Abstract
Objective
The aim of this study is to evaluate variations in cortical activation in early and late Uygur-Chinese bilinguals from the Xinjiang Uygur Autonomous Region of China. Methodology: During a semantic judgment task with visual stimulation by a single Chinese or Uygur word, functional magnetic resonance imaging (fMRI) was performed. The fMRI data regarding activated cortical areas and volumes by both languages were analyzed.
Results
The first language (L1) and second language (L2) activated language-related hemispheric regions, including the left inferior frontal and parietal cortices, and L1 specifically activated the left middle temporal gyrus. For both L1 and L2, cortical activation was greater in the left hemisphere, and there was no significant difference in the lateralization index (LI) between the two languages (p > 0.05). Although the total activated cortical areas were larger in early than late bilinguals, the activation volumes were not significantly different.
Conclusion
Activated brains areas in early and late fluent bilinguals largely overlapped. However, these areas were more scattered upon presentation of L2 than L1, and L1 had a more specific pattern of activation than L2. For both languages, the left hemisphere was dominant. We found that L2 proficiency level rather than age of acquisition had a greater influence on which brain areas were activated with semantic processing.
Keywords: Uygur-Chinese bilinguals, Uygur language, Chinese language, Semantic processing
Introduction
The ability to acquire and use language is a unique and fundamental characteristic of humans. Bilinguals can be categorized based on the age of acquisition of their second language (L2). Early bilinguals learn both languages starting from infancy, whereas late bilinguals learn their L2 later than their first language (L1). Magnetic resonance imaging (MRI) studies have found that different cortical areas are activated during semantic processing of L1 and L2 [1–3]. In contrast, some functional MRI (fMRI) studies showed that there is a shared neurological network for L1 and L2 in bilinguals [4–6].
Nearly 9 million Uygurs reside in China’s northwestern Xinjiang Uygur Autonomous Region. Uygurs mainly reside in the southern area of the Tianshan Mountain Range in the Kashgar (Kashi), Hotan (Hetian), and Aksu (Akesu) regions. The Uygur language belongs to the Turkic Language Branch of the Altaic Language Family and was formed by the integration of languages among ancient Uygur tribes and other ethnic groups. The modern Uygur language includes Central, Hotan (Hetian), and Lop (Luobu) dialects and uses the Yili-Urumqi pronunciation as the standard for the modern Uygur language. The Uygur language is an alphabetic language with eight vowels and 24 consonants and is written from right to left [7]. The Chinese language is a visually ideographic language written in a rectangular space, where the shape, sound, and meaning are all integrated. Thus, Chinese and Uygur languages are significantly different in the way they are written and read.
In our experience with the Uygur-speaking population, Uygurs exhibit an increased propensity for learning English compared to the Chinese-speaking population. This may be because English and Uygur are both alphabetic languages. In this study, we compared cortical regions activated by semantic judgment tasks after visual stimulation with Uygur and Chinese words in Uygur-Chinese bilinguals. In addition, we investigated whether the age of L2 acquisition affected patterns of cortical activation. We focused specifically on native Uygur (L1) speakers with Chinese (L2) as their second language. Our findings provide the foundation for future research regarding the processing of the Uygur, Chinese, and English languages in multilinguals.
Material and methods
Subjects
Highly competent Uygur-Chinese bilinguals from central Uygur dialect regions were enrolled in this study. Subjects were divided into two groups. Early bilinguals consisted of 18 (nine male, nine female) bilinguals who started learning Chinese before the age of six (mean age of 25.1 years, range 20–29). Late bilinguals included 21 (9 males, 12 females) bilinguals who started learning Chinese after the age of 12 (mean age of 26.8 years, range 20–31). All subjects passed eighth grade in the Advanced Chinese Language Test. All bilinguals were native Uygur speakers with a college degree or higher, were right-handed with normal visual acuity (naked or corrected), were equally fluent in both languages, were from central dialect regions, and spoke Uygur approximately 80% of the time. The Wechsler Intelligence Scale was used to assess intelligence quotient (IQ), and all subjects had normal IQ (IQ > 85). This study was performed in accordance with local cultural traditions and the ethical guidelines of the Xinjiang Medical University ethics committee. Prior to the start of experiments, procedures were explained, and informed consent was obtained from all subjects. fMRI images were acquired at the Department of Imaging Science, Image Center at the Second Affiliated Hospital of Xinjiang Medical University.
