Abstract
Convergent atrial fibrillation ablation involves extensive epicardial as well as endocardial ablation of the left atrium. We examined whether it changes the morphology of the surface P wave. We reviewed electrocardiograms of 29 patients who underwent convergent ablation for atrial fibrillation. In leads V1, II and III, we measured P wave duration, area and amplitude before ablation, and at 1, 3 and 6 months from ablation.
After ablation, there were no significant changes in P wave amplitude, area, or duration in leads II and III. There was a significant reduction in the area of the terminal negative deflection of the P wave in V1 from 0.38 mm2 to 0.13 mm2 (p = 0.03). There is also an acute increase in the amplitude and duration of the positive component of the P wave in V1 followed by a reduction in both by 6 months. Before ablation, 62.5% of the patients had biphasic P waves in V1. In 6 months, only 39.2% of them had biphasic P waves.
Hybrid ablation causes a reduction of the terminal negative deflection of the P wave in V1 as well as temporal changes in the duration and amplitude of the positive component of the P wave in V1. This likely reflects the reduced electrical contribution of the posterior left atrium after ablation as well as anatomical and autonomic remodeling. Recognition of this altered sinus P wave morphology is useful in the diagnosis of atrial arrhythmias in this patient population.
Keywords: Convergent ablation, Atrial fibrillation, P wave area, P wave duration
Introduction
Atrial fibrillation (AF) is a common form of supraventricular tachyarrythmia affecting more than 5 million people in the US alone [1]. Current understanding suggests that AF results from anatomic substrate capable of both initiation and perpetuation of fibrillatory waves [2], [3]. Most of these foci are found at the orifices of pulmonary veins and the posterior atrial wall [4]. Thus, ablation of these foci has emerged as the treatment for persistent and paroxysmal AF not responding to medical therapy.
Convergent AF ablation employs both trans-diaphragmatic epicardial and catheter induced endocardial ablation to electrically isolate the posterior wall of the left atrium and the area around the pulmonary veins. Ablation is associated with changes in architecture and the electrical progression across the LA. Additionally, autonomic ganglionated plexi are concomitantly ablated as they exist in the epicardial surface. These changes translate into changes in the 12-lead surface electrocardiography (ECG) and may correlate with freedom from AF.
Current literature shows P wave duration (PWD) significantly decreases after circumferential pulmonary vein isolation (CPVI), and that the decrease in PWD correlates with freedom from AF [5], [6], [7], [8], [9], [10], [11]. Some studies have assessed the change in P wave area post CPVI, however, the results are inconsistent.
Convergent ablation results in more extensive scarring and may lead to more distinct ECG changes. Data on ECG changes after this procedure is scarce. One study involving 41 patients undergoing convergent ablation reported reduction in PWD consistent with the studies involving CPVI [12]. We have assessed changes in P wave duration and P wave area (PWA) after 1 month, 3 months and 6 months of convergent ablation.
Materials and methods
Twenty-nine patients who underwent convergent ablation were included in the study. These patients had persistent AF or paroxysmal AF with enlarged atria, which did not respond to medical therapy including rate control or antiarrhythmic therapy. Six of them had undergone CPVI as well in the past.
Ablation technique
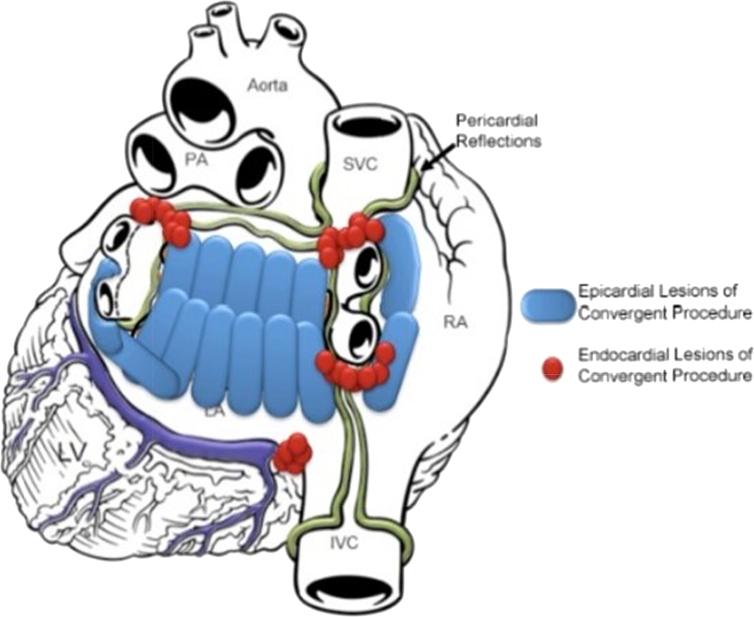
Convergent ablation procedure utilizes a transdiaphragmatic cannula and the EPi-Sense™ Guided Coagulation System with VisiTrax (nContact, Inc.). The EPi-Sense device is used to create epicardial lesions along the entire posterior wall and around the pulmonary veins (PV). The pericardial reflections limit the epicardial ablation of the superior aspect of the veins and the region below the right inferior PV. The lesion set is therefore completed endocardially by an electrophysiologist and bidirectional block is confirmed across the veins (Fig. 1). A right cavotricuspid isthmus line is also created.
