Abstract
Background
Short-latency afferent inhibition (SAI) results when somatosensory afferent input inhibits the corticospinal output from primary motor cortex (M1). The present study examined SAI in the flexor carpi radialis (FCR) muscle in individuals with spinal cord injury (SCI) and uninjured controls.
Methods
Short-latency afferent inhibition (SAI) was evoked by stimulating the median nerve at the elbow at intervals of 15, 20 and 25 ms in advance of a transcranial magnetic stimulation (TMS) pulse over M1. SAI was tested with the FCR at rest and also during ~20% of maximum voluntary contraction. Corticospinal output was assessed through measuring both motor thresholds and motor evoked potential (MEP) recruitment curves. The afferent volley was assessed via the N20–P25 amplitude of the somatosensory evoked potential (SEP) and the amplitude of sensory nerve action potentials (SNAP) recorded over the median nerve at the elbow.
Results
SAI is reduced in SCI in both the contracted and non-contracted FCR muscle. MEP recruitment curves and thresholds were decreased in SCI only in the active state and not the resting state. N20–P25 amplitude was similar between groups in both the resting and active states although SNAP was significantly reduced in SCI at rest.
Conclusions
We conclude that reduced SAI in SCI is likely attributed to neuroplasticity altering the intrinsic M1 circuitry mediating SAI and/or reduced afferent input traversing a direct thalamocortical route to M1. These data provide a new avenue of research aimed at identifying therapeutic approaches to alter SAI to improve upper limb function in individuals with SCI.
Keywords: Afferent pathways, Motor evoked potential, Spinal cord injury, Cervical spinal cord, Short-latency afferent inhibition, Corticospinal tracts
Introduction
Neuroplasticity in sensorimotor cortex is likely to follow spinal cord injury (SCI) and would reflect changes in the integrity of afferent and efferent pathways. In humans, the integrity of sensorimotor cortical paths may be assessed via short-latency afferent inhibition (SAI) whereby the afferent volley elicited by peripheral nerve stimulation reduces the amplitude of the motor evoked potential (MEP) elicited by transcranial magnetic stimulation (TMS) over the primary motor cortex (M1) [1,2]. SAI is considered a cortically generated circuit evoked by the arrival of the peripheral afferent volley in the cortex and is mediated via neuronal circuitry within M1 [1].
The magnitude of SAI depends on the integrity of afferent transmission and the activity within the cortical circuitry that mediates SAI. If, in SCI, the afferent volley arriving at the cortex is reduced due to damage to the ascending pathway, a reduction in SAI is expected as the depth of SAI decreases with lower nerve stimulation intensities [3]. Additionally, substantial plastic changes may occur after SCI in either M1 or primary somatosensory cortex (S1). Within M1 the cortical territory responsible for controlling the muscles below the level of injury decrease in size and the muscles above the level of injury increase in size [4]. Decreasing the number of neurons responsible for controlling muscles below the level of injury may decrease the ability for an afferent stimulus to condition those neurons and elicit SAI. Following upper limb deafferentation, somatosensory and thalamic areas atrophy in primates [5] and may decrease extensive projections from primary somatosensory cortex (S1) to M1 [6, 7], thus decreasing SAI. One study in chronic SCI demonstrated a reduction in SAI in the contracted anterior tibial muscle [8], although the mechanism(s) for this effect remain unclear. At present, there are several unstudied questions regarding SAI in SCI that include whether abnormalities exist in upper limb muscles, whether they occur when the muscle is in the relaxed, non-contracted state, and whether the magnitude of SAI is different in SCI versus uninjured controls.
Determining whether SAI is reduced in SCI compared to controls is important since SAI is a sensorimotor circuit that is implicated in movement and plays an important role in the concept of surround inhibition [9–11]. Further, reductions in SAI in other clinical populations have been implicated in sensory driven long-term potentiation to motor cortex, which may promote motor learning and recovery [12]. In the present study, SAI was examined in the flexor carpi radialis (FCR) muscle in chronic cervical SCI and uninjured controls in both the non-contracted and contracted muscle states. In uninjured controls, SAI is observed in the FCR muscle when the interval between the nerve and cortical stimulation is between 13–20 ms [13]. Relative to other muscles of the upper limb, FCR function is often partially retained in individuals with SCI [14], which offers the opportunity to study its afferent regulation in both the contracted and non-contracted states. Moreover, we explored whether alterations in the SAI circuit would be due to changes in the transmission through afferent pathways by measuring the amplitude of sensory nerve action potentials (SNAP) and somatosensory evoked potentials (SEP), and transmission of efferent pathways through MEP. Our data indicate that SAI is indeed reduced in SCI compared to uninjured controls in both the contracted and non-contracted muscle. However, our data also suggest that an impaired afferent volley may contribute to reduced SAI via a route that acts independently of S1 (i.e. direct thalamocortical projection to M1) and/or changes associated with neuroplasticity within S1 and/or M1.
Subjects and methods
Participants
Thirteen limbs from eight adults with cervical spinal cord injury were studied (7 males; mean age = 30.8 ± 2.4; The American Spinal Injury Association (ASIA) impairment scale categories B–D). Table 1 provides demographic, lesion and medical information for all SCI participants. All SCI participants were capable of performing volitional wrist flexion in the limbs tested. One participant, P003, dropped out due to medical reasons and was not able to finish the study. Therefore, twelve limbs from seven adults with SCI (6 males; mean age = 30.7 ± 2.6, ASIA impairment scale categories B–D) were included in our final results. Twelve dominant limbs from aged-matched uninjured participants were studied for comparison (8 males; mean age = 29.15 ± 5.33). The Hamilton Integrated Research Ethics Board approved the study. All participants provided written consent prior to participation. Where applicable SCI subjects were tested bilaterally, and limbs were treated as separate individuals as performed elsewhere [8, 15].
