Abstract
Background. Vaccination and passive antibody therapies are critical for controlling infectious diseases. Passive antibody administration has limitations, including the necessity for purification and multiple injections for efficacy. Vaccination is associated with a lag phase before generation of immunity. Novel approaches reported here utilize the benefits of both methods for the rapid generation of effective immunity.
Methods. A novel antibody-based prophylaxis/therapy entailing the electroporation-mediated delivery of synthetic DNA plasmids encoding biologically active anti–chikungunya virus (CHIKV) envelope monoclonal antibody (dMAb) was designed and evaluated for antiviral efficacy, as well as for the ability to overcome shortcomings inherent with conventional active vaccination and passive immunotherapy.
Results. One intramuscular injection of dMAb produced antibodies in vivo more rapidly than active vaccination with an anti-CHIKV DNA vaccine. This dMAb neutralized diverse CHIKV clinical isolates and protected mice from viral challenge. Combination of dMAb and the CHIKV DNA vaccine afforded rapid and long-lived protection.
Conclusions. A DNA-based dMAb strategy induced rapid protection against an emerging viral infection. This method can be combined with DNA vaccination as a novel strategy to provide both short- and long-term protection against this emerging infectious disease. These studies have implications for pathogen treatment and control strategies.
Keywords: passive antibody prophylaxis and therapy, monoclonal antibody, DNA plasmid, dMAb, chikungunya virus
Active vaccination and passive immunotherapy rank among the greatest medical achievements. However, improvements in these immune-based medical interventions are required. This article describes a novel strategy to develop short- and long-term protective immunity against chikungunya virus (CHIKV) infection, using a relevant emerging infectious disease model. CHIKV is a mosquito-borne RNA pathogen that has infected millions [1, 2]. A precipitous increase in cases of CHIKV infection and disease has been recently reported [3, 4], along with an increase in morbidity and mortality, suggesting increased virulence [5, 6]. These findings underscore the importance for developing anti-CHIKV prophylaxis and therapies [1, 7]. To date, however, no CHIKV vaccine has been licensed, although a variety of strategies are being evaluated [8–13]. Importantly, anti-CHIKV neutralizing antibody titers may be a protective immune correlate [14–16]. Passive immunotherapy has been an important short-term intervention against several infectious diseases, including monoclonal antibody (mAb) prophylaxis against respiratory syncytial virus [17]. However, passive antibody delivery has limitations because of the short half-life of immunoglobulins [18–21].
Conventional vaccines typically require a lag phase before antibody generation, in addition to multiple immunizations, to be effective [18, 22]. Furthermore, vaccination-induced protection can be problematic in some populations (ie, immunocompromised individuals), limiting immune control of infection outbreaks in these groups. The rapid local spread of CHIKV underscores the importance of conferring effective and timely immune protection [7, 23]. While a passive antibody therapy strategy is an attractive method for a short-term intervention against viruses such as CHIKV [2, 14, 24], the cost, production complexity, and cold chain requirements limit this approach. Therefore, the development of novel immunotherapeutic/prophylactic modalities that overcome these limitations is warranted. One such strategy is the in vivo delivery of expression plasmids encoding genes for the immunoglobulin chains of established functional mAbs. This approach bypasses conventional antibody production and may present unique opportunities for therapy, including combination with vaccines.
Our group has recently described an in vivo–delivery method that involves electroporation of DNA plasmids encoding mAb (designated dMAb), rather than viral vectors [25]. Compared with viral vector–mediated platforms (ie, adeno-associated viral vectors) for mAb delivery [26, 27], naked DNA plasmids represent a nonlive, nonintegrating, and noninfectious platform that does not generate antivector immunity [28–30]. Accordingly, it may have advantages for rapid antibody production and readministration because of the lack of serological interference often encountered with conventional immune-based strategies.
In this study, we demonstrate that in vivo production and delivery of a CHIKV dMAb derived from an established anti-CHIKV envelope (Env) human neutralizing mAb resulted in seroconversion, which could protect against lethal in vivo viral challenge. The effectiveness of dMAb delivery, when coadministered with a CHIKV Env antigen–based DNA vaccine, was also evaluated. This combination approach resulted in both short- and long-term protection from lethal CHIKV challenge. This strategy may have implications against CHIKV and other infectious diseases.
METHODS
Construction and Expression of CHIKV Specific dMAbs
Gene sequence information for an established anti–Env-specific CHIKV neutralizing human mAb were obtained from the National Center for Biotechnology Information database [23]. Human embryonic kidney 293T cells and Vero cells, used for expression confirmation studies, were maintained as described previously [12]. The variable heavy (VH) and variable light (VL) chain segments for the CHIKV Env dMAb preparation were generated by using synthetic oligonucleotides with several modifications and were constructed as either a full-length immunoglobulin G (IgG; designated “CVM1-IgG”) or Fab fragment (designated “CVM1-Fab”) [31]. For cloning of CVM1-IgG, a single open reading frame was assembled containing the heavy and light chain genes, separated by a furin cleavage site coupled with a P2A self-processing peptide sequence. This transgene was cloned into the pVax1 expression vector [31]. The CVM1-Fab VH and VL chains were cloned into separate pVax1 vectors. For tissue culture transfection, 100 μg of pVax1 DNA, CVM1-IgG, or CVM1-Fab (100 μg of each VH and VL construct) was used. The CHIKV Env–based DNA vaccine used in the study was developed and characterized as previously described [11, 12].
CHIKV dMAb Generated IgG Quantification and Binding Assays
Enzyme-linked immunosorbent assays (ELISAs) were performed with sera, collected and measured in duplicate, from mice administered CMV1-IgG or pVax1, to quantify expression kinetics and target antigen binding. These measurements and analyses were performed as previously described [32].
