Abstract
Background. Interleukin 6 (IL-6), high-sensitivity C-reactive protein (hsCRP), and D-dimer levels are linked to adverse outcomes in human immunodeficiency virus (HIV) infection, but the strength of their associations with different clinical end points warrants investigation.
Methods. Participants receiving standard of care in 2 HIV trials with measured biomarker levels were followed to ascertain all-cause death, non–AIDS-related death, AIDS, cardiovascular disease (CVD), and non–AIDS-defining malignancies. Hazard ratios (HRs) and 95% confidence intervals (CIs) of each end point for quartiles and log2-transformed IL-6, hsCRP, and D-dimer levels were calculated using Cox models. Marginal models modelling multiple events tested for equal effects of biomarker levels on different end points.
Results. Among 4304 participants, there were 157 all-cause deaths, 117 non–AIDS-related deaths, 101 AIDS cases, 121 CVD cases, and 99 non–AIDS-defining malignancies. IL-6 was more strongly associated with most end points, compared with hsCRP. IL-6 appeared to be a stronger predictor than D-dimer for CVD and non–AIDS-defining malignancies, but 95% CIs overlapped. Independent associations of IL-6 were stronger for non–AIDS-related death (HR, 1.71; 95% CI, 1.43–2.04) and all-cause death (HR, 1.56; 95% CI, 1.33–1.84) and similar for CVD (HR, 1.35; 95% CI, 1.12–1.62) and non–AIDS-defining malignancies (HR, 1.30; 95% CI, 1.06–1.61). There was heterogeneity of IL-6 (P < .001) but not hsCRP (P = .15) or D-dimer (P = .20) as a predictor for different end points.
Conclusions. IL-6 is a stronger predictor of fatal events than of CVD and non–AIDS-defining malignancies. Adjuvant antiinflammatory and antithrombotic therapies should be tested in HIV-infected individuals.
Keywords: HIV, inflammation, IL-6, hsCRP, D-dimer, cardiovascular disease, cancer
Activated inflammation and hypercoagulation have consistently been linked to many adverse clinical outcomes during human immunodeficiency virus (HIV) infection, including progression to AIDS [1, 2], anemia [3], cancer [4], cardiovascular disease (CVD) [5, 6], diabetes [7], and all-cause mortality [8–10]. This observation is drawn from several studies that reported independent associations between raised plasma levels of inflammatory and coagulation biomarkers and subsequent risk of clinical outcomes [11]. In the Strategies for Management of Anti-Retroviral Therapy (SMART) trial, episodic antiretroviral therapy (ART) use increased all-cause mortality, compared with continuous ART, with the majority of the deaths attributable to CVD and cancer [12]. Of a panel of 6 biomarkers tested in SMART participants at study entry, elevated levels of the following 3 were independently and strongly associated with a subsequent risk of death during follow-up [8]: interleukin-6 (IL-6), a proinflammatory cytokine that is a proximal mediator or upstream inflammatory marker [13]; high-sensitivity C-reactive protein (hsCRP), a downstream, acute-phase reactant whose hepatic production is stimulated, among other factors, by IL-6 [14]; and D-dimer, a degradation product of cross-linked intravascular fibrin that is a marker of hypercoagulation [15].
Many previous studies of IL-6, hsCRP, and D-dimer have investigated associations with all-cause death and different diseases in the setting of HIV infection [11], but the strength of the associations between levels of these biomarkers and different types of clinical end points is not well understood. For instance, it remains to be determined which biomarker best predicts AIDS-defining and non–AIDS-defining morbidity among HIV-positive persons receiving standard of care [16]. This poses an important research question as to whether activated inflammation and hypercoagulation contribute in a general way to the development of multiple diseases or are instead more strongly associated with the development of specific pathologies [17]. Here, we set out to evaluate the relative value of IL-6, hsCRP, and D-dimer levels as predictors of different clinical end points in a large cohort of HIV-positive persons.
