Abstract
Background. Bacillus Calmette-Guerin (BCG) vaccine is widely used for the prevention of tuberculosis, despite limited efficacy. Most immunological studies of BCG or Mycobacterium tuberculosis strains grow bacteria in the presence of detergent, which also strips the mycobacterial capsule. The impact of the capsule on vaccine efficacy has not been explored.
Methods. We tested the influence of detergent in cultures of BCG and M. tuberculosis strains on the outcome of vaccination experiments on mice and transcriptional responses on M. tuberculosis.
Results. Vaccination of mice with encapsulated BCG promoted a more potent immune response relative to vaccination with unencapsulated BCG, including higher polysaccharide-specific capsule antibody titers, higher interferon γ and interleukin 17 splenic responses, and more multifunctional CD4+ T cells. These differences correlated with variability in the bacterial burden in lung and spleen of mice infected with encapsulated or unencapsulated M. tuberculosis. The combination of vaccination and challenge with encapsulated strains resulted in the greatest protection efficacy. The transcriptome of encapsulated M. tuberculosis was similar to that of starvation, hypoxia, stationary phase, or nonreplicating persistence.
Conclusions. The presence of detergent in growth media and a capsule on BCG were associated with differences in the outcome of vaccination, implying that these are important variables in immunological studies.
Keywords: BCG, vaccine, Mycobacterium tuberculosis, capsule
A wide range of bacterial pathogens produce capsular polysaccharides, which provide a defensive barrier during the initial interaction with the host [1]. Numerous studies have documented the presence of a polysaccharide capsule in mycobacteria [2–4]. Mycobacterium tuberculosis, the causative agent of tuberculosis, has a remarkable capacity to establish infection and persist in hosts with intact immunity. There is strong evidence that the mycobacterial cell envelope contributes to survival in the host [2]. The mycobacterial capsule is loosely attached to the surface and can be visualized by electron microscopy as an electron-transparent zone. The presence of a capsule in mycobacterial cells is influenced by the culture conditions, including the supplementation of the culture medium with detergent to prevent clumping [5]. Addition of detergent to culture medium is associated with the shedding of the capsule into the medium [4, 6–8]. The mycobacterial capsule is composed primarily of proteins and polysaccharides [2]. The major surface-exposed capsule polysaccharides are glycogen-like α-glucan, arabinomannan, and mannan [9]. Both arabinomannan and mannan are structurally related to lipoarabinomannan, the major lipopolysaccharide of the mycobacterial cell wall.
The only licensed vaccine against tuberculosis, bacillus Calmette-Guerin (BCG), continues to be used today in regions with a high tuberculosis burden to prevent infant disease, with limited and variable efficacy [10, 11]. Several hypotheses have been proposed to explain this variable efficacy, which are not mutually exclusive: vaccine tolerance because of the exposure to environmental mycobacteria [12]; rapid clearance of BCG, preventing the establishment of a long-lasting immunity [13]; and mutations introduced during passage of BCG cultures, resulting in waning protective immunity. The current BCG organisms are produced for human use as pellicles on the surface of Sauton medium [14], suggesting it is used in an encapsulated form. Remarkably, most if not all of the preclinical experimental vaccine studies that included live strains have used cultures supplemented with detergent and consequently involve infection with unencapsulated cells [15–19]. To date, no study has assessed the role of the mycobacterial capsule in vaccination, despite the precedent that polysaccharide capsules are critically important to immune responses.
MATERIALS AND METHODS
Bacterial Strains, Medium, and Culture
Mycobacterium bovis BCG Danish 1331 strain was obtained from Statens Serum Institute (Copenhagen, Denmark), and virulent M. tuberculosis strain H37Rv was obtained from the Trudeau Institute (Saranac Lake, New York). Mycobacterial strains were grown in minimal medium as previously reported [34]. Bacterial colony-forming unit (CFU) titers were determined as described before [34]. BCG and M. tuberculosis clumps were disrupted as previously described [20]. M. tuberculosis lysates were obtained as previously reported [35]. Bacterial mass was calculated by determining the dry weight of BCG grown with and without detergent. Protein concentration was determined by using the bicinchoninic method.
Murine Immunization
C57BL/6 female mice aged 6–8 weeks were purchased from Jackson Laboratories (Bar Harbor, Minnesota). All procedures involving mice were reviewed and approved by the Animal Use and Care Committee of the Albert Einstein College of Medicine. Animals were maintained in a specific-pathogen-free animal facility under animal biosafety level 2 conditions for all experiments except those involving infection with virulent M. tuberculosis, which required animal biosafety level 3 conditions. Animals were initially immunized subcutaneously with 1 million encapsulated or unencapsulated BCG in protection efficacy experiments for 6 weeks.
Murine Infection
Aerogenic challenge was done using a whole-body exposure aerosol chamber (University of Wisconsin Mechanical Engineering Workshop) custom fitted to a class III biosafety cabinet (Baker) to deliver approximately 100 CFU per animal of encapsulated or unencapsulated M. tuberculosis (H37Rv). Mice were euthanized 4 weeks after challenge, and CFU were assessed in lungs and spleen as previously reported [34]. Animals infected with M. tuberculosis H37Rv were observed at least twice daily until they became moribund and were euthanized.
Transmission Electron Microscopy
Preparation of mycobacterial cells for transmission electron microscopy was performed as previously described [20]. Thin sections were created on an Ultracut UCT (Reichert) and stained with 0.5% uranyl acetate and 0.5% lead citrate (Reichart, Depew, New York). Samples were observed in a JEOL 1200EX transmission electron microscope operating at 80 kV.
Enzyme-Linked Immunosorbent Assay (ELISA)
Two types of ELISA were used in this study. In one assay, polystyrene microtiter plate wells were coated with 50 μL of 20 μg/mL of an encapsulated or unencapsulated M. tuberculosis whole-cell lysate in carbonate buffer (0.015 M Na2CO3, 0.035 M NaHCO3, and 0.003 M NaN3; pH 9.8) and incubated for 2 hours at room temperature. The wells were then blocked by adding 200 μL of 2% bovine serum albumin in TBS and incubated at 37°C for 1 hour. Serum from mice immunized with encapsulated or unencapsulated BCG was added to the wells and incubated for 1 hour at 37°C. The plates were then washed and incubated with immunoglobulin G1 (IgG1), IgG2c, IgG2b, and IgG3 AP-conjugated secondary antibodies (Southern Biotechnologies; 1:1000) for 1 hour at 37°C. Color was developed with p-nitrophenyl phosphate (Sigma-Aldrich).
