The significance and often centrality of reactive oxygen species (ROS)- and redox-related signaling are now established for most processes in plant development and acclimation. Each cell possesses a redox regulatory network whose state is adjusted by ROS and virtually controls all processes such as gene expression and translation, metabolism, and turnover. Recent years have witnessed significant advancements in understanding the cross talk between organelles in orchestrating the cellular redox state temporally and spatially. ROS function as oxidants of proteins altering their function and of lipids releasing signal-active compounds. This Focus Issue combines 18 publications that either summarize recent advances in the format of topical Focus Reviews or provide novel insight into ROS-associated processes. Our most detailed knowledge of ROS functions still comes from work with photosynthesizing leaves; therefore, the majority of reports in this special issue focus on leaf processes. Other contributions address ROS-dependent regulation of flower senescence (Rogers and Munné-Bosch, 2016), polar growth of pollen and root hairs (Mangano et al., 2016), and other root processes, including root architecture formation (Evans et al., 2016; Liu et al., 2016).
In vivo ROS imaging in cells and tissues has contributed significantly to many of the recent advances in understanding ROS signaling. Historically, 3,3′-diaminobenzidine for H2O2 and nitroblue tetrazolium for O2•− were used as the main workhorses for in situ imaging. Both dyes provided preliminary indications for ROS accumulation, but in most cases, more sensitive and less invasive probes should be used nowadays. Thus, the majority of studies presented in this Focus Issue employed fluorescein-based probes such as the 1O2 sensory fluorescein anthracene Singlet Oxygen Sensor Green (Koh et al., 2016) or the H2O2 probe OxyBurst Green (2′,7′-dichlorodihydrofluorescein diacetate; Evans et al., 2016; Zhang et al., 2016). These newer probes offer sensitive and more reliable readouts but are difficult to apply to some tissues like leaves, and their specificity is still disputed (Winterbourn, 2014). Genetically encoded sensors such as the ratiometric HyPer and HyPerRed were also used for ROS and redox detection in previous studies (Chiu et al., 2014; Ermakova et al., 2014). A major advantage for future plant ROS research would be to standardize protocols for in vivo cell imaging. Standardization would enable comparing ROS images and measurements between different studies and different labs.
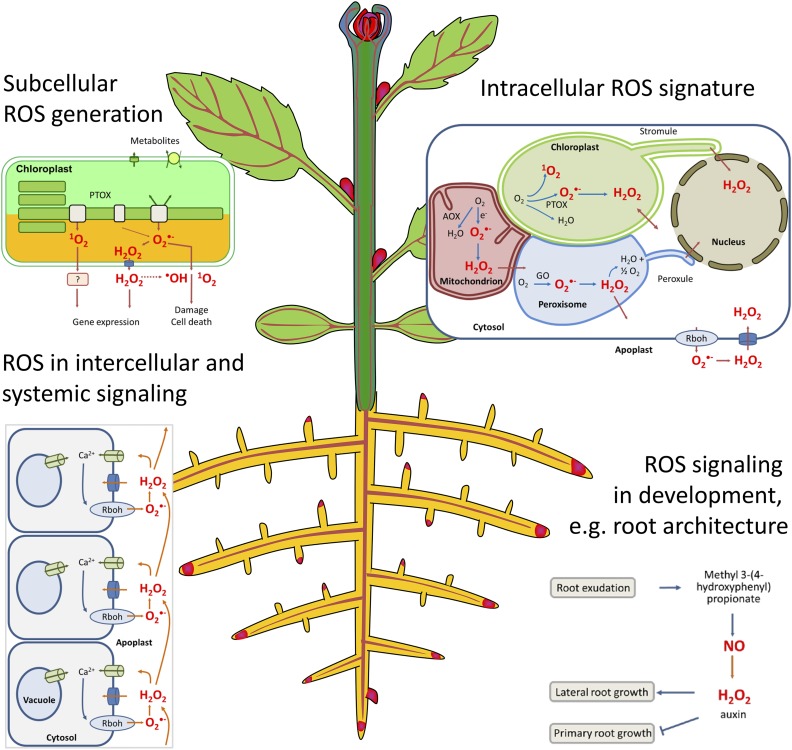
ROS are conditionally generated in various subcellular compartments and cellular environments (Fig. 1). The spatial and temporal pattern of ROS in combination with their reaction specificity is exploited for specific sensing and signaling in the cell. Chloroplasts (Dietz et al., 2016; Takagi et al., 2016), mitochondria (Huang et al., 2016), peroxisomes (Kerchev et al., 2016; Rodriguez-Serrano et al., 2016), and plasma membrane-associated NADPH oxidases (Respiratory burst oxidase homologs, RBOH; Gilroy et al., 2016) and the ROS-dependent dynamics of organellar shape are addressed in this Focus Issue.
