-trisphosphate5/6-kinase | 6.8 | 10.2 | 4.8 | 2.4 | 2.6 | 3.6 | 26.0 | |
| Mt0036_00256 | Medtr7g116710 | Cytoskeleton | ABIL (ABI-like) | S255 | 3.9 | 66.1 | 4.5 | 1.4 | 1.7 | 20.3 | |
| Mt0006_10532 | Medtr3g078623 | Cytoskeleton | Actin-binding formin-like protein8 | 579.1 | (NF) | 755.6 | |||||
| Mt0013_00466 | Medtr3g060900 | Cytoskeleton | Kinesin-4 microtubule binding | 136.1 | 1.3 | 4.3 | 20.2 | ||||
| Mt0036_00045 | Medtr7g112420 | Cytoskeleton | Kinesin-4 microtubule binding | S277 | 4.7 | 6.2 | 3.1 | 2.2 | |||
| Mt0054_10146 | Medtr6g061690 | Cytoskeleton | Microtubule-associated protein MAP65 | NF | (22.1) | 1,699.6 | |||||
| Mt0001_10069 | Medtr4g133890 | Cytoskeleton | Microtubule-associated protein MAP70 | 10.7 | 37.7 | 11.2 | 4.7 | 3.4 | 8.0 | 15.0 | |
| Mt0031_00057 | Medtr7g099290 | Cytoskeleton | MYOSIN2 | S1117 | 2.0 | 10.9 | |||||
| Mt0040_10068 | Medtr3g110720 | Cytoskeleton | Tubulin α-4 chain | S439 | 7.1 | 2.7 | |||||
| Mt0001_10832 | Medtr4g116870 | Cell cycle | DNA replication licensing factor | 316.1 | 2.6 | 8.1 |
RH swelling and branching require cell wall relaxation followed by a redirection of cell wall material secretion. Acidification of the apoplast caused by P-ATPases triggers hydration and cell wall loosening, a process facilitated by expansins and different enzymes (Sablowski and Carnier Dornelas, 2014). The genes encoding the H+-ATPase MtHA1 and the NODULIN26 aquaporin are rapidly induced by NF and thus could play a role in RH swelling, along with genes encoding expansins and enzymes involved in cell wall remodeling (pectin methyl esterases, cellulase, xyloglucan transglycosylases, pectin lyases, polygalacturonases, and endoglucanases), as well as specific Pro-rich proteins (ENOD11 and ENOD12 and the Pro-rich protein MtPRP4-like; Table II; Supplemental Table S3). Interestingly, a gene likely encoding a plasmodesmata-located protein (PDLP3-like; Table II) also was up-regulated at 4 h, suggesting early modifications of cell-to-cell communication, potentially involved in the preparation of neighboring outer cortical cells for infection.
Annexins are calcium-binding proteins involved in membrane organization, vesicle trafficking, and signaling (Clark et al., 2012). Two tandem annexin genes, MtAnn1 and MtAnn2, are known to be involved in symbiotic interactions in M. truncatula, with MtAnn1 up-regulated by NF (de Carvalho Niebel et al., 1998, 2002) and MtAnn2 expressed in nodule primordia and vasculature (Manthey et al., 2004). We found here that MtAnn2 also is strongly, but transiently, up-regulated 4 h after NF treatment in epidermal cells (Table II). Two small GTPase Ras-related proteins, potentially involved in polarized membrane trafficking (Vernoud et al., 2003), and three patellins (Sec14 and GOLD domain proteins, also implicated in membrane trafficking; Peterman et al., 2004), were additionally identified as NF-induced genes. Arabidopsis (Arabidopsis thaliana) PATELLIN1 is thought to bind phosphatidylinositol and its phosphorylated derivatives, themselves known to regulate membrane trafficking (Peterman et al., 2004). Noteworthy, two genes (Medtr1g105930 and Medtr3g073100) likely involved in the production of phosphoinositides were found to be NF induced (Table II; Supplemental Table S3).
Transporter and channel genes represented one of the largest classes of NF up- and down-regulated genes in epidermal cells (53 and 29 genes, respectively), with a variety of gene families (Supplemental Tables S3 and S4). Many of the transport genes are transcriptionally activated both strongly and early (4 h) by NF, even though some (e.g. the sugar transporter SWEET13) were described previously as only activated at the later rhizobium infection stage (Breakspear et al., 2014). Six corresponding proteins were reported to be phosphorylated 1 h after NF addition (Rose et al., 2012; Table II; Supplemental Table S3). Those included the previously mentioned MtHA1 ATPase, while a paralogous gene, MtAHA5, also encoding a protein rapidly phosphorylated upon NF addition (Nguyen et al., 2015), was down-regulated. Three NITRATE TRANSPORTER1/PEPTIDE TRANSPORTER (PTR) proteins are encoded by NF-induced genes and likely transport oligopeptides, similar to other PTR1- and PTR3-like proteins (Léran et al., 2014; von Wittgenstein et al., 2014). Interestingly, the M. truncatula Gene Atlas (MtGEA; http://mtgea.noble.org/v3/) indicates that the NF-induced PTR3-like gene (Mtr.31737.1.S1_s_at) is almost nodule specific.
Other components of NF-induced epidermal cell dynamics included genes involved in the S-phase of the cell cycle (coding for cyclin D1 and three minichromosome maintenance proteins, MCM2, MCM5, and MCM6, consistent with Breakspear et al., 2014; Table II; Supplemental Table S3). MetExplore (Cottret et al., 2010) was used as an unbiased approach to predict bioinformatically which metabolic pathways were NF regulated (Supplemental Table S5). This analysis notably revealed the NF induction (at 24 h) of genes involved in GA (Bonferroni = 1.1E−07; Supplemental Fig. S6) and trans-zeatin CK biosynthesis (Bonferroni = 7.7E−03; Supplemental Fig. S7). Down-regulation of the indole-3-acetyl-amide conjugate biosynthesis pathway was additionally identified transiently for the 4-h NF treatment (Bonferroni = 1.0E−05) and of the CK degradation pathway after 24 h of NF treatment (Bonferroni = 2.1E−06). Overall, this suggests that an increase in GA, auxin, and CK biosynthesis/accumulation occurs in the epidermis in response to NFs.
Genes Encoding Proteins Involved in Perception and Signaling Pathways
Numerous receptor-like and protein kinase genes (33 and 29, respectively) were found to be induced by NF. Expression of the NF receptor gene NFP was apparently not affected by the NF treatment, in contrast to what was reported by Breakspear et al. (2014), while the closely related LYK2 and LYK3 genes were down-regulated (Supplemental Table S4). Four other LYSM-RLK genes from three distinct subgroups (Arrighi et al., 2006) were up-regulated (Table III; Supplemental Table S3): LYR3, which encodes a high-affinity LCO-binding protein (Fliegmann et al., 2013; Fliegmann and Bono, 2015); LYR6, of unknown function, a chitin receptor CERK1-like gene; and, more strikingly, LYK10, the ortholog of LjEPR3 recently described as encoding a receptor of Rhizobium spp. exopolysaccharide (Kawaharada et al., 2015), required for rhizobial infection in L. japonicus. LjEPR3 is expressed in epidermal cells exclusively in response to NF or Rhizobium spp. (Kawaharada et al., 2015), consistent with data obtained for MtLYK10 in our study. Other up-regulated genes encoded different types of RKs, including 10 lectin domain receptors, 16 LRR-RLKs, two wall-associated RKs, and five Cys-rich RKs (CRKs). Interestingly, those included MtSymCRK (Table III), required at late stages of nodule development and proposed to be involved in the control of symbiotic immunity (Berrabah et al., 2014). A second gene thought to control immunity in nodules, MtDNF2 (Bourcy et al., 2013), also was identified as NF induced in the root epidermis (Table III). MtSymCRK and nine other NF up-regulated RKs exhibit a non-RD kinase motif, characteristic of kinases involved in innate immune signaling (Schwessinger and Ronald, 2012; Table III; Supplemental Table S3). Noteworthy, 41 defense-related genes were NF up-regulated, including 16 classified in the defense response category GO:0006952, whereas 28 defense-related genes, including six genes from the GO:0006952 class, were NF down-regulated (Fig. 2; Supplemental Table S4). Genes from the PR-1 and PR-10 families showed either up- or down-regulation depending on the members.
Table III. Transcriptomic data for selected NF-induced genes involved in perception, signaling, and gene expression.
The seven right columns indicate the regulation levels (fold change) following NF treatment for the indicated times or S. meliloti inoculation at 5 d post inoculation (RH-5dpi; skl mutant background) determined in this study versus previous studies: a, van Zeijl et al. (2015); b, Czaja et al. (2012); and c, Breakspear et al. (2014). ART, All root tissues from 1- to 2-cm-long root segments; ND = not detected; NF = RNAseq reads detected only in the NF-treated samples.
| Mt20120830 | MtV4.0 | Functional Category | Annotation | LCM-4h | LCM-24h | ART-3h (a) | ART-6h (b) | ART-24h (b) | RH-24h (c) | RH-5 dpi (c) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mt0012_10650 | Medtr5g033490 | Receptor-like (RD) kinase | MtLYK10/EPR3 | NF | NF | 10.8 | 8.7 | 7.8 | 13.9 | 50.8 |
| Mt0057_10185 | Medtr1g104890 | Receptor-like (RD) kinase | Cys-rich RLK | 160.8 | 3.6 | 8.0 | 1.3 | 2.7 | ||
| Mt0006_00443 | Receptor-like (non-RD) kinase | MtSymCRK | 101.7 | NF | 2.8 | 1.6 | 8.3 | 40.0 | ||
| Mt0039_00125 | Medtr1g088930 | Receptor-like (non-RD) kinase | LRR RLK | 51.5 | NF | 10.6 | ||||
| Mt0077_10096 | Medtr2g068650 | Receptor-like (non-RD) kinase | G-type lectin S RLK | 66.7 | 15.8 | 19.1 | ||||
| Mt0036_00102 | Medtr7g113490 | Protein kinase | MAPKKK1 ANP1-like | 16.6 | 3.5 | 2.7 | ||||
| Mt0068_00014 | Medtr2g023890 | Protein kinase | MAPKKK15-like | 17.8 | 19.8 | 4.2 | 6.9 | 3.4 | 12.0 | |
| Mt0016_00257 | Medtr2g100290 | Protein kinase | Protein kinase | 9.0 | NF | 31.8 | 1.8 | 1.6 | ||
| Mt0020_00194 | Medtr8g074920 | Protein kinase | MtSPK2 protein kinase2 | NF | 988.0 | 236.6 | 38.6 | 20.8 | 20.2 | 118.7 |
| Mt0015_10342 | Medtr8g012795 | Defense | Defensin-like protein | 1,025.5 | 559.3 | 1,292.0 | 21.5 | 63.7 | 46.7 | 25.5 |
| Mt0003_11544 | Medtr4g085800 | Immunity control | MtDNF2 | 87.9 | NF | 4.1 | ||||
| Mt0101_10077 | Medtr2g437800 | Signaling | CLE-related peptide | 55.6 | ND | |||||
| Mt0020_10418 | Signaling | MtCEP7 peptide | 162.0 | 13.6 | ||||||
| Mt0004_01114 | Medtr1g075730 | Signaling | Protein phosphatase2C | 27.8 | 7.9 | 15.3 | ||||
| Mt0026_00412 | Medtr7g075900 | Signaling | Subtilase, AtSBT1.1 ortholog | NF | NF | |||||
| Mt0003_00135 | Medtr4g053630 | Signaling | Subtilase, LjSBTS/AtAIR3-like | 3.6 | 13.7 | 7.4 | 17.8 | 4.8 | 3.2 | 25.6 |
| Mt0001_11381 | Medtr4g102400 | Signaling | Subtilase, P69C-like | 39.4 | 1,373.4 | 17.1 | 75.9 | 41.1 | 36.7 | 53.4 |
| Mt0036_10297 | Medtr7g117415 | Proteasome | E3 ubiquitin-protein ligase | 7.6 | 1.8 | 4.7 | 1.5 | 1.7 | 5.6 | |
| Mt0008_00403 | Medtr5g061290 | Proteasome | Seven-in-absentia (SINA) protein | NF | NF | 134.6 | 2.8 | 3.4 | 72.6 | |
| Mt0011_00804 | Medtr7g078150 | Proteasome | Zinc finger, RING/FYVE/PHD-type | 136.5 | 77.7 | 4.3 | 2.5 | 2.1 | 10.2 | 33.6 |
| Mt0009_00840 | Medtr8g103227 | Proteasome | MtLIN-like E3 ligase | NF | NF | 21.0 | ||||
| Mt0042_00049 | Medtr7g106340 | Proteasome | Plant U-box22 (PUB22) | 1.6 | 3.6 | 2.8 | 2.3 | 2.1 | ||
| Mt0012_00744 | Medtr5g031880 | Transcriptional regulation | MtPLT3 ethylene response factor | NF | NF | 13.2 | 1.1 | 1.7 | ||
| Mt0004_10921 | Medtr1g069725 | Transcriptional regulation | GRAS SCARECROW-like TF | 40.7 | NF | 11.8 | 2.4 | 7.5 | ||
| Mt0043_10032 | Medtr7g096530 | Transcriptional regulation | Lateral organ boundaries domain TF | 53.6 | 285.3 | 4.6 | ||||
| Mt0010_00697 | Medtr5g090970 | Transcriptional regulation | No apical meristem TF (ANAC048-like) | 9.5 | 3.4 | 3.5 | 2.2 |
Twenty NF up-regulated protein kinases were identified, including three calcium-dependent protein kinases and two orthologs of Arabidopsis MAPKK kinases (MAPKKK), namely MAPKKK15 and Arabidopsis NPK1-RELATED KINASE1 (ANP1; Table III; Supplemental Table S3). Five of these are phosphorylated upon a 1-h NF treatment, including the ANP1-like protein (Rose et al., 2012). SYMBIOTIC PROTEIN KINASE1 (MtSPK1; Table I) was shown previously to be also induced by exogenous hydrogen peroxide (H2O2) and relevant for nodulation as well as for NIN and MtNF-YA1 expression (Andrio et al., 2013). Three protein phosphatases (PP) also were identified as early induced by NF (Table III; Supplemental Table S3). They all belong to the PP2C family that emerged as a major player in abiotic and biotic stress signaling (Fuchs et al., 2013). One of them (Medtr5g080680) is the predicted ortholog of Arabidopsis PP2CA, a major negative regulator of abscisic acid signaling (Fuchs et al., 2013).