MRI scanning
An Achieva Nova Dual 1.5T (Philips Healthcare, Best, Netherlands) superconducting MRI system with a head quadrature coil and brain function stimulator (SA-9800) was used. An axial scan was performed with the axial slices parallel to the anterior commissure (AC)-posterior commissure (PC) line. Using a routine spin-echo (SE) series and a 24-layer axial scan, T1 weighted imaging anatomical images (24 slices, 5 mm slice thickness, 0 mm gap) were collected. The following parameters were used: FOV, 23 cm × 23 cm; TR, 3000 ms; TE, 5 ms; no gap; and data matrix, 64 × 64. A gradient echo planar imaging (EPI) sequence was applied to acquire blood oxygen level dependent (BOLD) functional images with a total scan time of 246 s.
Study design and language stimulation scheme
All subjects were test-naïve prior to the start of the study. On day one, subjects were visually stimulated with monosyllabic notional Uygur words, and on day two, they were visually stimulated with Chinese characters delivered in the same manner. In the fMRI task presentation, a block was composed of alternating resting phases (with a non-word cognitive task) and stimulation phases (with a word cognitive task). Each block consisted of 16 randomly presented Chinese characters or Uygur words (24s) and the control condition (24 s). Each word/character was presented for 250 ms at 1,250 ms intervals. A “+” sign was shown on the screen during the intervals, and the subjects were required not to move or think during the intervals. A total of 80 words/characters were shown to subjects during a single EPI serial scan. Eighty commonly used Uygur monosyllabic-content words from the Great Uygur-Chinese Dictionary and Chinese verb or noun characters from the Modern Chinese Frequency Dictionary were selected (Fig. 1).
Figure 1.

Sample Uygur and Chinese characters and words used in the present study: (a) semantically precise Uygur single-words, (b) semantically precise Chinese single-character. The meaning of each character and word, in English, is also shown in this figure.
Image processing and analysis
After completion of the fMRI scan, raw Digital Imaging and Communications in Medicine (DICOM) data were transferred to a PC workstation and analyzed using Matlab 7.4 (Mathworks, Natick, MA, USA). SPM5 software (http://www.fil.ion.ycl.ac.uk/spm) was used to correct for head motion and global MRI signal shift. Images were spatially normalized to the Montreal Neurological Institute (MNI) space, and spatial smoothing of standardized functional images was performed using a 10 mm full weight at half maximum (FWHM) Gaussian kernel. After parametrically assessing a time series of functional images using a General Linear Model (GLM), stochastic-effects analysis was performed to obtain statistical parametric maps. For the design matrix for parameter estimation and statistical tests, stimulation mode parameters convolved with hemodynamic response function (HRF). Clusters with >10 voxels and a t-value of 3.6 (p < 0.001, uncorrected) were considered significant and overlaid on the corresponding T1 images of the standard MNI brain. For each condition, spatial coordinates of the center-of-mass and volume (mm3) of the activation clusters were determined based on averaged activation maps. Activated images of both languages were converted to a T-map (1 for activated areas and 0 for non-activated areas) before they were converted to stacked activation images.
The dominant hemisphere for each language was determined by the laterality index (LI) using the formula (L−R)/(L+R) [8], where L and R represented the left and right activated cortical volumes, respectively. Left lateralization was defined as LI ≥ 0.10, while right lateralization was defined as LI ≤ −0.10.
Statistical analysis
SPSS17.0 statistical software (Chicago, IL, USA) was used for all statistical analyses. Normality of data distribution was confirmed with the Kolmogorov-Smirnov test. After fulfillment of normal distribution requirements, paired-sample t-test was used for within-group comparisons of activated cortical volume after Uygur and Chinese language stimulation. Unpaired-sample t-test was used to compare LI between early and late bilinguals after Uygur and Chinese language stimulation. For all statistical analyses, p < 0.05 was considered statistically significant.