Table 1.
Participant identification provided with level of injury, time after injury, cause of injury and The American Spinal Injury Association (ASIA) impairment scale categories to provide information regarding completeness of injury (below level of injury): A = complete; no motor or sensory function is preserved; B = incomplete; sensory but not motor function is preserved below level of injury; C = incomplete; motor function is preserved below level of injury, and more than half of key muscles below level of injury have a muscle grade of less than 3; D = incomplete; motor function is preserved below level of injury, and at least half of the key muscles below the neurological level have a muscle grade of 3 or more; E = motor and sensory functions are normal. Information about medications participants are currently taking and the limbs tests are also provided.
| Subject ID | Injury level | Years after injury | Cause of injury | ASIA score | Medications | Limb tested |
|---|---|---|---|---|---|---|
| P001 | C4–C5 | 11 | traumatic | C | baclofen | right & left |
| P002 | C3 | 2 | surgical | C | baclofen | right & left |
| P003 | C6–C7 | 2 | traumatic | B | baclofen, combination of acetaminophen and oxycodone | right |
| P004 | C5–C6 | 3 | traumatic | C | baclofen, docusate, gabapentin, pantoprazole, senna glycoside, tolterodine | right & left |
| P005 | C4 | 16 | traumatic | C | oxycodone, dihydromorphine, glucosamine | right |
| P006 | C5 | 14 | traumatic | B | nizatidine, docusate, oxybutynin, baclofen | right & left |
| P007 | C3–C4 | 7 | traumatic | B | fentanyl (transdermal patches), pregabalin, baclofen, zonadine (transdermal patches), oxybutynin, dihydromorphine | right |
| P008 | C5 | 9 | traumatic | D | diphenhydramine, combination of acetaminophen and oxycodone | right & left |
Electromyography (EMG) recordings
EMG was collected from the FCR using 9 mm diameter Ag-AgCl surface electrodes in a belly-tendon fashion. Manual palpation was performed during contraction and rest in all participants to locate the muscle belly. The active electrode was placed on average 3 cm distal and 2 cm lateral to the medial epicondyle. The reference electrode was placed over the tendons of the wrist and the ground electrode was placed over the medial styloid process of the wrist. EMG signals were amplified at a gain of 1000, band pass filtered with a high pass of 20 Hz and low pass of 25000 Hz and sampled at 5000 Hz (using CED 1401 data acquisition device, Cambridge Electronic Design Ltd., Cambridge, UK) for offline analysis (Signal v5).
Transcranial magnetic stimulation and Neuronavigation
Transcranial magnetic stimulation (TMS) was performed using a 50 mm inner diameter figure-of-eight branding coil connected to a Magstim 2002 stimulator (Magstim, Whitland, UK). Motor hotspot for FCR was determined as the location providing the most reproducible and largest MEP in the contralateral FCR muscle. The coil was positioned 45 degrees to the mid-sagittal line to induce current in a posterior to anterior direction. Resting motor threshold (RMT) was determined at this location and was defined as the lowest stimulator intensity required to elicit MEP ≥ 50 μV in 5 out of 10 consecutive trials. Active motor threshold (AMT) was defined as the lowest stimulator intensity required to elicit MEP of distinguishable from background contraction levels as determined by visual inspection in 5 out of 10 trials. During AMT participants contracted the FCR muscle to ~20% of their maximum voluntary contraction (MVC, described below) using visual feedback of their FCR EMG that was displayed as a bright line on an oscilloscope. Participants matched the position of their EMG controlled line to a second target line that represented 20% MVC. Brainsight Neuronavigation (Rogue Resolutions Ltd., Cardiff, UK) was used to target and track the location of these motor hotspots.
Corticospinal output
MEP recruitment curves were obtained in the active and resting FCR by stimulating the FCR hotspot at TMS intensities as a percentage of maximum stimulator output (MSO). The initial TMS intensity was set to 10% MSO and increased in increments of 10% every three stimulations. The MEP at each MSO was determined as the average of the three stimulations. MEPhalfmax was identified from the recruitment curve as the stimulation intensity where half of the maximal response was recorded. This intensity was further refined and confirmed through ten subsequent trials.
Short-latency afferent inhibition
The median nerve (MN) at the elbow was stimulated just lateral and proximal to the medial epicondyle, slightly medial to the medial edge of biceps. The MN was stimulated at 1.2 × motor threshold (for twitch in FCR) using a bar electrode with the cathode proximal (0.2 ms square wave monophasic pulse, SD9 stimulator, Grass Technologies, Warwick, RI, USA). TMS was delivered over the FCR hotspot at an intensity to evoke MEPhalfmax in the active or resting FCR. This intensity was adjusted online to maintain a half-max response in the active or resting FCR. To test SAI of FCR, the MN was stimulated at three interstimulus intervals in advance of the TMS pulse: 15, 20, 25 ms. These interstimulus intervals (ISI) approximate the arrival of the afferent volley in somatosensory cortex at ~15–18 ms following MN stimulation at the elbow [13] and account for additional delays due to traumatic injury to the ascending pathways. SAI was tested in each limb while the FCR was relaxed (i.e. rest) or actively contracted to ~20% MVC (active). Each ISI was repeated 10 times and ‘TMS only’ was repeated 20 times.