As mentioned previously, various secondary signals are generated in response to NF treatment, notably calcium fluxes and ROS production, as well as modifications in phytohormone accumulation and/or responses. Five calcium-binding protein genes, including a calcineurin B and MtCAML4 (a nodule-specific calmodulin-like protein; Liu et al., 2006), were rapidly induced by NF, along with genes suggesting the production of CK, auxin, ethylene, GA, strigolactones, and brassinosteroids (Supplemental Table S3), consistent with results from Breakspear et al. (2014). In addition, two genes encoding signaling peptides were strongly induced, encoding a CLE and the C-TERMINALLY ENCODED PEPTIDE7 (CEP7; Table III). These two genes have not been characterized so far but belong to families known to regulate nodulation (Mortier et al., 2012b; Imin et al., 2013).
Subtilases (Ser peptidases) were classified here as signaling proteins, since they belong to a large family of proteins among which some play important roles in signaling/developmental processes (Schaller et al., 2012). Seven subtilases were found to be NF induced (Table III; Supplemental Table S3). Two belong to a subgroup closely related to AUXIN-INDUCED ROOT PROTEIN3 (AtAIR3), expressed at sites of lateral root emergence (Neuteboom et al., 1999), and LjSbtS (subtilase S), induced by mycorrhizal fungi and NF (Kistner et al., 2005). The other five belong to the so-called SBT1 subgroup (Schaller et al., 2012), including one close homolog of the tomato (Solanum lycopersicum) PR-P69C protein (up-regulated by biotic stresses; Jordá et al., 1999) and the predicted ortholog of AtSBT1.1, required for the processing of phytosulfokine precursors (Schaller et al., 2012). Phytosulfokines are small sulfated peptide hormones that activate cell proliferation (Matsubayashi and Sakagami, 2006), and some of them seem to positively regulate nodulation in L. japonicus (Wang et al., 2015). The seven subtilase genes exhibit various expression patterns based on MtGEA data, but two of them (SBT1 subgroup) are clearly symbiosis specific, with one (Mtr.45771.1.S1_at) expressed only in nodules and the other (Mtr.13963.1.S1_at) induced both by rhizobium and mycorrhizal fungi or their signals.
The ubiquitin proteasome machinery comprises many genes involved in the regulation of signaling/developmental processes, including response to phytohormones and positive or negative roles for rhizobium infection and nodule development (Vinardell et al., 2003; Shimomura et al., 2006; Kiss et al., 2009; Mbengue et al., 2010; Den Herder et al., 2012; Yuan et al., 2012). Nineteen putative ubiquitin proteasome genes were scored as NF up-regulated, among which two genes encoding, respectively, a close homolog of LUMPY INFECTIONS (MtLIN), primarily required for infection thread growth in RH (Kiss et al., 2009), and a nodulation-specific SEVEN-IN-ABSENTIA (SINA) domain protein, very distantly related to LjSINA4, involved in SYMRK turnover (Den Herder et al., 2012; Table III; Supplemental Table S3). The ortholog of AtPUB22, encoding a negative regulator of immunity (Trujillo et al., 2008), also was found to be moderately but significantly induced by NF at 24 h (Table III).
A large number (38) of NF-induced TFs were identified (Table III; Supplemental Table S3). Six of them were expected: NSP1, NIN, ERN1, ERN2, NF-YA1, as well as NF-YC2, recently shown to interact with NF-YA1 (Baudin et al., 2015). Only one of the remaining NF-induced TFs, a noncharacterized SCARECROW-like GRAS factor (Table III), was found to be expressed specifically in symbiotic samples (mycorrhizal and nodulated roots), based on MtGEA data. Three NF-induced TFs belong to the ERF family in addition to ERN1 and ERN2, namely PLETHORA3 (MtPLT3), associated with both nodule and root meristems together with three other PLT proteins (Franssen et al., 2015), and two ERFs belonging to subgroup VIII, which carries an ERF-associated amphiphilic repression motif (Nakano et al., 2006). The expression of PLT1, PLT2, and PLT4 was not detected in the epidermis, suggesting a specific role of PLT3 at this early stage. Other NF-induced TFs notably included the ortholog of ANAC042, a NAC TF induced by H2O2 (Wu et al., 2012), two auxin-response factors (ARFs), five basic helix-loop-helix, one MADS box, four MYB-like, and two lateral organ boundaries domain (LBD) TFs. Among the latter was the predicted ortholog of AtLBD16, which regulates lateral root formation via direct activation by ARFs (Okushima et al., 2007). Interestingly, six homeodomain protein genes also were NF up-regulated, including MtKNOX3, a gene up-regulated during nodulation and recently shown to act depending on CK pathways (Azarakhsh et al., 2015).
CK Pathways Are Activated during NF Signaling in the Epidermis
The role of CK during nodule initiation in the root cortex is well demonstrated, whereas it remains poorly documented in epidermal cells. In this study, several genes involved in CK biosynthesis (Supplemental Fig. S7), perception (MtCHK1/CRE1), and response (MtRRA2, MtRRA8, and MtRRA9) were found to be up-regulated by NF in the epidermis, mostly at 4 h for CK perception and signaling genes. In mock-treated epidermal cells, MtCHK1/CRE1 was expressed at a much higher level than MtCHK2 and at a similar level to MtCHK3 and MtCHK4, while in NF-treated samples (4 h), MtCHK1 was the only (transiently) induced putative CK receptor gene (becoming expressed about 10-fold more than MtCHK3 and MtCHK4; Supplemental Table S2).
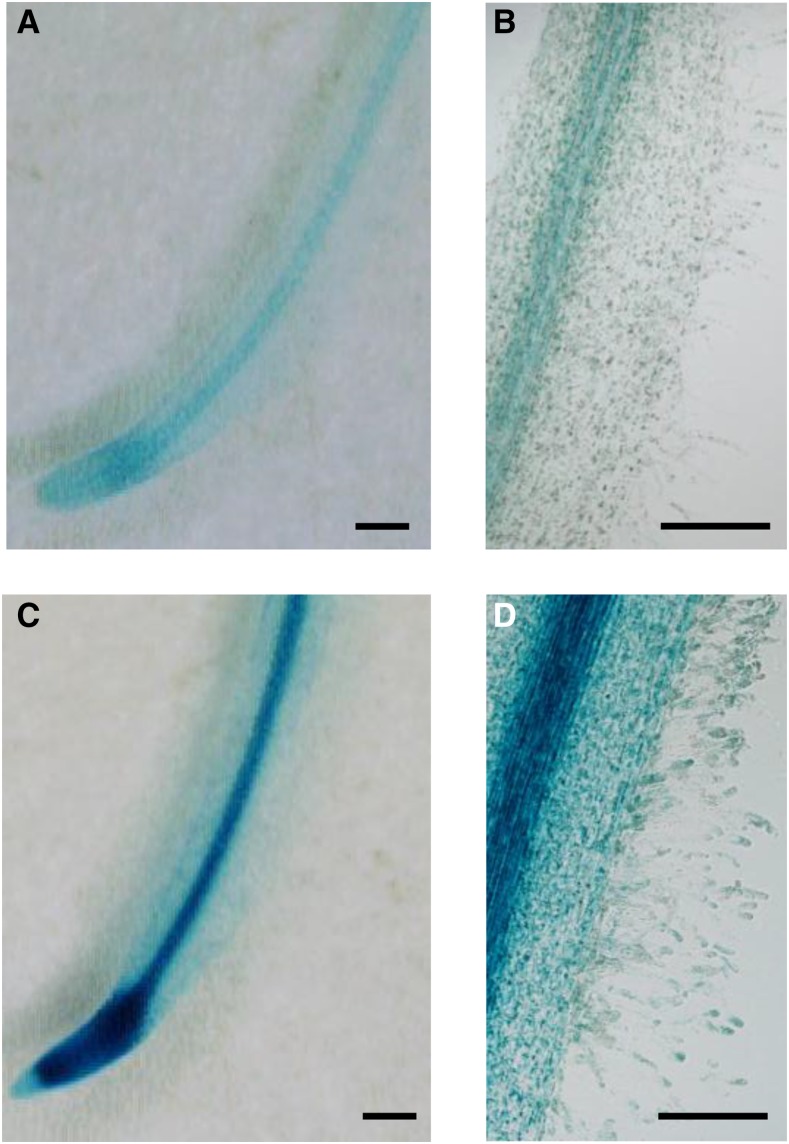
Knowing the essential role of CK in nodulation, we conducted promoter:GUS analyses to support the LCM-RNAseq data. We first analyzed the expression of a pMtCRE1:GUS transcriptional fusion treated or not for 4 h with NFs at 10−8 and 10−9 m. The pMtCRE1:GUS fusion showed a strong expression in the root stele and the apex, as described previously (Lohar et al., 2006), which was increased by the NF treatment (Fig. 3). A prolonged staining revealed, in contrast to the previous study, a weaker pMtCRE1:GUS signal in RHs, detected only in response to NF. This CRE1 spatial expression pattern is in agreement with the RH and LCM transcriptome data sets (Breakspear et al., 2014; this study).
Figure 3.
NFs induce pMtCRE1:GUS in M. truncatula RHs. A and B, GUS staining of untreated roots transformed with a pMtCRE1:GUS fusion. C and D, GUS staining of roots transformed with a pMtCRE1:GUS fusion, treated with NFs (10−9 m) for 4 h. Similar results were obtained with 10−8 m NFs. Bars = 300 µm.