MATERIAL AND METHODS
Study Population
This cohort study combined the control arms of 2 international HIV trials conducted by the International Network for Strategic Initiatives in Global HIV Trials: the SMART (clinical trials registration NCT00027352) [12] and the Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT; clinical trials registration NCT00004978) [18]. Participants who had consented to storing blood for future research and whose plasma levels of IL-6, hsCRP and D-dimer were measured at study entry, before randomization, were included (n = 4304). All participants in the control arms received standard of care according to HIV-treatment guidelines and were to be continuously administered ART. Before the enrolment of participants in SMART and ESPRIT, the institutional review board at each site had approved the original study protocols, and written informed consent was obtained from all participants. SMART and ESPRIT were conducted in compliance with the Declaration of Helsinki Guidelines, were registered on clinical trials databases and reviewed by independent data and safety interim monitoring boards.
Biomarker Measurements
For SMART participants, biomarker levels were measured at the Laboratory for Clinical Biochemistry Research at the University of Vermont (Burlington). In ESPRIT, laboratory measurements were performed at SAIC-Frederick (Frederick, Maryland). IL-6 levels were measured by the same method at each laboratory (chemiluminescent sandwich enzyme-linked immunosorbent assay [ELISA], R&D Systems). hsCRP levels were determined by means of highly sensitive ELISAs at both laboratories. For SMART participants, a NBTMII nephelometer, N Antiserum to Human CRP (Siemens Diagnostics), was used. For ESPRIT, an R&D Systems ELISA was used. D-dimer levels were determined by ELISA on the Sta-R analyzer, Liatest D-DI (Diagnostic Stago), for SMART participants and on a VIDAS instrument (BioMerieux, Durham, NC), for ESPRIT participants. As described in detail elsewhere [6, 19], the assays for determining hsCRP and D-dimer levels, while different, compared well, and measurements on duplicate samples were well correlated.
Follow-up and End Point Ascertainment
Patients were followed from study entry until death, loss to follow-up, or the closing date of each study. The following clinical end points were considered: (1) all-cause mortality; (2) non–AIDS-related, nonviolent, and unintentional death; (3) fatal and nonfatal progression to AIDS (Centers for Disease Control and Prevention category C events); (4) fatal and nonfatal CVD (defined as myocardial infarction, stroke, or coronary artery disease requiring a surgical procedure); and (5) fatal and nonfatal non–AIDS-defining malignancies (excluding basal and squamous cell skin cancers). All clinical end points were systematically reported to and centrally adjudicated by an end point review committee, using prespecified criteria [20]. Causes of death were determined using CoDe [21]. Clinical assessment intervals and total follow-up time varied by study. Median follow-up time was 29 months in SMART and 81 months in ESPRIT; the median follow-up time was 48 months for the entire cohort.
Statistical Analyses
Hazard ratios (HRs) with 95% confidence intervals (CIs) of each study end point for IL-6, hsCRP, and D-dimer levels, modeled as log2-transformed values (ie, per 2-fold higher), were calculated and used to compare the association of plasma levels of each biomarker with the risk of developing different clinical end points. Models for each biomarker were stratified by study to account for differences in underlying risk for the different cohorts. This stratification also grouped participants by the 2 laboratories that measured biomarker levels. We used sequential adjustment to fit 3 Cox proportional hazards regression models (models 1–3) for each of the 5 clinical end points, and we repeated these 3 models for levels of all 3 biomarkers. The Cox proportional hazards models were fit as follows: model 1 was univariable; model was 2 adjusted for demographic characteristics (age, sex, race, and body mass index), nadir and baseline CD4+ T-cell counts, baseline ART use, baseline HIV RNA level, prior AIDS and CVD, diabetes mellitus, and hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfection; model 3 adjusted for the factors in model 2, with further adjustment that included fitting biomarkers of inflammation and coagulation simultaneously. However, we did not include both biomarkers of inflammation in the same model because IL-6 and hsCRP have biological redundancy. The hepatic production of hsCRP is determined by IL-6 [14]. Therefore, elevated plasma levels of both biomarkers could have indicated similar pathophysiological processes.