Fig. 1.
Schematic of the convergent ablation lesion pattern relative to the high stress regions. By anatomically targeting high stress regions associated with atrial remodeling, AF substrate is ablated or isolated.
ECG parameters measurement
We analyzed ECGs before (up to 6 months prior to ablation), and 1, 3 and 6 months after ablation. P wave duration, area and amplitude were measured in lead V1, II and III as previous studies found ECG changes in these leads. Measurements were performed with use of Sketchandcalc, an online software designed for precise 2 dimensional measurements [13].
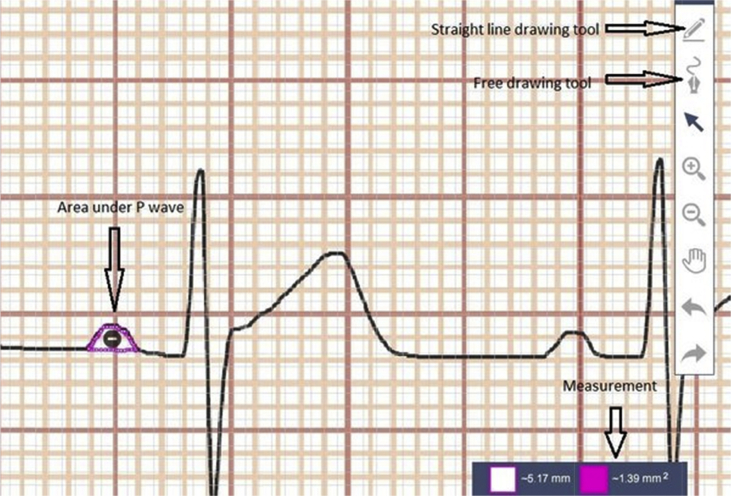
We uploaded the electronic copy of the standard 12-lead ECG onto the software, magnified it to 25 times, and made the measurements. We set the scale in the software so that one small box of the ECG strip after magnification would be equivalent to 1 mm which is the standard. We used the free drawing tool in the software, and drew under the curve of the P wave to get the area (Fig. 2). For each parameter, two consecutive P waves in ECG were measured and the average value was used.
Fig. 2.
Measurement of P wave area using sketchandcalc.
During the six months period after ablation, any episode of AF lasting more than 30 s during follow-up ECG, 24–48 h Holter, or 30 day event monitoring was considered recurrence of AF.
Statistical analysis
We used repeated measures analysis of variance (ANOVA) to evaluate the change in P wave duration, amplitude and area before and at 1 month, 3 months and 6 months after ablation.
Results
Twenty-nine patients who underwent convergent ablation of atrial fibrillation were included in the study (Table 1). The mean age was 62, and 21 of them were male. Five patients did not have any ECG demonstrating normal sinus rhythm within 6 months prior to ablation.
Table 1.
Patient demographics.
| Mean age | 62 ± 10 years |
| Male | 21 (72%) |
| BMI | 33.93 ± 6.41 |
| Ejection fraction | |
| ≤ 35% | 4 (13.7%) |
| > 35% | 25 (86.2%) |
| Pre-existing medical conditions | |
| Hypertension | 24 |
| Diabetes Mellitus | 10 |
| Coronary artery disease | 7 |
| Hyperlipidemia | 16 |
| Average time since first AF diagnosis | 50.7 months |
| Left atrium diameter | 50.3 ± 5.7 mm |
| Recurrence | 3 (10.3%) |
After convergent ablation, only lead V1 had some statistically significant changes. P wave area decreased significantly in the negative component of V1 (0.38 ± 0.4 mm2 to 0.13 ± 0.3 mm2, p = 0.03) in six months. Likewise, P wave duration also decreased significantly in the positive component of V1 (39.1 ± 19 ms to 29.42 ± 25 ms, P = 0.004), and positive P wave amplitude decreased from 0.46 ± 0.6 mm to 0.33 ± 0.5 mm, p = 0.05 (Table 2).
