Abstract
The aim of this study was to examine the role played by substance P and calcitonin gene-related peptide (CGRP) within the dorsal horn of the spinal cord in engagement of antinociception evoked by dexmedetomidine (DEX). Paw withdrawal threshold (PWT) to mechanical stimulation was determined after chronic intrathecal infusion of DEX and enzyme-linked immunosorbent assay (ELISA) was employed to examine the levels of spinal substance P and CGRP. Our results show that PWT was significantly increased by intrathecal administration of DEX in rats (P < 0.05 vs. vehicle control, n = 20 in each group). Also, intrathecal infusion of DEX significantly decreased the concentrations of substance P and CGRP as compared with vehicle control (P < 0.05 DEX vs. vehicle control, n = 20 in each group). Blocking α2-adrenoreceptors (α2-AR) blunted the decreases of substance P and CGRP levels and the enhancement of PWT evoked by DEX. Additionally, a linear relationship was observed between PWT and the levels of spinal substance P (r = 0.87; P < 0.005) and CGRP (r = 0.85; P < 0.005). Moreover, blocking individual substance P and CGRP receptors amplified PWT without altering substance P and CGRP levels. Thus, DEX plays a role in stimulating α2-AR receptors, which thereby decreases substance P and CGRP levels within the dorsal horn. This contributes to DEX-evoked antinociception.
Keywords: Dexmedetomidine, Pain modulation, Substance P, Calcitonin gene-related peptide (CGRP), Dorsal horn
Introduction
Dexmedetomidine (DEX) is often used by intensive care units physicians and anesthesiologists because of its sedative effect on critically ill or injured patients [1, 2]. There are minimal contraindications to the use of DEX. This drug has recently been applied to non-intubated patients who require sedation for short-term surgeries or procedures [3–5]. DEX is also useful as an adjunct for sedation and general anesthesia in the setting of certain operations and invasive medical procedures, such as colonoscopy [3–5].
Similar to clonidine, DEX is an agonist of α2-adrenoreceptors (α2-AR) in the central nervous system, and both drugs have been used clinically for sedation and anesthesia [6]. They have been administered through various approaches, including systemic, intrathecal, epidural, or peripheral perineural injections [7]. In addition, prior studies have demonstrated that α2-AR are expressed in brainstem nuclei as well as in the superficial laminae of the spinal cord and primary afferent terminals [8–10]. These findings support the notion that DEX likely exerts an antinociceptive effect via α2-AR not only at the brainstem level, but also at the spinal and sensory nerve levels [11, 12]. Nevertheless, the precise mechanisms responsible for the antinociceptive effect of DEX at the spinal cord level require further investigation.
The superficial dorsal horn of the spinal cord is the first synaptic site for pain transmission from peripheral afferent nerves. In general, the levels of neurotransmitter release from the primary afferent nerve terminals into the dorsal horn play an important role in modulating pain [13]. Among many neurotransmitters, accumulated evidence suggests that substance P and calcitonin gene-related peptide (CGRP) within the superficial dorsal horn are particularly important for pain transmission to the central nervous system [13, 14].
Therefore, in our current study, we first examined the effects of chronic intrathecal infusion of DEX on paw withdrawal threshold (PWT) to mechanical stimulation as well as the concentrations of substance P and CGRP within the dorsal horn of the spinal cord of rats. We further examined if blocking α2-AR altered the PWT enhancement evoked by DEX and the levels of substance P and CGRP. A linear relationship was also analyzed between the levels of substance P/CGRP and PWT. Moreover, we examined if selectively blocking substance P, neurokinin 1 (NK-1) or CGRP receptors altered PWT and the levels of substance P and CGRP within the dorsal horn. We hypothesized that intrathecal administration of DEX stimulates α2-AR and thereby amplifies PWT via decreasing the levels of spinal substance P and/or CGRP and engagement of their receptors.
Methods
All animal protocols were approved by the Animal Care and Use Committee of Shandong University and were carried out in accordance with the guidelines of the International Association for the Study of Pain. Wistar rats (both male and female) weighing 200–250 g were obtained from the University Experimental Animal Center. The rats were housed in individual cages with free access to food and water and were kept in a temperature-controlled room (24 ± 1°C) on a 12/12 h light/dark cycle.