We computed crude incidence rates and HRs (with 95% CIs) of clinical end points for each quartile of biomarker levels. To control for any differences in biomarker level distributions among studies and interlaboratory variability, quartiles were defined separately for participants in SMART and ESPRIT.
To compare the strength of associations of IL-6, hsCRP, and D-dimer levels with each end point investigated, competing risk or marginal Cox models described by Wei et al [22] were used to model multiple unordered events and to test for equal effects of biomarker levels on different types of end points. With this approach, the fit of a model that assumed a common association for biomarker level with each end point was compared with the fit of a model that allowed the association to vary. The corresponding P value is the probability that the magnitude of differences in estimated HRs for the end points could have arisen by chance if there were no real differences in the actual HRs. We also evaluated the strength of associations of each biomarker with specific end points by estimating the HRs of the highest versus the lowest biomarker quartiles and the HRs per 2-fold higher biomarker levels.
Secondary Analyses
To test the robustness of our main findings and adjust for other biologically plausible confounders, we performed 2 preplanned secondary analyses. First, we recalculated HRs of clinical end points for quartiles of biomarker levels, excluding events in the first 2 years of follow-up. This analysis was aimed at addressing the possibility of bias by reverse causality. Because CVD and cancer may have a long subclinical period before diagnosis, there is a possibility that elevated biomarkers levels were a consequence of the disease outcome instead of vice versa. Second, among SMART participants for whom information on smoking at entry was collected, analyses further adjusting for smoking were performed because smoking is causally related to cancer and CVD.
RESULTS
There were approximately 19 000 person-years of follow-up (PYFU) among 4304 participants (median age, 42 years; median baseline CD4+ T-cell count, 526 cells/mm3; 77% men), including 157 all-cause deaths (crude incidence rate, 8.25 cases/1000 person-years of follow-up; 95% CI, 6.96–9.53), 117 nonviolent, unintentional, and non–AIDS-related deaths (crude incidence rate, 6.14 deaths/1000 PYFU; 95% CI, 5.03–7.25), 101 progressions to AIDS (70 opportunistic infections and 32 AIDS-defining cancers; 1 participant in ESPRIT had both types of AIDS events; crude incidence rate, 5.39 events/1000 PYFU; 95% CI, 4.34–6.44), 121 CVD events (crude incidence rate, 6.47 events/1000 PYFU; 95% CI, 5.32–7.62), and 99 non–AIDS-defining malignancies (crude incidence rate, 5.27 cases/1000 PYFU; 95% CI, 4.23–6.30).