Western Blot and Immunofluorescence Analysis of dMAb-Generated IgG
For Western blot analysis of IgG expression CHIKV (viral isolate PC08) infected cells were lysed two days post infection and evaluated by previously published methods [12, 32]. For immunofluorescence analysis, chamber slides (Nalgene Nunc, Penfield, New York) were seeded with Vero cells (1 × 104) and infected for 2 hours with the viral isolate CHIKV PC08 at a multiplicity of infection of 1. Immunofluorescence analysis was performed as previously described [32], with slides being visually evaluated by confocal microscopy (LSM710; Carl Zeiss). The resulting images were semiquantitatively analyzed using Zen software (Carl Zeiss).
dMAb DNA Plasmid Administration and In Vivo Analysis
CVM1-Fab and CVM1-IgG expression kinetics and functionality were evaluated in B6.Cg-Foxn1nu/J mice (Jackson Laboratory) following intramuscular injection of 100 μg control pVax1, CVM1-IgG, or 100 μg of each plasmid chain of CVM1-Fab. For studies that include the DNA vaccine, 25 μg of the CHIKV Env plasmid were injected 3 times at 2-week intervals. All injections were followed immediately by delivery of CHIKV dMAb DNA plasmid via electroporation [25, 32, 33]. Animal studies were approved by the Committee on Animal Care, University of Pennsylvania.
CHIKV Challenge Study
BALB/c mice received a single (100 μg) electroporation-enhanced intramuscular injection of CVM1-IgG, CMV-Fab (VH and VL), or control pVax1 plasmids. The CHIKV Env DNA vaccine was delivered as described above. Two or 35 days after DNA delivery, mice were challenged with 107 plaque-forming units (25 μL) of the viral isolate CHIKV Del-03 (JN578247) [34] either subcutaneously (in the dorsal side of each hind foot) or intranasally [12]. Mouse foot swelling (height by breadth) was measured daily up to 14 days after infection. In addition, the animals were monitored daily (for up to 20 days after infection) for survival and signs of infection (ie, changes in body weight and lethargy). Animals losing >30% of their body mass were euthanized, and serum samples were collected for cytokine quantification and other immune analysis. Blood samples were collected from the tail on days 7–14 after infection, and viremia levels were measured by a plaque assay.
Neutralizing Antibody Analysis
Anti-CHIKV neutralizing antibody titers from mice administered CVM1-IgG were determined by previously described methods [10, 12], using Vero cells infected with the following CHIKV isolates: LR2006-OPY1 (Indian Ocean Outbreak), IND-63WB1 and SL-CH1 (Asian-clade), Ross (ECSA-clade), and PC08 and DRDE-06 (ECSA-clade). Neutralization titers were calculated as the reciprocal of the highest dilution mediating 100% reduction of the cytopathic effects in the Vero cell monolayer. Data were generated and statistical analyses performed using the GraphPad Prism 5 software package (GraphPad Software). Nonlinear regression fitting with sigmoidal dose response was used to determine the level of antibody mediating 50% inhibition of infection (IC50). CHIKV Env pseudotype production and fluorescence-activated cell-sorting (FACS) analysis were performed as described previously [35].
Cytokine Quantitative Analysis
Sera were collected from mice injected with CVM1-Fab, CVM1-IgG, or CHIKV Env, as well as those challenged with CHIKV (1 week after challenge). Tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) levels in sera were measured using ELISA kits according to the manufacturer's instructions (R&D Systems).
Statistical Analysis
A Student t test or a nonparametric Spearman correlation test were performed using GraphPad Prism software (Prism, La Jolla, California). Correlations between the variables in the control and experimental groups were statistically evaluated using the Spearman rank correlation test, with P values of <.05 considered to be statistically significant for all tests.
RESULTS
Anti-CHIKV dMAbs Design and Confirmation of Expression
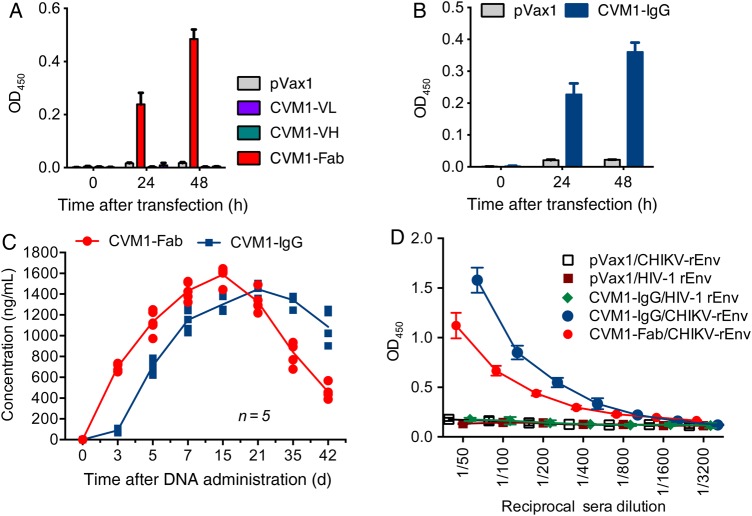
Viral entry into host cells by CHIKV is mediated by Env, against which the majority of neutralizing antibodies are generated [12, 36]. Thus, a DNA plasmid (dMAb) expressing the light and heavy immunoglobulin chains of a neutralizing anti-CHIKV mAb recognizing both E1 and E2 Env proteins was designed [23, 24]. The complementary DNAs for the coding sequences of the VL and VH immunoglobulin chains for full-length anti-CHIKV dMAb were optimized for increased expression and cloned into a pVax1 vector, using previously described methods [25, 31]. For the constructs expressing anti–CHIKV-Fab, the VH and VL genes were cloned separately. The optimized synthetic plasmids constructed from the anti-Env–specific CHIKV-neutralizing mAb were designated CVM1-IgG or CVM1-Fab, for the IgG and Fab antibodies, respectively. Human 293T cells were transfected with either the CVM1-IgG plasmid or the CVM1-Fab (VL, VH, or combined) plasmids to validate expression in vitro. As indicated in Figure 1A and 1B , anti-CHIKV antibody levels were measured by ELISA with recombinant CHIKV Env used as the binding antigen. These data indicate that the CVM1-Fab and CVM1-IgG expressed antibodies in the muscle that appeared to be properly assembled and biologically functional in vitro.