Eighty-five rats were anesthetized by intraperitoneal injection of sodium pentobarbital (60 mg/kg) in order to implant an intrathecal catheter for administration of drugs. An additional dose (10 mg/kg) was given during the surgery if needed. Briefly, one end of polyethylene-10 tubing was inserted intrathecally through an incision in the cisternal membrane and advanced 7–9 cm caudally until the tip of the catheter was positioned at the lumbar spinal level (L5 to L6). The other end of the intrathecal tubing was sutured to the musculature and skin at the incision site and externalized to the back of the rat. An osmotic mini pump (Durect Corporation, Cupertino, CA, USA) was implanted subcutaneously and connected to the intrathecal tubing. The pump was filled with either 200 μl of artificial cerebrospinal fluid (aCSF, n = 20) as control, DEX (n = 20, 50 μM; obtained from Sigma-Aldrich, St. Louis, MO, USA) or a combination of DEX and α2-AR antagonist BRL44408 (n = 15, 50 μM; obtained from Tocris Bioscience, Ellisville, MO, USA) [15, 16], respectively.
In a subset of studies (n = 10 in each of following experiments), using the same methods, an osmotic mini pump was used to intrathecally deliver NK-1 receptor antagonist CP-96345 (300 nM, Pfizer Research, Groton, CT, USA) and CGRP antagonist CGRP 8-37 (2 mg/ml, Bachem, Torrance, CA, USA), respectively [17]. These experiments were conducted to examine the pathways by which DEX acts.
The pump was designed to constantly deliver the vehicle or the antagonists at 1 μl/h for 7 days after an initial activation period of 4 hours inside the animal. Thus, the rats were allowed to receive the vehicle or the antagonists for 7 days following the procedure. Only animals that showed no evidence of neurological deficits after catheter implantation were used for this study.
The PWT value was determined after each intrathecal infusion. After measuring PWT, the superficial dorsal horn tissues of the spinal cord (at the L4–L6 levels) were obtained under an anatomic microscope from each group of rats for enzyme-linked immunosorbent assay (ELISA) measurements.
To quantify the mechanical sensitivity of the hindpaw, rats were placed in individual plastic boxes and allowed to acclimate for > 30 min. Mechanical PWT of the rat hindpaw in response to the stimulation of von Frey filaments was determined. A series of calibrated von Frey filaments (ranging from 0.5 to 20 g) were applied perpendicularly to the plantar surface of the hindpaw with sufficient force to bend the filament for 60 s or until the paw withdrew. In the presence of a response, the filament of next lower force was applied. In the absence of a response, the filament of next greater force was applied. To avoid injury during testing, the cutoff strength of the von Frey filament was 15 g. A tactile stimulus producing a 50% likelihood of withdrawal was determined using the “up-down” method [18]. Each trial was repeated 2 times at approximately 2 min intervals. The mean value was used as the force to produce a withdrawal response. A total of three trials in each animal were performed and 10 min were allowed between two consecutive trials. An interval time of 10 min was selected since it was sufficient to obtain a recovery of PWT. All the studies were performed using blind testing.
To examine the levels of substance P and CGRP in the dorsal horn of the spinal cord, ELISA was used. Substance P was measured using a substance P ELISA kit following the manufacturer’s instructions (Enzo Life Sci Inc., Farmingdale, NY, USA). Briefly, the diluted tissue supernatant (100 μl) was placed in a 96-well goat anti-mouse IgG-coated plate and incubated for 2 h. After incubation, the plate was washed using the provided washing buffer, and the color was developed by adding p-nitrophenyl phosphate (pNPP) (200 μl) substrate after 45 min and determined by an ELISA plate reader. The amount of substance P was calculated by using a substance P standard curve. In a similar way, the CGRP content of the samples (100 μl supernatant) was determined using a commercial CGRP ELISA kit (Cayman Chemical Co., Ann Arbor, MI, USA). In summary, the diluted samples were placed in a 96-well plate incubated with precoated anti-rat IgG antibody overnight, washed and developed, and quantified [19].