Results
Uygur language stimulation
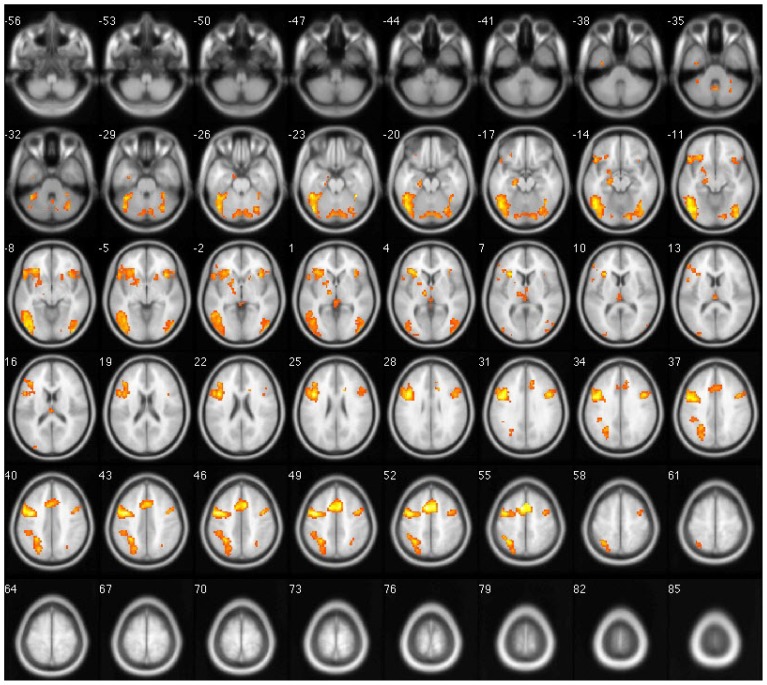
The primary cortical areas activated during the Uygur language stimulation task in both early and late bilinguals were bilateral middle frontal gyri (BA9), inferior frontal gyrus (BA47/46), precentral gyrus (BA6), left superior and inferior parietal lobules (BA7/40), left middle temporal gyrus (BA21), right superior temporal gyrus (BA22), bilateral fusiform gyri (BA37), left precuneus (BA7), right lingual gyrus (BA18), bilateral middle occipital gyri (BA19), and some areas in the cerebellum. In late bilinguals, Uygur language tasks activated the right superior and inferior parietal lobules (BA7/40) and the right middle temporal gyrus (BA21 (p < 0.001). In all subjects, the left hemispheric activation volume was significantly larger than the right (p < 0.05) (Fig. 2).
Figure 2.
Overlapped brain images of averaged activation areas with Uygur language stimulation. Red indicates activated areas in early bilinguals, orange indicates activated areas in late bilinguals, and yellow indicates activated areas in both groups.