Figure 1.
CVM1–immunoglobulin G (IgG) and CVM-1–Fab dMAb plasmid design and expression. A and B, In vitro expression of CVM1-IgG and CVM1-Fab. The CVM1-IgG, CVM1-Fab, CVM1–variable heavy chain (VH), and CVM1–variable light chain (VL) constructs were transfected into 293T cells to determine in vitro expression through binding enzyme-linked immunosorbent assays (ELISAs). Samples were analyzed at 0, 24, and 48 hours. post-transfection. Cells transfected with an empty backbone pVax1 plasmid served as a negative control. C, In vivo expression of CVM1-IgG and CVM1-Fab. Mice (B6.Cg-Foxn1nu/J) aged 5–6 weeks received a single, 100-μg intramuscular injection of CVM1-IgG, CVM1-VH, CVM1-VL, or CVM1-Fab plasmids, followed by electroporation (5 mice per group). Injection of a pVax1 vector was used a negative control. Sera IgG levels were measured at various time points in mice injected intramuscularly as described in “Materials and Methods” section. D, Sera from CVM1-IgG–administered mice binds chikungunya virus (CHIKV) envelope protein (Env). ELISA plates were coated with recombinant CHIKV envelope or human immunodeficiency virus type 1 (HIV-1) (subtype B; MN) envelope protein, and sera obtained on day 15 from mice given a single injection of CVM1-IgG, CVM1-Fab, or pVax1 were tested. For A, B and D mean OD450 values are shown ±SD.
In Vivo Expression and Quantification of CVM1-IgG and CVM1-Fab
Following confirmation of in vitro expression, the ability of CVM1-Fab or CVM1-IgG to produce anti-CHIKV antibodies in vivo was measured. B6.Cg-Foxn1nu/J mice aged 5–6 weeks were administered 100 μg of CVM1-IgG (CVM1-IgG is 1 plasmid), 100 μg each of CVM1 VH and VL (CVM1-Fab consists of 2 plasmids), or control vector by a single intramuscular electroporation-mediated injection. Sera were collected at indicated time points, and target antigen binding was measured by IgG quantification, using ELISA. Although mAbs generated from CVM1-Fab appeared more rapidly (ie, within 3 days after injection) than those from CVM1-IgG, both constructs generated similar mAb levels by day 15 (mean sera levels [±SD], 1587.23 ± 73.23 ng/mL of CVM1-Fab and 1341.29 ± 82.07 ng/mL of CVM1-IgG; Figure 1C). Mice were administered either CVM1-IgG or CVM1-Fab, and sera antibody levels were evaluated through a binding ELISA. Sera collected 15 days after injection from both CVM1-IgG and CVM1-Fab bound to CHIKV Env protein but not to an unrelated control antigen, human immunodeficiency virus type 1 Env (Figure 1D). These data indicate that in vivo produced anti-CHIKV antibodies from CVM1-IgG or CVM1-Fab constructs have similar biological characteristics to conventionally produced antigen specific antibodies.
In Vivo Specificity and Broadly Neutralizing Activity in Sera From Mice Injected With CVM1-IgG
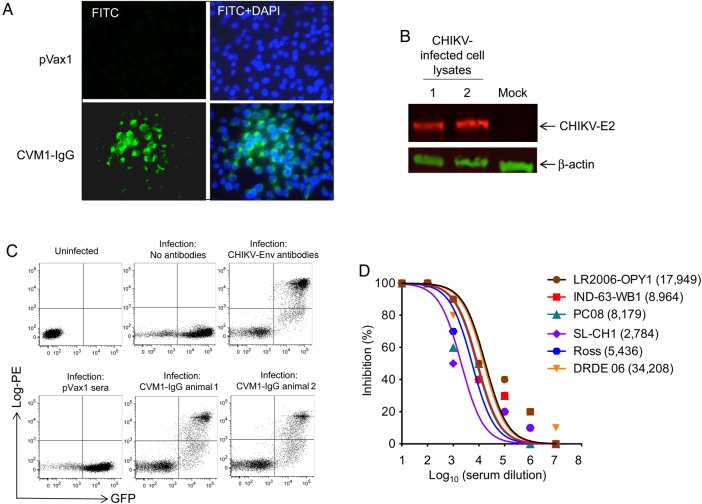
The anti-CHIKV dMAb generated mAbs were tested for binding specificity and anti-CHIKV neutralizing activity. Sera from mice injected with CVM1-IgG were tested against fixed CHIKV PC08–infected Vero cells by immunofluorescence assays. The results indicated binding of the sera antibodies to the CHIKV-infected cells (Figure 2A). Confirmation of binding of sera from CVM1-IgG–injected mice to target proteins was tested by Western blot analysis. The detection of CHIKV E2 protein (50 kDa) expression in total cell lysate from the CHIKV-infected cells indicates specificity of CVM1-IgG expression (Figure 2B). The specificity of in vivo–produced CVM1-IgG antibody was further demonstrated through FACS analysis against cells infected with green fluorescent protein–encoded CHIKV (Figure 2C). Moreover, CVM1-Fab binding, demonstrated by immunohistochemical analysis and FACS analysis, was similar to that of the generated full-length CVM1-IgG (data not shown). Together, these findings indicate a strong specificity of the antibody generated from the CVM1-IgG plasmid.
Figure 2.