Table 2.
Change in P wave parameter after convergent ablation at different time interval.
| Source | P wave parameters | Before ablation | One month after | 3 months after | 6 months after | p-Value |
|---|---|---|---|---|---|---|
| V1 | Duration of positive segment (ms) | 39.1 ± 19 | 46.63 ± 23 | 33.58 ± 25 | 29.42 ± 25 | 0.004 |
| Amplitude of positive segment (mv) | 0.46 ± 0.6 | 0.71 ± 0.6 | 0.58 ± 0.6 | 0.33 ± 0.5 | 0.05 | |
| Area of positive segment (mm2) | 0.25 ± 0.4 | 0.54 ± 0.6 | 0.42 ± 0.5 | 0.25 ± 0.5 | 0.07 | |
| Duration of negative segment (ms) | 45.1 ± 33 | 28.5 ± 32 | 31.7 ± 27 | 32.1 ± 29 | 0.1 | |
| Amplitude of negative segment (mv) | 0.25 ± 0.4 | 0.13 ± 0.3 | 0.17 ± 0.3 | 0.17 ± 0.3 | 0.58 | |
| Area of negative segment (mm2) | 0.38 ± 0.4 | 0.17 ± 0.3 | 0.08 ± 0.2 | 0.13 ± 0.3 | 0.03 | |
| Lead III | Duration of positive segment (ms) | 56.7 ± 48 | 52.3 ± 30 | 49.4 ± 32 | 56.5 ± 28 | 0.7 |
| Amplitude of positive segment (mv) | 0.50 ± 0.6 | 0.42 ± 0.5 | 0.38 ± 0.4 | 0.37 ± 0.4 | 0.78 | |
| Area of positive segment (mm2) | 0.79 ± 1.6 | 0.42 ± 0.5 | 0.37 ± 0.4 | 0.46 ± 0.5 | 0.36 | |
| Duration of negative segment (ms) | 18.4 ± 24 | 22.4 ± 25 | 19.6 ± 23 | 17.5 ± 25 | 0.87 | |
| Amplitude of negative segment (mv) | 0 | 0.04 ± 0.2 | 0.04 ± 0.2 | 0.08 ± 0.2 | 0.54 | |
| Area of negative segment (mm2) | 0 | 0.04 ± 0.2 | 0.04 ± 0.2 | 0.04 ± 0.2 | 0.59 | |
| Lead II | Duration of P wave (ms) | 91.65 ± 36 | 80.3 ± 19 | 80.3 ± 15 | 78.7 ± 16 | 0.21 |
| Amplitude of P wave (mv) | 1.1 ± 0.6 | 0.9 ± 0.3 | 0.87 ± 0.4 | 0.83 ± 0.3 | 0.09 | |
| Area of P wave (mm2) | 1.55 ± 1.4 | 1.02 ± 0.5 | 1.13 ± 0.6 | 1 ± 0.5 | 0.12 |
Discussion
Main findings
We found that after convergent ablation, in lead V1, there is a statistically significant reduction in P wave area in the terminal portion from 0.38 mm2 to 0.13 mm2. Furthermore, a significant portion of biphasic morphology of P wave seen in leads V1 (62.5%) decreased (39.2% at 6 months) to a mono-phasic morphology suggesting a decrease in the negative terminal portion of P wave as a result of the electrical debulking of the left atrium (Table 3). We noticed qualitatively that patients would frequently have very tall, upright P waves in V1 immediately post-ablation which was statistically significant. At elevated heart rates, this made it more challenging to distinguish these sinus P waves from an atrial tachycardia or atrial flutter given the fact the ECG differed from the pre-ablation baseline ECG. Interestingly, this increase in amplitude decreases over the 6 month follow-up period and ultimately becomes smaller than the pre-ablation amplitude. A similar temporal pattern was also noted regarding the duration of the positive component of the P wave in V1 (Table 2).
