Abstract
Developmental coordination disorder (DCD) and attention-deficit hyperactivity disorder (ADHD) are highly comorbid neurodevelopmental disorders; however, the neural mechanisms of this comorbidity are poorly understood. Previous research has demonstrated that children with DCD and ADHD have altered brain region communication, particularly within the motor network. The structure and function of the motor network in a typically developing brain exhibits hemispheric dominance. It is plausible that functional deficits observed in children with DCD and ADHD are associated with neurodevelopmental alterations in within- and between-hemisphere motor network functional connection strength that disrupt this hemispheric dominance. We used resting-state functional magnetic resonance imaging to examine functional connections of the left and right primary and sensory motor (SM1) cortices in children with DCD, ADHD and DCD + ADHD, relative to typically developing children. Our findings revealed that children with DCD, ADHD and DCD + ADHD exhibit atypical within- and between-hemisphere functional connection strength between SM1 and regions of the basal ganglia, as well as the cerebellum. Our findings further support the assertion that development of atypical motor network connections represents common and distinct neural mechanisms underlying DCD and ADHD. In children with DCD and DCD + ADHD (but not ADHD), a significant correlation was observed between clinical assessment of motor function and the strength of functional connections between right SM1 and anterior cingulate cortex, supplementary motor area, and regions involved in visuospatial processing. This latter finding suggests that behavioral phenotypes associated with atypical motor network development differ between individuals with DCD and those with ADHD.
Keywords: Motor network, Developmental coordination disorder, Attention-deficit/hyperactivity disorder, Resting-state fMRI
Highlights
-
•
Resting-state fMRI was used to examine motor networks of children with DCD and ADHD.
-
•
DCD and ADHD exhibited atypical within- and between-hemisphere motor network connections.
-
•
Neuromuscular development was associated with functional connection strength in DCD, but not ADHD.
-
•
Resting-state fMRI can identify shared and distinct neural mechanisms that underlie ADHD and DCD.
1. Introduction
Developmental coordination disorder (DCD) is one of the most common neurodevelopmental disorders of childhood, affecting 5–6% of school-aged children (American Psychiatric Association, 2013, Blank, 2012). It is characterized by impairments in motor coordination that significantly interferes with activities of daily living, and also impacts academic productivity, prevocational and vocational activities, as well as leisure and play (American Psychiatric Association, 2013, Blank, 2012). The clinical presentation of DCD is diverse (Kaplan et al., 1998, Schoemaker et al., 2013, Vaivre-Douret, 2014, Visser, 2003), and up to 50% of children with DCD also meet diagnostic criteria for attention-deficit/hyperactivity disorder (ADHD) (Kadesjo and Gillberg, 1998, Pitcher et al., 2003). ADHD occurs in approximately 5% of children (Kadesjo and Gillberg, 2001, American Psychiatric Association, 2013) and is associated with age-inappropriate levels of inattention, hyperactivity and/or impulsivity (American Psychiatric Association, 2013). The etiology of the comorbidity of DCD and ADHD, however, is not well understood.
Children with DCD often exhibit functional deficits in cross-modal integration (i.e., integration of information from different sensory modalities), specifically during tasks that demand visual feedback for motor control (Wilson and McKenzie, 1998), internal/forward modeling (e.g., movement planning), gait and postural control, visual perception and motor coordination (Dewey et al., 2007, Wilson et al., 2013). Deficits in cross-modal integration have also been observed in children with ADHD (Hale et al., 2009). Studies of typical individuals and split-brain patients indicate that cross-modal integration requires successful communication between a number of brain regions, both within and between hemispheres (Compton et al., 2008, Sauerwein and Lassonde, 1997, Toro et al., 2008), especially as tasks become more complex (Mostofsky et al., 2006, Scholz et al., 2000, Solodkin et al., 2001, Weissman and Banich, 2000).
Neuroimaging studies support the contention that brain region communication is disrupted in children with DCD and ADHD. Diffusion tensor imaging (DTI) studies have implicated the corpus callosum (Langevin et al., 2014, Roessner et al., 2004, Valera et al., 2007), a structure responsible for inter-hemispheric communication, as well as the right forceps minor and the anterior corona radiate (Van Ewijk et al., 2012) in these disorders. Functional magnetic resonance imaging (fMRI) studies of children with DCD have demonstrated atypical activity within visuospatial regions during complex tracing tasks (Kashiwagi et al., 2009, Zwicker et al., 2010, Zwicker et al., 2011). In children with ADHD, fMRI studies have noted reduced activity of the right inferior frontal gyrus (IFG) during right-hand (i.e., left-hemisphere) response inhibition when performing a Go/No-Go task (Booth et al., 2005, Garrett et al., 2008, Rubia et al., 2005). Thus, it is important that the motor network exhibit appropriate within- and between-hemisphere connections.
In the typically developing brain, the structure and function of motor regions actually differs between hemispheres. Structurally, right-handed individuals have a deeper left central sulcus than left-handed individuals (Amunts et al., 2000). The left motor cortex in right-handed individuals also has greater neuropil volume, reflecting the number of dendrites, axons and synapses (Amunts et al., 1996). A DTI study demonstrated that the left corticospinal tract in infants is more structurally developed than the right (Dubois et al., 2009). Functionally, an fMRI study of right-handed adults demonstrated that the left motor cortex exhibits greater activation with both ipsilateral and contralateral movements compared to the right motor cortex (Kim et al., 1993). Whether these structural and functional differences are associated with disrupted within-hemisphere and/or between-hemisphere functional connections between cortical and subcortical regions of the motor network has not been established. Furthermore, this has not been explored in children with DCD and ADHD, and could represent an underlying mechanism for the functional deficits observed in these children.
Using resting-state fMRI, our recent study observed reduced functional connectivity (i.e., reduced temporal synchrony between distinct brain regions and an indicator of functional connection strength) between left motor cortex and structures of the basal ganglia, including the caudate, putamen and globus pallidus in children with DCD, ADHD, and DCD + ADHD, compared to typically developing children (McLeod et al., 2014). It is plausible these disruptions of functional connectivity observed in our previous study could also be associated with alterations in how cortical and subcortical regions of the motor network are connected within and between hemispheres. Thus, in the present study, we reexamined the data from the right-handed individuals of our previous study in order to investigate the hypothesis that within- and between-hemisphere functional connections of the motor network are altered in children with DCD, ADHD, and DCD + ADHD, when compared to typically developing children. Furthermore, we hypothesized that ADHD and DCD children would exhibit similar alterations in within- and between-hemisphere connections to support the comorbidity of these disorders, as well as alterations specific to DCD alone.