We also used a BCG whole-cell ELISA to measure the relative abundances of arabinomannan and α-glucan on BCG cells [36]. Coated plates were blocked as described above and incubated with α-glucan–specific monoclonal antibody (24c5) [8] and BCG arabinomannan-specific mouse serum (obtained from mice immunized with arabinomannan-conjugates; Prados-Rosales, unpublished data). The plates were then processed as described above.
Enzyme-Linked Immunospot (ELISPOT) Assay
Splenocytes were collected 6 weeks after BCG immunization and cultured in Roswell Park Memorial Institute medium in ELISPOT plates (5 × 105/well; Millipore, Danvers, Massachusetts) coated with interferon γ (IFN-γ) capture antibody (clone R4.6A2; BD Biosciences) or interleukin 17 (IL-17) capture antibody (clone 50101; R&D Systems) in phosphate-buffered saline (PBS). The samples were stimulated with 20 µg/mL of either encapsulated or unencapsulated M. tuberculosis whole-cell lysate and with 10 µg/mL of each of the following synthetic peptide antigens (Invitrogen): FQDAYNAAGGHNAVF (Ag85B-P25; residues 240–254 of M. tuberculosis/BCG Ag85B, I-Ab restricted) and ESSAAFQAAHARFVAA (residues 46–61 of M. tuberculosis/BCG TB9.8, I-Ab restricted). Then, plates were incubated at 37°C for 24 hours. After removal of cells, the plates were washed and incubated with biotinylated anti–IFN-γ detection antibody (clone 4S.B3; BD Biosciences) and biotinylated anti–IL-17 detection antibody (R&D Systems) for 2 hours at 37°C. Development and analysis was performed as previously described [35].
Intracellular Cytokine Analysis
Analysis of cytokine-producing CD4+ T cells was performed 6 weeks after BCG immunization or 4 weeks after challenge, as reported before [35]. Stimuli included the same as those in the ELISPOT assay.
Microarray Analysis
M. tuberculosis was grown in minimal medium with Tyloxapol for 6 days and then subcultured in fresh minimal medium with or without Tyloxapol for 5 days. Cultures were harvested when samples with detergent reached an OD600 of 0.3 and were resuspended in Trizol (Ambion, Carlsbad, California). Cells were further processed for RNA purification, and complementary DNA probes were prepared and hybridized to DNA microarrays (Microarrays, Huntsville, Alabama), which were scanned and analyzed as described previously [37]. Microarray data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus (available at: http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE72745.
Histology
Hematoxylin-eosin staining of lung sections were performed as previously described [35]. Slides were scanned with a Perkin Elmer P250 High Capacity Slide Scanner (Waltham, Massachusetts) at approximately 787 dots/cm (2000 dots/inch). The total disease area for the entire lung and the number of granulomas per lung were calculated as previously described [35]. Five lungs were analyzed for each treatment group. The person who performed the histopathologic analysis was blind to the experimental groups.
Statistics
Standard 1-way analysis of variance followed by the Tukey multiple comparisons test of the means was used to determine the statistical significance of immune responses and the protective efficacies of the immunizations. A P value of < .05 was considered statistically significant.
RESULTS
Antibody Response to BCG Grown With or Without Tyloxapol
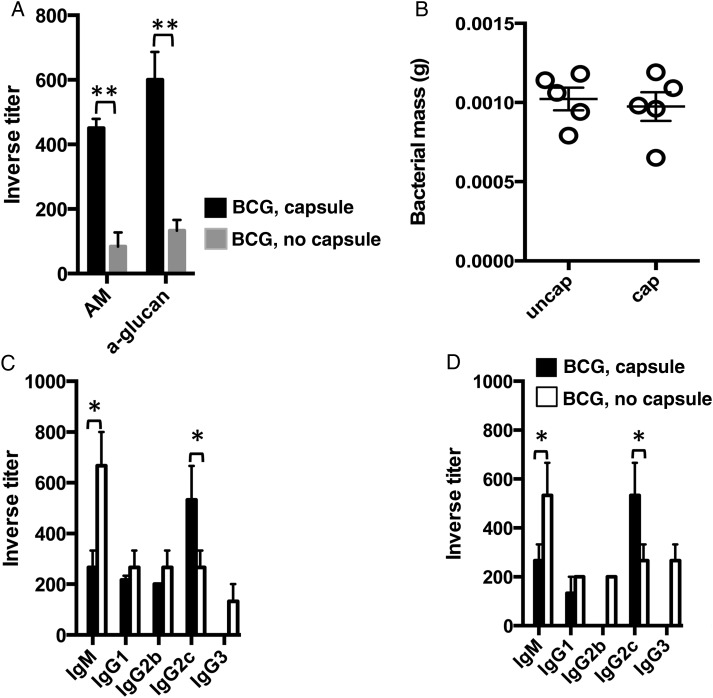
We explored whether immunizing mice with BCG grown in the presence or absence of detergent resulted in different immune responses. As previously reported, detergent reduced clumping (Supplementary Figure 1A) and induced the release of the capsule into the media (Supplementary Figure 1B) [4]. To disrupt clumps, we passed aggregated BCG through a syringe, which preserved the mycobacterial capsule (Supplementary Figure 1C) [20]. We next performed a whole-cell ELISA on BCG cells grown with or without detergent, using specific antibodies to the 2 major capsule polysaccharides, arabinomannan and α-glucan, to confirm that the majority of the capsule is not retained in BCG cells upon detergent treatment (Figure 1A). Since the mycobacterial capsule is enriched in polysaccharides and lipids and includes some immunologically active secreted proteins [4], we hypothesized that immunization with BCG with or without capsule would trigger different immune responses. We prepared encapsulated and unencapsulated BCG as previously reported [20]. We found no significant difference in the bacterial mass recovered from mice that received encapsulated BCG and those that received unencapsulated BCG (Figure 1B), indicating similar immunization amounts. Next, we used ELISA to measure titers of immunoglobulin M (IgM) and IgG antibody subclasses specific to M. tuberculosis encapsulated (Figure 1C) or unencapsulated (Figure 1D) cell lysates in serum from mice immunized for 6 weeks earlier with unencapsulated BCG or encapsulated BCG cells. We observed a predominant M. tuberculosis capsule–specific IgG2c antibody response in encapsulated BCG immune serum, whereas in unencapsulated BCG immune serum, IgM was predominant (Figure 1C). The same was true when we measured unencapsulated M. tuberculosis–specific antibody titers (Figure 1D), suggesting that the presence of a capsule influences the M. tuberculosis–specific antibody response to mycobacterial immunization.