Table 3.
Comparing V1 P wave morphology before and after ablation.
| Morphology of P wave in V1 | Frequency before ablation | One month post-ablation | Three months post-ablation | Six months post-ablation |
|---|---|---|---|---|
| Biphasic | 15 (62.5%) | 15 (51.7%) | 11 (39.3%) | 11 (39.2%) |
| Only positive | 6 (25%) | 11 (37.9%) | 9 (32.1%) | 7 (25%) |
| Flat | 0 | 2 (6.9%) | 1 (3.5%) | 2 (7.1%) |
| Only negative | 3 (12.5%) | 1 (3.4%) | 7 (25%) | 8 (28.5%) |
| Total | 24 | 29 | 28 | 28 |
The biphasic morphology of P wave decreased after ablation in V1.
Change in P wave area
Convergent ablation involves both epicardial and endocardial ablation. It encompasses the posterior wall and therefore removes the AF triggers. This approach increases the chance for durable trans-mural isolation. In addition, the epicardial ablation across the posterior wall creates an effective roof line and posterior box, and eliminates rotors in the posterior wall and around the pulmonary veins. Because of the extensive ablation, this procedure has higher success rate. In a study of 73 patients, Gersak et al. reported that 80% remained in sinus rhythm after a year of convergent ablation [14]. Other studies also had similar results [15], [16].
The terminal portion of P wave is generally accepted as a reflection of left atrial activation. After convergent ablation, the P wave area in the negative terminal portion of V1 decreased significantly from 0.38 mm2 to 0.13 mm2. This reduction in the PWA could be explained by the fact that convergent ablation eliminates a significant portion of LA electrical activity. After ablation, reverse morphological remodeling occurs, which may also further decrease the LA dimension [17], which is reflected as the decrease in P wave area. In a study by Beeuman et al., a significant decrease in PWA in lead II (2.32 to 1.62 mvms) was reported after VATS pulmonary vein isolation. The decrease was more pronounced in the terminal portion of P wave [5].
Change in P wave duration
P wave duration has been reported to correlate with LA size and studies have shown association of prolonged P wave duration with presence of paroxysmal AF [4], [5]. In a study, a cut off PWD of 140 ms had a sensitivity and specificity of 71% and 61% respectively in prediction of AF [7].
Previous studies have reported a decrease in PWD, mostly in lead II, with both convergent and conventional PVI [5], [6], [7], [8], [9], [10], [11], [12]. In one study involving convergent ablation, Kumar et al. reported a decrease in PWD from 104.4 ms to 84.7 ms after the procedure [12]. In our study, over a follow-up of six months, there was a trend towards decrease in PWD in lead II before and after ablation (91.6 ms to 78.7 ms). However, it did not meet statistical significance. The difference in the outcome can be explained by the fact that the mean pre-ablation PWD in our group was 91 ms, considerably shorter compared to the pre-ablation duration reported previously. Furthermore, 20% of our patients had already undergone the traditional catheter ablation, whereas in Kumar et al.'s study, all the participants were first time ablation patients. This history of prior intervention may have lessened the P wave shortening effect of ablation in our patient group.
Limitations of the study
Our study is limited by the relatively small sample size. However, using a pre-validated and precise method of evaluating P wave parameters, we had findings in line with prior studies.
Conclusions
Convergent ablation causes a reduction of the terminal negative deflection of the P wave in V1. This reflects the reduced electrical contribution of the ablated posterior left atrium. The majority of patients no longer met criteria for left atrial enlargement in V1 after ablation. The duration and amplitude of the positive segment of the P wave in V1 also acutely increased following ablation and then decreased over 6 months. In addition to anatomical remodeling, there may be autonomic remodeling given the collateral ablation of ganglionated plexi. The normal sinus P wave in these patients is significantly different than expected and may exhibit temporal changes in morphology. Recognizing the change in sinus P wave morphology post-ablation has implications in the diagnosis of atrial arrhythmias since many algorithms involve V1 morphology.
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Contributor Information
Suvash Shrestha, Email: sshrestha@maimonidesmed.org, suvashsht@gmail.com.
On Chen, Email: ochen@maimonidesmed.org.
Mary Greene, Email: MAgreene@maimonidesmed.org.
Jinu Jacob John, Email: Jjohn3@maimonidesmed.org, jinu.john@beaumont.edu.