2. Methods
This study was conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. The institutional review board for the ethics of human research approved the study. Consent and verbal assent were obtained from parents and children, respectively, after study procedures were fully explained.
2.1. Participants and assessment
As described in our previous study (McLeod et al., 2014), participants were recruited from local schools and through community advertisements in locations such as hospitals and physician's offices in Calgary, Alberta, Canada. Children were classified as DCD if they met the following criteria, which are consistent with the Diagnostic and Statistical Manual IV-TR diagnostic criteria: they scored below the 16th percentile on The Movement Assessment Battery for Children – Second Edition (MABC-2; Henderson et al., 2007) (Criterion A), were reported by their parents as exhibiting motor difficulties that interfered significantly with daily functioning (as indicated on the Developmental Coordination Questionnaire (Wilson et al., 2000)) (Criterion B), did not evidence a visual impairment or other neurological/medical (e.g., epilepsy, cerebral plays, muscular dystrophy) condition that would affect movement and did not meet criteria for a diagnosis of Pervasive Developmental Disorder (Criterion C) and did not display an intellectual impairment as evidenced by performance on a standardized measure of cognitive function (Criterion D). Scores greater than the 5th percentile and less than the 16th percentile on the MABC-2 are associated with mild to moderate motor impairment, whereas scores less than the 5th percentile are associated with severe motor impairment. Children were classified as ADHD if they met diagnostic criteria on the Diagnostic Interview for Children and Adolescents-IV (Reich et al., 1997) or had a t-score above the 95th percentile on the Conners Parent Rating Scale-Revised (Conners et al., 1998) and were diagnosed by a physician as having ADHD based on DSM-IV criteria. Children meeting criteria for both DCD and ADHD were classified as DCD + ADHD. Children not meeting the criteria for DCD, ADHD or DCD + ADHD were assigned to the typically developing group. All children were required to be right handed. This assessment resulted in 19 ADHD alone children, 6 DCD alone children, 14 DCD + ADHD children and 21 typically developing children. These group sizes are less than our previous study, as we selected only right-handed children for the present analysis. Demographic and clinical characteristics of each group are summarized in Table 1.
Table 1.
Participant Characteristics. CPRSC-C/H = Connor's Parent Rating Scale Revised Children Cognitive Problems/Inattention (C), Hyperactivity (H); MABC-2 = Movement Assessment Battery for Children - Second Edition. * indicates a significant difference between patient group and controls (Student's t-test, p < 0.05, corrected for multiple comparisons, or Tukey HSD test). CPRSC was not available for one child with ADHD. All errors are reported as the standard deviation of the mean.
| Controls | ADHD | DCD | ADHD + DCD | |
|---|---|---|---|---|
| Age (years) | 11.0 ± 2.8 | 12.4 ± 3.1 | 13.0 ± 2.8 | 11.3 ± 3.8 |
| N (females) | 21 (11) | 19 (1)* | 6 (1)* | 14 (3)* |
| IQ | 111.7 ± 13.3 | 107.1 ± 11.0 | 108.3 ± 13.7 | 103.5 ± 16.5 |
| CPRSC-C | 51.9 ± 9.8 | 73.7 ± 8.0* | 50.0 ± 3.5 | 70.6 ± 12.6* |
| CPRSC-H | 50.7 ± 8.0 | 72.0 ± 14.5* | 49.3 ± 3.5 | 64.1 ± 14.5* |
| MABC-2 | 10.3 ± 2.2 | 9.5 ± 1.6 | 5.5 ± 1.9* | 4.1 ± 2.4* |
| NDI | 98.8 ± 17.1 | 92.5 ± 15.2 | 71.0 ± 8.2* | 62.1 ± 9.9* |
Exclusion criteria for all groups included a history of a diagnosed metabolic or genetic condition, epilepsy or other seizure disorder, cerebral palsy, psychiatric disorder other than ADHD, intellectual disability, autism spectrum disorder, fetal alcohol spectrum disorder, prematurity (born at < 36 weeks gestation), very low birth weight (< 1500 g), or severe traumatic brain injury. There were no group differences for age or IQ (see Table 1); however, the typically developing group had a significantly higher proportion of females compared to the other groups [χ(1, N = 60) = 13.1, p < 0.05]. Children on stimulant treatment for ADHD were asked to refrain from taking medication on the day of imaging. All children in the ADHD group, and all but one in the DCD + ADHD group were on stimulant medication.
As there is no gold standard for identifying DCD, in addition to the MABC-2, all participants were assessed using a second measure of motor skills, the McCarron Assessment of Neuromuscular Development (MAND; McCarron, 1997). The MAND is a standardized measure of fine and gross motor skills in individuals from 3 years into adulthood. It includes 10 items. The raw scores on each item are converted to a scaled score with a mean of 10 and standard deviation (SD) of 3. A Neurodevelopmental Index score (NDI) with a mean of 100 (SD = 15) is derived from the sum of the scaled scores. The MAND has been found to have good test-retest reliability and to be a valid measure of motor function in children (Caeyenberghs et al., 2009, Hands et al., 2013). NDI scores of each group are summarized in Table 1.
2.2. Image acquisition and analysis
Images were collected using a 3 Tesla GE MR scanner (Signa VH/i, GE Healthcare, Waukesha, WI) with an eight-channel phased-array radiofrequency (RF) head coil. Resting-state fMRI consisted of five minutes of a T2*-weighted gradient-recalled echo, echo planar imaging (EPI) sequence (TR/TE = 2000/30 ms, flip angle = 70°, matrix size 64 × 64, FOV = 220 mm × 220 mm, 4-mm slice thickness, 26 slices). Foam padding was fitted between the RF coil and the head at the temples and the forehead to restrict head movement. Participants were asked to look at a fixation cross at the center of a screen during imaging. T1-weighted images were obtained for anatomical registration of the fMRI data (multi-slice fast spoiled gradient echo; TR/TE = 200/2.5 ms, flip angle = 18°, matrix size = 128 × 128, FOV = 220 mm × 220 mm, 4-mm slice thickness, 40 slices).