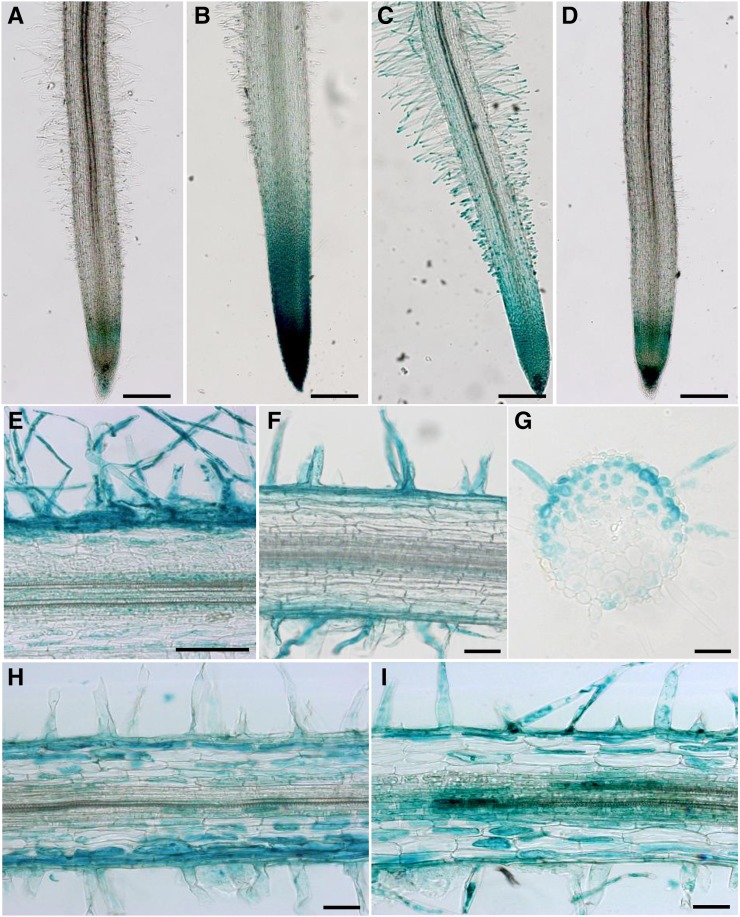
We then used TCSn:GUS, a newly improved version of the TCS reporter consisting of repeated cis-elements from the promoter of an RRA CK primary response gene, and therefore used as a proxy to monitor the activation of CK signaling pathways (Zürcher et al., 2013). We also used in parallel pENOD11:GUS as a positive control for the NF response. To validate the TCSn:GUS reporter, never described in M. truncatula, we first examined its expression pattern in nontreated roots versus roots treated with 10−7 m 6-benzylaminopurine (BAP; a CK; Fig. 4, A and B). In nontreated roots, TCSn:GUS was mostly expressed in the columella and the differentiation region of the root meristem, while it was strongly induced by the BAP treatment, as expected for a CK response reporter. Roots treated with 10−8 m NF (4 h) exhibited a strong induction of GUS activity in RHs of the region competent for nodulation in about 40% of the roots (Fig. 4C), consistent with the LCM-RNAseq data. The NF induction of TCSn:GUS was not detected in an M. truncatula nfp mutant (Fig. 4D). Root sections indicated that the TCSn:GUS induction takes place in epidermal cells as well as subtending cells from the outer cortex (Fig. 4E), while no induction was observed in the inner cortex. The NF-induced pENOD11:GUS expression seemed to be more restricted to epidermal cells (Fig. 4F). Roots inoculated with S. meliloti similarly revealed a strong activation of GUS expression in the nodulation-competent root region 24 to 48 h post inoculation (Supplemental Fig. S8). No TCSn:GUS or pENOD11:GUS activation was observed using a nodA S. meliloti mutant (Supplemental Fig. S8), indicating that the inductions observed were strictly dependent on NF. To define in which cell layers TCSn:GUS was activated first, we conducted a kinetic analysis from 4 to 72 h following S. meliloti inoculation. The TCSn:GUS up-regulation was detected at 4 and 8 h post inoculation, with maximal expression in epidermal and outer cortical cells (Fig. 4, G and H). The TCSn:GUS activation became strong in inner cortical cells only at 2 d post inoculation (Fig. 4I).
Figure 4.
CKs, NFs, and S. meliloti induce TCSn:GUS in M. truncatula root tissues. A to D, GUS staining of whole roots transformed with TCSn:GUS fusion via Agrobacterium rhizogenes. Shown are mock-treated roots (A), roots treated with CKs (10−7 m BAP for 4 h; B), and wild type (C) and nfp (D) roots treated with NFs (10−8 m for 4 h). Bars = 500 μm. E to I, GUS staining of longitudinal 25-μm sections (E, F, H, and I) or a transverse 10-μm section (G) of roots transformed via A. rhizogenes with TCSn:GUS (E, G, H, and I) or pENOD11:GUS (F) and treated with NFs (10−8 m for 4 h; E and F) or inoculated with S. meliloti at 8 h (G and H) and 72 h (H) post inoculation, respectively. Bars = 50 μm.
In conclusion, the TCSn:GUS expression pattern suggests an activation of the CK signaling pathway in response to NF treatment or S. meliloti inoculation, first in the outer root tissues (epidermis and outer cortex) and then in inner root tissues.
The Epidermal MtCRE1 Signaling Pathway Negatively Regulates the NF Induction of ENOD11 Expression and the Number of Nodules
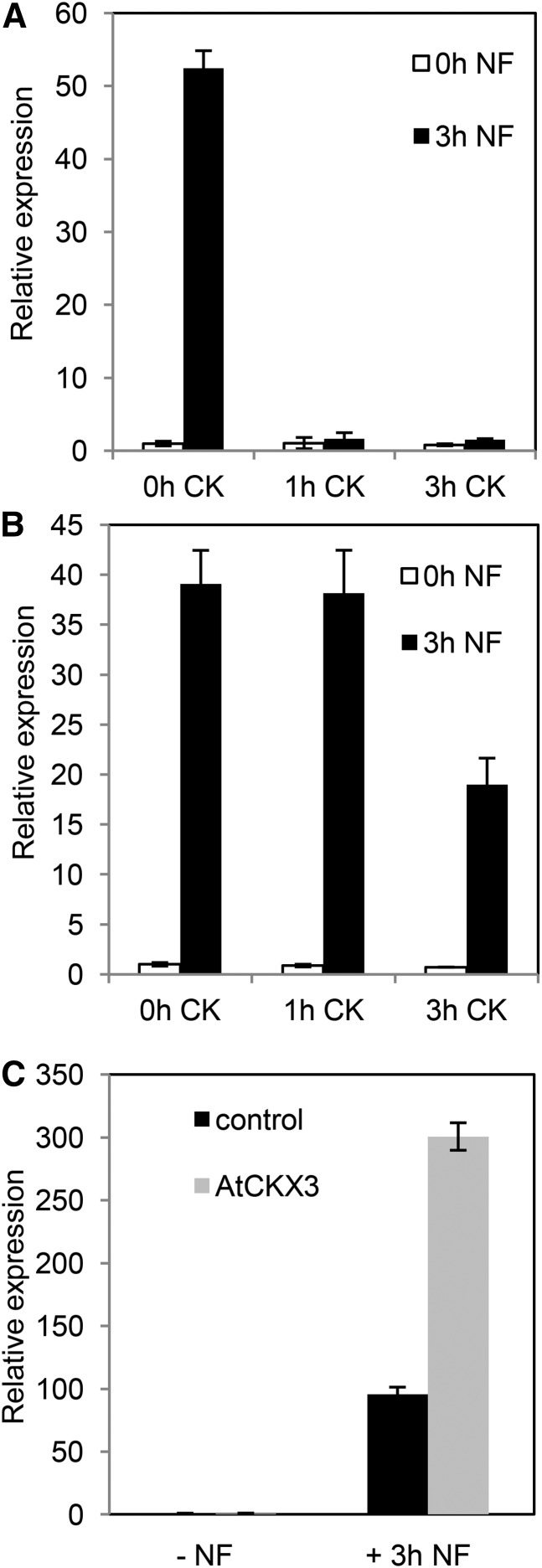
To determine the functional relevance of the observed NF induction of CK signaling in the epidermis, we first analyzed by quantitative reverse transcription-PCR the impact of a root pretreatment with exogenous CK on the level of ENOD11 induction by NF. ENOD11 was selected as a marker because it is strongly and rapidly induced in the epidermis by NFs (Fig. 4F). Wild-type and cre1 mutant plants were pretreated or not with CK (10−7 m BAP for 1 or 3 h) and then treated or not with NFs (10−9 m for 3 h). While ENOD11 induction in response to NFs was detected in the wild type as expected, this induction was strongly reduced after a 1- or 3-h CK pretreatment (Fig. 5A; Supplemental Fig. S9A). In the cre1 mutant, the NF induction of ENOD11 expression was similar in the absence of CK and after a 1-h CK pretreatment, indicating that the CRE1 signaling pathway is required for the inhibition of ENOD11 NF induction (Fig. 5B; Supplemental Fig. S9B). A reduction of the NF induction of ENOD11 expression was observed in cre1 after a 3-h CK pretreatment, suggesting that the other M. truncatula CK CHK receptors, the expression of which is detected in the epidermis and RHs (Supplemental Table S3; Breakspear et al., 2014), are likely functionally redundant with CRE1.
Figure 5.
The NF induction of ENOD11 is rapidly repressed by CKs via CRE1 and can be increased by expressing AtCKX3 in the root epidermis. A, Real-time reverse transcription-PCR analysis of ENOD11 relative expression in response to a 10−7 m BAP treatment (1 or 3 h) followed by a 10−8 m NF treatment (3 h) in wild-type M. truncatula roots. B, Real-time reverse transcription-PCR analysis of ENOD11 relative expression in response to a 10−7 m BAP treatment (1 or 3 h) followed by a 10−8 m NF treatment (3 h) in a cre1 mutant. C, Real-time reverse transcription-PCR analysis of ENOD11 relative expression in pEPI:AtCKX3 roots treated or not with 10−8 m NFs for 3 h. One biological replicate is shown out of two independent experiments (for the other biological replicate, see Supplemental Fig. S9). Error bars indicate sd of two technical replicates.
To independently evaluate the role of CK in regulating NF signaling in the epidermis, we expressed the Arabidopsis cytokinin oxidase/dehydrogenase gene (AtCKX3) from the pLeEXT1 promoter specifically acting in the epidermis of M. truncatula roots (Mirabella et al., 2004; Rival et al., 2012) and referred to henceforth as pEPI. Expression of the pEPI:AtCKX3 construct was checked in transformed roots by quantitative reverse transcription-PCR analysis (Supplemental Fig. S10). To directly assess the impact of the pEPI:AtCKX3 construct on CK accumulation, root CKs were profiled by liquid chromatography-tandem mass spectrometry (LC-MS/MS), using root fragments corresponding to the nodulation-competent region analyzed by RNAseq, with or without a 10−8 m NF (4-h) treatment. Three different nucleotide CK types were detected, namely trans-zeatin nucleotide (tZNT), cis-zeatin nucleotide, and isopentenyl adenine nucleotide (iPNT; Supplemental Table S6). Similar CK levels were detected for the NF- and mock-treated roots, but all pEPI:CKX3 samples presented significantly lower levels (P ≤ 0.05) of iPNT and tZNT (Supplemental Fig. S11), while no differences were observed for cis-zeatin nucleotide levels. This indicates that the pEPI:CKX3 construct efficiently reduced CK accumulation in the transformed roots, thereby demonstrating that a portion of the root CK pool locates in the epidermis.
We then assessed the impact of the pEPI:AtCKX3 construct on the induction of ENOD11 by NF. While AtCKX3 expressed from the strong constitutive 35S promoter previously revealed a positive role of CK in nodulation (Lohar et al., 2004), roots transformed with pEPI:AtCKX3 exhibited a stronger ENOD11 up-regulation by a 3-h NF treatment compared with control roots (Fig. 5C; Supplemental Fig. S9C). This suggested that the NF response is enhanced when iPNT and/or tZNT are depleted in the epidermis.
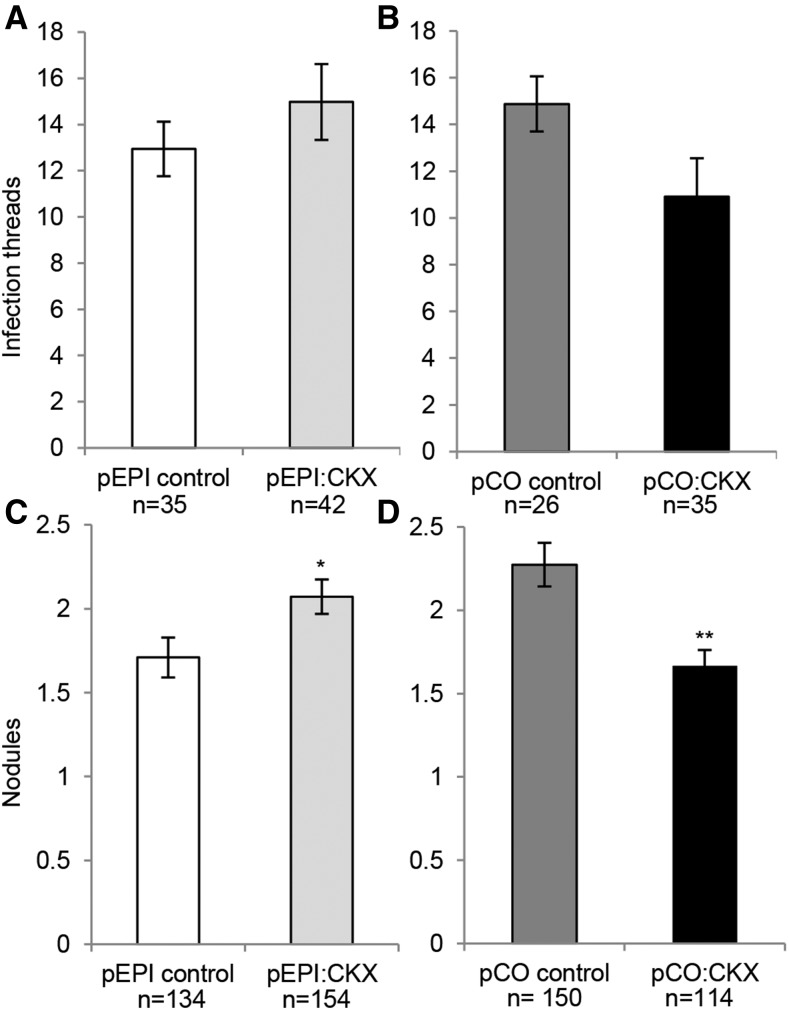
Finally, we compared rhizobium infection and nodulation in pEPI:AtCKX3 versus control roots at 6 and 14 d post inoculation with S. meliloti, respectively. Although there was a trend suggesting possible increased infection in pEPI:AtCKX3 versus control roots and decreased infection when expressing AtCKX3 from a cortex-specific promoter (pCO; Rival et al., 2012), the differences were not statistically significant (Fig. 6, A and B). However, using a larger population of transformed roots, we observed enhanced nodulation of pEPI:AtCKX3 versus control roots (P = 0.008, one-sided Welch’s test; Fig. 6C). Increased nodulation also was observed with pEPI:AtCKX3 roots inoculated with S. medicae (Supplemental Fig. S12). By contrast, when AtCKX3 was expressed from the pCO, nodulation was decreased (P = 0.0001; Fig. 6D), consistent with data obtained with the 35S promoter (Lohar et al., 2004). Altogether, these results suggest a possible negative role of the epidermal CK/CRE1 pathway in the NF induction of ENOD11 and nodulation.