Figure 1.
Immune response to encapsulated bacillus Calmette-Guerin (BCG). A, Findings of whole-cell enzyme-linked immunosorbent assay (ELISA) of encapsulated or unencapsulated BCG cells, using immune serum against BCG arabinomannan (AM) or a monoclonal antibody against α-glucan. B, Determination of the total mass of BCG grown with or without detergent. C and D, ELISA-determined inverse titers of immunoglobulin M (IgM) and immunoglobulin G (IgG) subclasses specific to encapsulated (C) or unencapsulated (D) Mycobacterium tuberculosis strain H37Rv in serum from 4 C57BL/6 mice 6 weeks after subcutaneous immunization with 1 × 106 encapsulated or unencapsulated BCG. The results are representative of 2 independent and similar experiments. *P < .05, **P < .01 one-way ANOVA with Tukey post-test.
T-Cell Responses After BCG Immunization
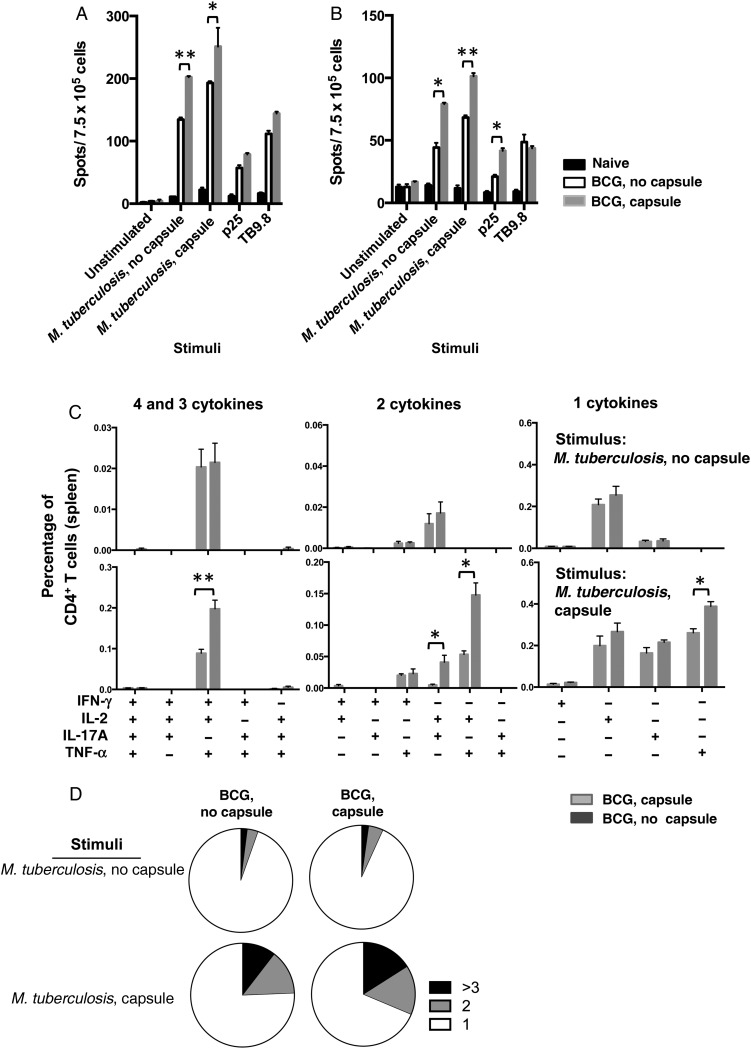
The same immunized mice were used to measure the splenic responses upon different restimulation (Figure 2A and 2B). The number of IFN-γ+ cells in spleens from mice immunized with encapsulated BCG was higher than in mice immunized with unencapsulated BCG and PBS-vaccinated control groups during all of the restimulations. However, significant differences were observed only with M. tuberculosis lysate stimulation (Figure 2A). We observed the same trend for IL-17+ splenocytes (Figure 2B).
Figure 2.