Prior to statistical analysis, resting-state fMRI data underwent pre-processing using with the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl), which included scalp and skull removal using the Brain Extraction Tool (BET) (Smith, 2002), motion correction using MCFLIRT (Jenkinson et al., 2002), interleaved slice timing correction, temporal high pass filtering (> 0.01 Hz), spatial smoothing using a Gaussian kernel of 6 mm, and registration to the Montreal Neurological Institute standard template with resampling to 2 × 2 × 2 mm3. T1-weighted images were segmented into grey matter, white matter and cerebrospinal fluid using FMRIB's Automated Segmentation Tool (FAST) (Zhang et al., 2001). All participants' data were examined for head motion, and data were excluded from analysis if head movement exceeded 2 mm. In addition, given the potential for head motion in the ADHD/DCD groups, an analysis of variance (ANOVA) was performed to determine if there were any differences in the degree of head motion between groups.
Masks of left and right SM1 were manually drawn on each participant's anatomical images using the FSLView drawing tool, with the omega-shaped anatomical landmark of the motor cortex as a guide (Yousry et al., 1995). Masks were registered to the participant's native resting-state fMRI data space using FLIRT, and then reduced to a final volume of 100 contiguous voxels using the process of inter-voxel temporal cross-correlation, which identifies the region within the mask with the greatest homogeneity in terms of temporal synchrony (Golestani and Goodyear, 2011). Prior to analysis of data using this mask, the center of gravity of the anatomical location of the mask and the degree of cross-correlation within masks were each tested between subject groups using a Student's t-test. This analysis revealed there were no differences between groups; thus, there were no group biases in this method of mask generation.
The average time series of the voxels in left SM1 was computed from the preprocessed resting-state data to act as the regressor of interest in a time-series analysis using the general linear model (GLM), as implemented in FEAT v6.0 (FSL, http://www.fmrib.ox.ac.uk/fsl). The average time series from white matter and CSF were used as nuisance regressors. The GLM analysis generated a map of the functional connectivity of left SM1 with the rest of the brain. This procedure was repeated using the average time series of the voxels in right SM1 to give a map of the functional connectivity of right SM1 with the rest of the brain. A mixed effects GLM analysis was then performed using all participants' left SM1 and right SM1 functional connectivity maps. Maps were created for each group to show: 1) the average connectivity of left SM1 and right SM1, as well as brain voxels that overlapped in these two maps, and 2) brain voxels that exhibited a significant difference in their connectivity with left SM1 and right SM1. These maps were computed as Z-statistic images and corrected for multiple comparisons based on a family-wise error rate to a significance level of p = 0.05, using AlphaSim of the AFNI analysis package (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). Brain regions were anatomically identified by Brodmann's area and using the Harvard-Oxford cortical and subcortical structural atlases within FSLView (Lancaster et al., 2007, Lancaster et al., 2000). Because the proportion of males and females significantly differed between groups, we performed a preliminary image analysis, which revealed no group differences for sex. Thus, sex was not included as a factor in the main group analysis, which helped to maximize the available degrees of freedom.
2.3. Behavioural analysis
NDI scores were compared between groups by ANOVA. For groups exhibiting a significant difference from typically developing children, NDI scores were entered into a correlation analysis with functional connectivity (left SM1 and right SM1, separately) on a pixel-by-pixel basis, to identify brain regions whose strength of functional connections with SM1 significantly varied with NDI score.
3. Results
No datasets needed to be discarded due to excessive head motion during imaging. ANOVA revealed no statistical difference between groups in terms of maximum head motion (in mm) during imaging [F(3,56) = 1.47, p = 0.23], and thus all data sets were included in the analysis.
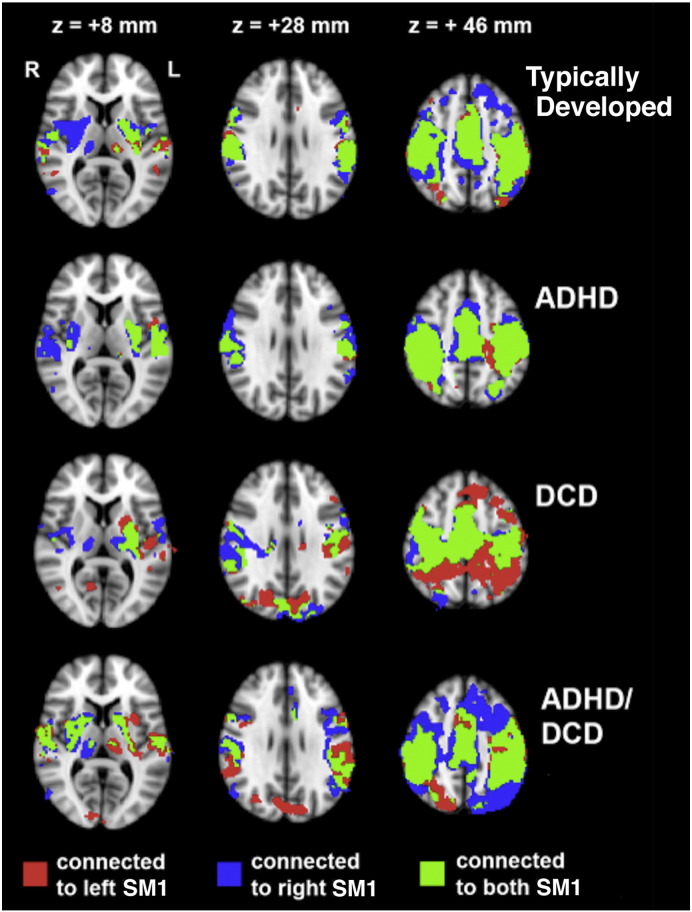
Group functional connectivity maps for left SM1 and right SM1, as well as overlapping voxels, are shown in Fig. 1. Significant functional connections with SM1 were observed for all groups and were consistent with those reported previously, including the contralateral and ipsilateral primary motor, somatosensory and premotor cortices, as well as the putamen, thalamus and cerebellum (Biswal et al., 1995, Deco and Corbetta, 2011). As expected, there was considerable overlap of the left and right SM1 connectivity maps (shown in green in Fig. 1) for all groups. Visual inspection suggests there were differences between the DCD, ADHD, DCD + ADHD, and typically developing groups in the degree of this overlap. These differences were tested statistically on a voxel-by-voxel basis and corrected for multiple comparisons, as described above.
Fig. 1.
Functional connectivity maps for each group demonstrating significant connections (p < 0.05) with left SM1 (red), right SM1 (blue) and to both left and right SM1 (green).