Figure 6.
Expression of the AtCKX3 gene leads to an increased nodulation in the root epidermis and to a decreased nodulation in the root cortex. Infection threads and nodules were counted on individual roots at 6 and 14 d post inoculation with S. meliloti, respectively, using roots transformed by A. rhizogenes with pEPI:CKX3 specifically expressing AtCKX3 in the root epidermis, or the corresponding control vector (A and C), or with pCO:CKX3 specifically expressing AtCKX3 in the root cortex, or the corresponding control vector (B and D). P = 0.1592 and 0.1658 in A and B respectively, and 0.00786 and 0.00010 in C and D, respectively (one-sided Welch’s test). Values shown are means of three biological replicates ± se.
DISCUSSION
In past years, transcriptomics approaches have enabled the identification of genes playing major roles in the control of nodulation, such as NF-YA or NCR, and therefore are complementary to forward genetics, especially in the case of functional redundancies or when used to identify genes for which mutation leads to lethal phenotypes. One challenge in transcriptomics now consists of analyzing single tissues rather than a mixture of tissues, in order to uncover the roles of regulatory and metabolic pathways in specific tissues. In this study, NF-induced gene expression reprogramming was analyzed by laser microdissection of epidermal cells from the root region that is competent for nodulation and responds to NF. This was combined with high-depth RNAseq and mapping of the RNAseq reads on the most recent M. truncatula genome sequence, which thereby enabled a sensitive genome-wide analysis.
More than 300 genes showed a greater than 10-fold level of NF activation, reflecting both dramatic modifications in gene expression and increased sensitivity of single-tissue studies. Forty-four of the NF-induced genes were found to be symbiosis specific based on MtGEA data (Supplemental Table S3) and corresponding to a variety of functions (receptors, kinases, TFs, proteases and proteasome elements, transporters, defense-related proteins, etc.), including 18 genes also up-regulated during mycorrhizal interactions. Surprisingly, these 44 genes include three NCR genes (NCR117, NCR150, and NCR160, the last two also being induced in mycorrhizal roots) not yet listed among the very few NCR genes that are known to be not exclusively expressed in the nodule (five out of 334 analyzed in 267 experimental conditions; Guefrachi et al., 2014). NCR genes are currently only known for their key roles in later nodulation stages for bacteroid differentiation, so this symbiotic induction in the absence of rhizobia suggests a role independent of bacterial infections. As NCRs are defensin-like proteins with antimicrobial properties demonstrated for some of them (Maróti et al., 2015), this early induction might be related to transient defense responses induced during symbiotic interactions (see below).
The complexity of NF-induced responses just within a few hours and a single tissue may seem, at first sight, somewhat surprising. However, the dissection of another plant-microbe signaling pathway, associated with the perception of microbe-associated molecular patterns, also shows great complexity, with a variety of players involved in positive or negative regulation (Macho and Zipfel, 2014). It is very likely that NF signaling is just as complex, leading to the production of secondary signals of various natures (ion fluxes, ROS, flavonoids, phytohormones, and peptides). A number of genes identified are certainly associated with gene networks required for their production, transport, perception, signaling, and action. This probably explains the many (145) NF-induced signaling genes identified, notably encoding receptors, protein kinases, and phosphatases, as well as regulators of gene expression at the transcriptional or posttranscriptional level. For example, several genes are good candidates for being part of a possible ROS pathway, such as the ortholog of ANAC042, a NAC TF gene induced by H2O2 (Wu et al., 2012), and a gene encoding a MAPKKK closely related to ANP1 kinase (Table III). ANP1 is known to be activated by exogenous H2O2, while constitutively active ANP1 mimics the H2O2 effect, with activation of the MAPK cascade and up-regulation of specific genes (Kovtun et al., 2000). This potential link between NF signaling and ROS is supported by the fact that 102 NF up-regulated genes (Supplemental Table S3) were shown previously to be affected (directly or indirectly) by the inhibition of H2O2 production in S. meliloti-inoculated roots (Andrio et al., 2013). Moreover, up-regulation by an exogenous H2O2 treatment was shown for five NF-induced genes: MtSPK1, MtSPK2, MtSRL1, MtABlL, and MtRIP1 (Ramu et al., 2002; Andrio et al., 2013).
Biological functions associated with the NF signaling/preinfection stage include both cell-autonomous and non-cell-autonomous processes, with the preparation of RHs for rhizobial infection involving the activation of cell cycle genes, the regulation of defense responses, and the induction of cell-cell communication with inner root cell layers. The non-cell-autonomous effects range from the preparation of the outer cortical cell to enable infection thread progression, to the production of an unknown signal leading to the initiation of nodule organogenesis by cell divisions in the inner cortex and pericycle cells, to a negative control in epidermal cells to prevent overinfection and control the number of nodules formed. A number of NF-regulated candidate genes that have potential to take part in these different processes were described in “Results.” For example, numerous genes, coding for proteins involved in the cell wall and cytoskeleton structure or dynamics, as well as membrane or vesicular trafficking, were identified in line with the major subcellular remodeling associated with infection thread formation (Fournier et al., 2015). The strong NF up-regulation of MtLYK10, for which the expression is not detectable in control epidermal cells, probably also contributes to the preparation for infection, since the orthologous L. japonicus EPR3 gene encodes a receptor for Rhizobium spp. exopolysaccharidic signals, a key factor for successful infections (Kawaharada et al., 2015). Down-regulation of MtLYK3, also observed using isolated RHs (Breakspear et al., 2014), is more surprising, since MtLYK3 is known to be involved in infection. MtLYK3 expression might concentrate in certain epidermal cells of the nodulation-competent root region, in preparation for infection, and decrease in others. Alternatively MtLYK3 down-regulation may be part of a negative feedback to prevent overinfection.
Successful rhizobial infection requires a tight regulation of plant immune responses in cells that get infected by the symbiont. The transient induction of defense responses following Rhizobium spp. inoculation has been reported often (for review, see Gourion et al., 2015; Limpens et al., 2015), and it was suggested that NF may actively suppress plant immunity, based on decreased ROS production and PR2-like (TC 78899 = Medtr4g076430) expression upon NF addition in M. truncatula (Shaw and Long, 2003; Mitra and Long, 2004). However, while Medtr4g076430 is indeed down-regulated by NF, as described previously, a paralogous gene (Medtr4g076470), located close to Medtr4g076430 in the genome, is up-regulated by NF, along with several other defense-related genes (PR1, PR3, PR10, MLO8, WRKY, etc; Supplemental Table S3), in agreement with several other transcriptomic studies (Nakagawa et al., 2011; Rose et al., 2012; Breakspear et al., 2014). Recent data additionally showed a dual function for the LCO receptors LjNFR1/MtLYK3 and LjNFR5/MtNFP and for orthologous genes in rice (Oryza sativa) in symbiosis and in defense responses (for review, see Limpens et al., 2015). This dual functioning may involve distinct LYSM RLK receptor complexes, and the fact that several LYSM RLK genes are expressed at different levels in the epidermis, with three of them NF induced in addition to LYK10 (MtLYR3, MtLYR6, and CERK1-like), suggests that several pathways could be activated. It also has been proposed that immune responses (e.g. ROS production) were recruited to facilitate and regulate symbiotic infections. We observed the activation of several non-RD kinases, which are thought to recognize conserved microbial signatures and to be involved in immunity (Schwessinger and Ronald, 2012). This raises the additional possibility that NF might activate a set of genes, including receptors of microbe-associated molecular patterns, that are useful for preventing nonrhizobium microbes from penetrating into root tissues during the infection stage. This could be important under natural conditions, where rhizobia are competing with numerous microorganisms. Intriguingly, we found out that two genes thought to down-regulate the plant immune response in functional nodules, MtDNF2 and MtSYMRK (Bourcy et al., 2013; Berrabah et al., 2014), are induced by NF at 4 and 24 h. This implies that MtDNF2 and MtSYMRK have an early symbiotic function in addition to their role at later nodulation stages, and this might contribute to the control of the transient NF-induced defense responses in the root. The predicted ortholog of PUB22, a gene known to negatively control the plant immune response (Trujillo et al., 2008), is NF induced and also could contribute to the control of defense reactions.
NF signaling induces several plant secondary signals, such as phytohormones, which can be involved in cell-cell communication. Our study generated evidence for the NF regulation of classic hormone pathways such as those for auxins, GAs, strigolactones, and CK (consistent with Breakspear et al., 2014), as well as peptide hormones such as CLE and CEP (Table III). We were particularly interested in CKs, known for their critical role in the initiation of nodule organogenesis and shown to accumulate rapidly in response to NF (van Zeijl et al., 2015; Reid et al., 2016) but not considered as early epidermal signals. Indeed, in Rhizobium spp.-inoculated L. japonicus roots, TCS:GUS expression is detected first in the cortex (Held et al., 2014). Yet, our RNAseq data coupled to metabolic network modeling suggest that CK biosynthesis is activated rapidly by NF in the epidermis. In addition, by LC-MS/MS quantitative analysis, we established that significantly lower amounts of two root CKs (tZNT and iPNT) are found in pEPI:AtCKX3-transformed roots that express a CK-degrading enzyme specifically in the root epidermis. This indicates that a portion of the root CK, in particular tZNT, is located in the epidermis. LC-MS/MS analysis revealed the presence of only the nucleotide forms of CK, which are known to be the precursors of active CK. The production of bioactive free base CK then requires a reaction catalyzed by phosphoribohydrolase of the LOG family (Sakakibara, 2006; Kamada-Nobusada and Sakakibara, 2009). Our RNAseq data indicate that several LOG-like genes (Medtr3g113710, Medtr1g015830, Medtr4g058740, and Medtr1g057020) are expressed in the root epidermis, suggesting that active CKs can be produced in this tissue even if not detected by LC-MS/MS. As a matter of fact, the activation of CK signaling pathways by NF was indicated by the transient induction of MtCRE1 and, more importantly, three RRA genes (MtRRA2, MtRRA8, and MtRRA9), in agreement with Op den Camp et al. (2011) for MtRRA9 (formerly MtRR9), based on GUS transcriptional fusions and other transcriptomic analyses performed on isolated RHs under symbiotic conditions (Breakspear et al., 2014; Liu et al., 2015). This symbiotic activation of CK signaling in the root epidermis was further validated by the use of a pCRE1:GUS fusion and a new version of the CK-responsive reporter, TCSn:GUS (Zürcher et al., 2013), which both gave signals in the epidermis of the nodulation-competent zone in response to NF and S. meliloti. The discrepancy with the pattern of the TCS:GUS reporter described by van Zeijl et al. (2015) likely relates to the increased CK sensitivity of the TCSn:GUS variant. A detailed kinetic analysis revealed that, in M. truncatula, the TCSn:GUS reporter expression is activated by Rhizobium spp. first in the epidermis and subtending cortical cells and becomes strong in the inner cortex only at later time points, in striking contrast to the TCS:GUS pattern in L. japonicus (Held et al., 2014). Once more, this could reflect differences between the CK sensitivity of the TCS and TCSn reporters (Zürcher et al., 2013), but alternatively, CK signaling activation could differ between M. truncatula and L. japonicus (i.e. indeterminate versus determinate nodulation types).