Chinese language stimulation
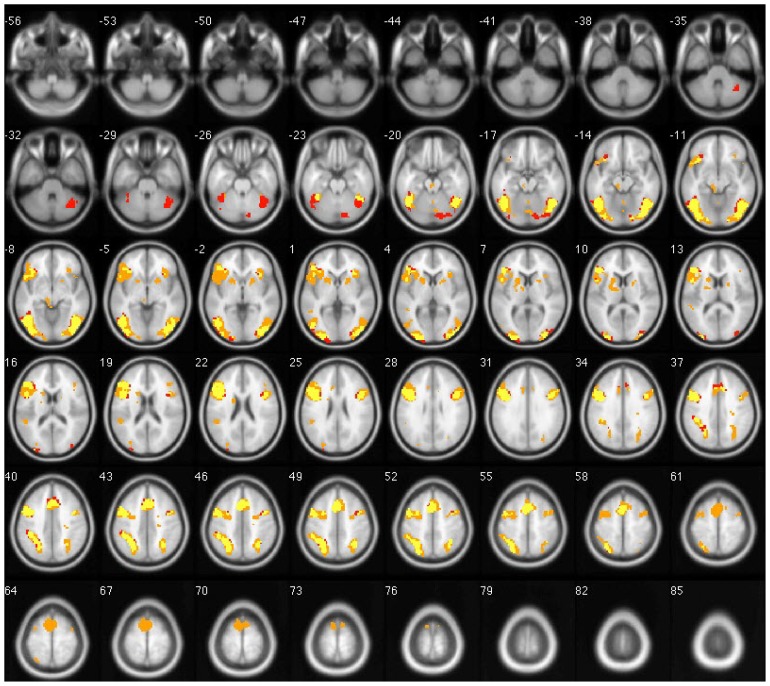
The primary cortical areas activated during Chinese stimulation tasks among both groups were bilateral middle frontal gyri (BA9), inferior frontal gyrus (BA47/46), precentralgyrus (BA6), medial frontal gyrus (BA6), bilateral superior and inferior parietal lobules (BA7/40), bilateral fusiform gyri (BA37), bilateral middle occipital gyri (BA19), left inferior occipital gyrus (BA18), and precuneus (BA7). The cingulated gyrus was partly activated (p < 0.001). Early bilinguals also exhibited activation in bilateral superior frontal gyri (BA6), right inferior occipital gyrus (BA18), and the insular cortex. The left hemisphere activation volume was significantly larger than the right (p < 0.05) (Fig. 3).
Figure 3.
Overlapped brain images of averaged activation areas with Chinese language stimulation. Note: early bilinguals are red; late bilinguals are orange; yellow indicates voxels that passed all statistical criteria for activation in both groups.
Comparison of brain activation with the Uygur or Chinese language
In early and late bilinguals, there was extensive overlap in cortical areas activated by both L1 and L2 stimulation (Table 1), but some specific regions were also activated. For example, the middle temporal gyrus and the superior temporal gyrus were specifically activated by L1.
Table 1.
Regions of significant difference between early and late bilinguals across Uygur and Chinese.
| Activation area | Activation Voxel | T | Talairach coordinates X, Y, Z |
BA |
|---|---|---|---|---|
| Comparison of L1 and L2 in early bilingual | ||||
| Early bilingual L1 | ||||
| L. middle temporal gyrus | 91 | 7.73 | −48 −70 −11 | 21 |
| R. superior temporal gyrus | 9 | 6.18 | 36 29 1 | 22 |
| Early bilingual L2 | ||||
| L. superior frontal gyrus | 122 | 10.21 | −6 2 67 | 6 |
| R. superior frontal gyrus | 102 | 8.93 | 0 11 49 | 6 |