Binding analyses and neutralization activity of CVM1–immunoglobulin G (IgG) antibodies. A, An immunofluorescence assay demonstrated that IgG generated from CVM1-IgG–administered mice was capable of binding to chikungunya virus (CHIKV) envelope protein (Env). CHIKV-infected Vero cells were fixed 24 hours after infection and evaluated by an immunofluorescence assay to detect CHIKV Env antigen expression (green). Cell nuclei were stained with DAPI (blue). Sera from control mice injected with pVax1 were used as a negative control. B, The binding affinity of sera from CVM1-IgG–injected mice (day 15) to target proteins was tested by Western blot, using cell lysates from CHIKV- or mock-infected cells as described in “Materials and Methods” section. Protein transferred membranes were reprobed with antibody against β-actin was used as a loading control. The image presented here was cropped from an original image and is representative of several gels. C, Fluorescence-activated cell-sorting analysis of the binding of sera from plasmid-injected mice to CHIKV-infected cells. The x-axis indicates green fluorescent protein (GFP) staining, using the lentiviral GFP pseudovirus complemented with CHIKV Env. The y-axis demonstrates staining of infected cells by human IgG produced in mice 15 days after injection with CVM1-IgG. Staining with a control anti-CHIKV antibody (Env antibody) is also shown, as well as staining with no antibodies and pVax1. The presence and number of double-positive cells indicate presence and level of sera binding to the CHIKV-infected cells. D, Sera from mice injected with CVM1-IgG via electroporation possess neutralizing activity against multiple CHIKV strains (ie, Ross, LR2006-OPY1, IND-63-WB1, PC-08, DRDE-06, and SL-CH1). Neutralizing antibody titers are plotted, and 50% inhibitory concentrations (IC50 values; parenthesis) were calculated with Prism GraphPad software. Similar results were observed in 2 independent experiments with at least 10 mice per group for each experiment.
Furthermore, the anti-CHIKV neutralizing activity in sera from mice that received CVM1-IgG was measured against that in 6 divergent CHIKV strains: LR2006-OPY1 (Indian Ocean Outbreak), IND-63WB1 (Asian-clade), Ross (ECSA-clade), PC08 (ECSA-clade), SL-CH1 (Asian-clade) and DRDE-06 (ECSA-clade) [37]. IC50 values were determined for each viral isolate. Sera from CVM1-IgG–injected mice effectively neutralized all 6 CHIKV isolates, demonstrating that a single injection can produce significant neutralizing levels of human anti-CHIKV IgG in mice (Figure 2D). Similar results were observed using sera from CVM1-Fab–injected mice (data not shown). These data indicate that antibodies produced in vivo by CVM1-IgG constructs have relevant biological activity (ie, binding and neutralizing activity against CHIKV).
CVM1-IgG Injection Protects Mice From Lethal CHIKV Challenge
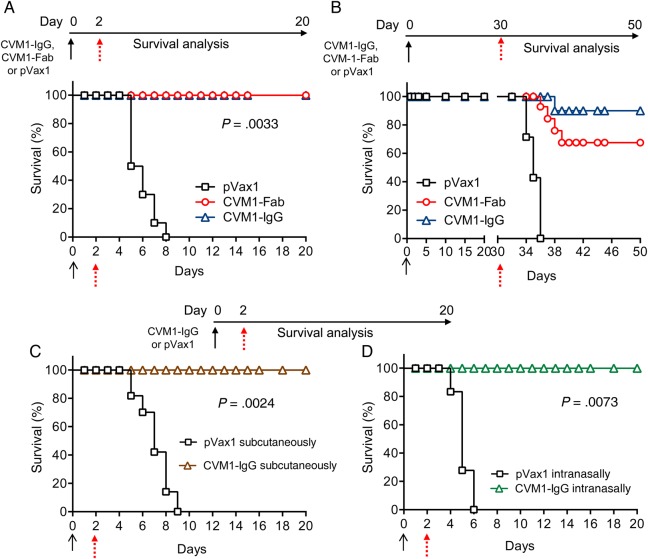
Previous studies demonstrated that early immunity against viruses is a key factor for controlling infections [22, 38, 39]. To determine whether antibodies generated from CVM1-IgG or CVM1-Fab provide protection against early exposure to CHIKV, groups of 10 mice received a single administration of pVax1, CVM1-IgG, or CVM1-Fab on day 0. Each group subsequently was challenged subcutaneously with virus on day 2 to mimic natural CHIKV infection (Figure 3A). Animal survival and weight changes were subsequently recorded for 20 days. All mice injected with pVax1 control plasmid died within a week of viral challenge. Conversely, 100% survival was observed in mice administered either CVM1-IgG or CVM1-Fab, compared with 0% survival among mice that received pVax1 plasmid (P = .0033), demonstrating that CVM1-IgG and CVM1-Fab plasmids confer protective immunity within 2 days after delivery.
Figure 3.
Characterization of in vivo immune protection conferred by CVM1-Fab and CVM1–immunoglobulin G (IgG). As described in the schematic representation for each panel, BALB/c mice were injected with 100 μg of pVax1 (negative control), CVM1-IgG, CVM1–variable heavy chain, and CVM1–variable light chain on day 0 and challenged on day 2 (A) or day 30 (B) with chikungunya virus (CHIKV) as described in “Materials and Methods” section. Mice were monitored daily, and survival rates were recorded for 20 days after viral challenge. C and D, Protection of mice from different routes of CHIKV challenge. Two groups of mice were injected with 100 μg of CVM1-IgG by the intramuscular route, followed by viral challenge on day 2 with either subcutaneous (C) or intranasal (D) inoculation. Mice were monitored daily, and survival rates were recorded for 20 days after the viral challenge. The black arrow indicates plasmid injections; the red arrow indicates the time of viral challenge. Each group consisted of 10 mice, and the results were representative of 2 independent experiments. P values for statistical comparisons between appropriate groups are indicated in panels A–D.