In spite of evidence for the activation of CK signaling pathways, we were not able to detect by LC-MS/MS an NF-induced accumulation of CK. This might be due to higher global CK concentration in A. rhizogenes-transformed roots as compared with nontransformed roots, which could mask a moderate NF-induced CK accumulation (only a 2-fold CK increase was reported by van Zeijl et al. [2015] in response to NF in nontransformed roots in the absence of aminoethoxyvinylglycine). A possible candidate for the direct or indirect regulation of CK pathways is MtKNOX3, which we found here to be NF induced in the epidermis, in addition to being up-regulated in nodule primordia (Azarakhsh et al., 2015). KNOX genes are indeed known to control CK metabolism in the shoot apical meristem, and MtKNOX3-RNAi lines exhibit reduced expression of IPT, LOG and RRA genes involved in CK biosynthesis, activation and response respectively (Azarakhsh et al., 2015).
It was demonstrated recently that CRE1 is activated in the root cortex in response to an elusive epidermal symbiotic signal, leading to NIN expression itself amplifying CRE1 expression via a positive feedback loop and eventually leading to nodule primordium formation (Vernié et al., 2015). The presence of CK in the epidermis and the activation of CK signaling in epidermal and subtending cortical cells at very early symbiotic stages make CKs attractive candidate molecules for the mobile signal that activates CRE1 in the cortex. In this regard, it is interesting that several genes belonging to families proposed to be involved in CK translocation (encoding ATP-binding cassette transporters and equilibrative nucleoside transporters; Sakakibara, 2006; Ko et al., 2014) are strongly up-regulated in NF-treated epidermal cells (e.g. Medtr2g011570; Table II).
A possible positive role of epidermal CK as a mobile signal activating cortical responses may seem difficult to reconcile with the increased nodulation observed when AtCKX3 is expressed in the epidermis. However, our study also suggested a distinct, cell-autonomous, and possibly antagonistic function of CK in the epidermis. A negative role of CK in the NF induction of MtENOD11 expression was identified, depending on the CRE1 CK receptor, either by performing a CK pretreatment before the NF treatment or by expressing the pEPI:AtCKX3 construct (Fig. 5). Bearing in mind that an exogenous CK treatment induces ERN1, NIN, and NSP2 (Plet et al., 2011) and that CRE1 is required for the NF induction of many genes (van Zeijl et al., 2015), CK might have both positive and negative roles in the root. This would be similar to NIN, which is required for symbiotic gene induction in the epidermis and cortex, as well as Rhizobium spp. infection and nodule initiation, while restricting ENOD11 expression on the one hand and nodulation on the other hand, via CLE peptides (Marsh et al., 2007; Soyano et al., 2013, 2014; Laloum et al., 2014; Yoro et al., 2014; Fournier et al., 2015; Vernié et al., 2015). Consequently, the impact of the pEPI:AtCKX3 construct might depend on the relative efficiency of different processes (e.g. speed of CK translocation to the cortex versus degradation by AtCKX3 in the epidermis). In L. japonicus, the mutation of a CKX3 gene expressed in nodule primordia but not in the epidermis leads to a reduction of both nodulation and infection thread formation (Reid et al., 2016), suggesting that CK overproduction in the cortex induces a negative feedback on infection and that CK may have temporally and spatially restricted antagonistic functions. In M. truncatula, the opposite impact on nodulation observed for the epidermal pEPI:AtCKX3 and cortical pCO:AtCKX3 constructs suggests distinct predominant roles for CK in outer and inner root tissues during NF signaling and nodulation, with a stronger negative component in the epidermis.
It remains an open question which downstream factors mediate the various effects of CK in different root tissues. Among possible candidates, beyond the NIN transcriptional regulator itself, are the flavonoid biosynthesis and auxin-responsive genes, as demonstrated previously for the activation of nodule organogenesis depending on MtCRE1 (Mathesius et al., 2000; Plet et al., 2011; Ng et al., 2015) as well as genes of the ethylene and GA pathways, activated both in response to a CK treatment (Ariel et al., 2012) and to NF (van Zeijl et al., 2015), notably in the epidermis (Breakspear et al., 2014; this study).
CONCLUSION
Investigations of tisue-specific responses have only just begun at the genome-wide scale. This represents a major challenge, now made accessible by recent tremendous technical progress, such as new-generation sequencing coupled to laser dissection approaches. Here, NF signaling could be investigated in the root epidermis, revealing the extent and complexity of gene expression reprogramming, with numerous NF-induced genes never identified before. This notably revealed the early activation of CK metabolic and signaling pathways in this tissue, thereby suggesting possible epidermis-specific cell-autonomous and non-cell-autonomous functions during early rhizobium-legume interactions. Interestingly, the activation of the CK-signaling pathway in the epidermis was correlated to a negative regulation of ENOD11 gene expression and nodulation, whereas the same pathway acts positively in the cortex for the stimulation of cell divisions. The possible dual (positive and negative) control of nodulation and infection by the same signal could contribute to the fine regulation and coordination of these two key symbiotic processes. In any case, our study emphasizes the critical importance of tissue-specific analyses needed to decipher the complex regulations that occur during the rhizobium-legume symbiosis and, more generally, in host-microbe interactions.
MATERIALS AND METHODS
Plant Growth and Treatment
To generate root samples for laser dissection, Medicago truncatula ‘Jemalong A17’ plants were grown in aeroponic caissons for 3 d in nitrogen-free aeroponic nutrient medium (Barker et al., 2007) prior the NF treatment (chamber conditions: temperature, 22°C; 75% hygrometry; light intensity, 200 μE m−2 s−1; and light/dark photoperiod, 16/8 h). Plantlets were then immersed for either 4 or 24 h in 10−8 m NFs (in Fahraeus medium, without aminoethoxyvinylglycine) or in Fahraeus medium only (mock treatment), using five plantlets per 50-mL Falcon tube. Twenty-five plants per time point and per biological repetition were treated and used for laser dissection. Each biological repetition (more than 50 plants) corresponded to an independent caisson. The cre1-1 and nfp-1 mutants are described by Plet et al. (2011) and Ben Amor et al. (2003), respectively. Plant growth conditions for promoter:GUS analyses and CK quantitative analysis are described below.
Laser Microdissection, RNAseq, and RNAseq Data Analysis
One-centimeter-long root fragments (without root tips) were collected and treated as described previously (Roux et al., 2014; for details, see Supplemental Materials and Methods S1). Pooled RNAs were ethanol precipitated, ribosomal RNA was eliminated by oligocapture, and remaining RNA was amplified by in vitro transcription as described (Roux et al., 2014). One microgram of amplified RNA per sample was used for oriented paired-end RNA sequencing (2 × 50 bases) by Fasteris using an Illumina HiSeq 2000 platform. Three biological repetitions were used per sample except for the 24-h NF treatment, for which only two repetitions were retained, based on quality criteria (r > 0.90).
Expressed genes were defined using data-based global filtering (Rau et al., 2013), giving a threshold of 28,255 normalized reads for one library. Differential expression analysis of RNAseq data was performed with the DESeq software version 1.20.0 (Anders and Huber, 2010). Dispersions were estimated using the pooled method with the fit-only criteria. P values were adjusted for multiple testing using the Benjamini and Hochberg false discovery rate (Benjamini and Hochberg, 1995).
The LEGoo knowledge base (https://www.legoo.org) Nicknames tool was used to find correspondences between various gene, transcript, and microarray oligonucleotide identifiers. Orthologous genes were predicted based on reciprocal best hits (BLASTP).
Metabolic Network Analyses
M. truncatula metabolic reactions and pathways were built from transcripts obtained in previous experiments (Roux et al., 2014). The relation between transcripts and biochemical reactions was established by comparing predicted protein sequences with the protein sequences found in the pathway genome databases (PGDBs) available in Pathway Tools (http://bioinformatics.ai.sri.com/ptools/; Karp et al., 2002). The resulting M. truncatula PGDB is available at https://pathway-tools.toulouse.inra.fr/MEDICAGO2 (login/password, guest/guest). From the PGDB and thanks to ad hoc bioinformatics tools, we built a metabolic graph that links reactions by their substrates and their products, not considering any a priori classification into pathways (Lacroix et al., 2008). We stored metabolic graphs in the MetExplore Web server (Cottret et al., 2010). Metabolic networks are directly available at http://metexplore.toulouse.inra.fr/metexplore2/index.html?idBioSource=3423. Thanks to the MetExplore mapping functions, we established a list of reactions for which corresponding genes are down-regulated or up-regulated by NF at 4 and 24 h. Pathway reaction enrichment significance was computed for each condition in MetExplore using the Bonferroni test. Metabolic networks composed of whole sets of reactions corresponding to up- or down-regulated genes were displayed using Cytoscape (Shannon et al., 2003). Then, we visually located interesting subnetworks crossing pathways of interest.
Transcriptional Fusion Constructs
For the transcriptional fusion between the MtCRE1 promoter, defined as 2,500 nucleotides upstream of the ATG, and the uidA reporter gene, the pMtCRE1 region was amplified by PCR using the following primers: pCRE1_F, 5′-ggtaccTAACATAAGGACCTAGAACCAATATAAAGA-3′, and pCRE1_R, 5′-gacgtcTACAACACCAACTAACACCAAATCTC-3′ (lowercase letters indicate KpnI and AatII restriction sites used for cloning). The PCR product was cloned into the pFRN-GUS vector (derived from the pFGC5941 plasmid; National Center for Biotechnology Information accession no. AY310901) using the above-mentioned restriction sites.
For epidermis-specific expression, the pFRN-RNAi vector (Gonzalez-Rizzo et al., 2006) was cut with EcoRI and SmaI to remove the RNAi cassette and religated with a linker containing EcoRI and KpnI sites. The pLeEXT1 promoter was excised from a pGEM-T vector (Rival et al., 2012) and cloned into these sites to generate the pFRN-pEpi vector. An AtCKX3 PCR fragment was amplified from a pUC19 plasmid described by Werner et al. (2001) with primers AtCKX3_F, 5′-ggatcATGGCGAGTTATAATCTTCGTTC-3′, and AtCKX3_R, 5′-aggcctACTCGAGTTTATTTTTTGAAATATATTTTG-3′ (lowercase letters indicate BamHI and StuI restriction sites used for cloning) and inserted into the pFRN-pEpi vector to generate the pEPI:AtCKX3 construct.
The TCSn reporter was amplified by PCR from the TCSn:GFP vector (Zürcher et al., 2013) using the following primers: TCSn F, 5′-aggtctccaaatCAAAGATCTTTAAAAGATTTTGAAAG-3′, and TCSn R, 5′-aggtctcccaatTGTTATATCTCCTTGGATCGATCCCC-3′ (lowercase letters indicate BsaI restriction site and spacer used for cloning). The PCR product was Golden Gate cloned (Engler and Marillonnet, 2011) into a modified pCAMBIA2200 vector (Fliegmann et al., 2013) using BsaI sites to generate the TCSn:GUS binary vector.
Plant Transformation, Promoter:GUS Analyses, CK Quantitative Analysis, and Nodulation Assays
M. truncatula roots were transformed using Agrobacterium rhizogenes as described (Boisson-Dernier et al., 2001). All transformations were performed in at least three biological repetitions. Composite plants were selected for 2 to 3 weeks on 25 mg L−1 kanamycin (25°C; day/night, 12/12 h) and then transferred into slanted square petri dishes (12 × 12 or 24 × 24 cm) on Fahraeus agar medium without nitrogen covered with autoclaved seed germination paper (38# Seed Germination Paper 2M/CTN; Anchor Paper).
For composite plants expressing the TCSn:GUS or the pENOD11:GUS construct, analyses were conducted following a 4-h treatment with 10−8 m NF, 10−7 m BAP, or Fahraeus medium without nitrogen (mock), or Sinorhizobium meliloti 2011 inoculation (pXLGD4 phemA:lacZ [GMI6526]; Ardourel et al., 1994) or nodA::Tn5#2208 at optical density at 600 nm = 0.01. Roots were collected and prefixed in 0.4% paraformaldehyde for 1 h, stained for GUS activity 4 h at 37°C, and fixed in 2% glutaraldehyde. Longitudinal sections were made from roots embedded in 8% agarose, using a vibrating microtome (Leica VT 1000S), and observed with a Zeiss Axiophot light microscope. For CK LC-MS/MS analysis, approximately 1-cm-long root fragments were collected before or after 4 h of treatment with 10−8 m NF versus mock, after cutting off root tips (approximately 1–2 mm long) to remove a potential source of non-NF-regulated CK. Samples of 180 mg fresh weight (about 50 root systems per sample) were lyophilized and then processed as described by Kisiala et al. (2013); for details see Supplemental Materials and Methods S1.