Yisachar Greenberg, Email: ygreenberg@maimonidesmed.org.
Felix Yang, Email: fyang@maimonidesmed.org.
References
- 1.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013 Oct 15;112(8):1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Shiroshita-Takeshita A., Brundel B.J., Nattel S. Atrial fibrillation: basic mechanisms, remodeling and triggers. J Interv Card Electrophysiol. 2005;13:181–193. doi: 10.1007/s10840-005-2362-y. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H., Brugada J., Packer D.L., Cappato R., Chen S.A., Crijns H.J. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow up. Europace. 2007;9(6):335–379. doi: 10.1093/europace/eum120. http://dx.doi.org/10.1093/europace/eum120 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.J., Chen S.A. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 5.Beeumen K.V., Houben R., Tavernier R., Ketels S., Duytschaever M. Changes in P wave area and P wave duration after circumferential pulmonary vein isolation. Europace. 2010;12:798–804. doi: 10.1093/europace/eup410. [DOI] [PubMed] [Google Scholar]
- 6.Chang S.L., Tai C.T., Lin Y.J., Wongcharoen W., Lo L.W., Tuan T.C. Biatrial substrate properties in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18(11):1134–1139. doi: 10.1111/j.1540-8167.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa M., Kumagai K., Vakulenko M., Yasuda T., Siegerman C., Garfinkel A. Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007 Sep;18(9):931–938. doi: 10.1111/j.1540-8167.2007.00890.x. [Epub 2007 Jul 27] [DOI] [PubMed] [Google Scholar]
- 8.Nassif M., Krul S.P., Driessen A.H., Deneke T., Wilde A.A., de Bakker J.M. Electrocardiographic P wave changes after thoracoscopic pulmonary vein isolation for atrial fibrillation. J Interv Card Electrophysiol. 2013 Sep;37(3):275–282. doi: 10.1007/s10840-013-9802-x. http://dx.doi.org/10.1007/s10840-013-9802-x [DOI] [PubMed] [Google Scholar]
- 9.Pappone C., Santinelli V., Manguso F., Vicedomini G., Gugliotta F., Augello G. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004 Jan 27;109(3):327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 10.Zhao L., Jiang W.F., Zhou L., Liu X. Early phase changes of P wave characteristics after circumferential pulmonary vein isolation. Chin Med J. 2013;126:2607–2612. [PubMed] [Google Scholar]
- 11.Udyavar A.R., Huang S.H., Chang S.L., Lin Y.J., Tai C.T., Lo L.W. Acute effect of circumferential pulmonary vein isolation on left atrial substrate. J Cardiovasc Electrophysiol. 2009 Jul;20(7):715–722. doi: 10.1111/j.1540-8167.2008.01411.x. http://dx.doi.org/10.1111/j.1540-8167.2008.01411.x [DOI] [PubMed] [Google Scholar]
- 12.Kumar N., Bonizzi P., Pison L., Phan K., Lankveld T., Maesen B. Impact of hybrid procedure on P wave duration for atrial fibrillation ablation. J Interv Card Electrophysiol. 2015 Mar;42(2):91–99. doi: 10.1007/s10840-014-9969-9. http://dx.doi.org/10.1007/s10840-014-9969-9 [DOI] [PubMed] [Google Scholar]
- 13.Dobbs, Elliott. SketchAndCalc™. Area Calculator. http://sketchandcalc.com. Vers 4.1.3.
- 14.Geršak B., Zembala M.O., Müller D., Folliguet T., Jan M., Kowalski O. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg. 2014 Apr;147(4):1411–1416. doi: 10.1016/j.jtcvs.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan D.M., Joyner C.A., Bundy G.M. Multidisciplinary collaboration for the treatment of atrial fibrillation: convergent procedure outcomes from a single center. J Innov Cardiac Rhythm Manag. 2013:1–8. [Google Scholar]
- 16.Zembala M., Filipiak K., Kowalski O., Boidol J., Sokal A., Lenarczyk R. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol. 2012;70(8):819–828. [PubMed] [Google Scholar]
- 17.Choi J.I., Park S.M., Park J.S., Hong S.J., Pak H.N., Lim do S. Changes in left atrial structure and function after catheter ablation and electrical cardioversion for atrial fibrillation. Circ J. 2008 Dec;72(12):2051–2057. doi: 10.1253/circj.cj-08-0428. [DOI] [PubMed] [Google Scholar]