All data were analyzed using a one-way repeated-measures analysis of variance (ANOVA). Values were presented as means ± standard deviation (SD). For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SAS for Windows version 9.13 (SAS Institute, Cary, NC, USA).
Results
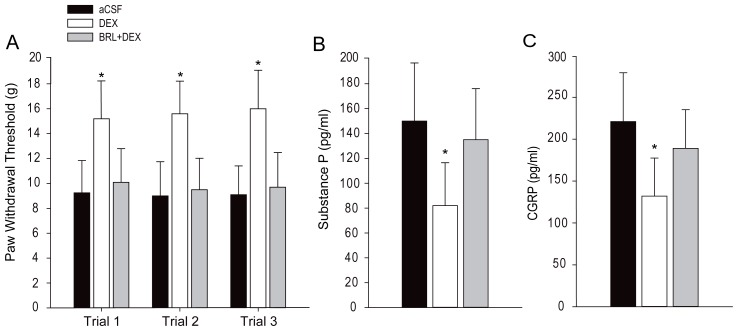
Figure 1A shows that intrathecal infusion of 50 μM of DEX (n = 20) significantly increased PWT as compared with aCSF (n = 20; P < 0.05, DEX vs. aCSF). No significant differences for the effects of DEX were observed among the three trials (P > 0.05). In addition, co-injection of α2-AR antagonist BRL44408 (50 μM) significantly blunted the enhanced PWT evoked by DEX (n = 15; P < 0.05, DEX vs. DEX plus BRL44408). Figure 1B and C shows that DEX significantly decreased the levels of substance P and CGRP within the dorsal horn and blocking α2-AR increased the levels of substance P and CGRP.
Figure 1.
Effects of dexmedetomidine (DEX). (A) The effects of DEX on paw withdrawal threshold (PWT). Intrathecal administration of DEX (50 μM) increased PWT in rats as compared with aCSF infusion. Blocking α2-AR blunted DEX-evoked effects. (B and C) Intrathecal adminstration of DEX decreased the concentrations of substance P and CGRP within the dorsal horn of the spinal cord. BRL44408 (50 μM), an antagonist of α2-AR, largely restored the levels of substance P and CGRP that were attenuated by DEX. Data are expressed as mean ± standard deviation. *P < 0.05 DEX vs. aCSF and DEX plus BRL44408. The number of rats = 20 in each of the aCSF and DEX groups; the number of rats in the group with DEX plus BRL44408 = 15. Legend: aCSF, artificial cerebrospinal fluid; CGRP, calcitonin gene-related peptide.
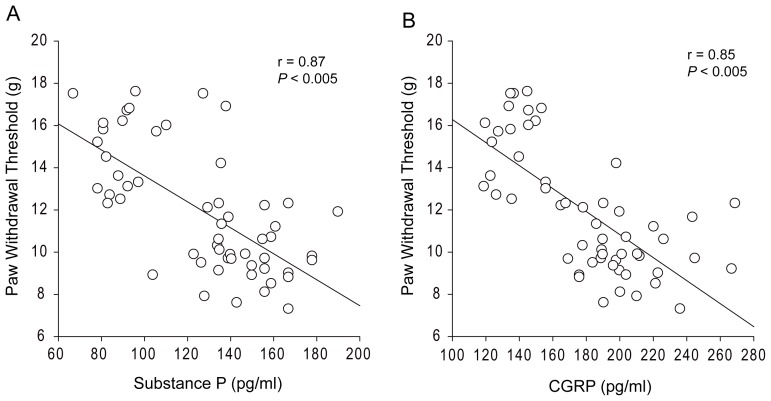
Moreover, a linear regression for mechanical withdrawal thresholds and the levels of substance P and CGRP was analyzed. Figure 2A and B further demonstrates that there was a close relationship between the levels of spinal substance P and PWT (r = 0.87 and P < 0.01), and levels of CGRP and PWT (r = 0.85 and P < 0.01).
Figure 2.