In nodulation assays, composite plants expressing the pEPI:AtCKX3 or pCO:AtCKX3 construct were grown in vitro on paper-covered Fahraeus medium without nitrogen and inoculated with S. meliloti 2011 as before; infection threads and nodules were counted at 6 and 14 d post inoculation, respectively, following lacZ staining performed as described previously (Vernié et al., 2008). In addition, plants grown in vitro on i medium (Gonzalez-Rizzo et al., 2006) were inoculated with S. medicae WSM419 (Terpolilli et al., 2008).
The Sequence Read Archive project accession number for original RNAseq data is SRP058185.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. LCM of root epidermal cells.
Supplemental Figure S2. Pairwise comparisons of NF-regulated genes detected in recent transcriptomic studies.
Supplemental Figure S3. Multiple comparisons (Venn diagrams) of NF down-regulated M. truncatula genes detected in recent transcriptomics experiments.
Supplemental Figure S4. topGo analysis of NF up- and down-regulated M. truncatula genes (4-h treatment).
Supplemental Figure S5. topGo analysis of NF up- and down-regulated M. truncatula genes (24-h treatment).
Supplemental Figure S6. Expression of the GA12 metabolic network in response to NFs (24 h).
Supplemental Figure S7. Expression of the trans-zeatin CK metabolic network in response to NFs (24 h).
Supplemental Figure S8. TCSn:GUS and pENOD11:GUS are up-regulated by wild-type S. meliloti in an NF-dependent way.
Supplemental Figure S9. Biological repetition of experiments shown in Figure 5.
Supplemental Figure S10. AtCKX3 is strongly expressed in M. truncatula roots transformed with the pEPI:AtCKX3 construct.
Supplemental Figure S11. Statistical analysis of CK quantitative analysis in root fragments, transformed with the pEPI:AtCKX3 construct or a control plasmid, and treated or not with 10−8 m NF for 4 h.
Supplemental Figure S12. Nodulation assays following S. medicae inoculation of roots transformed by A. rhizogenes with the pEPI:CKX3 construct.
Supplemental Table S1. Number of total and unambiguously mapped RNAseq read pairs.
Supplemental Table S2. Complete RNAseq and annotation data for mock- or NF-treated laser-dissected root epidermis samples.
Supplemental Table S3. List of genes (and annotation data) up-regulated in M. truncatula root epidermis in response to 10−8 m NFs, identified by laser microdissection coupled with RNAseq analysis.
Supplemental Table S4. List of genes (and annotation data) down-regulated in M. truncatula root epidermis in response to 10−8 m NFs, identified by laser microdissection coupled with RNAseq analysis.
Supplemental Table S5. List of metabolic networks regulated in M. truncatula root epidermis in response to 10−8 m NFs, identified using MetExplore.
Supplemental Table S6. Levels of CKs in roots transformed via A. rhizogenes with a control plasmid or a plasmid carrying the pEPI:CKX3 construct.
Supplemental Materials and Methods S1. Laser microdissection, RNA sequencing, and CK extraction and quantification.
Acknowledgments
We thank Bruno Müller (University of Zurich) for the TCSn:GFP construct, Thomas Schmuelling (Free University Berlin) for the AtCKX3 clone, Fabienne Maillet (LIPM) for providing NFs, Sandra Bensmihen (LIPM) for the pGEM-T:pLeEXT1 vector, Fernanda de Carvalho Niebel (LIPM) for valuable advice and supervising the NF treatments, Christine Hervé and Andreas Niebel (LIPM) for help in NF treatments, Sébastien Carrere (LIPM) for the SYMbiMICS Web site, Alain Jauneau and the Fédération de Recherche Agrobiosciences, Interactions, en Biodiversité microscopy platform for the laser microdissection equipment, and the IMAGIF cell biology platform.
Glossary
- RH
root hair
- LCO
lipochitooligosaccharide
- NF
Nod factor
- TF
transcription factor
- ROS
reactive oxygen species
- CK
cytokinin
- RNAseq
RNA sequencing
- LCM
laser-capture microdissection
- MtGEA
M. truncatula Gene Atlas
- H2O2
hydrogen peroxide
- BAP
6-benzylaminopurine
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- tZNT
trans-zeatin nucleotide
- iPNT
isopentenyl adenine nucleotide
- PGDB
pathway genome database
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, the ANR Labex Saclay Plant Science, and the Lidex Plant Phenotyping Pipeline; by the French Région Ile de France ASTREA program (Ph.D. fellowship to S.B.); by the ANR (grant no. ANR–08–GENO–106 to P.G.) and the French Laboratory of Excellence project TULIP (grant nos. ANR–10–LABX–41 and ANR–11–IDEX–0002–02); and by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to R.J.N.E.).
Articles can be viewed without a subscription.
References
- Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriankaja A, Boisson-Dernier A, Frances L, Sauviac L, Jauneau A, Barker DG, de Carvalho-Niebel F (2007) AP2-ERF transcription factors mediate Nod factor dependent Mt ENOD11 activation in root hairs via a novel cis-regulatory motif. Plant Cell 19: 2866–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrio E, Marino D, Marmeys A, de Segonzac MD, Damiani I, Genre A, Huguet S, Frendo P, Puppo A, Pauly N (2013) Hydrogen peroxide-regulated genes in the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytol 198: 179–189 [DOI] [PubMed] [Google Scholar]
- Ané JM, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GE, Ayax C, Lévy J, Debellé F, Baek JM, Kalo P, et al. (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303: 1364–1367 [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M, Ried MK, Parniske M (2014) Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr Biol 24: 422–427 [DOI] [PubMed] [Google Scholar]
- Ardourel M, Demont N, Debellé F, Maillet F, de Billy F, Promé JC, Dénarié J, Truchet G (1994) Rhizobium meliloti lipooligosaccharide nodulation factors: different structural requirements for bacterial entry into target root hair cells and induction of plant symbiotic developmental responses. Plant Cell 6: 1357–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel F, Brault-Hernandez M, Laffont C, Huault E, Brault M, Plet J, Moison M, Blanchet S, Ichanté JL, Chabaud M, Carrere S, Crespi M, Chan RL, Frugier F (2012) Two direct targets of cytokinin signaling regulate symbiotic nodulation in Medicago truncatula. Plant Cell 24: 3838–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi JF, Barre A, Ben Amor B, Bersoult A, Soriano LC, Mirabella R, de Carvalho-Niebel F, Journet EP, Ghérardi M, Huguet T, et al. (2006) The Medicago truncatula lysin [corrected] motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol 142: 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi JF, Godfroy O, de Billy F, Saurat O, Jauneau A, Gough C (2008) The RPG gene of Medicago truncatula controls Rhizobium-directed polar growth during infection. Proc Natl Acad Sci U S A 105: 9817–9822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarakhsh M, Kirienko AN, Zhukov VA, Lebedeva MA, Dolgikh EA, Lutova LA (2015) KNOTTED1-LIKE HOMEOBOX 3: a new regulator of symbiotic nodule development. J Exp Bot 66: 7181–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C (2014) jvenn: an interactive Venn diagram viewer. BMC Bioinformatics 15: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DG, Pfaff T, Moreau D, Groves E, Ruffel S, Lepetit M, Whitehand S, Maillet F, Nair RM (2006) Growing Medicago truncatula: Choice of Substrates and Growth Conditions. In The Medicago truncatula handbook. Mathesius U, Journet EP, Sumner LW (eds). ISBN 0-9754303-1-9 http://www.noble.org/MedicagoHandbook/
- Baudin M, Laloum T, Lepage A, Ripodas C, Ariel F, Frances L, Crespi M, Gamas PC, Blanco FA, Zanetti ME, et al. (2015) A phylogenetically conserved group of NF-Y transcription factors interact to control nodulation in legumes. Plant Physiol 169: 2761–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amor B, Shaw SL, Oldroyd GE, Maillet F, Penmetsa RV, Cook D, Long SR, Dénarié J, Gough C (2003) The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J 34: 495–506 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approch to multiple testing. J Roy Statist Soc Ser B 57: 289–300 [Google Scholar]
- Berrabah F, Bourcy M, Eschstruth A, Cayrel A, Guefrachi I, Mergaert P, Wen J, Jean V, Mysore KS, Gourion B, et al. (2014) A nonRD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol 203: 1305–1314 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG (2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14: 695–700 [DOI] [PubMed] [Google Scholar]
- Boivin S, Kazmierczak T, Brault M, Wen J, Gamas P, Mysore KS, Frugier F. (2016) Different cytokinin CHK receptors regulate nodule initiation as well as later nodule developmental stages in Medicago truncatula. Plant Cell Environ, in press [DOI] [PubMed] [Google Scholar]
- Bourcy M, Brocard L, Pislariu CI, Cosson V, Mergaert P, Tadege M, Mysore KS, Udvardi MK, Gourion B, Ratet P (2013) Medicago truncatula DNF2 is a PI-PLC-XD-containing protein required for bacteroid persistence and prevention of nodule early senescence and defense-like reactions. New Phytol 197: 1250–1261 [DOI] [PubMed] [Google Scholar]
- Breakspear A, Liu C, Roy S, Stacey N, Rogers C, Trick M, Morieri G, Mysore KS, Wen J, Oldroyd GE, et al. (2014) The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 26: 4680–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoen W, Sun J, Wysham D, Otegui MS, Venkateshwaran M, Hirsch S, Miwa H, Downie JA, Morris RJ, Ané JM, et al. (2011) Nuclear membranes control symbiotic calcium signaling of legumes. Proc Natl Acad Sci USA 108: 14348–14353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Martínez A, Sánchez F, Quinto C (2008) Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J 56: 802–813 [DOI] [PubMed] [Google Scholar]
- Cerri MR, Frances L, Laloum T, Auriac MC, Niebel A, Oldroyd GE, Barker DG, Fournier J, de Carvalho-Niebel F (2012) Medicago truncatula ERN transcription factors: regulatory interplay with NSP1/NSP2 GRAS factors and expression dynamics throughout rhizobial infection. Plant Physiol 160: 2155–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M, Bredemeier R, Wanner G, Takeda N, Schleiff E, Parniske M (2008) Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. Plant Cell 20: 3467–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zhu H, Ke D, Cai K, Wang C, Gou H, Hong Z, Zhang Z (2012) A MAP kinase kinase interacts with SymRK and regulates nodule organogenesis in Lotus japonicus. Plant Cell 24: 823–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen W, Li X, Jiang H, Wu P, Xia K, Yang Y, Wu G (2014) Knockdown of LjIPT3 influences nodule development in Lotus japonicus. Plant Cell Physiol 55: 183–193 [DOI] [PubMed] [Google Scholar]
- Clark GB, Morgan RO, Fernandez MP, Roux SJ (2012) Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol 196: 695–712 [DOI] [PubMed] [Google Scholar]
- Combier JP, Frugier F, de Billy F, Boualem A, El-Yahyaoui F, Moreau S, Vernié T, Ott T, Gamas P, Crespi M, et al. (2006) MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev 20: 3084–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Dreyer D, Bonnet D, Howell M, Nony E, VandenBosch K (1995) Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell 7: 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottret L, Wildridge D, Vinson F, Barrett MP, Charles H, Sagot MF, Jourdan F (2010) MetExplore: a web server to link metabolomic experiments and genome-scale metabolic networks. Nucleic Acids Res 38: W132–W137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja LF, Hogekamp C, Lamm P, Maillet F, Martinez EA, Samain E, Dénarié J, Küster H, Hohnjec N (2012) Transcriptional responses toward diffusible signals from symbiotic microbes reveal MtNFP- and MtDMI3-dependent reprogramming of host gene expression by arbuscular mycorrhizal fungal lipochitooligosaccharides. Plant Physiol 159: 1671–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Niebel F, Lescure N, Cullimore JV, Gamas P (1998) The Medicago truncatula MtAnn1 gene encoding an annexin is induced by Nod factors and during the symbiotic interaction with Rhizobium meliloti. Mol Plant Microbe Interact 11: 504–513 [DOI] [PubMed] [Google Scholar]
- De Carvalho-Niebel F, Timmers AC, Chabaud M, Defaux-Petras A, Barker DG (2002) The Nod factor-elicited annexin MtAnn1 is preferentially localised at the nuclear periphery in symbiotically activated root tissues of Medicago truncatula. Plant J 32: 343–352 [DOI] [PubMed] [Google Scholar]
- Den Herder G, Yoshida S, Antolín-Llovera M, Ried MK, Parniske M (2012) Lotus japonicus E3 ligase SEVEN IN ABSENTIA4 destabilizes the symbiosis receptor-like kinase SYMRK and negatively regulates rhizobial infection. Plant Cell 24: 1691–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbrosses GJ, Stougaard J (2011) Root nodulation: a paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 10: 348–358 [DOI] [PubMed] [Google Scholar]