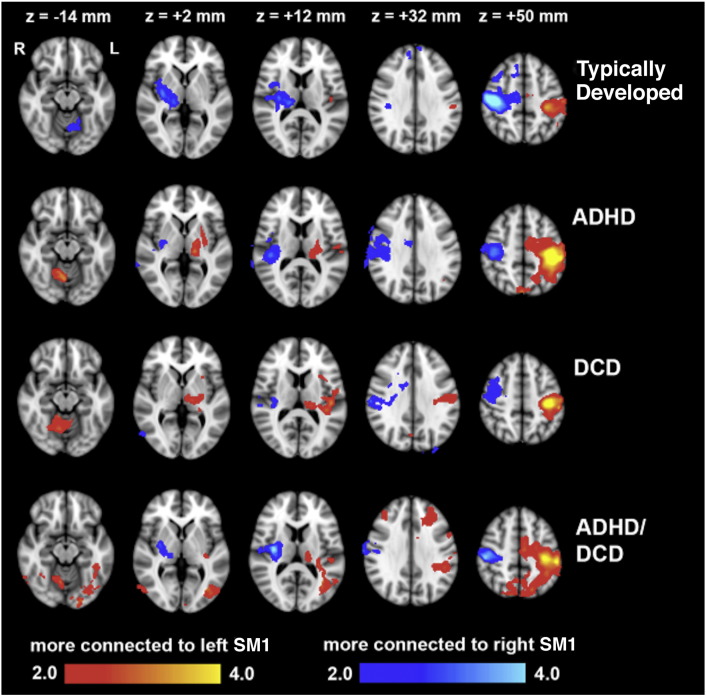
Brain regions whose functional connectivity with left SM1 significantly differed from that with right SM1 are shown in Fig. 2 and are listed in Table 2 and Table 3. For all groups, the left precentral and postcentral gryi and left posterior insula were more strongly connected with left SM1 than with right SM1, and the right precentral and postcentral gryi and right posterior insula were more strongly connected with right SM1 than with left SM1. In other words, the motor cortex of typically developing children possessed stronger within-hemisphere connections with other motor cortices and the posterior insula than between-hemisphere connections, and this was preserved in children with ADHD or DCD.
Fig. 2.
Brain regions exhibiting greater functional connectivity with left SM1 than right SM1 (red/yellow), and regions exhibiting greater functional connectivity with right SM1 than left SM1 (blue/light blue), for each group. Colors represent statistical significance, expressed as a Z-score. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Brain regions exhibiting significantly stronger functional connections with right SM1 than left SM1. Coordinates are given in mm of the MNI standard template brain atlas; BA = Brodmann's Area.
| Right SM1 > left SM1 | Brain region | Max Z-score | x | y | z | BA |
|---|---|---|---|---|---|---|
| Controls | Right precentral gyrus | 5.0 | 44 | − 16 | 58 | 4 |
| Right postcentral gyrus | 5.0 | 46 | − 24 | 52 | 2 | |
| Right posterior insula | 2.7 | 36 | − 14 | 12 | 13 | |
| Right thalamus | 3.3 | 14 | − 24 | 0 | − | |
| Right putamen | 3.1 | 32 | − 12 | − 2 | − | |
| Left cerebellum lobule V | 2.5 | − 6 | − 62 | − 14 | − | |
| ADHD | Right precentral gyrus | 3.7 | 40 | − 18 | 54 | 4 |
| Right postcentral Gyrus | 3.6 | 46 | − 20 | 46 | 2 | |
| Right Posterior insula | 2.7 | 40 | − 22 | 12 | 13 | |
| Right putamen | 2.3 | 30 | − 12 | 2 | − | |
| DCD | Right Precentral gyrus | 2.5 | 48 | − 8 | 48 | 4 |
| Right postcentral gyrus | 3.3 | 52 | − 20 | 40 | 2 | |
| Right posterior insula | 2.8 | 36 | − 20 | 14 | 13 | |
| ADHD + DCD | Right precentral gyrus | 4.3 | 42 | − 20 | 58 | 4 |
| Right postcentral gyrus | 4.1 | 48 | − 22 | 52 | 2 | |
| Right posterior insula | 2.7 | 40 | − 12 | 8 | 13 | |
| Right putamen | 2.5 | 28 | − 14 | 8 | − |
Table 3.
Brain regions exhibiting significantly stronger functional connections with left SM1 than right SM1. Coordinates are given in mm of the MNI standard template brain atlas; BA = Brodmann's Area.
| Left SM1 > right SM1 | Brain region | Max Z-score | x | y | z | BA |
|---|---|---|---|---|---|---|
| Controls | Left precentral gyrus | 3.8 | − 38 | − 22 | 60 | 4 |
| Left postcentral gyrus | 2.9 | − 46 | − 32 | 48 | 2 | |
| Left posterior insula | 2.9 | − 40 | − 24 | 18 | 13 | |
| ADHD | Left precentral gyrus | 4.9 | − 34 | − 22 | 58 | 4 |
| Left postcentral gyrus | 5.2 | − 44 | − 32 | 50 | 2 | |
| Left posterior insula | 2.6 | − 44 | − 20 | 14 | 13 | |
| Left thalamus | 3.3 | − 14 | − 26 | 6 | − | |
| Left putamen | 2.6 | − 28 | − 6 | 2 | − | |
| Right cerebellum lobule V | 3.3 | 8 | − 60 | − 12 | − | |
| DCD | Left precentral gyrus | 4.1 | − 38 | − 18 | 52 | 4 |
| Left postcentral gyrus | 4.6 | − 40 | − 28 | 50 | 2 | |
| Left posterior insula | 2.9 | − 42 | − 18 | 14 | 13 | |
| Left thalamus | 2.7 | − 10 | − 20 | 2 | − | |
| Right cerebellum lobule V | 3.1 | 10 | − 62 | − 14 | − | |
| Left precentral gyrus | 4.1 | − 36 | − 22 | 60 | 4 | |
| ADHD + DCD | Left postcentral gyrus | 4.4 | − 46 | − 28 | 52 | 2 |
| Left posterior insula | 3.7 | − 44 | − 24 | 18 | 13 | |
| Left thalamus | 2.3 | − 14 | − 26 | 10 | − | |
| Right cerebellum lobule V | 2.9 | 10 | − 62 | − 14 | − | |
| Left precuneus | 3.1 | − 18 | − 70 | 50 | 7 | |
| Right precuneus | 2.7 | 10 | − 74 | 50 | 7 | |
| Left middle frontal gyrus | 2.5 | − 32 | 36 | 30 | 9 | |
| Right middle frontal gyrus | 2.4 | 40 | 38 | 30 | 9 | |
| Left inferior lateral occipital cortex | 2.9 | − 46 | − 78 | 0 | 19 | |
| Right inferior lateral occipital cortex | 2.7 | 54 | − 72 | 0 | 19 |
In typically developing children, the right thalamus and left lobule V of the cerebellum were more strongly connected with right SM1 than with left SM1. This was not the case for children with ADHD or DCD. For these children, the left thalamus and right lobule V of the cerebellum were more strongly connected with left SM1 than with right SM1. Thus, the right motor cortex of typically developing children had stronger within-hemisphere connections with the thalamus and contralateral connections with the cerebellum. For children with ADHD or DCD, it is the left motor cortex that possessed these stronger connections.