| R. medial frontal gyrus | 32 | 8.8 | 0 17 43 | 32 |
| R. superior/inferior parietal lobule | 84 | 8.14 | 30 −61 46 | 7/40 |
| 26 | 4.18 | 30 −73 | 31 | |
| Comparison of L1 and L2 in late bilingual | ||||
| Late bilingual L1 | ||||
| L. middle temporal gyrus | 24 | 5.12 | −48 −49 −2 | 21 |
| R. middle temporal gyrus | 22 | 7.73 | 48 −70 −11 | 21 |
| Late bilingual L2 | ||||
| R. medial frontal gyrus | 52 | 7.1 | −9 26 37 | 32 |
| L. inferior occipital gyrus | 81 | 7.34 | −21 −103 4 | 18 |
Comparison of activated cortical volume with the Uygur or Chinese language
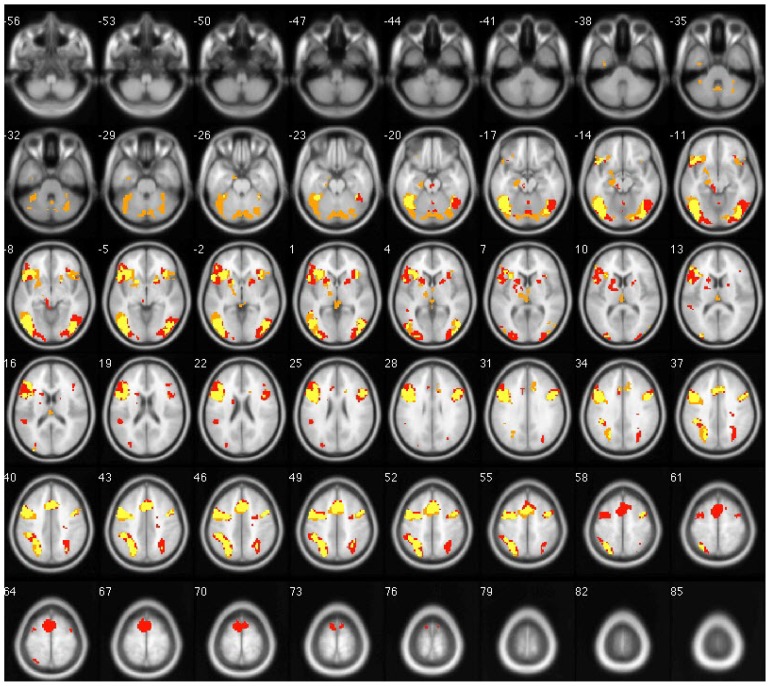
Early Uygur-Chinese bilinguals performed semantic judgment tasks in Uygur and Chinese with a calculated LI of 0.39 and 0.35, respectively. The activated left cortical volume was larger than the right for both languages (t = 5.057 and t = 5.054, p < 0.05), indicating left hemisphere dominance with semantic processing of L1 and L2 among early Uygur-Chinese bilinguals. There was no significant difference in LI between Uygur and Chinese language stimulation (t = 0.850, p > 0.05).
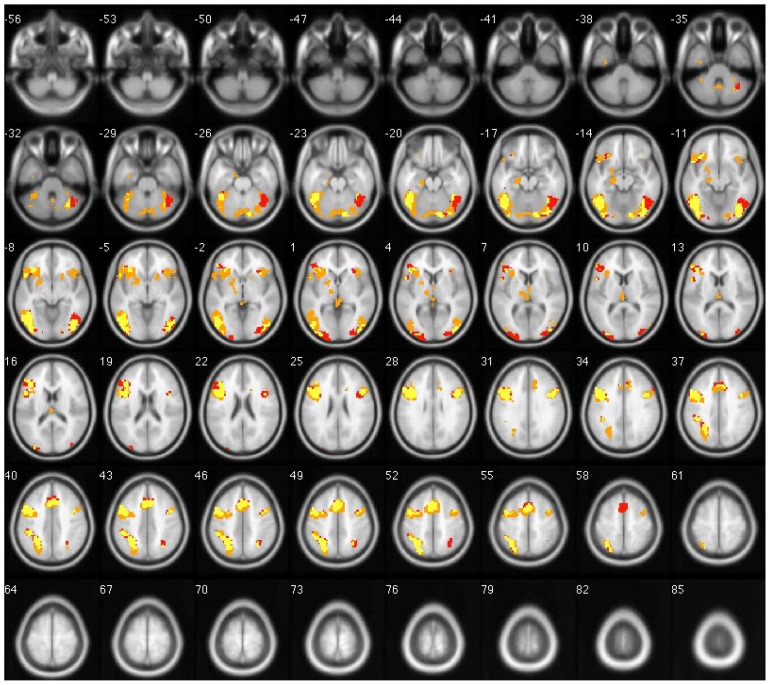
Late Uygur-Chinese bilinguals performed semantic judgment tasks in Uygur and Chinese with a calculated LI of 0.44 and 0.32, respectively. The activated left cortical volume was larger than the right for both languages (t = 4.989 and t = 5.130, respectively, p < 0.05), indicating left hemisphere dominance with semantic processing of L1 and L2 among late Uygur-Chinese bilinguals. There was no significant difference in LI between Uygur and Chinese language stimulation (t = 0.895, p > 0.05, Figs. 3 and 4).
Figure 4.
Overlapped brain images of averaged activation areas with Uygur and Chinese language stimulation in early bilinguals. Note: Chinese language is red; Uygur language is orange; yellow indicates voxels that passed all statistical criteria for activation during both Uygur and Chinese language tasks.