The longevity of immune protection was next evaluated. A second group of mice (n = 10) was challenged with CHIKV 30 days after a single injection with CVM1-IgG, CVM1-Fab, or pVax1 on day 0 (Figure 3B). Mice were monitored for survival over the next 20 days. Mice injected with CVM1-Fab or CVM1-IgG demonstrated 70% and 90% survival, respectively, compared with no survival among pVax1-injected mice (P = .0120), indicating that CVM1-IgG provides a more durable degree of immune protection (Figure 3B).
To assess the ability of the CVM1-IgG plasmid to protect against infection at a mucosal surface, the protective efficacy of CVM1-IgG against subcutaneous versus intranasal viral challenge, previously demonstrated to produce visible CHIKV pathogenesis such as limb muscle weakness, footpad swelling, lethargy, and high mortality within 6–10 days of infection, was evaluated [12, 40]. For simplicity, studies focused on the CVM1-IgG construct. Groups of 20 mice received a single administration of pVax1 or CVM1-IgG, with half (ie, 10) being challenged with CHIKV via a subcutaneous or intranasal route 2 days after injection. CVM1-IgG protected mice from both subcutaneous viral challenge (P = .0024; Figure 3C) and intranasal viral challenge (P = .0073; Figure 3D), compared with pVax1-injected mice, demonstrating that it can protect against systemic and mucosal infection.
Comparison Between In Vivo Protective Immunity Conferred by CHIKV-IgG Administration and CHIKV Env DNA Vaccination
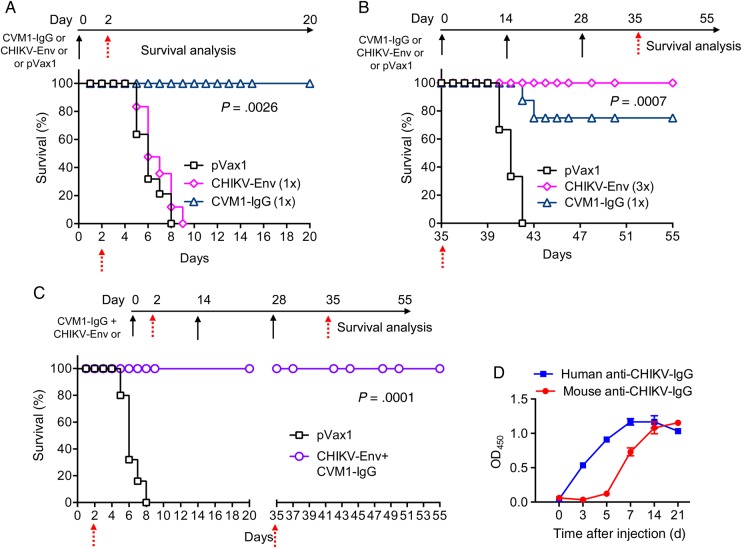
A study comparing the protective efficacy of CVM1-IgG administration vs a CHIKV Env–expressing DNA vaccine (CHIKV Env) was next performed. A novel consensus-based DNA vaccine was developed by our laboratory and was capable of providing protection against CHIKV challenge in mice. The DNA vaccine also induced both measurable cellular immune responses, as well as potent neutralizing antibody responses in rhesus macaques [11, 12]. Groups of mice were administered a single injection of CVM1-IgG, CHIKV Env, or the pVax1, followed by viral challenge on 2 days after injection. Mice that received a single immunization of CHIKV Env or pVax1 died within 6 days of viral challenge, whereas a single immunization of CVM1-IgG provided 100% protection (Figure 4A). CVM1-IgG clearly conferred protective immunity more rapidly than the CHIKV Env DNA vaccine (P = .0026).
Figure 4.
Comparative and combination studies with CVM1–immunoglobulin G (IgG) and the chikungunya virus (CHIKV) envelope protein (Env) DNA vaccine. Schematic representation of CVM1-IgG injection and the CHIKV Env DNA vaccination time course and challenge studies are shown for each study. A, BALB/c mice were injected with 100 μg of CVM1-IgG, 100 μg of pVax1 (negative control), or 25 μg of CHIKV-Env DNA on day 0 and challenged on day 2 with CHIKV Del-03 (JN578247; 1 × 107 plaque-forming units in a total volume of 25 μL). B, BALB/c mice were administered either a single injection of 100 μg of CVM1-IgG on day 0 or 3 immunizations of 25 μg of CHIKV Env DNA on day 0, day 14, and day 28 and then challenged on day 35 under the same conditions and with the same CHIKV isolate. C, Groups of 20 BALB/c mice were administered a single 100 μg injection of CVM1-IgG on day 0 and 3 immunizations with CHIKV-Env DNA (25 μg) on day 0, day 14, and day 28. Half of the mice were then challenged on day 2, and the remaining half were challenged on day 35 under the same conditions and with the same CHIKV isolate challenge described above. The black arrow indicates plasmid injection, and the red arrow indicates the time of viral challenge. For each study, mice were monitored for 20 days after challenge, and survival rates were recorded. D, Induction of persistent and systemic anti-CHIKV Env antibodies following a single CVM1-IgG (human anti-CHIKV Env) injection and CHIKV-Env immunization (mouse anti-CHIKV Env) 1 week after the second immunization in mice. Binding enzyme-linked immunosorbent assays were performed as described in “Material and Methods” section. For D mean OD450 values are shown ±SD.