Figure 1.
Some of the multiple regulatory roles of ROS in plants addressed in this Focus Issue. Subcellular sites of ROS generation, e.g. in the chloroplast or mitochondrion, determine processes such as gene expression, cellular damage, and cell death. Progress has been made in understanding intracellular ROS transfer; formation of protrusions such as stromules, peroxules, and matrixules; and the ROS network of cells. ROS play a profound role in intercellular and long-distance communication in plants. In addition to their roles in environmental acclimation, ROS are receiving increasing awareness for their function in developmental processes such as the regulation of root architecture, polar growth, and organ senescence. Abbreviations not defined in the text: AOX, alternative oxidase; GO, glycolate oxidase; PTOX, plastid terminal oxidase.
Redox interactions between intracellular compartments are key to stress responses and developmental processes. Organelles such as chloroplasts, mitochondria, and peroxisomes are powerful generators of ROS and other redox signals through core processes such as photosynthesis, photorespiration, and respiration. Coordination of gene expression between the three genomes of the plant cell requires monitoring of chloroplast and mitochondrial status, to allow appropriate retrograde signaling to the nucleus (Kleine and Leister, 2016). In this issue, the latest developments in understanding ROS and redox signals originating in chloroplasts and mitochondria are discussed by Dietz et al. (2016) and Huang et al. (2016). An outstanding question is how such signals are transmitted between compartments. This question is discussed by Noctor and Foyer (2016) in light of recent advances such as the identification of chloroplast envelope ascorbate transporters (Miyaji et al., 2015) and the roles of stromules and other organellar extensions in ensuring communication from redox-active locations (Caplan et al., 2015; Rodriguez-Serrano et al., 2016).
A powerful and complex antioxidant system means that, despite their high capacity for ROS production, many intracellular compartments are generally much more reduced than the apoplast. However, plentiful reductant not only favors ROS removal but also can promote ROS production (Foyer and Noctor, 2016). Consequently, there is a delicate balance between the reductant-dependent production of ROS and the metabolism of these oxidizing compounds. As discussed in this issue, our knowledge of ROS concentrations at specific locations is still very incomplete (Noctor and Foyer, 2016). It may be that ROS accumulation in many intracellular compartments is less marked than in the apoplast, with enhanced ROS production being largely sensed by secondary effects on the status of antioxidants. Examples in plants are the oxidation products that are generated by the reaction of carotenoids with singlet oxygen (Ramel et al., 2012). Compounds such as ascorbate and glutathione are more than simple antioxidants, not least because they consist of both reducing and oxidizing forms. Oxidation-triggered adjustments in compounds such as glutathione may be important in ROS signaling (Han et al., 2013). This underscores the complexity of ROS and redox homeostasis inside the cell, a view reinforced by the finding that accepted antioxidant enzymes such as monodehydroascorbate reductase may have pro-oxidant roles under some conditions (Johnston et al., 2015). Nevertheless, there is communication between intracellular and apoplastic ROS pools, notably through certain aquaporins. In this issue, Tian et al. (2016) describe a role for an Arabidopsis (Arabidopsis thaliana) aquaporin (AtPIP1;4) in importing extracellular H2O2 into the cytoplasm and in activating immune responses induced by bacteria.
ROS generating systems in the various subcellular sites are of utmost importance for the adjustment of the thiol redox network and are intensively covered in the literature. However, often there exists a lack of understanding of the involved ROS-sensitive switches and their regulation by electron donors and electron acceptors, which may be ROS by immediate reaction or redox-sensitive mediators. Among the most sensitive targets for peroxides are thiol peroxidases like glutathione peroxidase and peroxiredoxins. Oxidized peroxiredoxins oxidize redox transmitters such as thioredoxins, which in turn oxidize target proteins. In addition, they may serve as proximity-based oxidase and redox-dependent interaction partners (Dietz, 2016). Highly sensitive, untargeted or targeted, mass spectrometry combined with flexible use of blocking, labeling, and detection represent important steps forward for dissecting the dynamic and early modifications of redox proteins (Jones and Sies, 2015).