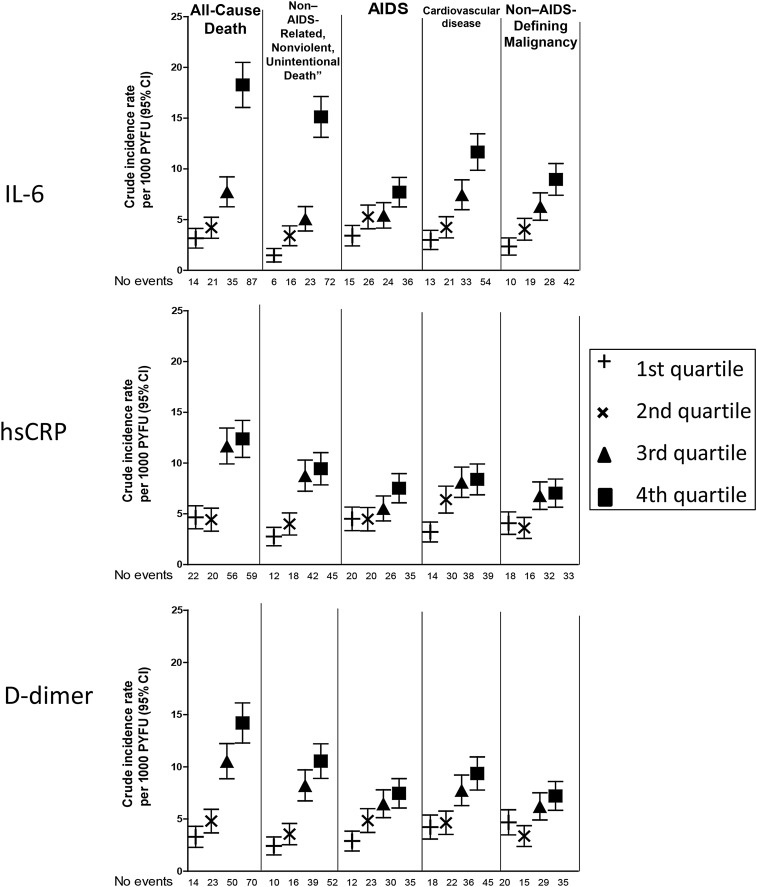
Baseline characteristics are presented for the entire cohort and separately for people who experienced the different clinical end points and are shown in Table 1. Levels of IL-6, hsCRP, and D-dimer at study entry were higher among participants experiencing each clinical end point when compared to the entire cohort (Table 1). Crude incidence rates of all clinical end points increased across higher quartiles of all biomarkers (Figure 1). This was particularly true for IL-6 and D-dimer quartiles, for which a clearly graded response pattern was observed.
Table 1.
Baseline Characteristics Participants in the Control Arms of SMART and ESPRIT
| Characteristic | Overall (n = 4304) | All-Cause Death (n = 157) | Non–AIDS-Related, Nonviolent, Unintentional Death (n = 117) | AIDS (n = 101) | CVD (n = 121) | Non–AIDS-Defining Malignancy (n = 99) |
|---|---|---|---|---|---|---|
| Age, y | 42 [36, 49] | 48 [40, 54] | 48 [41, 55] | 44 [38, 50] | 49 [43, 56] | 50 [44, 57] |
| Female sex | 1002 (23.3) | 28 (17.8) | 17 (14.5) | 22 (21.8) | 14 (11.6) | 14 (14.1) |
| Black race | 907 (21.1) | 29 (18.5) | 25 (21.4) | 18 (17.8) | 26 (21.5) | 26 (26.3) |
| BMIa | 24.4 [22.1, 27.1] | 23.5 [21.4, 27.3] | 23.3 [21.3, 27.1] | 25.2 [22.6, 29.0] | 24.0 [22.1, 27.4] | 23.9 [21.7, 25.8] |
| Prior AIDS | 1093 (25.4) | 46 (29.3) | 32 (27.4) | 42 (41.6) | 47 (38.8) | 21 (21.2) |
| Hepatitis B/C | 761 (17.7) | 54 (34.4) | 41 (35.0) | 16 (15.8) | 18 (14.9) | 27 (27.3) |
| Prior CVD | 112 (2.6) | 14 (9.0) | 12 (10.3) | 1 (1.0) | 16 (13.3) | 5 (5.1) |
| Diabetes | 217 (5.1) | 14 (9.0) | 10 (8.6) | 7 (6.9) | 14 (11.7) | 9 (9.1) |
| PI-based ART | 1478 (34.3) | 53 (33.8) | 45 (38.5) | 31 (30.7) | 52 (43.0) | 41 (41.4) |
| NNRTI-based ART | 1643 (38.2) | 52 (33.1) | 39 (33.3) | 34 (33.7) | 30 (24.8) | 30 (30.3) |
| CD4+ T-cell count, cells/mm3 | ||||||
| Baseline | 526 [415, 701] | 451 [370, 594] | 470 [384, 639] | 466 [376, 599] | 515 [401, 673] | 526 [404, 679] |
| Nadir | 230 [120, 337] | 194 [93, 297] | 194 [85, 282] | 190 [90, 298] | 187 [74, 301] | 219 [97, 311] |
| HIV RNA load ≤500 copies/mL | 3263 (75.8) | 97 (61.8) | 77 (65.8) | 51 (50.5) | 88 (72.7) | 75 (75.8) |