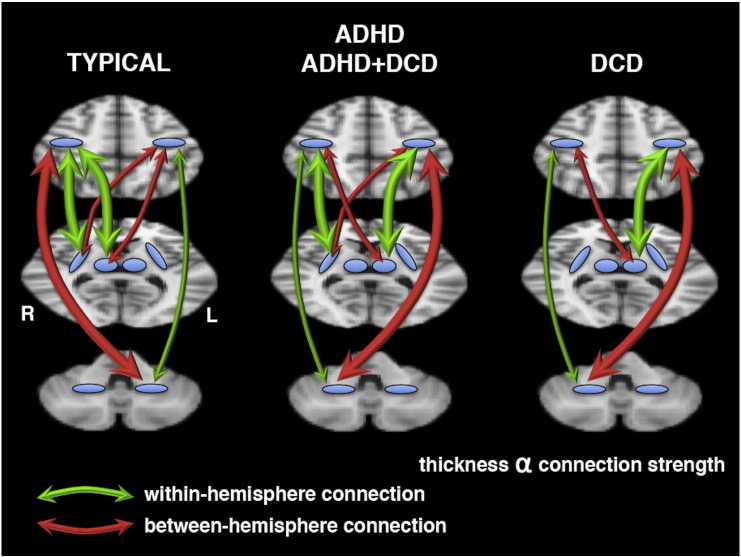
Our results also demonstrated altered functional connections specific to children with DCD. The right putamen was more strongly connected with right SM1 than left SM1 in typically developing children and children with ADHD. For children with DCD, however, the right putamen did not possess stronger within-hemisphere connections with motor cortex; the right putamen in these children was equally connected to right and left SM1. A summary highlighting the group differences in within- and between-hemisphere functional connections with SM1 is provided in Fig. 4.
Fig. 4.
A summary of the within-hemisphere (green) and between-hemisphere (red) functional connections with SM1, in typically developing children and children with ADHD, DCD, and DCD + ADHD. The thickness of the arrows is proportional to the strength of the functional connection. The top brain slice passes through SM1. The middle slice passes through the putamen (lateral blue regions) and the thalamus (medial blue regions). The bottom slice passes through the cerebellum. A region without arrows indicates that within- and between-hemisphere functional connections are of equal strength. See the Discussion section for a description of these differences between groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Children with DCD + ADHD exhibited additional alterations in motor network connectivity, including stronger connections of bilateral precuneus, middle frontal gyri and inferior lateral occipital cortices with the left SM1 than with the right SM1. These regions were equally connected to left and right SM1 in typically developing children and children with only ADHD or DCD,
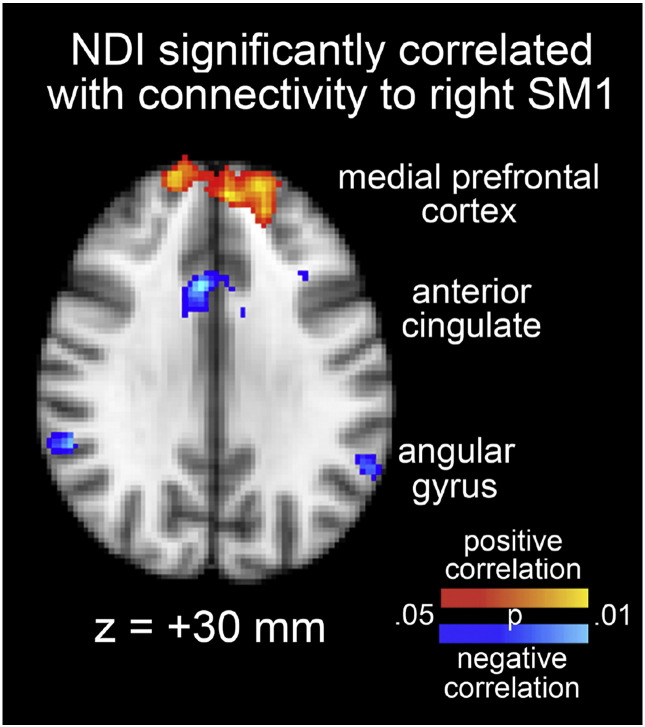
ANOVA of NDI scores revealed a significant difference between groups [F(3,56) = 21.87, p < 0.001]. Follow-up Tukey HSD tests demonstrated that the typically developing group significantly differed from the DCD and DCD + ADHD groups [HSD > HSD(0.01) = 19.34, p < 0.01]; however, typically developing children did not differ significantly from children with ADHD [HSD < HSD(0.05) = 15.75, p > 0.05]. This is in agreement with a previous study (Loh et al., 2011). Since the NDI scores for children with ADHD children were in the average range, it appears that the difference from typically developing children was predominantly due to DCD and not ADHD. Thus, the DCD and DCD + ADHD groups were combined in order to investigate the correlation between NDI scores and each of left and right SM1 connectivity in all children with DCD. Fig. 3 shows the results of this analysis. Connectivity with right SM1 was significantly correlated with NDI score in regions of the default mode network, including a positive correlation with the medial prefrontal cortex and a negative correlation with the anterior cingulate cortex and bilateral angular gyri. No brain regions exhibited a significant correlation between NDI score and connectivity with left SM1.
Fig. 3.
Brain regions of DCD children exhibiting a significant correlation between Neurodevelopment Index (NDI) and connectivity with the right SM1. Red-yellow (blue-light blue) indicates a significant positive (negative) correlation (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Within- and between-hemisphere functional connections amongst cortical motor areas, subcortical motor areas and the cerebellum (e.g., cerebello-thalamo-cortical pathway connections) are necessary to integrate sensory information from both sides of the body when coordinating, planning and executing movements. Our findings demonstrate that the functional connections between these regions are altered in children with DCD and ADHD, lending support to our hypothesis that within- and between-hemisphere motor functional connections are altered in children with DCD and ADHD, and that some of these are neural mechanisms common to ADHD and DCD and some are mechanisms specific to DCD.