Recent years have seen an increase in the number of studies describing the regulation of several different biological processes at the level of protein-protein interactions linking ROS with metabolic and environmental responses. Maintaining the balance between labile iron and ROS is critical for the growth and health of plants preventing the formation of the highly toxic hydroxyl radicals. A recent study explored the link between the ROS-response ZAT12 zinc finger protein and iron regulation in cells. Thus, Le et al. (2016) reported that ZAT12 interacts with and suppresses the function of a central regulator of iron deficiency responses, the basic helix-loop-helix transcription factor FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR. When ZAT12 is up-regulated in response to ROS accumulation, it therefore suppresses iron uptake and prevents the risk of hydroxyl radical formation. Another example of an important biological regulatory circuit was reported by Adachi et al. (2015). These authors identified a W-box in the promoter of tobacco (Nicotiana tabacum) RBOH, as well as a WRKY transcription factor that is phosphorylated by MITOGEN-ACTIVATED PROTEIN KINASE (MAPK), linking MAPK phosphorylation events in response to pathogen recognition with accumulation of RBOH protein. Several members of the FAR-RED-IMPAIRED RESPONSE1-related sequence family of transcription factors were also recently implicated in integrating ROS with different developmental and environmental responses (Wang and Wang, 2015). Additional ROS-response regulatory proteins identified in recent years include the APETALA2/ethylene response transcription factor REDOX RESPONSIVE TRANSCRIPTION FACTOR1 that is regulated by different WRKYs (Matsuo et al., 2015), different members of the NAC family of transcription factors (e.g. Fang et al., 2015; Chen et al., 2016; Zhu et al., 2016), and different zinc-finger proteins such OXIDATIVE STRESS2 (He et al., 2016).
The previous Focus Issue on ROS that appeared in Plant Physiology integrated different transcriptome studies describing the response of plants to ROS stress (Gadjev et al., 2006). At least two new efforts have recently been made to follow up on this analysis and identify transcript expression signatures that define the response of plants to different types of ROS and their production site (Vaahtera et al., 2014; Willems et al., 2016). These studies refine our understanding of regulatory networks activated in response to the accumulation of ROS in different cellular compartments and cell types. In addition to the accumulation of H2O2 and O2•− in different cellular compartments, recent years have seen the appearance of studies describing an important role for singlet oxygen in mediating responses to different cellular and subcellular signals (e.g. Mor et al., 2014). These studies highlight the importance of different types of ROS and the integration of signals generated by different types of ROS within cells.
ROS have been implicated in generating and mediating whole-plant systemic signals in response to biotic or abiotic stresses (Gilroy et al., 2016). ROS and calcium waves constitute important components of rapid systemic signaling in plants (e.g. Suzuki et al., 2013; Choi et al., 2016; Evans et al., 2016; Carmody et al., 2016). In addition to RBOHs, which play a key role in mediating systemic signaling, a role for xanthine dehydrogenase was recently proposed in this response (Ma et al., 2016). In addition, growing evidence point to a possible role for nitric oxide and abscisic acid in mediating systemic responses to pathogens and abiotic stress (e.g. Wendehenne et al., 2014; Mittler and Blumwald, 2015), and a new report by Carmody et al., (2016) in this Focus Issue proposes a role for singlet oxygen as an initiator of systemic ROS signals. The growing interest in systemic signaling mechanisms and their potential to enhance crop tolerance to biotic and abiotic stresses is likely to generate many more interesting studies in years to come.
RBOH-dependent O2•− production appears to be the most tightly regulated ROS-signaling hub directly linking ROS signaling with calcium signaling and protein phosphorylation events. Thus, it is not surprising that RBOH participates in many of the ROS-signaling pathways described in this Focus Issue. RBOH activation is an early event in stomatal closure (Sierla et al., 2016). In addition, RBOH controls systemic signaling waves between the different plant organs in response to light and salinity stress (Evans et al., 2016; Gilroy et al., 2016). RBOH-dependent release of ROS was also found to modulate fiber growth in cotton (Gossypium hirsutum; Zhang et al., 2016) and peroxule outgrowth from peroxisomes (Rodriguez-Serrano et al., 2016). Furthermore, the meta-analysis of ROS-linked transcriptomes by Willems et al. (2016) identified AtRBOHF as central component in ROS-dependent regulation. These findings, together with earlier results, as summarized e.g. by Baxter et al. (2014), define RBOH as a central hub in the cellular ROS-signaling network. Linked to RBOH function, MAP kinase pathways integrate ROS signals orchestrating a cellular response to abiotic and biotic stress, as well as control hormone-signaling pathways and stomatal closure (Sierla et al., 2016) and modulate cell-to-cell signal propagation in local and systemic signaling (Evans et al., 2016; Gilroy et al., 2016). MAP kinase pathways participate in retrograde signaling from the chloroplast to the nucleus (Vogel et al., 2014; Dietz et al., 2016). Thus, MAP kinase pathways are of fundamental and far-reaching significance in converting ROS signals into protein phosphorylation.