| IL-6, pg/mL | 1.80 [1.18, 2.90] | 3.09 [2.10, 4.40] | 3.17 [2.10, 4.49] | 2.42 [1.40, 3.33] | 2.60 [1.78, 4.30] | 2.50 [1.81, 3.58] |
| hsCRP, µg/mL | 1.60 [0.69, 3.67] | 2.83 [1.53, 6.27] | 2.70 [1.57, 6.18] | 2.03 [0.83, 4.50] | 2.33 [1.02, 5.05] | 2.54 [1.13, 4.93] |
| D-dimer, µg/mL | 0.24 [0.15, 0.38] | 0.33 [0.23, 0.55] | 0.35 [0.23, 0.55] | 0.31 [0.22, 0.53] | 0.31 [0.20, 0.51] | 0.28 [0.18, 0.52] |
Data are no. (%) of subjects or median value (interquartile range).
Abbreviations: ART, antiretroviral therapy; CVD, cardiovascular disease; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Figure 1.
Crude incidence rates of clinical end points across biomarker quartiles. Quartile cut points were defined differently for each study, as described in Supplementary Table 2. Abbreviations: CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; PYFU, person-years of follow-up.
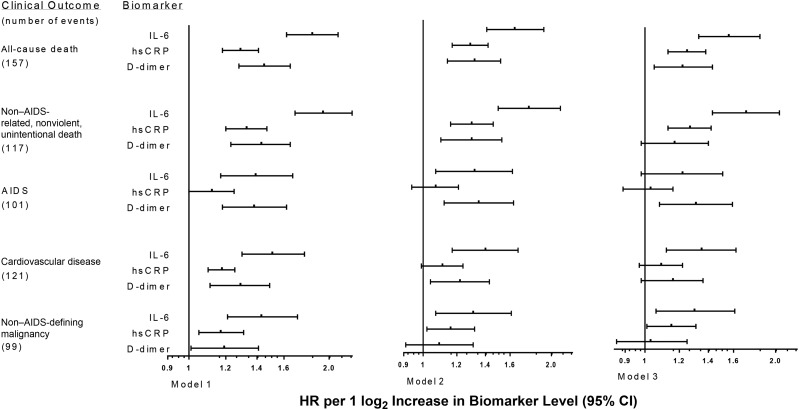
When compared to hsCRP, IL-6 was more strongly associated with most outcomes investigated both in univariable and multivariable models that considered log2-transformed biomarkers. IL-6 was a stronger predictor than D-dimer level for CVD events and non–AIDS-defining malignancies (Figure 2, Table 2, and Supplementary Table 1), but 95% CIs overlapped.
Figure 2.
Hazard ratios (HRs) for clinical end points associated with baseline levels of biomarkers. Model 1: unadjusted associations, stratified by study. Model 2: adjusted for demographic characteristics (age, sex, race, and body mass index [calculated as the weight in kilograms divided by the height in meters squared]), antiretroviral therapy use, nadir and baseline CD4+ T-cell counts, baseline human immunodeficiency virus RNA level, prior AIDS and cardiovascular disease, diabetes mellitus, and hepatitis B virus and hepatitis C virus coinfection, stratified by study. Model 3: HRs for interleukin 6 (IL-6) and D-dimer levels, using model 2 and including both biomarkers simultaneously, and HRs for high-sensitivity C-reactive protein (hsCRP) level, using model 2 and also adjusted for D-dimer level, stratified by study. Abbreviation: CI, confidence interval.
Table 2.