To assess the background EMG during afferent regulation measured at rest, the pre-stimulus (38 ms) area of the rectified EMG was measured for each epoch. This value was averaged for each ISI and TMS only trials. To assess the background EMG during afferent regulation measured in active FCR, the pre-stimulus (38 ms) area of the rectified EMG was measured for each epoch and normalized to a 38 ms window of the individual’s maximum voluntary contraction. To determine the MVC for FCR, participants were asked to maximally contract the forearm during wrist flexion. Three trials (each 3 s) were performed and separated by ~20 s to allow EMG to fall back to baseline while allowing participants to reach maximum EMG responses in each subsequent trial. To confirm that each trial reached a similar level between trials, and to test whether significant differences existed between our groups, a 2-way analysis of variance (ANOVA) was performed using within-subject factor TRIAL (first, second, third) and between-subject factor GROUP (SCI, group). This analysis revealed no significant differences across groups, trials, or their interaction indicating that participants did not fatigue during MVC acquisition and were performed similarly across groups. As described earlier, MVC was marked on an oscilloscope and was used to calculate 20% MVC to which a bright line was displayed. During afferent regulation in active FCR (which required 20% MVC), the EMG from FCR was displayed as bright line on the oscilloscope and participants would match the position of the FCR EMG line to the experimenter-defined 20% MVC target line.
Somatosensory evoked potentials and sensory nerve action potentials
SEP were acquired at C3′ contralateral to the MN stimulation and referenced to Fz. The ground electrode was placed over the clavicle. SEP were evoked through MN stimulation at the elbow just lateral and proximal to the medial epicondyle, slightly medial to the medial edge of biceps. The MN was stimulated at 1.2 × motor threshold (for twitch in FCR) using a bar electrode with the cathode proximal (0.2 ms square wave pulse, DS7AH, Digitimer Ltd., Hertfordshire, UK) delivered at a frequency of 3 Hz and were averaged over 500 sweeps. Sensory nerve action potentials (SNAP) were evoked by stimulation of the MN at the wrist and recorded using surface electrodes placed at the elbow with one located above the medial epicondyle and the other just below the bicipital groove. The MN was stimulated at 1.2 × motor threshold (for twitch in the musculus abductor pollicis brevis) using a bar electrode with the cathode proximal (0.2 ms square wave pulse, DS7AH, Digitimer Ltd.) delivered at a frequency of 3 Hz and were averaged over 500 sweeps. Both EEG and SNAP signals were sampled at a rate of 5000 Hz, amplified at a gain of 10000, band pass filtered with a high pass of 2 Hz and a low pass of 25000 Hz and collected (CED 1401) for offline analysis of the amplitude (N20–P25, SNAP) and latency (Signal v5). Since MN is stimulated at the elbow the latency of the N20 is expected to fall around 15 ms as observed elsewhere [13]. Where applicable, SEP were collected bilaterally.
Data analysis
All data was collected and stored for offline analysis using Signal v5 (Cambridge Electronic Design Ltd., Cambridge, UK). The area of the rectified MEP was measured. Afferent regulation was expressed as the ratio of the conditioned to unconditioned MEP (i.e. MEPnerve-TMS/MEPTMS). AMT and RMT were subject to unpaired one-tailed t-tests to test the hypotheses that each threshold measure would be greater in SCI compared to controls as observed elsewhere [16]. For MEP recruitment curves, a two-way ANOVA was performed with INTENSITY as the within-subject factor (10 levels; 10%–100%) and between-subject factor GROUP (2 levels; SCI, uninjured). For SAI, normalized MEP area (MEPnerve-TMS/MEPTMS) was subjected to a two-way ANOVA performed using within-subject factor ISI (4 levels; Test, 15, 20, 25) and between-subject factor GROUP (2 levels; SCI, uninjured). Background EMG was subject to two-way ANOVA with either ISI (4 levels; Test, 15, 20, 25) or INTENSITY (10 levels; 10, 20, 30, 40, 50, 60, 70, 80, 90, 100) as the within-subject factor and between-subject factor GROUP (2 levels; SCI, uninjured). SEP latency and amplitudes were subject to two-way ANOVA with STATE (2 levels; active, rest) as the within-subject factor and GROUP (2 levels; SCI, uninjured) as the between-subject factor. Group averaged SNAP amplitude was subject to unpaired two-tailed t-test. Post-hoc Tukey’s tests were conducted following significant ANOVA effects. Significance was set at p < 0.05 and if the assumptions of sphericity were not met, Greenhouse-Geisser corrections were used.
Results
Twelve limbs from seven adults with cervical SCI were studied. Reliable and robust MEP were obtainable in FCR during active contraction. However, only 9 limbs provided reliable, robust MEP in FCR during rest and subsequent analyses were performed only on these limbs for measures of RMT, MEP recruitment curve at rest, and afferent regulation at rest. Data from all twelve limbs were included in AMT, MEP recruitment curve during active contraction and afferent regulation during active contraction.
Threshold and corticospinal output
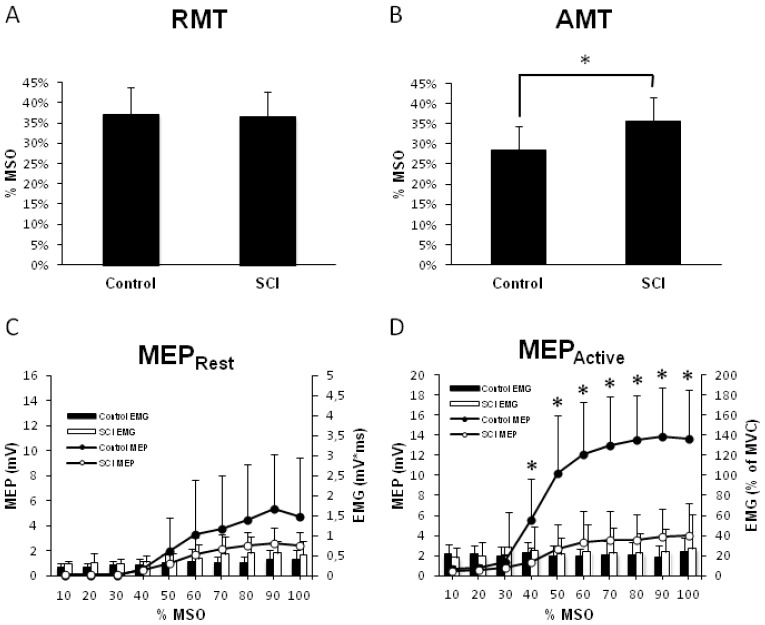
Group-averaged RMT and AMT are shown in Figure 1A and B. RMT was not different between groups (p = 0.87), and therefore we cannot reject the null hypothesis. AMT was significantly higher in SCI (p = 0.002).