Linear relation analysis. There is a close relationship between paw withdrawal threshold (PWT) and the levels of spinal substance P (A), and between PWT and the levels of spinal CGRP (B). The number of animals is 20 for the aCSF control; 20 for dexmedetomidine (DEX) injection and 15 for DEX injection with prior administration of α2-AR inhibitor. Legend: CGRP, calcitonin gene-related peptide; aCSF, artificial cerebrospinal fluid.
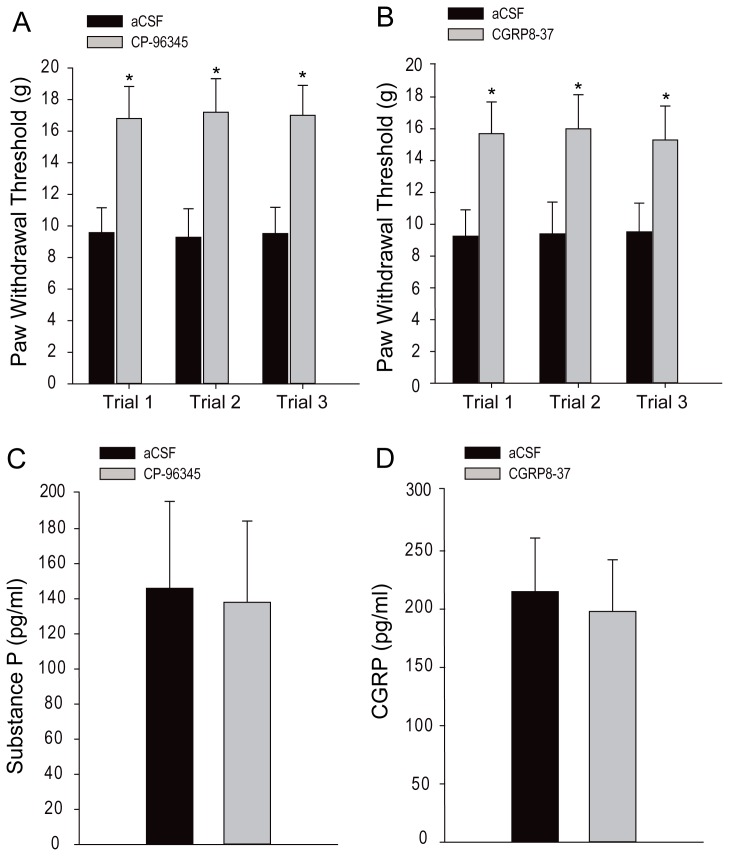
Figure 3A and B shows, respectively, the effects of blocking substance P and CGRP receptors on PWT and the levels of substance P and CGRP within the dorsal horn of the spinal cord. Chronic intrathecal administration of antagonists to NK-1 (n = 10, CP-96345 at 300 nM) and CGRP receptors (n = 10, CGRP 8-37 at 2 mg/ml) significantly increased PWT as compared with the aCSF injection (n = 10; P < 0.05 vs. both antagonists). However, the levels of substance P and CGRP within the dorsal horn were not significantly altered (P > 0.05, aCSF vs. both antagonists; shown in Figure 3C and D).
Figure 3.
Effects of blocking NK-1 and CGRP receptors on paw withdrawal threshold (PWT) and the levels of substance P and CGRP within the dorsal horn of the spinal cord. Intrathecal administration of antagonists to NK-1 (CP-96345, n = 10 at 300 nM) and CGRP receptors (n = 10, CGRP 8-37 at 2 mg/ml) significantly increased PWT as compared with only aCSF injection (n = 10) (A and B). However, the levels of substance P and CGRP within the dorsal horn were not significantly altered by blocking NK-1 and CGRP receptors (B and C). Data are expressed as mean ± standard deviation. *P < 0.05 vs. aCSF control. Legend: CGRP, calcitonin gene-related peptide; aCSF, artificial cerebrospinal fluid.