Hazard Ratios (HRs) of Study End Points for Plasma Levels of Interleukin 6 (IL-6), High-Sensitivity C-Reactive Protein (hsCRP), and D-Dimer
| End Point | IL-6, HR (95% CI) |
hsCRP, HR (95% CI) |
D-Dimer, HR (95% CI) |
|||
|---|---|---|---|---|---|---|
| 2-Fold Higher Level | 4th/1st Quartile | 2-Fold Higher Level | 4th/1st Quartile | 2-Fold Higher Level | 4th/1st Quartile | |
| All-cause death | 1.64 (1.41–1.92) | 3.07 (1.70–5.55) | 1.29 (1.17–1.42) | 2.73 (1.56–4.69) | 1.32 (1.14–1.52) | 3.00 (1.61–5.59) |
| Non–AIDS-related, nonviolent, unintentional death | 1.77 (1.50–2.10) | 6.31 (2.68–14.83) | 1.30 (1.16–1.46) | 3.10 (1.59–6.05) | 1.30 (1.10–1.53) | 3.38 (1.62–7.08) |
| Progression to AIDS | 1.32 (1.07–1.62) | 1.63 (.83–3.21) | 1.07 (.94–1.21) | 1.35 (.74–2.48) | 1.35 (1.12–1.63) | 2.20 (1.07–4.50) |
| Cardiovascular disease event | 1.40 (1.17–1.67) | 3.34 (1.66–6.70) | 1.11 (.99–1.24) | 1.89 (.99–3.63) | 1.22 (1.04–1.43) | 1.80 (.98–3.29) |
| Non–AIDS-defining malignancy | 1.31 (1.07–1.61) | 3.12 (1.42–6.84) | 1.16 (1.02–1.32) | 1.68 (.86–3.28) | 1.09 (.91–1.31) | 1.03 (.55–1.94) |
HRs were adjusted for demographic characteristics (age, sex, race, and body mass index [calculated as the weight in kilograms divided by the height in meters squared]), nadir and baseline CD4+ T-cell counts, antiretroviral therapy use, baseline human immunodeficiency virus RNA level, prior AIDS and cardiovascular disease, diabetes mellitus, and hepatitis B virus and hepatitis C virus coinfection, stratified by study (model 2). For detailed information on results using models 1 and 3, refer to Supplementary Tables 1 and 2.
Abbreviation: CI, confidence interval.
Independent associations between log2 IL-6 and clinical end points were strongest for fatal events and similar for fatal and nonfatal CVD and non–AIDS-defining malignancies in multivariable analyses. For instance, adjusted HRs calculated using model 3 were numerically higher for non–AIDS-related, nonviolent, and unintentional death (1.71 [95% CI, 1.43–2.04] per 2-fold higher IL-6 level) and for all-cause death (1.56; 95% CI, 1.33–1.84) than for fatal or nonfatal CVD events (1.35; 95% CI, 1.12–1.62) and non–AIDS-defining malignancies (1.30; 95% CI, 1.06–1.61; Figure 2 and Supplementary Table 1). The Wei-Lin-Weissfeld test found evidence of heterogeneity in the association of log2 IL-6 with different end points (P < .001), but not of log2 hsCRP (P = .15) or log2 D-dimer (P = .20).