Footnotes
Articles can be viewed without a subscription.
References
- Adachi H, Nakano T, Miyagawa N, Ishihama N, Yoshioka M, Katou Y, Yaeno T, Shirasu K, Yoshioka H (2015) WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell 27: 2645–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65: 1229–1240 [DOI] [PubMed] [Google Scholar]
- Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody M, Crisp PA, d’Alessandro S, Ganguly D, Gordon M, Havaux M, Albrecht-Borth V, Pogson BJ (2016) Uncoupling high light responses from singlet oxygen retrograde signaling and spatial-temporal systemic acquired acclimation in Arabidopsis. Plant Physiol 171: 1734–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SP, Lin IW, Chen X, Huang YH, Chang HC, Lo HS, Lu HH, Yeh KW (2016) Sweet potato NAC transcription factor, IbNAC1, upregulates sporamin gene expression by binding the SWRE motif against mechanical wounding and herbivore attack. Plant J 86: 234–248 [DOI] [PubMed] [Google Scholar]
- Chiu WK, Towheed A, Palladino MJ (2014) Genetically encoded redox sensors. Methods Enzymol 542: 263–287 [DOI] [PubMed] [Google Scholar]
- Choi WG, Hilleary R, Swanson SJ, Kim SH, Gilroy S (2016) Rapid, long-distance electrical and calcium signaling in plants. Annu Rev Plant Biol 67: 287–307 [DOI] [PubMed] [Google Scholar]
- Dietz KJ. (2016) Thiol-based peroxidases and ascorbate peroxidases: why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol Cells 39: 20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Turkan I, Krieger-Liszkay A (2016) Redox- and reactive oxygen species-dependent signaling in and from the photosynthesizing chloroplast. Plant Physiol 171: 1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova YG, Bilan DS, Matlashov ME, Mishina NM, Markvicheva KN, Subach OM, Subach FV, Bogeski I, Hoth M, Enikolopov G, et al. (2014) Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat Commun 5: 5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Choi WG, Gilroy S, Morris R (2016) A ROS-assisted calcium wave dependent on AtRBOHD and TPC1 propagates the systemic response to salt stress in Arabidopsis roots. Plant Physiol 171: 1771–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Liao K, Du H, Xu Y, Song H, Li X, Xiong L (2015) A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J Exp Bot 66: 6803–6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2016) Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ 39: 951–964 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171: 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G (2013) Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H(2)O(2) to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal 18: 2106–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Ma X, Li Z, Jiao Z, Li Y, Ow DW (2016) Maize OXIDATIVE STRESS2 homologs enhance cadmium tolerance in Arabidopsis through activation of a putative SAM-dependent methyltransferase gene. Plant Physiol 171: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Van Aken O, Schwarzländer M, Belt K, Millar AH (2016) The roles of mitochondrial reactive oxygen species in cellular signaling and stress responses in plants. Plant Physiol 171: 1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston EJ, Rylott EL, Beynon E, Lorenz A, Chechik V, Bruce NC (2015) Monodehydroascorbate reductase mediates TNT toxicity in plants. Science 349: 1072–1075 [DOI] [PubMed] [Google Scholar]
- Jones DP, Sies H (2015) The redox code. Antioxid Redox Signal 23: 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchev P, Waszczak C, Lewandowska A, Willems P, Shapiguzov A, Li Z, Alseekh S, Mühlenbock P, Hoebrichts F, Huang JJ, et al. (2016) Lack of GLYCOLATE OXIDASE1, but not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol 171: 1704–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T, Leister D (2016) Retrograde signaling: organelles go networking. Biochim Biophys Acta 1857: 1313–1325 [DOI] [PubMed] [Google Scholar]