Discussion
This study demonstrated that intrathecal administration of DEX significantly amplified mechanical PWT and decreased the concentrations of substance P and CGRP within the superficial dorsal horn of the spinal cord as compared with vehicle controls. Blocking α2-AR largely restored the decreased levels of substance P and CGRP and blunted the enhancement of PWT evoked by DEX. Furthermore, blocking the respective NK-1 and CGRP receptors increased PWT without altering the levels of substance P and CGRP. Thus, we suggest that substance P and CGRP within the dorsal horn play an important role in regulating DEX-evoked antinociception. Attenuation of NK-1 and CGRP receptors failed to alter substance P and CGRP levels within the dorsal horn per se, but resulted in antinociception. Also, the role played by DEX is via stimulating α2-AR and thereby decreasing substance P and CGRP within the dorsal horn.
Prior studies have demonstrated that α2-AR have 3 subtypes, namely α2A-, α2B- and α2C-AR [20]. The α2A- and α2C-AR are Gi/Go protein coupled receptors [20]. In general, activation of Gi/Go protein coupled receptors inhibits cyclic adenosine monophosphate production and results in the opening of K+ channels and the closing of voltage-gated Ca2+ channels, with consequent hyperpolarization and reduced rate of firing of excitable cells [20]. The α2-AR have been found in different areas involved in pain control, including the dorsal root ganglion and the dorsal horn of the spinal cord [8–10]. It should be noted that in the central and peripheral nervous systems, α2A-AR play a primary role in regulating DEX, but α2C-AR play a little role [21, 22].
With respect to pain treatment, peripheral, spinal and supraspinal α2-AR are responsible for the mechanism of DEX. Presynaptic stimulation of α2-AR inhibits neurotransmitter release from primary afferent fibers. Postsynaptic stimulation of α2-AR at the level of the spinal cord increases acetylcholine concentrations in the superficial dorsal horn and inhibits nociceptive neurotransmission by reducing the release of neurotransmitters such as substance P and glutamate [22–24]. Nevertheless, in our current study, we found that intrathecal administration of DEX decreased the levels of substance P and CGRP. It has been reported that α2-AR are expressed on presynaptic sites of the primary afferent nerve terminals and postsynaptic neurons of the dorsal horn of the spinal cord [25, 26]. Thus, it is well reasoned that stimulation of α2-AR attenuates the release of substance P and CGRP via either presynaptic or postsynaptic mechanisms. Interestingly, in the current study we found that blocking NK-1 and CGRP receptors failed to alter substance P and CGRP levels within the dorsal horn, but increased PWT. Thus, it is unlikely that stimulation of NK-1 and CGRP receptors leads to releases of substance P and CGRP in the dorsal horn under tonic conditions.
Prior studies showed that the activation of α2-AR causes potassium efflux from Gi-protein-gated potassium channels in neuronal cells and this results in hyperpolarization and suppressed neuronal firing [22, 27]. It has also been reported that activation of α2-AR by DEX results in Gs-protein-mediated facilitation of acetylcholine release from the spinal dorsal horn synaptosomes in vitro [28, 29]. In addition, spinal α2-AR stimulation produces analgesia in animals with neuropathic pain, and this effect is blocked after inhibition of the brain-derived neurotrophic factor (BDNF) signaling pathway [30]. DEX can also inhibit glial cell hypertrophy in the spinal dorsal horn and activate the extracellular signal-regulated kinase (ERK) signaling pathway [31, 32].
Evidence has further demonstrated synergistic interactions that result from co-application of DEX and opioids or local anesthetics. α2-AR are responsible for the analgesic function of DEX at systemic and regional levels [7]. Using acute and chronic rat pain models, studies have shown that the antinociceptive systemic synergism of opioids and DEX is mediated by peripheral mu (μ) and delta (δ) opioid receptors and α2-AR [1, 33, 34]. It has also been reported that a synergy between δ opioid receptors and α2-AR agonists inhibit the release of CGRP from the primary afferent nerve terminals [35]. Systemic administration of morphine also enhanced the analgesic effect of intrathecal DEX via up-regulation of α2A, α2B and α2C-AR in the lumbar dorsal root ganglion and dorsal horn [36]. Consistent with these prior results [35], we demonstrated the role played by CGRP in mediating the antinociceptive effects of DEX.