Next, a long-term CHIKV challenge protection study was performed on day 35 following vaccination with the CHIKV Env DNA vaccine or administration of CVM1-IgG on day 0. The multibooster delivery of the CHIKV Env DNA vaccine conferred 100% protection (Figure 4B), while 80% survival was observed in mice administered CVM1-IgG (P = .0007). The kinetics of the induced antibody responses was measurable within 2 days of a single injection of CVM1-IgG, with peak levels by day 15 (approximately 1400 ng/mL) and detectable mAb levels maintained for at least 45 days after injection (Supplementary Figure 1A). Although there is continued expression, these levels are decreased, compared with peak levels, supporting the partial protection noted in the experiment (Figure 4B).
Codelivery of CVM1-IgG and the CHIKV Env DNA Vaccine Produces Systemic Humoral Immunity, Cell-Mediated Immunity, and Protection In Vivo
One potential issue of combining antibody delivery with vaccination approaches is that the antibodies can neutralize many traditional vaccines [12, 25, 32, 41] and thus are incompatible platforms. The effect of coadminstration of CVM1-IgG and CHIKV Env on mouse survival in the context of CHIKV challenge was also evaluated. In this experiment, 20 mice were administered at day 0 a single dose of CVM1-IgG and 3 doses of CHIKV Env DNA as described above. Subsequently, half of the animals were challenged with CHIKV at day 2 and the other half at day 35. Survival in these groups was followed as a function of time. Not unexpectedly, both of the challenge groups had 100% long-term survival (Figure 4C). Specifically, results of the day 2 CHIKV challenge experiment indicated the utility of the CVM1-IgG reagent in mediating protection from infection, with the survival percentage decreasing to approximately 30% by 4 days after challenge in control (pVax1) animals. Figure 4D indicates levels of anti-CHIKV IgG, by time, generated in mice that received CVM1-IgG and CHIKV Env DNA vaccine; anti-CHIKV human IgG represents antibody produced by the CVM1-IgG plasmid and anti-CHIKV mouse IgG represents antibody induced by the CHIKV Env vaccine. Both human IgG and mouse IgG were detected and exhibited different expression kinetics. By 3 days after initial CHIKV Env DNA vaccination, mouse anti-Env antibody levels were essentially near 0 (mouse anti-CHIKV IgG). Conversely, 3 days after a single CVM1-IgG injection, human anti-Env antibody levels were significant (human anti-CHIKV IgG). These data underscore the importance of CVM1-IgG in mediating rapid protection from infection and death after CHIKV challenge.
Furthermore, T-cell responses induced in animals injected with CVM1-IgG, CHIKV Env, or CVM1-IgG plus CHIKV Env was evaluated by a quantitative enzyme-linked immunospot assay, which measures IFN-γ levels (Supplementary Figure 1B). CHIKV Env elicited strong T-cell responses irrespective of codelivery with CVM1-IgG, showing the lack of interference of these approaches. Conversely, animals administered only CVM1-IgG did not develop T-cell responses, as would be expected. These findings demonstrate that both CVM1-IgG and CHIKV Env DNA vaccine can be administered simultaneously without reciprocal interference, providing immediate and long-lived protection via systemic humoral and cellular immunity.
CVM1-IgG Administration Reduces CHIKV Loads and Proinflammatory Cytokine Levels
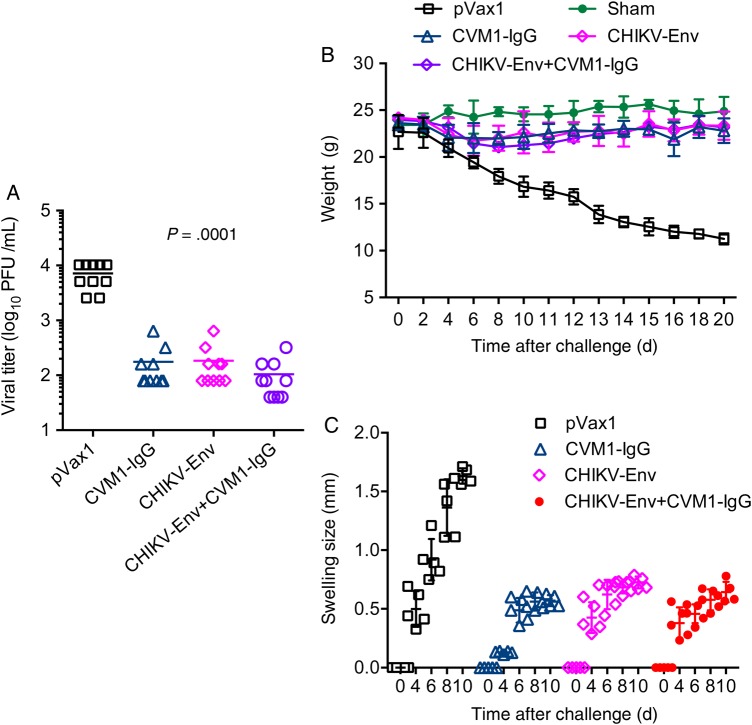
Previous studies identified molecular correlates of CHIKV-associated disease severity, including viral load and proinflammatory cytokine levels [42, 43]. Thus, the ability of CVM1 IgG to suppress these disease-associated markers at early and late time points after viral challenge was assessed. Mice immunized with CVM1 IgG, CVM1 Fab, CHIKV Env, or CVM1 IgG plus CHIKV Env DNA vaccine generated mAb and significantly reduced viral loads (Figure 5A). In addition to viral load reduction, these mice did not exhibit footpad swelling, compared with control (pVax1) immunized mice, and consistently gained body weight during the 20-day experimental period (Figure 5B and 5C). Also the CVM1-IgG–generated mAb and the CHIKV Env DNA vaccine exhibited significantly reduced levels of CHIKV-mediated proinflammatory cytokines (ie, TNF-α, IL-6, and IL-β), compared with pVax1, 10 days after viral challenge (Supplementary Figure 2). These findings suggest that a single injection with CVM1-IgG suppresses CHIKV-associated pathology to an extent comparable to that induced by protective vaccination [12].