Figure 1.
Corticospinal output to flexor carpi radialis (FCR) muscle. A) Group-averaged resting motor threshold (with standard deviations, SD) as a function of absolute TMS stimulator intensity in %MSO tested in both groups (n = 9 SCI, n = 9 controls). B) Group-averaged resting motor threshold (with SD) as a function of absolute TMS stimulator intensity in %MSO tested in both groups (n = 12 SCI, n = 12 controls). C) MEP recruitment curve in non-contracted FCR (n = 9 SCI, n = 9 controls). Group-averaged MEP amplitudes are represented in the line graphs (with SD). Histograms represent background activity in absolute terms (with SD). D) MEP recruitment curve in contracted FCR (n = 12 SCI, n = 12 controls). Group-averaged MEP amplitudes are represented in the line graph with SD. Asterisks represent group differences in MEP amplitude. Histograms represent background contraction expressed as a percent of their MVC (with SD). Abbreviations: FCR, flexor carpi radialis muscle; MEP, motor evoked potential; MSO, maximum stimulator output; MVC, maximum voluntary contraction; SCI, spinal cord injury; TMS, transcranial magnetic stimulation.
MEP recruitment curves with FCR at rest are shown in Figure 1C. Two-way ANOVA revealed a significant effect of INTENSITY (F1.29, 25.51 = 17.70, p < 0.001) with no GROUP effect (F1, 19 = 1.85, p = 0.189) or INTENSITY*GROUP interaction (F1.29, 25.51 = 2.085, p = 0.16). Background EMG was not different between groups (F1.085, 18.44 = 1.849, p = 0.19).
MEP recruitment curves with FCR contracted to ~20% MVC are shown in Figure 1D. Two-way ANOVA revealed significant effects of INTENSITY (F1.93, 42.38 = 67.42; p < 0.001), GROUP (F1, 22 = 32.82; p < 0.001) and an INTENSITY*GROUP interaction (F1.93, 42.38 = 23.91, p < 0.001). Post-hoc Tukeys test revealed MEP were greater from 40 to 100% MSO in controls compared to SCI (Tukey’s, p < 0.05). Background EMG was not different between groups (F3.056, 58.07 = 0.98, p = 0.41) showing both groups held a similar contraction. In summary, in SCI, AMT is increased and recruitment curves are reduced during active contraction.
SAI in FCR
Non-contracted, relaxed FCR
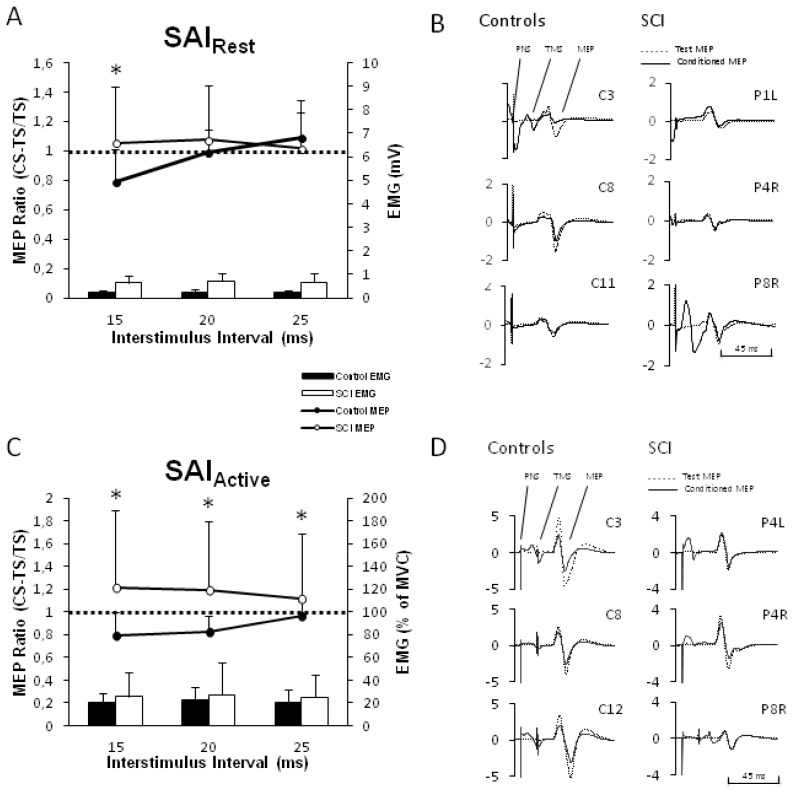
Figure 2A displays the group-averaged normalized SAI data in resting FCR for SCI and controls. Values below the horizontal line indicate afferent inhibition of the MEP. Two-way ANOVA revealed a significant ISI*GROUP interaction (F2, 32 = 4.31, p = 0.022). SAI was significantly reduced for SCI versus controls (Tukey’s, p < 0.05) at an ISI of 15 ms, which corresponds to the latency for SAI evoked by MN stimulation at the elbow [13]. Individual traces of SAI at the 15 ms ISI (conditioned and unconditioned MEP) from individual participants are shown in Figure 2B. Background EMG was not significantly different during SAI testing (GROUP (F1, 19 = 0.315, p = 0.581, ISI*GROUP (F1.44, 27.34 = 1.11, p = 0.326)). These indicate that SAI is indeed reduced in the relaxed FCR muscle in SCI. We note, however, that SAI is reduced in SCI but not facilitated as is seen in short-latency afferent facilitation (p = 0.37, 0.33, 0.45 for 15, 20, 25 ms, respectively).