Overall, there was no statistically significant difference of cortical activation volume with L2 stimulation among Uygur-Chinese bilinguals (p > 0.05).
Discussion
In this study, we investigated differences in activated cortical areas and volumes in Uygur-Chinese bilinguals from Xinjiang of China and found that Chinese and Uygur characters activated largely overlapping brain regions. However, Uygur had a specific activation region in both early and late bilinguals. The dominant hemisphere for both languages was the left.
Comparison of activated cortical areas of two languages
In both Uygur and Chinese semantic judgment processes, we found the left inferior frontal gyrus was the most strongly activated cortical area with the largest activation volume. Consistent with our data, a study comparing English-Spanish bilinguals during a semantic judgment task concluded that the same neurological structures were activated in bilinguals when semantically processing both L1 and L2 [5]. These results indicated that the left inferior frontal gyrus is a very important area for semantic implementation and semantic judgment processes.
In the current study, we found that the left middle temporal gyrus of both early and late bilinguals was activated during semantic processing of the Uygur language. Activation of the right temporal gyrus was different between bilinguals, with the right superior temporal gyrus activated in early bilinguals and the right middle temporal gyrus activated in late bilinguals. There was no meaningful activation of this area during semantic judgment with the Chinese language. By studying brain language function areas using a visual input vocabulary task and a listening vocabulary phonology task, Booth et al. [9] found that the superior temporal gyrus (BA22, BA42) was activated when processing text phonemes, whereas the left middle temporal gyrus was activated when performing semantic processing. In monolingual English language speakers, activation of the left middle temporal gyrus is linked to phoneme elements [10]. Modern Uygur uses the Arabic alphabet and it read left-to-right in the upper-to-lower direction and follows the rule of sound to shape transformation. When identifying alphabetic writings, pronunciation can be directly spelled out using the alphabetic composition before lexical processing. When performing semantic tasks, the phonetic system is automatically activated in order to activate, extract, and assist semantic judgment. The Uygur language is an alphabetic text and is therefore processed phonetically. In contrast, Chinese is a pictographic expression language, and its stimulation tends to automatically activate semantic processes and retrieve information. Uygur and English language processing specifically activate the left middle temporal gyrus [10], and this explains why Uygurs in Xinjiang have an increased ability to learn the English language compared to their Chinese counterparts. Interestingly, we also found that the right superior temporal gyrus and the right middle temporal gyrus were activated in a small portion of study subjects. We speculate that these areas may be involved in alphabetic script semantic processing.
An area known as the visual word form area (VWFA) in the left fusiform gyrus has been identified for processing visual words in both alphabetic writing systems, such as English and French, and non-alphabetic writing systems, like Chinese [11–17]. Interestingly, Chinese and Korean characters engage essentially the same VWFA in early Chinese-Korean bilinguals, even at the level of fine spatial patterns [11]. These findings suggest that similar populations of neurons are engaged in processing different scripts within the same VWFA in early bilinguals. We found that regardless of L2 age of acquisition, processing of alphabetic or pictographic scripts activated the VWFA, which is excellent evidence for the universality of language processing.
Laterality in Uygur-Chinese bilinguals
In this study, comparison of laterality indices in early and late bilinguals demonstrated no significant difference between the Uygur and Chinese languages. Although the group of late bilinguals started learning L2 at various ages, their proficiency in Chinese was relatively high. The laterality indices were not significantly different between groups in this study, likely because bilinguals did not need to recruit right cortical areas to process semantic judgment.
Comparison of activated cortical volume by L2 in early and late Uygur-Chinese bilinguals
Perhaps the extent and strength of cortical activation by L2 is not related to the age of acquisition but to the degree of L2 proficiency [5, 18, 19]. However, other studies have found that some cortical areas are activated in a language-dependent manner, because the L2 age of acquisition is different [20, 21]. All fluent Uygur-Chinese bilinguals in our study exhibited overlapping activated cortical areas when processing L2. This is consistent with the hypothesis that L2 proficiency level rather than age of acquisition affects activated brain areas during semantic processing. We did not detect any statistically significant differences in activated cortical volume between early and late bilinguals, possibly due to the relatively high level of Chinese language proficiency in both early and late bilinguals. Our data suggest that the degree of L2 proficiency plays an important role the study of L2 effects on cortical activation.