Conclusion
DEX activation of α2-AR at the spinal cord level has antinociceptive effects mediated by reduced substance P and CGRP levels and engagement of their receptors. Alterations in these receptors in patients have a clinical implication for using DEX. Results of this study provided a base for the mechanisms responsible for DEX regulating pain and will further offer a strategy to target the sensory nerve system for patients who receive DEX in the intensive care unit or during surgical procedures.
Acknowledgment
Conflict of interest statement: The authors declare that they have no conflicts of interest.
References
- 1.Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009;43:2064–2074. doi: 10.1345/aph.1M310. [DOI] [PubMed] [Google Scholar]
- 2.Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol. 2005;18:412–418. doi: 10.1097/01.aco.0000174958.05383.d5. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor M, Bucknall T, Manias E. Sedation management in Australian and New Zealand intensive care units: doctors’ and nurses’ practices and opinions. Am J Crit Care. 2010;19:285–295. doi: 10.4037/ajcc2009541. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 5.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 6.Cormack JR, Orme RM, Costello TG. The role of α2-agonists in neurosurgery. J Clin Neurosci. 2005;12:375–378. doi: 10.1016/j.jocn.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Bai X. New therapeutic uses for an α2 adrenergic receptor agonist - dexmedetomidine in pain management. Neurosci Lett. 2014;561:7–12. doi: 10.1016/j.neulet.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Happe HK, Coulter CL, Gerety ME, Sanders JD, O’Rourke M, Bylund DB, et al. α-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Stamer WD, Anthony TL, Kumar DV, St John PA, Regan JW. Expression of α2-adrenergic receptor subtypes in prenatal rat spinal cord. Dev Brain Res. 2002;133:93–104. doi: 10.1016/s0165-3806(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 10.Shi TS, Winzer-Serhan U, Leslie F, Hökfelt T. Distribution and regulation of α2-adrenoceptors in rat dorsal root ganglia. Pain. 2000;84:319–330. doi: 10.1016/s0304-3959(99)00224-9. [DOI] [PubMed] [Google Scholar]
- 11.Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, et al. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansikka H, Lahdesmaki J, Scheinin M, Pertovaara A. α2A adrenoceptors contribute to feedback inhibition of capsaicin-induced hyperalgesia. Anesthesiology. 2004;101:185–190. doi: 10.1097/00000542-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Hua X-Y, Yaksh TL. Dorsal horn substance P and NK1 receptors: study of a model system in spinal nociceptive processing. In: Malcangio M, editor. Synaptic plasticity in pain. Springer; London: 2009. pp. 109–138. [Google Scholar]
- 14.Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 15.de Souza GG, Duarte ID, de Castro Perez A. Differential involvement of central and peripheral α2 adrenoreceptors in the antinociception induced by aerobic and resistance exercise. Anesth Analg. 2013;116:703–711. doi: 10.1213/ANE.0b013e31827ab6e4. [DOI] [PubMed] [Google Scholar]
- 16.Takano M, Takano Y, Yaksh TL. Release of calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal polypeptide (VIP) from rat spinal cord: modulation by α2 agonists. Peptides. 1993;14:371–378. doi: 10.1016/0196-9781(93)90055-l. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Shenoy M, Pasricha PJ. Substance P and calcitonin gene related peptide mediate pain in chronic pancreatitis and their expression is driven by nerve growth factor. JOP. 2011;12:389–394. [PMC free article] [PubMed] [Google Scholar]