Figure 2.
Short-latency afferent inhibition (SAI) in flexor carpi radialis (FCR) muscle. A) Group-averaged SAI in non-contracted, resting FCR (n = 9 SCI, n = 9 controls) shown as the ratio of the conditioned to unconditioned (i.e. TS alone) is represented in the line graphs (with standard deviation, SD). Asterisks indicate where SAI is reduced in SCI compared to controls (at ISI of 15 ms). Histograms represent background activity in absolute terms (with SD). B) Individual examples of SAI at 15 ms ISI. Averaged MEP amplitude at 15 ms ISI and TS alone for three control and three SCI limbs. Conditioned MEP is shown as solid black and test MEP (i.e. TS alone) as dashed line. Participants’ codes are shown. C) Group-averaged SAI in contracted, active FCR (n = 12 SCI, n = 12 controls) shown as the ratio of the conditioned to unconditioned (i.e. TS alone) is represented in the line graphs (with SD). Asterisks indicate where SAI is reduced in SCI compared to controls (at ISI of 15, 20 and 25 ms). Histograms represent background contraction expressed as a percent of their MVC with SD. D) Individual examples of SAI at 15 ms ISI. Averaged MEP amplitude at 15 ms ISI and TS alone for three control and three SCI limbs are shown. Conditioned MEP is shown as solid black and test MEP (i.e. TS alone) as dashed line. Participants’ codes are shown. Abbreviations: FCR, flexor carpi radialis muscle; ISI, interstimulus interval; PNS, peripheral nerve stimulation; TMS, transcranial magnetic stimulation; MEP, motor evoked potential; SAI, short-latency afferent inhibition; SCI, spinal cord injury; TMS, transcranial magnetic stimulation; TS, test stimulation pulse.
Contracted, active FCR
Figure 2C displays the group-averaged normalized SAI data in the active FCR for SCI and controls. Values below the horizontal line indicate afferent inhibition of the MEP. Two-way ANOVA revealed a significant ISI*GROUP interaction (F1.54, 35.43 = 6.35, p = 0.008). SAI was significantly reduced in SCI versus controls at the 15, 20 and 25 ms ISI (Tukey’s, p < 0.05). Individual traces of SAI at the 15 ms ISI from individual participants are plotted in Figure 2D. Background EMG was not significantly different during SAI testing (GROUP (F1, 17 = 2.42, p = 0.138), ISI*GROUP (F1.08, 18.45 = 3.9, p = 0.061). Similar to the findings at rest, these indicate that SAI is also reduced in the contracted FCR muscle in SCI.
Somatosensory evoked potentials and sensory nerve action potentials
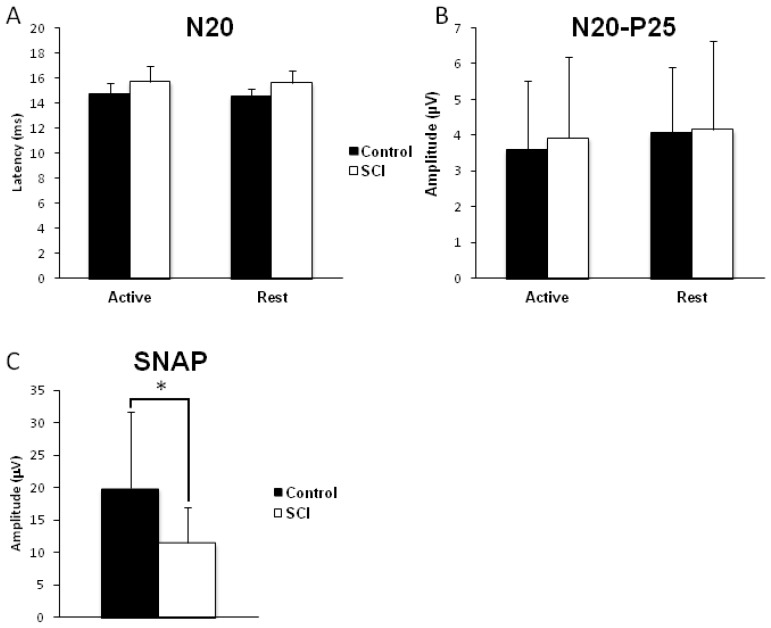
Figure 3A displays the group-averaged N20–P25 SEP latency in both the active and resting FCR for SCI and controls. Two-way ANOVA revealed no STATE*GROUP interaction (F1, 12 = 0.153, p = 0.702), no effect of STATE (F1, 12 = 0.646, p = 0.437) and no effect of GROUP (F1, 12 = 4.009, p = 0.068). The latencies observed in the SCI and control groups are similar to the latency observed in other studies performing MN stimulation at the elbow, ~15 ms [13]. Figure 3B displays the group-averaged N20–P25 SEP amplitude in both the active and resting FCR for SCI and controls. Two-way ANOVA revealed no STATE*GROUP interaction (F1, 12 = 0.2, p = 0.662), no effect of STATE (F1, 12 = 1.82, p = 0.202) and no effect of GROUP (F1, 12 = 0.038, p = 0.849). Figure 3C displays the group-averaged SNAP amplitude for SCI and control only in the resting state. SNAP amplitude was significantly reduced in the SCI group compared to controls (unpaired two-tailed t-test, p = 0.045).