Figure 5.
Characterization of pathologic footpad swelling and changes in weight in viral-challenged mice vaccinated with CVM1–immunoglobulin G (IgG) and/or chikungunya virus (CHIKV) envelope protein (Env) DNA. A, Viral titers 1 week after CHIKV challenge in mice that received CVM1-IgG, CHIKV-Env, CVM1-IgG plus CHIKV-Env, or pVax1 (control). Each data point represents the average viral titers from 10 mice. Error bars indicate standard errors of the means. B, Mean daily weight gain (±standard deviation [SD]) after subcutaneous inoculation with the CHIKV isolate among mice that received CVM1-IgG, CHIKV-Env, CVM1-IgG plus CHIKV-Env, or pVax1. Mice were weighed on the specified days after inoculation. Results are presented as mean body weights (±SD). C, Swelling of the hind feet was quantified using calipers on the specified days among mice that received CVM1-IgG, CHIKV-Env, CVM1-IgG plus CHIKV-Env, or pVax1. Data are mean values (±SD). Abbreviation: PFU, plaque-forming units.
DISCUSSION
Antigen-based vaccination requires a lag period during which the vaccine recipient remains susceptible to infection and disease [22, 44]. Use of passive antibody therapy has advantages in several high-risk populations that either respond poorly to active vaccination or manifest significant vaccine-related side effects [45]. It would be a major advantage to generate effective and specific in vivo immunity rapidly without the need for repeated administration of preformed antibodies or a significant lag time for immune response generation that follows conventional antigen-based immunization. In this study, a novel, synthetic DNA-delivery system (dMAb) for generating rapid immune protection was evaluated using the emerging CHIKV as a model.
The increased incidence and geographic spread of CHIKV infection and other emerging viral infections raises concerns for potential global outbreaks, underscoring the need for targeted antiviral interventions [15, 24]. Currently, neither a vaccine nor a therapy for CHIKV infection has been licensed [7], but evidence suggests that humoral immunity plays a critical role in protecting against CHIKV infection [14, 15, 24]. Our group previously demonstrated that passive transfer of sera from mice immunized with a CHIKV Env DNA vaccine protected naive mice from lethal CHIKV challenge [12], highlighting the utility of antibody-based therapy, as well as prompting interest in developing a novel approach for providing a source of anti-CHIKV antibodies generated directly in vivo.
This study demonstrates the utility of electroporation-mediated delivery of optimized DNA plasmids for the in vivo rapid production of biologically functional mAbs. Unlike viral vectors, DNA plasmids do not pose a risk of genome integration or generate antivector immunity, which allows for booster immunizations and co-vaccinations with multiple DNA plasmids [32, 46–48]. In addition, they are stable, which facilitates manufacturing and stockpiling and obviates the necessity for a refrigerated cold chain. The strategy could also be particularly useful to combat pathogens adept at escaping the immune response, since multiple plasmids encoding antibodies targeting different epitopes could be administered without serological interference [49].
This study demonstrates that mice injected with a single dose of CVM1 IgG were fully protected from viral challenge 2 days after administration, whereas no mice survived infection following a single immunization with CHIKV Env DNA vaccine, owing presumably to an insufficient time to mount protective immunity. However, complete protection was observed with CHIKV Env after a immunization regimen followed by challenge at later time points. A similar level of protection occurred in mice administered a single dose of CVM1-IgG, although protection waned to 80% over time. Notably, the codelivery of CVM1-IgG and CHIKV Env produced rapid and persistent humoral and cellular immunity, suggesting that a combination approach can have additive or synergistic effects. Importantly, codelivery of CVM1-IgG and CHIKV Env were not antagonistic in terms of the development of short- or long-term protective immune responses, providing a new important approach that provides infection resistance against this relevant pathogen. These studies likely have importance for a variety of other infectious and noninfectious diseases.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank members of the Weiner laboratory, for significant contributions and/or critical reading and editing of the manuscript; and the Penn Center for AIDS Research and the Abramson Cancer Center core facilities, for their support.
Disclaimer. The funders of the study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01-AI092843 to D. B. W.); DARPA-PROTECT (to D. B. W. and K. M.); and Inovio Pharmaceuticals (to D. B. W. and K. M.).