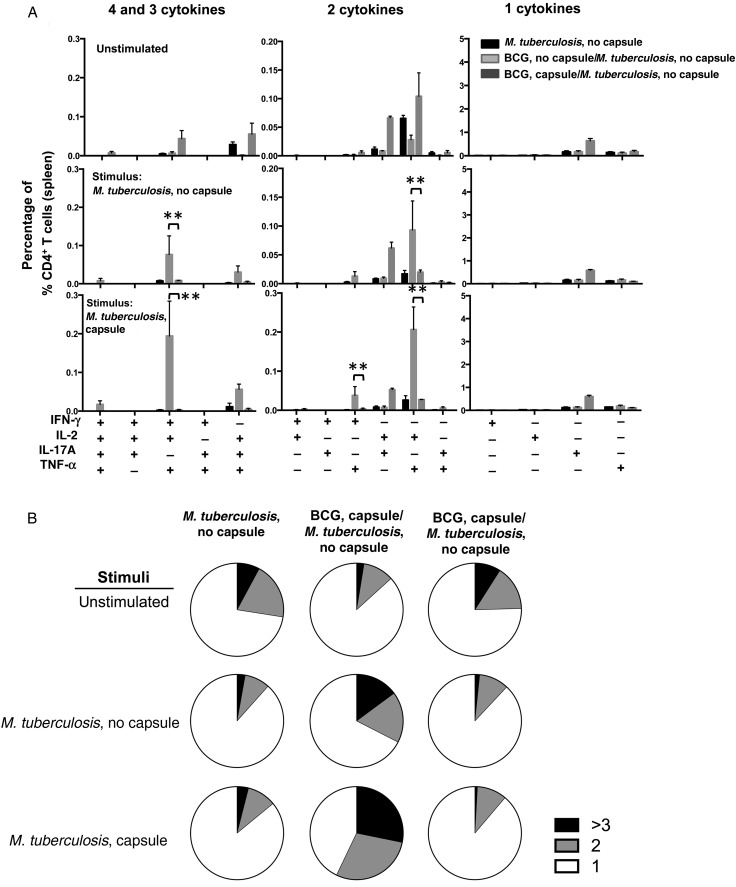
Analysis of T-cell responses upon immunization with bacillus Calmette-Guerin (BCG). A and B, Cellular immune response measured in spleens of 4 C57BL/6 mice 6 weeks after subcutaneous immunization with 1 × 106 encapsulated or unencapsulated BCG. Splenocytes were stimulated in vitro with the indicated antigen preparations. Splenocytes were tested by enzyme-linked immunospot analysis for the presence of interferon γ (IFN-γ; A) and interleukin 17 (IL-17; B) 24 hours after stimulation. Data are means ± standard errors of the mean. The results are representative of 2 independent and similar experiments. *P < .05 and **P < .01 by 1-way analysis of variance with the Tukey post hoc test. C, Multiparameter flow cytometry with intracellular staining for cytokines in splenocytes from 5 animals subcutaneously immunized 6 weeks previously with 1 × 106 colony-forming units of encapsulated or unencapsulated BCG cells and restimulated in vitro with whole-cell lysates from encapsulated or unencapsulated Mycobacterium tuberculosis plus soluble anti-CD28 monoclonal antibody. The graphs show the percentages of total CD4+ T cells producing IFN-γ, interleukin 2 (IL-2), tumor necrosis factor α (TNF-α) or IL-17A and combinations of these cytokines. *P < .05 and ***P < .001, by 1-way analysis of variance with the Tukey post hoc test. D, Pie charts summarizing frequencies of cells producing 1, 2, or 3 cytokines in the experiment shown in panel C. The results are representative of 2 independent experiments.
Next, we studied whether BCG capsule could influence the frequency of multifunctional CD4+ T cells, since T-helper type 1 cells with the capacity to secrete >1 cytokine are associated with protection in BCG-vaccinated mice upon M. tuberculosis challenge [21]. We used multiparameter flow cytometry to measure intracellular cytokine levels among CD4+ T cells from spleens of mice immunized with either encapsulated or unencapsulated BCG and CD4+ T-cell responses, which were restimulated with M. tuberculosis cell lysates and specific mycobacterial antigens (Figure 2C and 2D and Supplementary Figure 2) [21, 22]. We could not see any significant differences in antigen-specific CD4+ T-cell responses to a lysate from unencapsulated M. tuberculosis between mice vaccinated with both BCGs (Figure 2C). However, when the same splenocytes were stimulated with a lysate from encapsulated M. tuberculosis, we observed significant increase in responses among mice immunized with encapsulated BCG, relative to those immunized with unencapsulated BCG for the following subsets of multifunctional T cells: CD4+IFN-γ+IL-2+TNF-α+, CD4+IL-2+IL17A+, and CD4+IL-2+TNF-α+ (Figure 2C). The consolidation of the frequencies of CD4+ T cells producing ≥3, 2, or 1 cytokine suggested that immunization with encapsulated BCG promoted higher-quality CD4+ T-cell responses and that stimulation with an encapsulated M. tuberculosis lysate promotes a higher frequency of multifunctional CD4+ T cells (Figure 2D). Of note, no significant differences were observed when splenocytes were stimulated with peptides such as Ag85bP25 or TB9.8 (Supplementary Figure 2). Overall, mice immunized with encapsulated BCG showed a shift toward greater multifunctional cytokine secretion in the CD4+ T-cell compartment as compared to mice immunized with unencapsulated BCG.
Tyloxapol Influences the Protective Efficacy of BCG
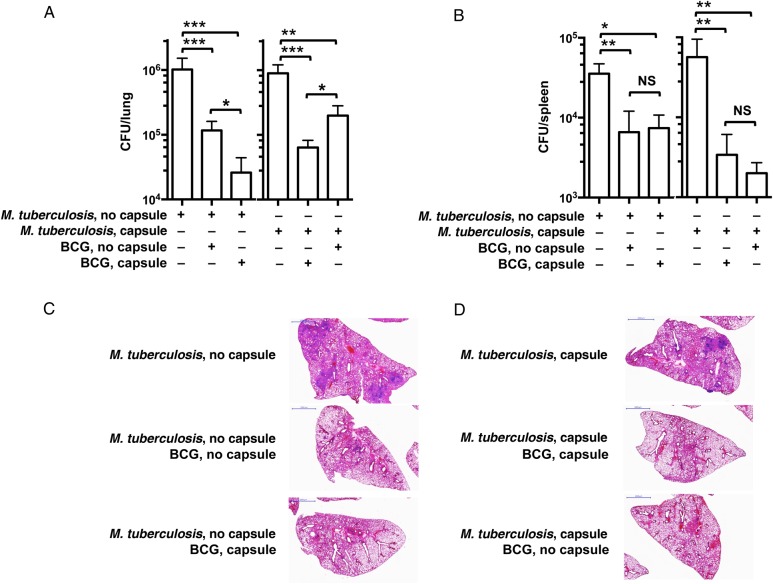
We next explored whether the presence of the capsule in both the vaccine and infecting strain influenced the degree of protection provided by the vaccine strain. Although at this time there is no direct evidence that M. tuberculosis is transmitted in its encapsulated form, for Mycobacterium lepraemurium and Mycobacterium avium this may be the case [3, 23]. To investigate protection against an inoculum with and one without capsules, we immunized mice with either encapsulated or unencapsulated BCG, infected them with either unencapsulated or encapsulated M. tuberculosis, and determined the level of protection by measuring the bacterial loads in lung and spleen (Figure 3). When mice were challenged with unencapsulated M. tuberculosis, we observed a significant reduction in lung CFU with both immunization regimens, relative to unvaccinated mice (1.01 log in unencapsulated BCG–immunized mice and 1.74 log in encapsulated BCG–immunized mice; Figure 3A). Of note, immunization with encapsulated BCG translated into significantly better protection after challenge than immunization with unencapsulated BCG. Both immunizations promoted the control of mycobacterial dissemination equally, as shown by comparable CFU counts in the spleen (Figure 3B). Combination of immunization and infection with encapsulated strains (encapsulated BCG and encapsulated M. tuberculosis) provided a significantly enhanced level of protection, relative to immunization with unencapsulated BCG (1.42 log vs 0.85 log), as measured by the reduction in the CFU counts (Figure 3A). Similarly, both immunization regimens were equally effective in controlling dissemination to the spleen when mice were challenged with encapsulated M. tuberculosis (Figure 3B).