- Ding Y, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, Harris JM, Oldroyd GE (2008) Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 20: 2681–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Marillonnet S (2011) Generation of families of construct variants using golden gate shuffling. Methods Mol Biol 729: 167–181 [DOI] [PubMed] [Google Scholar]
- Fang Y, Hirsch AM (1998) Studying early nodulin gene ENOD40 expression and induction by nodulation factor and cytokinin in transgenic alfalfa. Plant Physiol 116: 53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegmann J, Bono JJ (2015) Lipo-chitooligosaccharidic nodulation factors and their perception by plant receptors. Glycoconj J 32: 455–464 [DOI] [PubMed] [Google Scholar]
- Fliegmann J, Canova S, Lachaud C, Uhlenbroich S, Gasciolli V, Pichereaux C, Rossignol M, Rosenberg C, Cumener M, Pitorre D, et al. (2013) Lipo-chitooligosaccharidic symbiotic signals are recognized by LysM receptor-like kinase LYR3 in the legume Medicago truncatula. ACS Chem Biol 8: 1900–1906 [DOI] [PubMed] [Google Scholar]
- Fournier J, Teillet A, Chabaud M, Ivanov S, Genre A, Limpens E, de Carvalho-Niebel F, Barker DG (2015) Remodeling of the infection chamber before infection thread formation reveals a two-step mechanism for rhizobial entry into the host legume root hair. Plant Physiol 167: 1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen HJ, Xiao TT, Kulikova O, Wan X, Bisseling T, Scheres B, Heidstra R (2015) Root developmental programs shape the Medicago truncatula nodule meristem. Development 142: 2941–2950 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants. FEBS J 280: 681–693 [DOI] [PubMed] [Google Scholar]
- Gage DJ. (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68: 280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F (2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18: 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion B, Berrabah F, Ratet P, Stacey G (2015) Rhizobium-legume symbioses: the crucial role of plant immunity. Trends Plant Sci 20: 186–194 [DOI] [PubMed] [Google Scholar]
- Groth M, Takeda N, Perry J, Uchida H, Dräxl S, Brachmann A, Sato S, Tabata S, Kawaguchi M, Wang TL, et al. (2010) NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development. Plant Cell 22: 2509–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guefrachi I, Nagymihaly M, Pislariu CI, Van de Velde W, Ratet P, Mars M, Udvardi MK, Kondorosi E, Mergaert P, Alunni B (2014) Extreme specificity of NCR gene expression in Medicago truncatula. BMC Genomics 15: 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney CH, Riely BK, Tricoli DM, Cook DR, Ehrhardt DW, Long SR (2011) Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell 23: 2774–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann AB, Sandal N, Bek AS, Madsen LH, Jurkiewicz A, Nielsen MW, Tirichine L, Stougaard J (2011) Cytokinin induction of root nodule primordia in Lotus japonicus is regulated by a mechanism operating in the root cortex. Mol Plant Microbe Interact 24: 1385–1395 [DOI] [PubMed] [Google Scholar]
- Held M, Hou H, Miri M, Huynh C, Ross L, Hossain MS, Sato S, Tabata S, Perry J, Wang TL, et al. (2014) Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. Plant Cell 26: 678–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyl A, Brault M, Frugier F, Kuderova A, Lindner AC, Motyka V, Rashotte AM, Schwartzenberg KV, Vankova R, Schaller GE (2013) Nomenclature for members of the two-component signaling pathway of plants. Plant Physiol 161: 1063–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S, Kim J, Muñoz A, Heckmann AB, Downie JA, Oldroyd GE (2009) GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell 21: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA (2013) The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J Exp Bot 64: 5395–5409 [DOI] [PubMed] [Google Scholar]
- Jordá L, Coego A, Conejero V, Vera P (1999) A genomic cluster containing four differentially regulated subtilisin-like processing protease genes is in tomato plants. J Biol Chem 274: 2360–2365 [DOI] [PubMed] [Google Scholar]
- Journet EP, Pichon M, Dedieu A, de Billy F, Truchet G, Barker DG (1994) Rhizobium meliloti Nod factors elicit cell-specific transcription of the ENOD12 gene in transgenic alfalfa. Plant J 6: 241–249 [DOI] [PubMed] [Google Scholar]
- Journet EP, El-Gachtouli N, Vernoud V, de Billy F, Pichon M, Dedieu A, Arnould C, Morandi D, Barker DG, Gianinazzi-Pearson V (2001) Medicago truncatula ENOD11: a novel RPRP-encoding early nodulin gene expressed during mycorrhization in arbuscule-containing cells. Mol Plant Microbe Interact 14: 737–748 [DOI] [PubMed] [Google Scholar]
- Kaló P, Gleason C, Edwards A, Marsh J, Mitra RM, Hirsch S, Jakab J, Sims S, Long SR, Rogers J, et al. (2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308: 1786–1789 [DOI] [PubMed] [Google Scholar]
- Kamada-Nobusada T, Sakakibara H (2009) Molecular basis for cytokinin biosynthesis. Phytochemistry 70: 444–449 [DOI] [PubMed] [Google Scholar]
- Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, Miwa H, Downie JA, James EK, Felle HH, Haaning LL, et al. (2006) A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA 103: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp PD, Paley S, Romero P (2002) The Pathway Tools software. Bioinformatics (Suppl 1) 18: S225–S232 [DOI] [PubMed] [Google Scholar]
- Kawaharada Y, Kelly S, Nielsen MW, Hjuler CT, Gysel K, Muszyński A, Carlson RW, Thygesen MB, Sandal N, Asmussen MH, et al. (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523: 308–312 [DOI] [PubMed] [Google Scholar]
- Kevei Z, Lougnon G, Mergaert P, Horváth GV, Kereszt A, Jayaraman D, Zaman N, Marcel F, Regulski K, Kiss GB, et al. (2007) 3-Hydroxy-3-methylglutaryl coenzyme A reductase 1 interacts with NORK and is crucial for nodulation in Medicago truncatula. Plant Cell 19: 3974–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiala A, Laffont C, Emery RJ, Frugier F (2013) Bioactive cytokinins are selectively secreted by Sinorhizobium meliloti nodulating and nonnodulating strains. Mol Plant Microbe Interact 26: 1225–1231 [DOI] [PubMed] [Google Scholar]
- Kiss E, Oláh B, Kaló P, Morales M, Heckmann AB, Borbola A, Lózsa A, Kontár K, Middleton P, Downie JA, et al. (2009) LIN, a novel type of U-box/WD40 protein, controls early infection by rhizobia in legumes. Plant Physiol 151: 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner C, Winzer T, Pitzschke A, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Webb KJ, Szczyglowski K, Parniske M (2005) Seven Lotus japonicus genes required for transcriptional reprogramming of the root during fungal and bacterial symbiosis. Plant Cell 17: 2217–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D, Kang J, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song WY, et al. (2014) Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci USA 111: 7150–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix V, Cottret L, Thebault P, Sagot MF (2008) An introduction to metabolic networks and their structural analysis. IEEE/ACM Trans Comput Biol Bioinform 5: 594–617 [DOI] [PubMed] [Google Scholar]
- Laloum T, Baudin M, Frances L, Lepage A, Billault-Penneteau B, Cerri MR, Ariel F, Jardinaud MF, Gamas P, de Carvalho-Niebel F, et al. (2014) Two CCAAT-box-binding transcription factors redundantly regulate early steps of the legume-rhizobia endosymbiosis. Plant J 79: 757–768 [DOI] [PubMed] [Google Scholar]
- Laplaze L, Lucas M, Champion A (2015) Rhizobial root hair infection requires auxin signaling. Trends Plant Sci 20: 332–334 [DOI] [PubMed] [Google Scholar]
- Laporte P, Lepage A, Fournier J, Catrice O, Moreau S, Jardinaud MF, Mun JH, Larrainzar E, Cook DR, Gamas P, et al. (2014) The CCAAT box-binding transcription factor NF-YA1 controls rhizobial infection. J Exp Bot 65: 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrainzar E, Riely BK, Kim SC, Carrasquilla-Garcia N, Yu HJ, Hwang HJ, Oh M, Kim GB, Surendrarao AK, Chasman D, et al. (2015) Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between nodulation factor and ethylene signals. Plant Physiol 169: 233–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B, Timmers T, Mbengue M, Moreau S, Hervé C, Tóth K, Bittencourt-Silvestre J, Klaus D, Deslandes L, Godiard L, et al. (2010) A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc Natl Acad Sci USA 107: 2343–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léran S, Varala K, Boyer JC, Chiurazzi M, Crawford N, Daniel-Vedele F, David L, Dickstein R, Fernandez E, Forde B, et al. (2014) A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci 19: 5–9 [DOI] [PubMed] [Google Scholar]
- Libault M, Govindarajulu M, Berg RH, Ong YT, Puricelli K, Taylor CG, Xu D, Stacey G (2011) A dual-targeted soybean protein is involved in Bradyrhizobium japonicum infection of soybean root hair and cortical cells. Mol Plant Microbe Interact 24: 1051–1060 [DOI] [PubMed] [Google Scholar]
- Limpens E, Franken C, Smit P, Willemse J, Bisseling T, Geurts R (2003) LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302: 630–633 [DOI] [PubMed] [Google Scholar]
- Limpens E, van Zeijl A, Geurts R (2015) Lipochitooligosaccharides modulate plant host immunity to enable endosymbioses. Annu Rev Phytopathol 53: 311–334 [DOI] [PubMed] [Google Scholar]
- Liu CW, Breakspear A, Roy S, Murray JD (2015) Cytokinin responses counterpoint auxin signaling during rhizobial infection. Plant Signal Behav 10: e1019982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Miller SS, Graham M, Bucciarelli B, Catalano CM, Sherrier DJ, Samac DA, Ivashuta S, Fedorova M, Matsumoto P, et al. (2006) Recruitment of novel calcium-binding proteins for root nodule symbiosis in Medicago truncatula. Plant Physiol 141: 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J 38: 203–214 [DOI] [PubMed] [Google Scholar]
- Lohar DP, Sharopova N, Endre G, Peñuela S, Samac D, Town C, Silverstein KA, VandenBosch KA (2006) Transcript analysis of early nodulation events in Medicago truncatula. Plant Physiol 140: 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho AP, Zipfel C (2014) Plant PRRs and the activation of innate immune signaling. Mol Cell 54: 263–272 [DOI] [PubMed] [Google Scholar]
- Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, Sato S, Kaneko T, Tabata S, Sandal N, et al. (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425: 637–640 [DOI] [PubMed] [Google Scholar]
- Manthey K, Krajinski F, Hohnjec N, Firnhaber C, Pühler A, Perlick AM, Küster H (2004) Transcriptome profiling in root nodules and arbuscular mycorrhiza identifies a collection of novel genes induced during Medicago truncatula root endosymbioses. Mol Plant Microbe Interact 17: 1063–1077 [DOI] [PubMed] [Google Scholar]
- Maróti G, Downie JA, Kondorosi É (2015) Plant cysteine-rich peptides that inhibit pathogen growth and control rhizobial differentiation in legume nodules. Curr Opin Plant Biol 26: 57–63 [DOI] [PubMed] [Google Scholar]
- Marsh JF, Rakocevic A, Mitra RM, Brocard L, Sun J, Eschstruth A, Long SR, Schultze M, Ratet P, Oldroyd GE (2007) Medicago truncatula NIN is essential for rhizobial-independent nodule organogenesis induced by autoactive calcium/calmodulin-dependent protein kinase. Plant Physiol 144: 324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Charon C, Rolfe BG, Kondorosi A, Crespi M (2000) Temporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifolii inoculation or localized cytokinin addition. Mol Plant Microbe Interact 13: 617–628 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14: 23–34 [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Sakagami Y (2006) Peptide hormones in plants. Annu Rev Plant Biol 57: 649–674 [DOI] [PubMed] [Google Scholar]
- Mbengue M, Camut S, de Carvalho-Niebel F, Deslandes L, Froidure S, Klaus-Heisen D, Moreau S, Rivas S, Timmers T, Hervé C, et al. (2010) The Medicago truncatula E3 ubiquitin ligase PUB1 interacts with the LYK3 symbiotic receptor and negatively regulates infection and nodulation. Plant Cell 22: 3474–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton PH, Jakab J, Penmetsa RV, Starker CG, Doll J, Kalo P, Prabhu R, Marsh JF, Mitra RM, Kereszt A, Dudas B, Vandenbosch K, Long SR, Cook DR, Kiss GB, Oldroyd GE (2007) An ERF Transcription Factor in Medicago truncatula That Is Essential for Nod Factor Signal Transduction. Plant Cell 19: 1221–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella R, Franken C, van der Krogt GN, Bisseling T, Geurts R (2004) Use of the fluorescent timer DsRED-E5 as reporter to monitor dynamics of gene activity in plants. Plant Physiol 135: 1879–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Long SR (2004) Plant and bacterial symbiotic mutants define three transcriptionally distinct stages in the development of the Medicago truncatula/Sinorhizobium meliloti symbiosis. Plant Physiol 134: 595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra RM, Shaw SL, Long SR (2004) Six nonnodulating plant mutants defective for Nod factor-induced transcriptional changes associated with the legume-rhizobia symbiosis. Proc Natl Acad Sci USA 101: 10217–10222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier V, De Wever E, Vuylsteke M, Holsters M, Goormachtig S (2012a) Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling. Plant J 70: 367–376 [DOI] [PubMed] [Google Scholar]