- 18.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Zhao J, Wang J, Li J, Yu S, Guo X. Deficiency of female sex hormones augments PGE and CGRP levels within midbrain periaqueductal gray. J Neurol Sci. 2014;346:107–111. doi: 10.1016/j.jns.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald E, Kobilka BK, Scheinin M. Gene targeting - homing in on α2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 21.Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci. 2008;27:3182–3190. doi: 10.1111/j.1460-9568.2008.06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Cui S, Liu Y, Zhang J, Zhang W, Zhang J, et al. Dexmedetomidine prevents remifentanil-induced postoperative hyperalgesia and decreases spinal tyrosine phosphorylation of N-methyl-D-aspartate receptor 2B subunit. Brain Res Bull. 2012;87:427–431. doi: 10.1016/j.brainresbull.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Chiu KM, Lin TY, Lu CW, Wang SJ. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by α2 adrenoceptor agonist dexmedetomidine. Eur J Pharmacol. 2011;670:137–147. doi: 10.1016/j.ejphar.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett. 2012;529:70–74. doi: 10.1016/j.neulet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Kamisaki Y, Hamada T, Maeda K, Ishimura M, Itoh T. Presynaptic α2 adrenoceptors inhibit glutamate release from rat spinal cord synaptosomes. J Neurochem. 1993;60:522–526. doi: 10.1111/j.1471-4159.1993.tb03180.x. [DOI] [PubMed] [Google Scholar]
- 26.Pan YZ, Li DP, Pan HL. Inhibition of glutamatergic synaptic input to spinal lamina IIo neurons by presynaptic α2-adrenergic receptors. J Neurophysiol. 2002;87:1938–1947. doi: 10.1152/jn.00575.2001. [DOI] [PubMed] [Google Scholar]
- 27.Shirasaka T, Kannan H, Takasaki M. Activation of a G protein-coupled inwardly rectifying K+ current and suppression of Ih contribute to dexmedetomidine-induced inhibition of rat hypothalamic paraventricular nucleus neurons. Anesthesiology. 2007;107:605–615. doi: 10.1097/01.anes.0000281916.65365.4e. [DOI] [PubMed] [Google Scholar]
- 28.Hayashida K, Eisenach JC. Spinal α2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010;113:406–412. doi: 10.1097/ALN.0b013e3181de6d2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashida K, Peters CM, Gutierrez S, Eisenach JC. Depletion of endogenous noradrenaline does not prevent spinal cord plasticity following peripheral nerve injury. J Pain. 2012;13:49–57. doi: 10.1016/j.jpain.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashida K, Eisenach JC. A tropomyosine receptor kinase inhibitor blocks spinal neuroplasticity essential for the anti-hypersensitivity effects of gabapentin and clonidine in rats with peripheral nerve injury. J Pain. 2011;12:94–100. doi: 10.1016/j.jpain.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Zhao Z, Code WE, Hertz L. A correlation between dexmedetomidine-induced biphasic increases in free cytosolic calcium concentration and energy metabolism in astrocytes. Anesth Analg. 2000;91:353–357. doi: 10.1097/00000539-200008000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Ji F, Liang J, He H, Fu Y, Cao M. Inhibition by dexmedetomidine of the activation of spinal dorsal horn glias and the intracellular ERK signaling pathway induced by nerve injury. Brain Res. 2012;1427:1–9. doi: 10.1016/j.brainres.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Guneli E, Karabay Yavasoglu NU, Apaydin S, Uyar M, Uyar M. Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol Biochem Behav. 2007;88:9–17. doi: 10.1016/j.pbb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Ulger F, Bozkurt A, Bilge SS, Ilkaya F, Dilek A, Bostanci MO, et al. The antinociceptive effects of intravenous dexmedetomidine in colorectal distension-induced visceral pain in rats: the role of opioid receptors. Anesth Analg. 2009;109:616–622. doi: 10.1213/ane.0b013e3181a9fae2. [DOI] [PubMed] [Google Scholar]
- 35.Overland AC, Kitto KF, Chabot-Dore AJ, Rothwell PE, Fairbanks CA, Stone LS, et al. Protein kinase C mediates the synergistic interaction between agonists acting at α2-adrenergic and δ-opioid receptors in spinal cord. J Neurosci. 2009;29:13264–13273. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamagaki S, Suzuki T, Hagihira S, Hayashi Y, Mashimo T. Systemic daily morphine enhances the analgesic effect of intrathecal dexmedetomidine via up-regulation of α2 adrenergic receptor subtypes A, B and C in dorsal root ganglion and dorsal horn. J Pharm Pharmacol. 2010;62:1760–1767. doi: 10.1111/j.2042-7158.2010.01192.x. [DOI] [PubMed] [Google Scholar]