Figure 5.
Overlapped brain images of averaged activation areas with Uygur and Chinese language stimulation in late bilinguals. Note: Chinese language is red; Uygur language is orange; yellow indicates voxels that passed all statistical criteria for activation during both Uygur and Chinese language tasks.
Acknowledgments
Conflict of interest statement: The authors report no conflicts of interest. We would like to thank Prof. Weijun Tang for his contribution to data analysis. This research was supported by the Department of Radiology, Huashan Hospital, Fudan University.
References
- 1.Luders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, et al. Basal temporal language area. Brain. 1991;114:743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- 2.Pillai JJ, Araque JM, Allison JD, Sethuraman S, Loring DW, Thiruvaiyaru D, et al. Functional MRI study of semantic and phonological language processing in bilingual subjects: preliminary findings. Neuroimage. 2003;19:565–576. doi: 10.1016/s1053-8119(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 3.Suh S, Yoon HW, Lee S, Chung JY, Cho ZH, Park H. Effects of syntactic complexity in L1 and L2; an fMRI study of Korean-English bilinguals. Brain Res. 2007;1136:178–189. doi: 10.1016/j.brainres.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Halsband U. Bilingual and multilingual language processing. J Physiol Paris. 2006;99:355–369. doi: 10.1016/j.jphysparis.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Illes J, Francis WS, Desmond JE, Gabrieli JD, Glover GH, Poldrack R, et al. Convergent cortical representation of semantic processing in bilinguals. Brain Lang. 1999;70:347–363. doi: 10.1006/brln.1999.2186. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: an fMRI study. Neuroimage. 2001;14:510–520. doi: 10.1006/nimg.2001.0810. [DOI] [PubMed] [Google Scholar]
- 7.Yari A. Modern Uygur Language. 1st Ed. Xinjiang Education Publishing House; Ürümqi, Xinjiang Uyghur Autonomous Region of China: 2004. [Google Scholar]
- 8.Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 9.Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, et al. Specialization of phonological and semantic processing in Chinese word reading. Brain Res. 2006;1071:197–207. doi: 10.1016/j.brainres.2005.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Shi J, Jiang Y, He S, Weng X. Chinese and Korean characters engage the same visual word form area in proficient early Chinese-Korean bilinguals. PLoS One. 2011;6:e22765. doi: 10.1371/journal.pone.0022765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci USA. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 2004;23:1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 14.Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- 15.Kuo WJ, Yeh TC, Duann JR, Wu YT, Ho LT, Hung D, et al. A left-lateralized network for reading Chinese words: a 3 T fMRI study. Neuroreport. 2001;12:3997–4001. doi: 10.1097/00001756-200112210-00029. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Zhang WT, Tang YY, Mai XQ, Chen HC, Tardif T, et al. The Visual Word Form Area: evidence from an fMRI study of implicit processing of Chinese characters. Neuroimage. 2008;40:1350–1361. doi: 10.1016/j.neuroimage.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431:71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Carpenter PA, Just MA. An fMRI study of bilingual sentence comprehension and workload. Neuroimage. 2002;15:647–660. doi: 10.1006/nimg.2001.1001. [DOI] [PubMed] [Google Scholar]
- 19.Perani D, Paulesu E, Galles NS, Dupoux E, Dehaene S, Bettinardi V, et al. The bilingual brain. Proficiency and age of acquisition of the second language. Brain. 1998;121:1841–1852. doi: 10.1093/brain/121.10.1841. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JS, Newport EL. Critical period effects in second language learning: the influence of maturational state on the acquisition of English as a second language. Cogn Psychol. 1998:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- 21.Frenck-Mestre C, Anton JL, Roth M, Vaid J, Viallet F. Articulation in early and late bilinguals’ two languages: evidence from functional magnetic resonance imaging. Neuroreport. 2005;16:761–765. doi: 10.1097/00001756-200505120-00021. [DOI] [PubMed] [Google Scholar]