- Mortier V, Holsters M, Goormachtig S (2012b) Never too many? How legumes control nodule numbers. Plant Cell Environ 35: 245–258 [DOI] [PubMed] [Google Scholar]
- Mortier V, Wasson A, Jaworek P, De Keyser A, Decroos M, Holsters M, Tarkowski P, Mathesius U, Goormachtig S (2014) Role of LONELY GUY genes in indeterminate nodulation on Medicago truncatula. New Phytol 202: 582–593 [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K (2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315: 101–104 [DOI] [PubMed] [Google Scholar]
- Murray JD, Muni RR, Torres-Jerez I, Tang Y, Allen S, Andriankaja M, Li G, Laxmi A, Cheng X, Wen J, et al. (2011) Vapyrin, a gene essential for intracellular progression of arbuscular mycorrhizal symbiosis, is also essential for infection by rhizobia in the nodule symbiosis of Medicago truncatula. Plant J 65: 244–252 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kaku H, Shimoda Y, Sugiyama A, Shimamura M, Takanashi K, Yazaki K, Aoki T, Shibuya N, Kouchi H (2011) From defense to symbiosis: limited alterations in the kinase domain of LysM receptor-like kinases are crucial for evolution of legume-Rhizobium symbiosis. Plant J 65: 169–180 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuteboom LW, Veth-Tello LM, Clijdesdale OR, Hooykaas PJ, van der Zaal BJ (1999) A novel subtilisin-like protease gene from Arabidopsis thaliana is expressed at sites of lateral root emergence. DNA Res 6: 13–19 [DOI] [PubMed] [Google Scholar]
- Ng JL, Hassan S, Truong TT, Hocart CH, Laffont C, Frugier F, Mathesius U (2015) Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 27: 2210–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Volkening JD, Rose CM, Venkateshwaran M, Westphall MS, Coon JJ, Ané JM, Sussman MR (2015) Potential regulatory phosphorylation sites in a Medicago truncatula plasma membrane proton pump implicated during early symbiotic signaling in roots. FEBS Lett 589: 2186–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GE. (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11: 252–263 [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Engstrom EM, Long SR (2001) Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 13: 1835–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GE, Murray JD, Poole PS, Downie JA (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 45: 119–144 [DOI] [PubMed] [Google Scholar]
- Op den Camp RH, De Mita S, Lillo A, Cao Q, Limpens E, Bisseling T, Geurts R (2011) A phylogenetic strategy based on a legume-specific whole genome duplication yields symbiotic cytokinin type-A response regulators. Plant Physiol 157: 2013–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR (1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275: 527–530 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Uribe P, Anderson J, Lichtenzveig J, Gish JC, Nam YW, Engstrom E, Xu K, Sckisel G, Pereira M, et al. (2008) The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J 55: 580–595 [DOI] [PubMed] [Google Scholar]
- Peterman TK, Ohol YM, McReynolds LJ, Luna EJ (2004) Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol 136: 3080–3094, discussion 3001–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plet J, Wasson A, Ariel F, Le Signor C, Baker D, Mathesius U, Crespi M, Frugier F (2011) MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J 65: 622–633 [DOI] [PubMed] [Google Scholar]
- Popp C, Ott T (2011) Regulation of signal transduction and bacterial infection during root nodule symbiosis. Curr Opin Plant Biol 14: 458–467 [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Grønlund M, Sato S, Nakamura Y, Tabata S, Sandal N, et al. (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425: 585–592 [DOI] [PubMed] [Google Scholar]
- Ramu SK, Peng HM, Cook DR (2002) Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol Plant Microbe Interact 15: 522–528 [DOI] [PubMed] [Google Scholar]
- Rau A, Gallopin M, Celeux G, Jaffrézic F (2013) Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 29: 2146–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DE, Heckmann AB, Novák O, Kelly S, Stougaard J (2016) CYTOKININ OXIDASE/DEHYDROGENASE3 Maintains Cytokinin Homeostasis during Root and Nodule Development in Lotus japonicus. Plant Physiol 170: 1060–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival P, de Billy F, Bono JJ, Gough C, Rosenberg C, Bensmihen S (2012) Epidermal and cortical roles of NFP and DMI3 in coordinating early steps of nodulation in Medicago truncatula. Development 139: 3383–3391 [DOI] [PubMed] [Google Scholar]
- Rose CM, Venkateshwaran M, Volkening JD, Grimsrud PA, Maeda J, Bailey DJ, Park K, Howes-Podoll M, den Os D, Yeun LH, et al. (2012) Rapid phosphoproteomic and transcriptomic changes in the rhizobia-legume symbiosis. Mol Cell Proteomics 11: 724–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B, Rodde N, Jardinaud MF, Timmers T, Sauviac L, Cottret L, Carrère S, Sallet E, Courcelle E, Moreau S, et al. (2014) An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to RNA sequencing. Plant J 77: 817–837 [DOI] [PubMed] [Google Scholar]
- Sablowski R, Carnier Dornelas M (2014) Interplay between cell growth and cell cycle in plants. J Exp Bot 65: 2703–2714 [DOI] [PubMed] [Google Scholar]
- Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, Asamizu E, Sato S, Tabata S, Imaizumi-Anraku H, Umehara Y, et al. (2007) NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19: 610–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57: 431–449 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Suzaki T, Soyano T, Kojima M, Sakakibara H, Kawaguchi M (2014) Shoot-derived cytokinins systemically regulate root nodulation. Nat Commun 5: 4983. [DOI] [PubMed] [Google Scholar]
- Schaller A, Stintzi A, Graff L (2012) Subtilases: versatile tools for protein turnover, plant development, and interactions with the environment. Physiol Plant 145: 52–66 [DOI] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195 [DOI] [PubMed] [Google Scholar]
- Schwessinger B, Ronald PC (2012) Plant innate immunity: perception of conserved microbial signatures. Annu Rev Plant Biol 63: 451–482 [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL, Long SR (2003) Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol 132: 2196–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K, Nomura M, Tajima S, Kouchi H (2006) LjnsRING, a novel RING finger protein, is required for symbiotic interactions between Mesorhizobium loti and Lotus japonicus. Plant Cell Physiol 47: 1572–1581 [DOI] [PubMed] [Google Scholar]
- Singh S, Katzer K, Lambert J, Cerri M, Parniske M (2014) CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15: 139–152 [DOI] [PubMed] [Google Scholar]
- Smit P, Limpens E, Geurts R, Fedorova E, Dolgikh E, Gough C, Bisseling T (2007) Medicago LYK3, an entry receptor in rhizobial nodulation factor signaling. Plant Physiol 145: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit P, Raedts J, Portyanko V, Debellé F, Gough C, Bisseling T, Geurts R (2005) NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308: 1789–1791 [DOI] [PubMed] [Google Scholar]
- Soyano T, Hirakawa H, Sato S, Hayashi M, Kawaguchi M (2014) Nodule inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci USA 111: 14607–14612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Kouchi H, Hirota A, Hayashi M (2013) Nodule inception directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genet 9: e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Cardoza V, Mitchell DM, Bright L, Oldroyd G, Harris JM (2006) Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J 46: 961–970 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Akune M, Kogiso M, Imagama Y, Osuki K, Uchiumi T, Higashi S, Han SY, Yoshida S, Asami T, et al. (2004) Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol 45: 914–922 [DOI] [PubMed] [Google Scholar]
- Terpolilli JJ, O’Hara GW, Tiwari RP, Dilworth MJ, Howieson JG (2008) The model legume Medicago truncatula A17 is poorly matched for N2 fixation with the sequenced microsymbiont Sinorhizobium meliloti 1021. New Phytol 179: 62–66 [DOI] [PubMed] [Google Scholar]
- Tian Y, Liu W, Cai J, Zhang LY, Wong KB, Feddermann N, Boller T, Xie ZP, Staehelin C (2013) The nodulation factor hydrolase of Medicago truncatula: characterization of an enzyme specifically cleaving rhizobial nodulation signals. Plant Physiol 163: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J (2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315: 104–107 [DOI] [PubMed] [Google Scholar]
- Trujillo M, Ichimura K, Casais C, Shirasu K (2008) Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr Biol 18: 1396–1401 [DOI] [PubMed] [Google Scholar]
- van Zeijl A, Op den Camp RH, Deinum EE, Charnikhova T, Franssen H, Op den Camp HJ, Bouwmeester H, Kohlen W, Bisseling T, Geurts R (2015) Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol Plant 8: 1213–1226 [DOI] [PubMed] [Google Scholar]
- Venkateshwaran M, Cosme A, Han L, Banba M, Satyshur KA, Schleiff E, Parniske M, Imaizumi-Anraku H, Ané JM (2012) The recent evolution of a symbiotic ion channel in the legume family altered ion conductance and improved functionality in calcium signaling. Plant Cell 24: 2528–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshwaran M, Jayaraman D, Chabaud M, Genre A, Balloon AJ, Maeda J, Forshey K, den Os D, Kwiecien NW, Coon JJ, et al. (2015) A role for the mevalonate pathway in early plant symbiotic signaling. Proc Natl Acad Sci USA 112: 9781–9786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernié T, Kim J, Frances L, Ding Y, Sun J, Guan D, Niebel A, Gifford ML, de Carvalho-Niebel F, Oldroyd GE (2015) The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 27: 3410–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernié T, Moreau S, de Billy F, Plet J, Combier JP, Rogers C, Oldroyd G, Frugier F, Niebel A, Gamas P (2008) EFD is an ERF transcription factor involved in the control of nodule number and differentiation in Medicago truncatula. Plant Cell 20: 2696–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Horton AC, Yang Z, Nielsen E (2003) Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol 131: 1191–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinardell JM, Fedorova E, Cebolla A, Kevei Z, Horvath G, Kelemen Z, Tarayre S, Roudier F, Mergaert P, Kondorosi A, et al. (2003) Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15: 2093–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wittgenstein NJ, Le CH, Hawkins BJ, Ehlting J (2014) Evolutionary classification of ammonium, nitrate, and peptide transporters in land plants. BMC Evol Biol 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yu H, Zhang Z, Yu L, Xu X, Hong Z, Luo L (2015) Phytosulfokine is involved in positive regulation of Lotus japonicus nodulation. Mol Plant Microbe Interact 28: 847–855 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98: 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. (2012) JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 24: 482–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao TT, Schilderink S, Moling S, Deinum EE, Kondorosi E, Franssen H, Kulikova O, Niebel A, Bisseling T (2014) Fate map of Medicago truncatula root nodules. Development 141: 3517–3528 [DOI] [PubMed] [Google Scholar]
- Xie F, Murray JD, Kim J, Heckmann AB, Edwards A, Oldroyd GE, Downie JA (2012) Legume pectate lyase required for root infection by rhizobia. Proc Natl Acad Sci USA 109: 633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoro E, Suzaki T, Toyokura K, Miyazawa H, Fukaki H, Kawaguchi M (2014) A positive regulator of nodule organogenesis, NODULE INCEPTION, acts as a negative regulator of rhizobial infection in Lotus japonicus. Plant Physiol 165: 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Zhu H, Gou H, Fu W, Liu L, Chen T, Ke D, Kang H, Xie Q, Hong Z, et al. (2012) A ubiquitin ligase of symbiosis receptor kinase involved in nodule organogenesis. Plant Physiol 160: 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher E, Tavor-Deslex D, Lituiev D, Enkerli K, Tarr PT, Müller B (2013) A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol 161: 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]