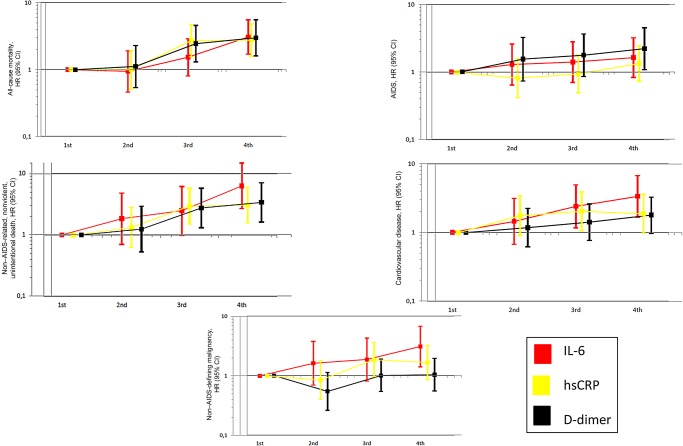
In multivariable analyses comparing the highest to the lowest biomarker quartiles (Table 2, Figure 3, and Supplementary Table 2), HRs for all-cause death were similar for IL-6, hsCRP, and D-dimer. However, higher quartiles of IL-6 were independently associated with steeper risk gradients than hsCRP for all other outcomes. When compared to D-dimer, IL-6 appeared to be associated with steeper risk gradients for CVD and non–AIDS-defining malignancies (Table 2 and Figure 3), but 95% CIs overlapped (Figure 3 and Supplementary Table 2). The strength of associations of IL-6 and D-dimer levels with the other end points was similar (Table 2).
Figure 3.
Adjusted hazard ratios (HRs) for clinical end points associated with biomarker quartiles. Quartile cut points were defined differently for each study, as described in Supplementary Table 2. HRs were adjusted for factors included in model 2, namely demographic characteristics (age, sex, race, and body mass index [calculated as the weight in kilograms divided by the height in meters squared]), antiretroviral therapy use, nadir and baseline CD4+ T-cell counts, baseline human immunodeficiency virus RNA level, prior AIDS and cardiovascular disease, diabetes mellitus, and hepatitis B virus and hepatitis C virus coinfection. HRs calculated as in models 1 and 3 showed consistent results (data not shown). Abbreviations: CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6.
Secondary Analyses
After censoring the events occurring in the first 2 years of follow-up, 95% CIs of HRs became larger owing to the smaller number of clinical end points (Supplementary Table 3). As a result, some associations between biomarker quartiles and certain types of clinical end points were no longer significant. This was the case, for instance, for the association between higher D-dimer quartile and increased risk of progression to AIDS. However, most associations followed the same direction of and were broadly consistent with results of the main analyses including all follow-up (Supplementary Table 2).
In the SMART, adjustment for smoking weakened the association between higher quartile of IL-6 and death end points but strengthened associations between higher quartile of IL-6 and increased risk of CVD and non–AIDS-defining malignancies (Supplementary Table 4).
DISCUSSION
In a large cohort of HIV-positive persons, we confirmed that activated inflammation and coagulation, as demonstrated by elevated levels of IL-6, hsCRP, and D-Dimer, respectively, are linked to a variety of clinical end points representative of AIDS-associated and non–AIDS-associated morbidity. These 3 biomarkers are of particular interest because they have similarly been linked to disease in the general population. We found that the pathophysiological pathways that involve raised plasma levels of IL-6, hsCRP, and D-dimer seem to be associated with different types of clinical end points. Higher IL-6 levels at study entry were more strongly associated with subsequent risk of non–AIDS-defining end points such as CVD and non–AIDS-defining malignancies, compared with hsCRP or D-dimer levels. Furthermore, higher IL-6 level was more strongly associated with fatal events than with fatal and nonfatal CVD or non–AIDS-defining malignancies. These findings were broadly consistent after adjustment for confounders and accounting for potential reverse causality.
We demonstrated that IL-6 was superior to hsCRP in predicting cardiovascular and non–AIDS-defining malignancies. Gene-association studies using principles of Mendelian randomization support a causal role of IL-6 in CVD [23] and cancer [24]. With respect to hsCRP, polymorphisms in genes that are associated with higher levels of hsCRP were not associated with increased risk of CVD [25, 26] or cancer [27]. hsCRP seems, therefore, to be more of a epiphenomenon of underlying pathophysiological processes than a biomarker causally linked to adverse end points. However, some studies in the general population have found hsCRP to be more strongly associated with CVD than IL-6, after adjustment for confounders [28, 29]. The reasons why hsCRP is a less informative biomarker than IL-6 in the setting of HIV infection are unclear. Since hsCRP is a terminal product of the inflammatory cascade produced by the liver, subclinical hepatic impairment determined by conditions that may affect HIV-positive persons disproportionally, such as HCV and/or HBV coinfection or hepatic steatosis, could play a role [30, 31].