- Koh E, Carmieli R, Mor A, Fluhr R (2016) Singlet oxygen-induced membrane disruption and serpin-protease balance in vacuolar-driven cell death in Arabidopsis. Plant Physiol 171: 1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le CT, Brumbarova T, Ivanov R, Stoof C, Weber E, Mohrbacher J, Fink-Straube C, Bauer P (2016) ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) linking iron deficiency and oxidative stress responses. Plant Physiol 170: 540–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang R, Zhang P, Chen Q, Luo I, Zhu Y, Xu J (2016) The nitrification inhibitor methyl 3-(4-hydroxyphenyl)propionate modulates root development by interfering with auxin signaling via the NO/ROS pathway in Arabidopsis. Plant Physiol 171: 1686–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang WM, Bittner F, Schmidt N, Berkey R, Zhang L, King H, Zhang Y, Feng J, Wen Y, et al. (2016) Dual and opposing roles of xanthine dehydrogenase in defense-associated reactive oxygen species metabolism in Arabidopsis. Plant Cell http://dx.doi.org/tpc.00880.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano S, Denita Juárez S, Estevez JM (2016) ROS regulation of polar growth in plant cells. Plant Physiol 171: 1593–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo M, Johnson JM, Hieno A, Tokizawa M, Nomoto M, Tada Y, Godfrey R, Obokata J, Sherameti I, Yamamoto YY, et al. (2015) High REDOX RESPONSIVE TRANSCRIPTION FACTOR1 levels result in accumulation of reactive oxygen species in Arabidopsis thaliana shoots and roots. Mol Plant 8: 1253–1273 [DOI] [PubMed] [Google Scholar]
- Mittler R, Blumwald E (2015) The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27: 64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T, Kuromori T, Takeuchi Y, Yamaji N, Yokosho K, Shimazawa A, Sugimoto E, Omote H, Ma JF, Shinozaki K, et al. (2015) AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat Commun 6: 5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Koh E, Weiner L, Rosenwasser S, Sibony-Benyamini H, Fluhr R (2014) Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol 165: 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (2016) Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol 171: 1581–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA 109: 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Sanz-Fernández M, Hu J, Sandalio LM (2016) Peroxisomes extend peroxules in a fast response to stress via a reactive oxygen species-mediated induction of the peroxin PEX11a. Plant Physiol 171: 1665–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H, Munné-Bosch S (2016) Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: similar but different. Plant Physiol 171: 1560–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierla M, Waszczak C, Vahisalu T, Kangasjärvi J (2016) Reactive oxygen species in the regulation of stomatal movements. Plant Physiol 171: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C (2016) Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol 171: 1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H (2016) Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol 171: 1635–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaahtera L, Brosché M, Wrzaczek M, Kangasjärvi J (2014) Specificity in ROS signaling and transcript signatures. Antioxid Redox Signal 21: 1422–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel MO, Moore M, König K, Pecher P, Alsharafa K, Lee J, Dietz KJ (2014) Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell 26: 1151–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang H (2015) Multifaceted roles of FHY3 and FAR1 in light signaling and beyond. Trends Plant Sci 20: 453–461 [DOI] [PubMed] [Google Scholar]
- Willems P, Mhamdi A, Stael S, Storme V, Kerchev P, Noctor G, Gevaert K, Van Breusegem F (2016) The ROS wheel: refining ROS transcriptional footprints in Arabidopsis. Plant Physiol 171: 1720–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Gao QM, Kachroo A, Kachroo P (2014) Free radical-mediated systemic immunity in plants. Curr Opin Plant Biol 20: 127–134 [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. (2014) The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 1840: 730–738 [DOI] [PubMed] [Google Scholar]
- Zhang F, Jin X, Wang L, Li S, Wu S, Cheng C, Zhang T, Guo W (2016) GhFAnnxA affects fiber elongation and secondary cell wall biosynthesis associated with Ca2+ influx, ROS homeostasis, and actin filament reorganization. Plant Physiol 171: 1750–1770 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhu Y, Yan J, Liu W, Liu L, Sheng Y, Sun Y, Li Y, Scheller HV, Jiang M, Hou X, Ni L, Zhang A (2016) Phosphorylation of a NAC transcription factor by ZmCCaMK regulates abscisic acid-induced antioxidant defense in maize. Plant Physiol 171: 1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]