Figure 3.
Somatosensory evoked potentials and sensory nerve action potentials. SEP were evoked via MN stimulation at the elbow, lateral and proximal to the medial epicondyle, slightly medial to the medial edge of biceps. SEP were collected from 7 limbs from 4 SCI participants and from 7 uninjured controls. SEP were collected and analyzed in both the active, where participants were asked to hold an isometric contraction equal to ~20% MVC and in the resting, where participants relaxed their forearm, states. SNAP were evoked via MN stimulation at the wrist just lateral to the palmar tendons of the wrist and were recorded over the MN at the elbow. A) Group-averaged SEP latency in both contracted (active) and non-contracted (resting) FCR for both SCI and controls are shown. ANOVA revealed no significant differences between the groups (p = 0.068) although a trend is emerging for longer SEP latency in the SCI group. The latency in both the SCI (~15.6 ms) and control (~14.7 ms) group are consistent with previous literature showing latency from this location being 15.4 ms [13]. B) Group-averaged N20–P25 amplitude in both contracted (active) and non-contracted (resting) FCR for both SCI and controls are shown. ANOVA revealed no significant differences between the groups (p = 0.849). C) Group-averaged SNAP amplitude in the non-contracted, resting, FCR for SCI and controls. Unpaired two-tailed t-tests revealed significant differences between the two groups (p = 0.045). Abbreviations: ANOVA, analysis of variance; FCR, flexor carpi radialis muscle; MN, median nerve; MVC, maximum voluntary contraction; SCI, spinal cord injury; SEP, somatosensory evoked potentials; SNAP, sensory nerve action potentials.
Summary of results
Individuals with SCI have reduced corticospinal excitability in the contracted muscle, reduced SNAP, reduced SAI and SEP similar to that in our control group. Collectively, these data may imply that reduced SAI is not mediated by changes in S1 activity but instead may result from alterations in the SAI circuitry within M1 and/or thalamocortical projections to M1.
Discussion
The present study revealed abnormalities in the magnitude of SAI in SCI. Compared to uninjured controls, SAI is reduced in SCI in both the contracted and relaxed FCR muscle. In SCI, corticospinal output to FCR is different from that of controls only during active contraction of the muscle. Further, in our sample of participants, SEP amplitude, which reflects the magnitude of the cortical afferent volley arriving at the primary somatosensory cortex, is not different between groups and therefore activation of S1 may not explain reduced SAI in SCI. However, measurements of the peripheral afferent volley (SNAP) show reductions in our SCI group and may imply that reduced afferents lead to altered SAI via a route that acts independently of S1 (i.e. direct thalamocortical projections to M1).
Mechanisms that mediate SAI and its reduction
The SAI circuit is very complex and the exact mechanisms that underpin its origin are relatively unknown. SAI is a cortically mediated circuitry modulated by the late I-wave generating neurons within M1 as peripheral nerve stimulation reduces the amplitude of the descending I3 wave [1]. SAI can be altered through many different mechanisms. SAI is increased with an increase in the peripheral afferent volley [3] and decreased with an increase in the amplitude of the descending efferent volley [17]. Further, any modifications to the corticocortical projection from S1 to M1 may potentially modify SAI, and changes to the late I-wave circuitry within M1 may alter SAI depth. Alternatively, the afferent volley responsible for conditioning the SAI circuitry may traverse a path that bypasses S1 altogether and projects directly from the thalamus to M1. Alterations to any one of these or their combination may result in changes to the strength of the SAI circuitry. In our study we observed a decrease in the peripheral afferent volley in our SCI group without changes in SEP amplitude. It is possible that our cohort had ~50% of their afferent tracts spared since SEP amplitudes saturate at approximately 50% of the afferent volley maximum [18]. Further, SCI may result in neuroplastic changes within S1 [5, 19] that may lead to alterations in the integrity of the corticocortical projection from S1 to M1, and possible neuroplastic changes to the I-wave circuitry within M1. Therefore, in our study the altered afferent volley still had a similar effect on the activation within S1, and may not explain the alterations seen in SAI. We propose that neuroplasticity associated with the corticocortical projections, or to the intrinsic M1 I-wave circuitry may explain the alterations seen in SAI and may be detected using alternate imaging methods. However, we cannot rule out the possibility that the reduced SNAP amplitude in SCI may result in a deficient afferent volley conveyed to M1 via a direct thalamocortical projection that bypasses S1.
Non-contracted FCR
With the FCR muscle relaxed, we observed similarities between SCI and controls in RMT, MEP recruitment curve and SEP. In contrast, our SCI group showed ~26% reduction in SAI compared to controls; in fact SAI was abolished in the SCI group and we observed ~43% reduction in SNAP in SCI compared to controls. This effect occurred specifically at the 15 ms interstimulus interval that corresponds to the latency of the cortical arrival of the afferent volley (Figure 3A). Reduced SAI does not result from abnormalities in the magnitude of the afferent volley terminating in S1 since the amplitude of the N20–P25 is within the normative range [20] and not different from our control group but may be explained by the reduced afferent volley that projects to M1 via a pathway independent of S1. Further, abolished SAI does not result from reduced corticospinal/ spinal output since the MEP recruitment curves are not substantially different between groups. Therefore, the absence of SAI is likely to result from changes in neuroplasticity altering the somatosensory to M1 projection (i.e. corticocortical) and/or the intrinsic M1 neuronal circuitry that mediates SAI or direct thalamocortical projections to M1.