Figure 3.
Analysis of vaccine efficacy of bacillus Calmette-Guerin (BCG) in a mouse model of aerosol infection with unencapsulated and encapsulated Mycobacterium tuberculosis. Five C57BL/6 mice were immunized subcutaneously with 1 × 106 colony-forming units (CFU) of encapsulated or unencapsulated BCG or sham vaccinated with phosphate-buffered saline. Six weeks later, animals were challenged by aerosol infection with 50–100 CFU of virulent unencapsulated or encapsulated M. tuberculosis strain H37Rv. A, Lung CFU in mice infected with unencapsulated (left) or encapsulated (right) M. tuberculosis at 4 weeks. B, Splenic CFU in mice infected with unencapsulated (left) or encapsulated (right) M. tuberculosis at 4 weeks. Bars represent means and standard errors of the mean. Results shown are representative of 2 independent experiments, each with 5 mice per group. *P < .05, **P < .01, and ***P < .001, by 1-way analysis of variance with the Tukey post hoc test. C and D, Representative images of hematoxylin-eosin–stained lungs of C57BL/6 mice immunized with encapsulated or unencapsulated BCG and challenged with encapsulated or unencapsulated M. tuberculosis. Abbreviation: NS, not significant.
Cross-sections of the lung lobes (Figure 3C and 3D) showed a higher number of lesions, frequently multifocal, in the lungs of unvaccinated mice infected with either unencapsulated M. tuberculosis or encapsulated M. tuberculosis. Lesions appeared larger in lungs of mice infected with unencapsulated M. tuberculosis, relative to those in lungs of mice infected with encapsulated M. tuberculosis (Figure 3C and 3D). These lesions, when present, were observed only in mice vaccinated with unencapsulated BCG and were always smaller, more compact, and never multifocal (Figure 3C and 3D). The percentage of diseased lung tissue was twice as large in the unvaccinated mice as compared to BCG-vaccinated mice, and there was no obvious difference between vaccinated groups when mice were challenged with unencapsulated M. tuberculosis (Supplementary Figure 3E). On the contrary, we measured a significant reduction in the percentage of diseased tissue in mice immunized with encapsulated BCG, relative to those immunized with unencapsulated BCG and infected with encapsulated M. tuberculosis (Supplementary Figure 3E), indicating better control of lung inflammation in the former.
Correlation of Protection Efficacy and T-Cell Responses After Infection
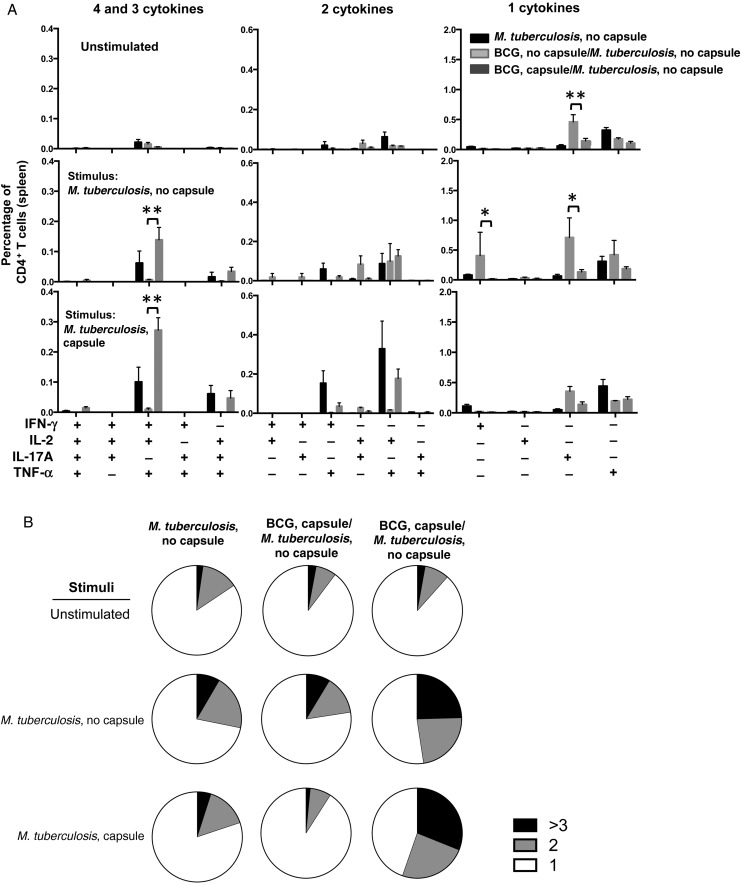
Spleens from unvaccinated and BCG-vaccinated mice were collected 4 weeks after infection with unencapsulated M. tuberculosis and stained for intracellular cytokine expression to determine the multifunctionality of M. tuberculosis–specific CD4+ T cells upon stimulation with either encapsulated M. tuberculosis, unencapsulated M. tuberculosis, or Ag85b-P25 and TB9.8 peptides (Figure 4 and Supplementary Figure 4). Notably, we observed an increase in the population of IFN-γ+IL-2+TNF-α+ CD4+ T cells in mice immunized with encapsulated BCG and infected with unencapsulated M. tuberculosis when restimulated with encapsulated or unencapsulated M. tuberculosis lysates (Figure 4A). An increased frequency of CD4+ T cells in mice immunized with unencapsulated BCG and challenged with unencapsulated M. tuberculosis was observed only for subpopulations of CD4+ T cells producing single cytokines, such as IFN-γ and IL-17. Analysis of the consolidated CD4+ T-cell responses upon stimulation with whole-cell lysate showed a marked increase in the percentage of multifunctional CD4+ T cells in mice immunized with encapsulated BCG and then challenged with unencapsulated M. tuberculosis (Figure 4B). We observed a significant increase in the frequency of multifunctional Ag85bP25-specific CD4+ T cells producing IFN-γ, IL-2, and TNF-α in splenocytes of mice immunized with unencapsulated BCG and infected with unencapsulated M. tuberculosis than in splenocytes of unvaccinated mice and encapsulated BCG–vaccinated mice (Supplementary Figure 4A). Of note, 3 of 6 subpopulations of CD4+ T cells producing 2 cytokines were significantly elevated in spleens of mice that had received vaccination and were infected with unencapsulated strains, relative to the other groups. In general, the results demonstrated a shift to more multifunctional splenic CD4+ T cells in mice vaccinated with encapsulated BCG and then challenged with unencapsulated M. tuberculosis, relative to mice vaccinated with encapsulated BCG, when splenocytes were stimulated with M. tuberculosis lysates. However, this trend was reversed when the same splenocytes were stimulated with M. tuberculosis–specific peptides such as Ag85B-P25. We observed a limited stimulation when using the M. tuberculosis–related peptide TB9.8 (Supplementary Figure 4A).