4.1. Preserved motor connections in ADHD and DCD
We observed stronger within-hemisphere connections of the posterior insula and SM1 in typically developing children and in children with ADHD and DCD, compared to between-hemisphere connections. This not been reported previously; however, one resting-state fMRI study has established significant connections between the posterior insula and SM1 (Cauda et al., 2011), supporting a role of the posterior insula in sensorimotor integration. Given that DCD is a sensory processing disorder, altered functional connections between the posterior insula and SM1 is a plausible mechanism for sensory processing deficits in DCD; however, our data suggest there is no alteration in the within- and between-hemisphere functional connections of the posterior insula with SM1.
4.2. Altered motor connections common to ADHD and DCD
Lobules V and VI of the cerebellum are key regions in the coordination, planning and execution of movements (Stoodley and Schmahmann, 2009). The thalamus fulfills a similar role, amongst others. Our typically developing children exhibited stronger functional connections between right SM1 and the right thalamus and left cerebellar lobule V, relative to their connections with left SM1. Stronger within-hemisphere connections between right SMI and right thalamus relative to between-hemisphere connections and stronger contralateral connections between right SM1 and the cerebellum have not been reported previously, and the reason for their existence is unclear. One possibility is that it is necessary to mitigate the non-dominance of the left hand for tasks that require dexterity and coordination; however, further studies to investigate the relationship between these functional connections and left hand function are required to substantiate this postulation.
Our results show that children with DCD and ADHD (including DCD + ADHD) exhibit a different pattern of within- and between-hemisphere connections of the thalamus and cerebellum, when compared to typically developing children: the left thalamus and the right lobule V of the cerebellum are more connected with left SM1 than with right SM1 in children with DCD and ADHD children (summarized in Fig. 4). Following the argument above regarding mitigation of the non-dominant hand in controls, it is plausible that stronger within-hemisphere connections of the left thalamus and stronger between-hemisphere connections of the right cerebellum may be an attempt to mitigate sensorimotor deficits of the dominant right hand in children with DCD and ADHD. Our findings also support a previous resting-state fMRI study of ADHD that demonstrated disruptions within frontal-striatal-cerebellar circuits (Cao et al., 2006), as well other studies that observed alterations in cognitive and attention brain networks (Lin et al., 2015, Carmona et al., 2015, Wang and Li, 2015, dos Santos Siqueira et al., 2014, Mattfeld et al., 2014); some of these studies suggest there is an interaction between these altered networks and changes within networks associated with motor and sensory functions (Carmona et al., 2015, dos Santos Siqueira et al., 2014). Our findings provide direct evidence of how the motor network is altered in DCD and ADHD, and serve to foster future studies of these interactions.
4.3. Altered motor connections in DCD, but preserved in ADHD
We observed that the within-hemisphere connections between the right putamen and SM1 were stronger than the between-hemisphere connections, in typically developing children and in children with ADHD (both ADHD alone and in combination with DCD). These connections thus appear to be part of typical development and are not impacted in ADHD. As discussed above, it is possible that stronger connections in the right hemisphere between subcortical regions and the motor cortex may be associated with mitigation of the non-dominant hand. In DCD alone children, however, the between-hemisphere and within-hemisphere connections of the right putamen with SM1 were of equivalent strength (summarized in Fig. 4). This finding suggests that DCD children lack the hemispheric dominance of functional connections between the right putamen and the motor cortex, and hence constitutes a neural mechanism specific to DCD. The lack of hemispheric dominance of right putamen functional connections may help explain the bimanual coordination deficits observed in children with DCD. This alteration in functional connections could also be associated with the observation of reduced diffusivity within the corticospinal tract and posterior thalamic radiation in children with DCD relative to typically developing children (Zwicker et al., 2011), which together may help explain the significant deficits observed across a wide range of motor functions, including implicit motor planning (Jarus et al., 2015).
4.4. Additional altered connections in DCD + ADHD
In our children with DCD + ADHD, the precuneus, middle frontal gyri and inferior lateral occipital cortex all exhibited stronger functional connections with left SM1 than with right SM1. This could be the result of weakened functional connections of these areas to right SM1. These regions have vital roles in spatial orientation and other higher order visual and sensory functions. Thus, our findings suggest that alteration in functional connections associated with higher order sensory processing represent a mechanism underlying the comorbidity of ADHD and DCD. These altered connections could possibly explain the visuospatial problems that have been associated with these disorders, particularly DCD + ADHD (Crawford and Dewey, 2008, Wilson and McKenzie, 1998).
4.5. Correlations between right SM1 connectivity and NDI scores in DCD children
These latter points are supported by our observation of a significant correlation between NDI scores and connectivity of the right SM1 with regions of the default mode network associated with visuospatial processing (i.e., the angular gyri), which has been implicated in DCD (Crawford and Dewey, 2008, Kashiwagi et al., 2009, Zwicker et al., 2010, Zwicker et al., 2011). A significant difference in NDI scores between DCD (either alone or in combination with ADHD) and typically developing children, but not ADHD alone children, is in agreement with previous studies (Loh et al., 2011). We, however, have extended this previous finding by demonstrating an underlying physiological mechanism for neurodevelopment severity in DCD (and DCD + ADHD) children in the form of altered functional connections of the motor network with visuospatial processing regions; this is highly clinically significant, and it also suggests that the behavioral phenotypes associated with atypical motor network development differ between individuals with DCD (alone or in combination with ADHD) and those with ADHD alone.
4.6. Limitations
Although we had 20 children with DCD in our sample, only six had DCD alone. Thus, sample size of the DCD alone group is a major limitation. However, the consistency between the findings in our DCD alone group with those of the other groups increases the confidence in our results. In addition, NDI only differed between typically developing children and DCD (either alone or in combination with ADHD) and not ADHD alone. This allowed us to perform our correlational analysis of NDI score and functional connection strength with our original DCD group size of 20. Nevertheless, our findings could be further substantiated by increasing the size of the DCD group, as well as by employing concurrent structural markers to examine the integrity of white matter tracts along motor pathways. Furthermore, comprehensive behavioral evaluation of the functional deficits experienced by these children could help elucidate the specific motor pathways altered by different clinical features of DCD and ADHD. Due to the cross-sectional design of the current study, we are unable to examine the impact of brain maturation on connectivity; however, future longitudinal studies could address this issue.
The children in the DCD group met only 3 of the 4 of the DSM-V criteria for the disorder. We did not specifically establish whether the motor coordination difficulties evidenced by the children in the DCD groups were present from an early age or of recent onset. However, children who evidenced history of traumatic brain injury or other neurological conditions, which could account for recent onset of the symptoms associated with DCD, were excluded from participation in this study.