Contracted FCR
With the FCR muscle voluntarily contracted, we observed several differences between SCI and controls. First, AMT was higher in SCI compared to controls. This effect may result if active contraction excites fewer neurons and/or the capacity to reduce the membrane potential is compromised in the SCI group. Increased AMT may relate to abnormalities in the function of voltage-gated Na+ channels [21] that regulate axon excitability [22]. Drugs that block voltage-gated Na+ channels increase motor threshold [23–26]. Further evidence to support alterations in voltage-gated Na+ channels is derived from animal models of SCI. Following SCI in rats, cortical motor neurons in layer V [27] and spinal motor neurons [29] have reduced expression of Na+ channels. At the spinal level, such changes are suggested to be a mechanism for the reduction in the H-reflex following SCI [28]. Second, compared to controls, MEP amplitude in SCI during active contraction was reduced from 40–100% MSO, and within this range MEP were not increased as a function of TMS intensity. One potential explanation for the reduction in MEP amplitude during active contraction may relate to alterations in the activity of the GABAA receptor. In support of this suggestion the GABAA mediated short-interval intracortical inhibition circuit is reduced in SCI participants during active contraction of the tibialis muscle in the lower limb [15]. Further, GABAA agonists reduce the slope and amplitude of MEP [29–34]. Therefore, it remains unclear whether GABAA receptor function is increased or decreased in our SCI population. Our MEP recruitment curve data, however, indicate that alterations likely exist in GABAA function in individuals with SCI. Further, considering that both AMT and active corticospinal output are reduced, individual with SCI may have abnormalities in the neural mechanisms required to bring upper and/or lower motor neurons closer to firing threshold through active contraction.
During active contraction, SAI in SCI was reduced compared to controls; in fact the SCI group appears to show short-latency afferent facilitation. However, paired t-tests on these data do not show significant facilitation (15 ms: p = 0.487; 20 ms: p = 0.468; 25 ms: p = 0.344). These data are consistent with SAI reductions in the anterior tibial muscle in SCI seen elsewhere [8]. SAI was abolished in SCI at latencies corresponding to the cortical termination of the afferent volley (i.e. 15 ms) and beyond, but the amplitude of N20–P25 was not different between groups. SNAP were unattainable during active contraction because muscle activity contaminated the SNAP traces. As such, we cannot comment on the integrity of the afferent volley during active contraction. Therefore, reduced SAI during active contraction may be explained by reduced integrity of the descending pathway, neuroplastic changes occuring within S1 [5], in the somatosensory to M1 projections and/or to the intrinsic motor circuitry that mediates SAI.
Limitations
We did not attempt to test individuals with SCI in their non-medicated state to avoid spasticity and other complications. We consider the contribution of baclofen, a GABAB agonist, minimal since the SAI circuitry is modulated via acetylcholine and/or GABAA [21, 35–37]. Our study has primarily focused on SAI and circuits that may better reflect changes in GABAB function, including the contralateral silent period and long-interval intracortical inhibition. Further, neuroimaging techniques such as high-field functional magnetic resonance imaging may reveal neuroplasticity effects within either S1 or M1 that may reduce SAI.
Conclusions
The data presented support the existence of neuroplasticity changes that have altered the cortical circuit that mediates SAI. Further, our data suggest that, in chronic tetraplegia, activating the upper and/or lower motorneurons via voluntary muscle contraction underestimates the magnitude of the residual corticospinal output to the FCR muscle. Artificial stimulation of the corticospinal tract via TMS in the relaxed muscle exposes the greater, residual capacity of the corticospinal tract. This suggestion is supported by the finding that MEP in SCI are obtainable via TMS in muscles that are incapable of voluntary contraction [14].
In conclusion, our study reveals novel findings of aberrant SAI cortical circuitry that outputs to the FCR muscle in chronic SCI. This circuit is abolished in both relaxed and contracted muscle, an effect that exists despite investigating a well recovered muscle and nerve combination. MVC, RMT, and SEP are all similar between the two groups and collectively argue for functional SAI. Moreover, reduced SAI is accompanied by reduced peripheral afferents that may alter SAI circuitry via thalamocortical projection to M1. However, reduced SAI may also be explained by neuroplasticity changes in cortical circuitry that mediates SAI. This work leads to a new avenue of research in chronic SCI populations aimed at increasing corticospinal output to active FCR and by increasing SAI, a sensorimotor circuit implicated in the control of movement [9, 11, 38], through altering either peripheral volley, projections from S1-M1 and/or the intrinsic neurons in M1 responsible for controlling SAI.