Figure 4.
Analysis of multifunctional CD4+ T cells after challenge with unencapsulated Mycobacterium tuberculosis strain H37Rv. A, Multiparameter flow cytometry with intracellular staining for cytokines in splenocytes from 5 animals immunized subcutaneously 6 weeks previously with 1 × 106 colony-forming units of encapsulated or unencapsulated bacillus Calmette-Guerin (BCG) cells and challenged with an unencapsulated M. tuberculosis strain. Splenocytes were restimulated in vitro with whole-cell lysates from encapsulated or unencapsulated M. tuberculosis plus soluble anti-CD28 monoclonal antibody. The graphs show the percentages of total CD4+ T cells producing interferon γ (IFN-γ), interleukin 2 (IL-2), tumor necrosis factor α (TNF-α), or interleukin 17 (IL-17) and combinations of these cytokines. *P < .05 and ***P < .001, by 1-way analysis of variance with the Tukey post hoc test. B, Pie charts summarizing frequencies of cells producing 1, 2, or 3 cytokines in the experiment shown in panel A. The results are representative of 2 independent experiments.
The same type of analysis was performed in mice infected with encapsulated M. tuberculosis. We measured an increased frequency of multifunctional CD4+ T cells in mice vaccinated with encapsulated BCG, when splenocytes were stimulated with either encapsulated or unencapsulated M. tuberculosis lysates (Figure 5A). This increase was manifested in a significantly higher frequency of subpopulations of IFN-γ+, IL-2+TNF-α+, IL-2+IL-17+TNF-α+, IFN-γ+TNF-α+, and IL-2+TNF-α+ CD4+ T cells. The combined analysis of these subpopulations revealed a higher frequency of M. tuberculosis–specific CD4+T cell populations producing ≥3 and 2 cytokines in mice immunized and infected with the encapsulated M. tuberculosis (Figure 5B). When splenocytes were stimulated with Ag85bP25, we observed an increase in the frequency of CD4+ T-cell subpopulation simultaneously producing IL-2, IL-17A, and TNF-α, relative to other groups (Supplementary Figure 4B). This was also true for CD4+ T-cell subpopulations producing 2 cytokines, consisting of IL-2, IL-17A, and/or TNF-α. Stimulation with TB9.8 induced a significant increase in the IFN-γ+IL-2+TNF-α+, IL-2+IL17+, and IL-2+TNF-a+ CD4+ T-cell subpopulations in mice immunized with unencapsulated BCG, relative to counts in unvaccinated and encapsulated BCG-vaccinated mice.
Figure 5.
Analysis of multifunctional CD4+ T cells after challenge with encapsulated Mycobacterium tuberculosis strain H37Rv. A, Multiparameter flow cytometry with intracellular staining for cytokines in splenocytes from 5 animals immunized subcutaneously 6 weeks previously with 1 × 106 colony-forming units of encapsulated or unencapsulated bacillus Calmette-Guerin (BCG) cells and challenged with an encapsulated M. tuberculosis strain. Splenocytes were restimulated in vitro with whole-cell lysates from encapsulated or unencapsulated M. tuberculosis plus soluble anti-CD28 monoclonal antibody. The graphs show the percentages of total CD4+ T cells producing interferon γ (IFN-γ), interleukin 2 (IL-2), tumor necrosis factor α (TNF-α), or interleukin 17A (IL-17A) and combinations of these cytokines. *P < .05 and ***P < .001, by 1-way analysis of variance with the Tukey post hoc test. B, Pie charts summarizing frequencies of cells producing 1, 2, or 3 cytokines in the experiment shown in panel A. The results are representative of 2 independent experiments.