All children with ADHD, but one, were on stimulant medication. Therefore, medication status could potentially explain some of the differences we observed between ADHD and DCD children. However, the consistency of our findings across all patient groups as well as the apparent specificity of NDI score to children with DCD (either alone or in combination with ADHD) strongly suggests that medication status had little or no effect on our findings.
In summary, our findings support the hypothesis that within- and between-hemisphere functional connections are altered in the motor networks of children with DCD and ADHD, some of which are associated with ADHD and DCD comorbidity, and others that are specific to DCD alone. Our results thus provide important clues as to the shared and distinct neural mechanisms that underlie these common neurodevelopmental disorders.
Funding
This study was supported by on operating grant from the Canadian Institutes of Health Research (grant number MOP-88588) to DD and BGG. KRM was the recipient of an Alberta Children's Hospital Research Institute Studentship through the CIHR Training Program in Genetics, Child Development and Health and a Katharina Zeigler Scholarship, University of Calgary. These funding agencies had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Acknowledgements
We thank Holly Johnston, University of Calgary, for her assistance in image acquisition, as well as Nadia Barnieh, Ashley Marsh and Sally Powis-Campbell, University of Calgary, for their assistance with neurocognitive assessments. We also thank the children who participated in this study.
Contributor Information
Kevin R. McLeod, Email: kmcleod@ucalgary.ca.
Lisa Marie Langevin, Email: lmlangev@ucalgary.ca.
Deborah Dewey, Email: dmdewey@ucalgary.ca.
Bradley G. Goodyear, Email: goodyear@ucalgary.ca.
References
- American Psychiatric Association . American Psychiatric Publishing; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition. [Google Scholar]
- Amunts K., Schlaug G., Schleicher A., Steinmetz H., Dabringhaus A., Roland P.E., Zilles K. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Amunts K., Jäncke L., Mohlberg H., Steinmetz H., Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–312. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blank R. European Academy of Childhood Disability (EACD): Recommendations on the definition, diagnosis and intervention of developmental coordination disorder (pocket version). German-Swiss interdisciplinary clinical practice guideline S3-standard according to the Association of the Scientific Medical Societies in Germany. Pocket version. Definition, diagnosis, assessment, and intervention of developmental coordination disorder (DCD). Dev. Med. Child Neurol. 2012;54:e1–e7. doi: 10.1111/j.1469-8749.2011.04175.x. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D., Li W., Parrish T.B., Gitelman D.R., Mesulam M.M. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD). J. Child Psychol. Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Tsoupas J., Wilson P.H., Smits-Engelsman B.C.M. Motor imagery development in primary school children. Dev. Neuropsych. 2009;34:103–121. doi: 10.1080/87565640802499183. [DOI] [PubMed] [Google Scholar]
- Cao Q., Zang Y., Sun L., Sui M., Long X., Zou Q., Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Cauda F., D'Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Carmona S., Hoekzema E., Castellanos F.X., García-García D., Lage-Castellanos A., Van Dijk K.R., Navas-Sánchez F.J., Martínez K., Desco M., Sepulcre J. Sensation-to-cognition cortical streams in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2015;36:2544–2557. doi: 10.1002/hbm.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton R.J., Carp J., Chaddock L., Fineman S.L., Quandt L.C., Ratliff J.B. Trouble Crossing the Bridge: Altered Interhemispheric Communication of Emotional Images in Anxiety. Emot. Wash. DC. 2008;8:684–692. doi: 10.1037/a0012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K., Sitarenios G., Parker J.D., Epstein J.N. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Crawford S.G., Dewey D. Co-occurring disorders: a possible key to visual perceptual deficits in children with developmental coordination disorder? Hum. Mov. Sci. 2008;27:154–169. doi: 10.1016/j.humov.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Deco G., Corbetta M. The dynamical balance of the brain at rest. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2011;17:107–123. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey D., Cantell M., Crawford S.G. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J. Int. Neuropsychol. Soc. JINS. 2007;13:246–256. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- dos Santos Siqueira A., Biazoli Junior C.E., Comfort W.E., Rohde L.A., Sato J.R. Abnormal functional resting-state networks in ADHD: graph theory and pattern recognition analysis of fMRI data. BioMed Res. Int. 2014;2014:380531. doi: 10.1155/2014/380531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Hertz-Pannier L., Cachia A., Mangin J.F., Le Bihan D., Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb. Cortex N. Y. N. 2009;1991(19):414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Garrett A., Penniman L., Epstein J.N., Casey B.J., Hinshaw S.P., Glover G., Tonev S., Vitolo A., Davidson M., Spicer J., Greenhill L.L., Reiss A.L. Neuroanatomical abnormalities in adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1321–1328. doi: 10.1097/CHI.0b013e318185d285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani A.M., Goodyear B.G. Regions of interest for resting-state fMRI analysis determined by inter-voxel cross-correlation. NeuroImage. 2011;56:246–251. doi: 10.1016/j.neuroimage.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Hale T.S., Loo S.K., Zaidel E., Hanada G., Macion J., Smalley S.L. Rethinking a right hemisphere deficit in ADHD. J. Atten. Disord. 2009;13:3–17. doi: 10.1177/1087054708323005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hands B., Larkin D., Rose E. The psychometric properties of the McCarron Assessment of Neuromuscular Development as a longitudinal measure with Australian youth. Hum. Mov. Sci. 2013;32:485–497. doi: 10.1016/j.humov.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Henderson S.E., Sugden D.A., Barnett A.L. Psychol. Corp.; Lond. UK: 2007. Movement assessment battery for children - 2 second edition (Movement ABC-2) [Google Scholar]
- Jarus T., Ghanouni P., Abel R.L., Fomenoff S.L., Lundberg J., Davidson S., Caswell S., Bickerton L., Zwicker J.G. Effect of internal versus external focus of attention on implicit motor learning in children with developmental coordination disorder. Res. Dev. Disabil. 2015;37:119–126. doi: 10.1016/j.ridd.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kadesjo B., Gillberg C. Attention deficits and clumsiness in Swedish 7-year-old children. Dev. Med. Child Neurol. 1998;40:796–804. doi: 10.1111/j.1469-8749.1998.tb12356.x. [DOI] [PubMed] [Google Scholar]