Potential conflicts of interest. K. M. reports receiving grants from DARPA and Inovio, receiving consulting fees from Inovio related to DNA vaccine development, and a pending patent application (to Inovio) for delivery of DNA-encoded monoclonal antibodies. A. S. K., N. Y. S., and J. J. K. are employees of Inovio Pharmaceuticals and as such receive salary and benefits, including ownership of stock and stock option, from the company. D. B. W. has received grant funding, participates in industry collaborations, has received speaking honoraria, and has received fees for consulting, including serving on scientific review committees and board services. Remuneration received by D. B. W. includes direct payments or stock or stock options, and in the interest of disclosure he notes potential conflicts associated with this work with Medimmune and Inovio and possibly others. In addition, he has a patent DNA vaccine delivery pending to Inovio. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet 2012; 379:662–71. [DOI] [PubMed] [Google Scholar]
- 2.Akahata W, Yang ZY, Andersen H et al. . A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med 2010; 16:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffey LL, Failloux AB, Weaver SC. Chikungunya virus-vector interactions. Viruses 2014; 6:4628–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks--the globalization of vectorborne diseases. N Engl J Med 2007; 356:769–71. [DOI] [PubMed] [Google Scholar]
- 5.Teo TH, Lum FM, Claser C et al. . A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J Immunol 2013; 190:259–69. [DOI] [PubMed] [Google Scholar]
- 6.Teng TS, Foo SS, Simamarta D et al. . Viperin restricts chikungunya virus replication and pathology. J Clin Invest 2012; 122:4447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines 2012; 11:1087–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg 2000; 62:681–5. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Mamidi P, Das I et al. . A novel 2006 Indian outbreak strain of Chikungunya virus exhibits different pattern of infection as compared to prototype strain. PLoS One 2014; 9:e85714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang E, Volkova E, Adams AP et al. . Chimeric alphavirus vaccine candidates for chikungunya. Vaccine 2008; 26:5030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthumani K, Lankaraman KM, Laddy DJ et al. . Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine 2008; 26:5128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallilankaraman K, Shedlock DJ, Bao H et al. . A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis 2011; 5:e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Arriaza J, Cepeda V, Hallengard D et al. . A novel poxvirus-based vaccine (MVA-CHIKV) is highly immunogenic and protects mice against chikungunya infection. J Virol 2014; 88:3527–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couderc T, Khandoudi N, Grandadam M et al. . Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis 2009; 200:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fric J, Bertin-Maghit S, Wang CI, Nardin A, Warter L. Use of human monoclonal antibodies to treat Chikungunya virus infection. J Infect Dis 2013; 207:319–22. [DOI] [PubMed] [Google Scholar]
- 16.Sheela PJ, Sumathy K. Serological correlates of immune protection conferred by Chikungunya virus infection. Acta Virol 2013; 57:471–3. [DOI] [PubMed] [Google Scholar]
- 17.Manohar A, Ahuja J, Crane JK. Immunotherapy for Infectious Diseases: Past, Present, and Future. Immunol Invest 2015; 44:731–7. [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34–47. [DOI] [PubMed] [Google Scholar]
- 19.Zheng K, Bantog C, Bayer R. The impact of glycosylation on monoclonal antibody conformation and stability. MAbs 2011; 3:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zalevsky J, Chamberlain AK, Horton HM et al. . Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010; 28:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strop P, Ho WH, Boustany LM et al. . Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 2012; 420:204–19. [DOI] [PubMed] [Google Scholar]
- 22.Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nat Rev Microbiol 2014; 12:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warter L, Lee CY, Thiagarajan R et al. . Chikungunya virus envelope-specific human monoclonal antibodies with broad neutralization potency. J Immunol 2011; 186:3258–64. [DOI] [PubMed] [Google Scholar]
- 24.Pal P, Dowd KA, Brien JD et al. . Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog 2013; 9:e1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flingai S, Plummer EM, Patel A et al. . Protection against dengue disease by synthetic nucleic acid antibody prophylaxis/immunotherapy. Sci Rep 2015; 5:12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson PR, Schnepp BC, Zhang J et al. . Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med 2009; 15:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 2012; 481:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manno CS, Pierce GF, Arruda VR et al. . Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12:342–7. [DOI] [PubMed] [Google Scholar]
- 29.Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther 2011; 11:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Calcedo R, Bell P et al. . Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011; 22:1389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthumani K, Flingai S, Wise M, Tingey C, Ugen KE, Weiner DB. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum Vaccin Immunother 2013; 9:2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthumani K, Falzarano D, Reuschel EL et al. . A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med 2015; 7:301ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broderick KE, Khan AS, Sardesai NY. DNA vaccination in skin enhanced by electroporation. Methods Mol Biol 2014; 1143:123–30. [DOI] [PubMed] [Google Scholar]
- 34.Muruganandam N, Chaaithanya IK, Senthil GS et al. . Isolation and molecular characterization of Chikungunya virus from the Andaman and Nicobar archipelago, India: evidence of an East, Central, and South African genotype. Can J Microbiol 2011; 57:1073–7. [DOI] [PubMed] [Google Scholar]
- 35.Muthumani K, Wise MC, Broderick KE et al. . HIV-1 Env DNA vaccine plus protein boost delivered by EP expands B- and T-cell responses and neutralizing phenotype in vivo. PLoS One 2013; 8:e84234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun S, Xiang Y, Akahata W et al. . Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization. eLife 2013; 2:e00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler SA, Lu L, da Rosa AP, Xiao SY, Tesh RB. An animal model for studying the pathogenesis of chikungunya virus infection. Am J Trop Med Hyg 2008; 79:133–9. [PubMed] [Google Scholar]
- 38.Hudson PJ, Souriau C. Engineered antibodies. Nat Med 2003; 9:129–34. [DOI] [PubMed] [Google Scholar]
- 39.Smith SA, Silva LA, Fox JM et al. . Isolation and Characterization of Broad and Ultrapotent Human Monoclonal Antibodies with Therapeutic Activity against Chikungunya Virus. Cell Host Microbe 2015; 18:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couderc T, Chretien F, Schilte C et al. . A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog 2008; 4:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laddy DJ, Yan J, Kutzler M et al. . Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS ONE 2008; 3:e2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng LF, Chow A, Sun YJ et al. . IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE 2009; 4:e4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaaitanya IK, Muruganandam N, Sundaram SG et al. . Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol 2011; 24:265–71. [DOI] [PubMed] [Google Scholar]
- 44.Krammer F, Palese P. Universal influenza virus vaccines: need for clinical trials. Nat Immunol 2014; 15:3–5. [DOI] [PubMed] [Google Scholar]
- 45.Walker BD, Ahmed R, Plotkin S. Moving ahead an HIV vaccine: use both arms to beat HIV. Nat Med 2011; 17:1194–5. [DOI] [PubMed] [Google Scholar]
- 46.Kalams SA, Parker S, Jin X et al. . Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One 2012; 7:e29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trimble CL, Morrow MP, Kraynyak KA et al. . Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015; 386:2078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lienert F, Lohmueller JJ, Garg A, Silver PA. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat Rev Mol Cell Biol 2014; 15:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutnick NA, Myles DJ, Ferraro B et al. . Intradermal DNA vaccination enhanced by low-current electroporation improves antigen expression and induces robust cellular and humoral immune responses. Hum Gene Ther 2012; 23:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.