Transcriptional Changes of M. tuberculosis Cultures With and Those Without Detergent
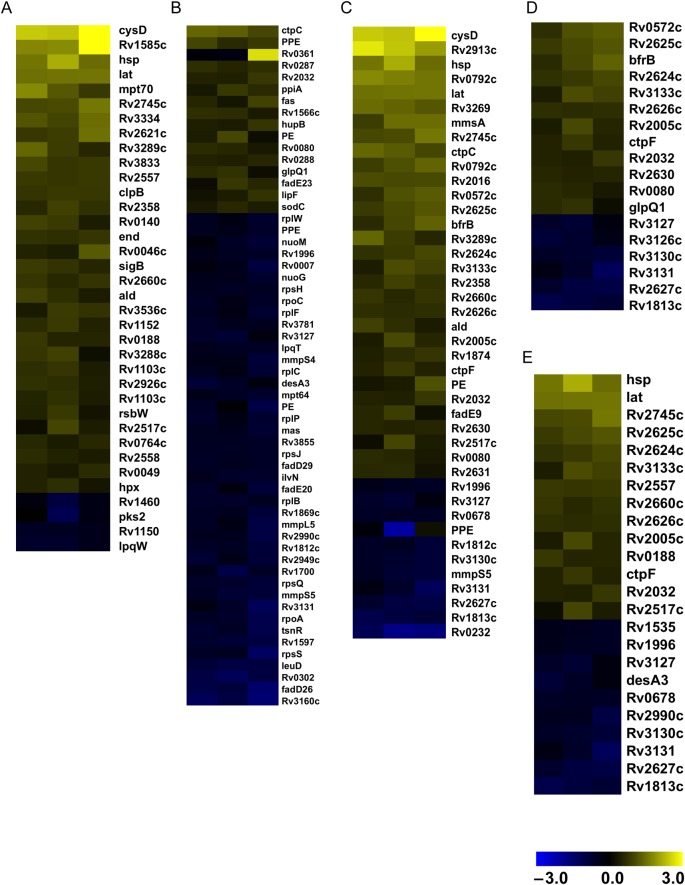
We also considered the possibility that these distinct responses were due to other differences in the composition and/or behavior of mycobacteria. Consequently, we compared the transcriptional response of M. tuberculosis cells grown in media with or without Tyloxapol. We observed a global shift indicative of a slowing metabolism, reflected in the downregulation of genes involved in transcription, translation, and cell division, and the induction of stress responses, as well as elements of the dosR regulon. The parallels for changes in gene expression during starvation and nonreplicating persistence were particularly striking and included upregulation of genes associated with the sulfur assimilation system encoded by cysDN and the lysine aminotransferase encoded by lat. To further explore these similarities in transcription profiles, we compared our results to those associated with conditions that may reflect the environment in the host, including starvation [24], hypoxia [25], nonreplicating persistence [26], stationary phase [26], and acidic conditions [27]. We observed a high percentage of coincidence in upregulated and downregulated genes between starvation and detergent-free cultures (Figure 6A and B). Similar parallels were observed with nonreplicating persistence (Figure 6C), hypoxia (Figure 6D), and stationary phase (Figure 6E). These results suggest that growing M. tuberculosis in the absence of Tyloxapol induces transcriptional changes that bear resemblance to those observed when M. tuberculosis is in the host.
Figure 6.
Effect of nondetergent condition on the transcriptional profile of genes differentially expressed in stress conditions. Samples from 3 biologically independent replicates of Mycobacterium tuberculosis grown in the presence and absence of detergent were harvested on day 5, and RNA was extracted. The pool of genes upregulated or downregulated in the presence of detergent was determined in conditions that may reflect the intracellular environment of host. A, Genes upregulated under starvation conditions (created by exposure to phosphate-buffered saline for 96 hours). B, Genes downregulated under starvation conditions. C, Genes upregulated under nonreplicating-persistence conditions. D, Genes upregulated under hypoxic conditions. E, Genes upregulated in stationary phase. Each column represents an independent biological replicate.
DISCUSSION
Growing BCG in different culture media is known to influence its efficacy as a vaccine [22, 28]. In this study, we show that another potential source of variability in BCG vaccine efficacy experimental studies is growth in detergent. Since human BCG vaccination is already done with mycobacteria grown the absence of detergent we do not believe that the effects shown for encapsulated and non-encapsulated strains described here are relevant to differences in BCG efficacy in humans. However, our findings could be highly relevant to conclusions from immunological studies, which often use mycobacteria grown with detergent. We confirmed a previously reported finding that growing BCG or M. tuberculosis in the presence of Tyloxapol resulted in cells with little or no capsule [4]. Considering that most if not all of the experimental immunological studies using live attenuated vaccines [15–19] have been performed with strains grown with detergent, we sought to investigate the impact of encapsulated BCG and M. tuberculosis in a typical vaccination-challenge experiment. We hypothesized that vaccination and infection with encapsulated strains would influence the protective efficacy of the immune responses elicited. Indeed, a more potent and diverse antibody response was observed when vaccinating mice with encapsulated BCG relative to that elicited by unencapsulated BCG. Arabinomannan- or lipoarabinomannan-specific monoclonal antibodies can contribute to the clearance of mycobacteria from the host [29, 30], since both polysaccharides can elicit robust antibody responses in infected hosts [31] and a lower lipoarabinomannan-specific antibody response in children with tuberculosis was associated with disseminated mycobacterial disease [32].
There were major differences in the transcriptional profile of M. tuberculosis cells grown with or without detergent. Most interesting was the observation that M. tuberculosis manifested a transcriptional program that is similar to that expressed by M. tuberculosis in the host when grown without detergent and suggestive of starvation or hypoxia. Of note and with relevance to these observations, the inclusion of M. tuberculosis antigens expressed during the chronic phase in subunit vaccines (multistage vaccines) was an effective strategy in eliciting immune responses that provided long-lasting protection [33].
The presence of a capsule in the vaccine and challenge mycobacterial strains was associated with differences in the level of protection. This suggests that the capsule influenced the type of immune response elicited at the time of vaccination and that this immune response is most efficiently directed to an encapsulated infecting strain. We acknowledge that a limitation of the interpretation assigning immunological changes to the presence and absence of a capsule is that we have compared mycobacterial strains grown with and those grown without detergent and inferred that the capsule is the relevant variable despite the fact that different growth conditions elicit other metabolic changes, as we have measured here in the transcriptional analysis. We do not believe that trace amounts of detergent in the inoculum were responsible for changes in inflammation since Tyloxapol is pro-inflammatory [33, 34] and the opposite was observed, since infection with bacteria grown with detergent induced less inflammation. Furthermore, we note that we have analyzed 1 BCG strain and that other strains may differ in their metabolic response to detergent and/or capsular antigens. In other bacteria capsules are notoriously variable in structure and antigenicity, leading to differences that allow the classification of strains into distinct serotypes. At present, we do not know whether the concept of serotype applies to M. tuberculosis. However, we note that the reactivity of a monoclonal antibody varied among several M. tuberculosis strains, suggesting a high likelihood that mycobacterial polysaccharides are also antigenically variable [7, 29].
In summary, our study provides evidence that the presence of detergent in the medium used for growth and the mycobacterial capsule is an important variable in eliciting protective responses during BCG vaccination. We propose that vaccination protocols should be adapted to reflect the clinical vaccination/infection situation in which both BCG and M. tuberculosis are possibly applied/transmitted in their encapsulated forms.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the staff at the Albert Einstein College of Medicine flow cytometry and analytical imaging facilities.