Acknowledgments
Conflict of interest statement: The authors report no conflict of interests. Aimee J. Nelson received funding from the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- 3.Fischer M, Orth M. Short-latency sensory afferent inhibition: conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. Brain Stimul. 2011;4:202–209. doi: 10.1016/j.brs.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Mikulis DJ, Jurkiewicz MT, McIlroy WE, Staines WR, Rickards L, Kalsi-Ryan S, et al. Adaptation in the motor cortex following cervical spinal cord injury. Neurology. 2002;58:794–801. doi: 10.1212/wnl.58.5.794. [DOI] [PubMed] [Google Scholar]
- 5.Jurkiewicz MT, Crawley AP, Verrier MC, Fehlings MG, Mikulis DJ. Somatosensory cortical atrophy after spinal cord injury: a voxel-based morphometry study. Neurology. 2006;66:762–764. doi: 10.1212/01.wnl.0000201276.28141.40. [DOI] [PubMed] [Google Scholar]
- 6.Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- 7.Jones EG, Powell TP. Connexions of the somatic sensory cortex of the rhesus monkey, I. Ipsilateral cortical connexions. Brain. 1969;92:477–502. doi: 10.1093/brain/92.3.477. [DOI] [PubMed] [Google Scholar]
- 8.Roy FD, Yang JF, Gorassini MA, Roy FD, Yang JF, Gorassini MA. Afferent regulation of leg motor cortex excitability after incomplete spinal cord injury. J Neurophysiol. 2010;103:2222–2233. doi: 10.1152/jn.00903.2009. [DOI] [PubMed] [Google Scholar]
- 9.Voller B, St Clair Gibson A, Dambrosia J, Pirio Richardson S, Lomarev M, Dang N, et al. Short-latency afferent inhibition during selective finger movement. Exp Brain Res. 2006;169:226–231. doi: 10.1007/s00221-005-0140-9. [DOI] [PubMed] [Google Scholar]
- 10.Asmussen MJ, Zapallow CM, Jacobs MF, Lee KGH, Tsang P, Nelson AJ. Modulation of short-latency afferent inhibition depends on digit and task-relevance. PLoS One. 2014;9:e104807. doi: 10.1371/journal.pone.0104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asmussen MJ, Jacobs MF, Lee KGH, Zapallow CM, Nelson AJ. Short-latency afferent inhibition modulation during finger movement. PLoS One. 2013;8:e60496. doi: 10.1371/journal.pone.0060496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lazzaro V, Profice P, Pilato F, Capone F, Ranieri F, Florio L, et al. The level of cortical afferent inhibition in acute stroke correlates with long-term functional recovery in humans. Stroke. 2012;43:250–252. doi: 10.1161/STROKEAHA.111.631085. [DOI] [PubMed] [Google Scholar]
- 13.Bertolasi L, Priori A, Tinazzi M, Bertasi V, Rothwell JC. Inhibitory action of forearm flexor muscle afferents on corticospinal outputs to antagonist muscles in humans. J Physiol. 1998;511:947–956. doi: 10.1111/j.1469-7793.1998.947bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards DJ, Cortes M, Thickbroom GW, Rykman A, Pascual-Leone A, Volpe BT. Preserved corticospinal conduction without voluntary movement after spinal cord injury. Spinal Cord. 2013;51:765–767. doi: 10.1038/sc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy FD, Zewdie ET, Gorassini MA. Short-interval intracortical inhibition with incomplete spinal cord injury. Clin Neurophysiol. 2011;122:1387–1395. doi: 10.1016/j.clinph.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Davey NJ, Smith HC, Savic G, Maskill DW, Ellaway PH, Frankel HL. Comparison of input-output patterns in the corticospinal system of normal subjects and incomplete spinal cord injured patients. Exp Brain Res. 1999;127:382–390. doi: 10.1007/s002210050806. [DOI] [PubMed] [Google Scholar]
- 17.Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh I-J, et al. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol. 2011;105:749–756. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- 18.Gandevia SC, Burke D. Saturation in human somatosensory pathways. Exp Brain Res. 1984;54:582–585. doi: 10.1007/BF00235486. [DOI] [PubMed] [Google Scholar]
- 19.Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair. 2007;21:527–538. doi: 10.1177/1545968307301872. [DOI] [PubMed] [Google Scholar]
- 20.Gott PS, Karnaze DS, Fisher M. Assessment of median nerve somatosensory evoked potentials in cerebral ischemia. Stroke. 1990;21:1167–1171. doi: 10.1161/01.str.21.8.1167. [DOI] [PubMed] [Google Scholar]
- 21.Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Bull Math Biol. 1990;52:25–71. doi: 10.1007/BF02459568. [DOI] [PubMed] [Google Scholar]
- 23.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 24.Kimiskidis VK, Papagiannopoulos S, Sotirakoglou K, Kazis DA, Kazis A, Mills KR. Silent period to transcranial magnetic stimulation: construction and properties of stimulus-response curves in healthy volunteers. Exp Brain Res. 2005;163:21–31. doi: 10.1007/s00221-004-2134-4. [DOI] [PubMed] [Google Scholar]
- 25.Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of diphenylhydantoin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 26.Chen R, Samii A, Caños M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- 27.Hains BC, Black JA, Waxman SG. Primary motor neurons fail to up-regulate voltage-gated sodium channel Nav1.3/brain type III following axotomy resulting from spinal cord injury. J Neurosci Res. 2002;70:546–552. doi: 10.1002/jnr.10402. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Pillai S, Wolpaw JR, Chen XY. Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci. 2006;23:141–150. doi: 10.1111/j.1460-9568.2005.04547.x. [DOI] [PubMed] [Google Scholar]
- 29.Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–937. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- 30.Schönle PW, Isenberg C, Crozier TA, Dressler D, Machetanz J, Conrad B. Changes of transcranially evoked motor responses in man by midazolam, a short acting benzodiazepine. Neurosci Lett. 1989;101:321–324. doi: 10.1016/0304-3940(89)90553-3. [DOI] [PubMed] [Google Scholar]
- 31.Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, et al. Lorazepam-induced effects on silent period and corticomotor excitability. Exp Brain Res. 2006;173:603–611. doi: 10.1007/s00221-006-0402-1. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadi B, Krampfl K, Petri S, Bogdanova D, Kossev A, Bufler J, et al. Selective and nonselective benzodiazepine agonists have different effects on motor cortex excitability. Muscle Nerve. 2006;33:778–784. doi: 10.1002/mus.20531. [DOI] [PubMed] [Google Scholar]
- 34.Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 35.Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol. 2005;569:315–323. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, et al. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- 37.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005;76:1064–1069. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voller B, St Clair Gibson A, Lomarev M, Kanchana S, Dambrosia J, Dang N, et al. Long-latency afferent inhibition during selective finger movement. J Neurophysiol. 2005;94:1115–1119. doi: 10.1152/jn.00333.2005. [DOI] [PubMed] [Google Scholar]