- Kadesjo B., Gillberg C. The comorbidity of ADHD in the general population of Swedish school-age children. J. Child Psychol. Psychiatry. 2001;42:487–492. [PubMed] [Google Scholar]
- Kaplan B.J., Wilson B.N., Dewey D., Crawford S.G. DCD may not be a discrete disorder. Hum. Mov. Sci. 1998:471–490. [Google Scholar]
- Kashiwagi M., Iwaki S., Narumi Y., Tamai H., Suzuki S. Parietal dysfunction in developmental coordination disorder: a functional MRI study. Neuroreport. 2009;20:1319–1324. doi: 10.1097/WNR.0b013e32832f4d87. [DOI] [PubMed] [Google Scholar]
- Kim S.G., Ashe J., Hendrich K., Ellermann J.M., Merkle H., Uğurbil K., Georgopoulos A.P. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kochunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin L.M., Macmaster F.P., Crawford S., Lebel C., Dewey D. Common white matter microstructure alterations in pediatric motor and attention disorders. J. Pediatr. 2014;164:1157–1164. doi: 10.1016/j.jpeds.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Tseng W.Y., Lai M.C., Matsuo K., Gau S.S. Altered resting-state frontoparietal control network in children with attention-deficit/hyperactivity disorder. J. Int. Neuropsychol. Soc. 2015;21:271–284. doi: 10.1017/S135561771500020X. [DOI] [PubMed] [Google Scholar]
- Loh R.R., Piek J.P., Barrett N.C. Comorbid ADHD and DCD: examining cognitive functions using the WISC-IV. Res. Dev. Disabil. 2011;32:1260–1269. doi: 10.1016/j.ridd.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Mattfeld A.T., Gabrieli J.D., Biederman J., Spencer T., Brown A., Kotte A., Kagan E., Whitfield-Gabrieli S. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 2014;137:2423–2428. doi: 10.1093/brain/awu137. [DOI] [PubMed] [Google Scholar]
- McCarron L.T. McCarron-Dial Systems; Dallas, TX: 1997. McCarron Assessment of Neuromuscular Development. [Google Scholar]
- McLeod K.R., Langevin L.M., Goodyear B.G., Dewey D. Functional connectivity of neural motor networks is disrupted in children with developmental coordination disorder and attention-deficit/hyperactivity disorder. NeuroImage Clin. 2014;4:566–575. doi: 10.1016/j.nicl.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S.H., Rimrodt S.L., Schafer J.G.B., Boyce A., Goldberg M.C., Pekar J.J., Denckla M.B. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol. Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Pitcher T.M., Piek J.P., Hay D.A. Fine and gross motor ability in males with ADHD. Dev. Med. Child Neurol. 2003;45:525–535. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- Reich W., Welner Z., Herjanic B. 1997. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda NY Multi-Health Syst. [Google Scholar]
- Roessner V., Banaschewski T., Uebel H., Becker A., Rothenberger A. Neuronal network models of ADHD – lateralization with respect to interhemispheric connectivity reconsidered. Eur. Child Adolesc. Psychiatry. 2004;13(Suppl. 1):I71–I79. doi: 10.1007/s00787-004-1007-5. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Toone B., Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Sauerwein H.C., Lassonde M. Neuropsychological alterations after split-brain surgery. J. Neurosurg. Sci. 1997;41:59–66. [PubMed] [Google Scholar]
- Schoemaker M.M., Lingam R., Jongmans M.J., van Heuvelen M.J.G., Emond A. Is severity of motor coordination difficulties related to co-morbidity in children at risk for developmental coordination disorder? Res. Dev. Disabil. 2013;34:3084–3091. doi: 10.1016/j.ridd.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Scholz V.H., Flaherty A.W., Kraft E., Keltner J.R., Kwong K.K., Chen Y.I., Rosen B.R., Jenkins B.G. Laterality, somatotopy and reproducibility of the basal ganglia and motor cortex during motor tasks. Brain Res. 2000;879:204–215. doi: 10.1016/s0006-8993(00)02749-9. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A., Hlustik P., Noll D.C., Small S.L. Lateralization of motor circuits and handedness during finger movements. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Toro R., Fox P.T., Paus T. Functional coactivation map of the human brain. Cereb. Cortex N. Y. N. 2008;1991(18):2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaivre-Douret L. Developmental coordination disorders: State of art. Neurophysiol. Clin. Clin. Neurophysiol. 2014;44:13–23. doi: 10.1016/j.neucli.2013.10.133. [DOI] [PubMed] [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Van Ewijk H., Heslenfeld D.J., Zwiers M.P., Buitelaar J.K., Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Visser J. Developmental coordination disorder: a review of research on subtypes and comorbidities. Hum. Mov. Sci. 2003;22:479–493. doi: 10.1016/j.humov.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Wang X.H., Li L. Altered temporal features of intrinsic connectivity networks in boys with combined type of attention deficit hyperactivity disorder. Eur. J. Radiol. 2015;84:947–954. doi: 10.1016/j.ejrad.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Weissman D.H., Banich M.T. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology. 2000;14:41–59. doi: 10.1037//0894-4105.14.1.41. [DOI] [PubMed] [Google Scholar]
- Wilson P.H., McKenzie B.E. Information processing deficits associated with developmental coordination disorder: a meta-analysis of research findings. J. Child Psychol. Psychiatry. 1998;39:829–840. [PubMed] [Google Scholar]
- Wilson B.N., Kaplan B.J., Crawford S.G., Campbell A., Dewey D. Reliability and validity of a parent questionnaire on childhood motor skills. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 2000;54:484–493. doi: 10.5014/ajot.54.5.484. [DOI] [PubMed] [Google Scholar]
- Wilson P.H., Ruddock S., Smits-Engelsman B., Polatajko H., Blank R. Understanding performance deficits in developmental coordination disorder: a meta-analysis of recent research. Dev. Med. Child Neurol. 2013;55:217–228. doi: 10.1111/j.1469-8749.2012.04436.x. [DOI] [PubMed] [Google Scholar]
- Yousry T.A., Schmid U.D., Jassoy A.G., Schmidt D., Eisner W.E., Reulen H.J., Reiser M.F., Lissner J. Topography of the cortical motor hand area: prospective study with functional MR imaging and direct motor mapping at surgery. Radiology. 1995;195:23–29. doi: 10.1148/radiology.195.1.7892475. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics. 2010;126:678–686. doi: 10.1542/peds.2010-0059. [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Brain activation associated with motor skill practice in children with developmental coordination disorder: an fMRI study. Int. J. Dev. Neurosci. 2011;29:145–152. doi: 10.1016/j.ijdevneu.2010.12.002. [DOI] [PubMed] [Google Scholar]