Financial support. This work was supported by the National Institutes of Health (awards 5R01HL059842, 5R01AI033774, 5R37AI033142, and 5R01AI052733 to A. C.; award 1R21AI115091 to R. P.-R.; program project grant P01AI063537 to S. A. P., W. R. J., and J. C.; award R01AI094745 to J. C.; and award R01AI093649 to S. A. P.), the Bill and Melinda Gates Foundation (award OPP1070514 to A. C.), the Ramon y Cajal Program from the Spanish Ministry of Economy and Competitiveness (to R. P.-R.), and the Pew Latin American Fellows Program (to L. J. C.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cress BF, Englaender JA, He W, Kasper D, Linhardt RJ, Koffas MA. Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev 2014; 38:660–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daffe M, Etienne G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber Lung Dis 1999; 79:153–69. [DOI] [PubMed] [Google Scholar]
- 3.Hanks JH. Capsules in electron micrographs of Mycobacterium leprae. Int J Lepr 1961; 29:84–7. [PubMed] [Google Scholar]
- 4.Sani M, Houben EN, Geurtsen J et al. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 2010; 6:e1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright EL, Pourshafie M, Barrow WW. Mycobacterium avium rough-to-smooth colony conversion resulting from growth in Tween 80 without presence of type-specific glycopeptidolipid antigens. FEMS Microbiol Lett 1992; 77:209–16. [DOI] [PubMed] [Google Scholar]
- 6.Glatman-Freedman A, Casadevall A, Dai Z et al. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol 2004; 42:3225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwebach JR, Casadevall A, Schneerson R et al. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect Immun 2001; 69:5671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwebach JR, Glatman-Freedman A, Gunther-Cummins L et al. Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect Immun 2002; 70:2566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortalo-Magne A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology 1995; 141:1609–20. [DOI] [PubMed] [Google Scholar]
- 10.Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis 1999; 79:243–50. [DOI] [PubMed] [Google Scholar]
- 11.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339–45. [DOI] [PubMed] [Google Scholar]
- 12.Smith D, Reeser P, Musa S.. Does infection with environmental mycobacteria suppress the protective response to subsequent vaccination with BCG? Tubercle 1985; 66:17–23. [DOI] [PubMed] [Google Scholar]
- 13.Russell DG, Barry CE III, Flynn JL. Tuberculosis: what we don't know can, and does, hurt us. Science 2010; 328:852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eickhoff TC. The current status of BCG immunization against tuberculosis. Annu Rev Med 1977; 28:411–23. [DOI] [PubMed] [Google Scholar]
- 15.Sambandamurthy VK, Wang X, Chen B et al. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat Med 2002; 8:1171–4. [DOI] [PubMed] [Google Scholar]
- 16.Arbues A, Aguilo JI, Gonzalo-Asensio J et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine 2013; 31:4867–73. [DOI] [PubMed] [Google Scholar]
- 17.Hess J, Miko D, Catic A, Lehmensiek V, Russell DG, Kaufmann SH. Mycobacterium bovis Bacille Calmette-Guerin strains secreting listeriolysin of Listeria monocytogenes. Proc Natl Acad Sci U S A 1998; 95:5299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun R, Skeiky YA, Izzo A et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 2009; 27:4412–23. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Fu R, Yuan X et al. Differential immune responses and protective effects in avirulent mycobacterial strains vaccinated BALB/c mice. Curr Microbiol 2015; 71:129–35. [DOI] [PubMed] [Google Scholar]
- 20.Stokes RW, Norris-Jones R, Brooks DE, Beveridge TJ, Doxsee D, Thorson LM. The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect Immun 2004; 72:5676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darrah PA, Patel DT, De Luca PM et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843–50. [DOI] [PubMed] [Google Scholar]
- 22.Venkataswamy MM, Goldberg MF, Baena A, Chan J, Jacobs WR Jr., Porcelli SA. In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine 2012; 30:1038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frehel C, Ryter A, Rastogi N, David H. The electron-transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann Inst Pasteur Microbiol 1986; 137B:239–57. [DOI] [PubMed] [Google Scholar]
- 24.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 2002; 43:717–31. [DOI] [PubMed] [Google Scholar]
- 25.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha -crystallin. Proc Natl Acad Sci U S A 2001; 98:7534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voskuil MI, Visconti KC, Schoolnik GK. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb) 2004; 84:218–27. [DOI] [PubMed] [Google Scholar]
- 27.Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE III. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 2004; 279:40174–84. [DOI] [PubMed] [Google Scholar]
- 28.Petricevich VL, Ueda C, Alves RC et al. A single strain of Mycobacterium bovis bacillus Calmette-Guerin (BCG) grown in two different media evokes distinct humoral immune responses in mice. Braz J Med Biol Res 2001; 34:81–92. [DOI] [PubMed] [Google Scholar]
- 29.Teitelbaum R, Glatman-Freedman A, Chen B et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A 1998; 95:15688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol 2004; 138:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X, Prados-Rosales R, Jenny-Avital ER, Sosa K, Casadevall A, Achkar JM. Comparative evaluation of profiles of antibodies to mycobacterial capsular polysaccharides in tuberculosis patients and controls stratified by HIV status. Clin Vaccine Immunol 2012; 19:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello AM, Kumar A, Narayan V et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg 1992; 86:686–92. [DOI] [PubMed] [Google Scholar]
- 33.Aagaard C, Hoang T, Dietrich J et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 2011; 17:189–94. [DOI] [PubMed] [Google Scholar]
- 34.Prados-Rosales R, Baena A, Martinez LR et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest 2011; 121:1471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prados-Rosales R, Carreno LJ, Batista-Gonzalez A et al. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. MBio 2014; 5:e01921–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glatman-Freedman A, Martin JM, Riska PF, Bloom BR, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol 1996; 34:2795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilcheze C, Weinrick B, Wong KW, Chen B, Jacobs WR Jr. NAD+ auxotrophy is bacteriocidal for the tubercle bacilli. Mol Microbiol 2010; 76:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.