In this study, IL-6 was more strongly associated than D-dimer with CVD and non–AIDS-defining malignancies. There is paucity of data on the relative associations of IL-6 and D-dimer with non–AIDS-defining outcomes during HIV infection. A previous report including SMART and ESPRIT participants who were receiving ART and virologically suppressed at baseline found that both IL-6 and D-dimer levels independently predicted the risk of a composite end point of serious non–AIDS-related morbidity or all-cause death [10]. Higher D-dimer levels also predicted a composite end point of non–AIDS-defining conditions in a case-control study involving HIV-positive persons participating in the AIDS Clinical Trials Group [32]. In other reports involving the general population and HIV-positive persons, D-dimer was found to predict CVD [33, 34] and cancer mortality [35].
We also observed that elevated levels of IL-6 and D-dimer were similarly linked to increased risk of progression to AIDS. Elevated D-dimer levels had been previously associated with increased risk of AIDS or death among participants in the Flexible Initial Retrovirus Suppression Therapies (FIRST) trial with advanced HIV infection (median CD4+ T-cell count, 163 cells/mm3) [2]. In a nested case-control study involving participants from both arms in SMART (ie, those randomized to continuous use of ART and to CD4+ T-cell count-guided ART interruptions), D-dimer was not associated with the development of opportunistic disease [1]. However, there appeared to be a stronger association between D-dimer and opportunistic disease among those receiving ART continually, although this was an underpowered analysis, given the low number of events [1]. The prognostic value of D-dimer in HIV-positive persons, particularly its relationship with AIDS-defining and non–AIDS-defining end points, deserves, therefore, further investigation.
Activated innate immunity seems to be a stronger determinant than adaptive immunity of IL-6 and D-dimer levels [32, 36, 37], but higher levels of each biomarker are related to different phenotypes of monocyte activation [36]. Furthermore, we had previously demonstrated that different host factors and HIV-specific variables are associated with levels of IL-6 [38] and D-dimer [19]. This is compatible with the hypothesis that circulating levels of IL-6 and D-dimer reflect 2 distinct pathophysiological processes that may lead to different clinical end points. Indeed, combined scores of IL-6 and D-dimer have proven more helpful than each biomarker individually in estimating the future risk of non–AIDS-related morbidity [10].
The Wei-Lin-Weissfeld test indicated that higher IL-6 levels were more strongly associated with death end points than CVD and cancer in our study. From our studies, higher levels of IL-6 at study entry were associated with greater risk of fatal CVD and greater risk of death after a nonfatal cardiovascular event [6]. As for non–AIDS-defining malignancies, we also found that the risk associated with elevated plasma levels of IL-6 was higher for fatal than for nonfatal events among HIV-positive persons (unpublished data). Furthermore, raised levels of inflammatory biomarkers were more strongly associated with cancer death than with nonfatal cancer in healthy elderly individuals [39] and with shorter survival following cancer diagnosis [40]. Taken together, these findings suggest that higher IL-6 level is not only a marker of disease risk, but also of disease severity.
Our study had several limitations. First, we only studied 3 biomarkers. Other biomarkers not tested might have provided useful information. Second, we studied IL-6, hsCRP, and D-dimer levels at study entry only because data on follow-up biomarkers were only available for a subset of participants; therefore, it was not possible to investigate the effects of changes in plasma levels of these biomarkers over time. The fact that biomarker levels may have changed over time during follow-up means that the association between levels of these biomarkers and the clinical end points could be greater than those observed. Third, associations of each biomarker level were investigated for several clinical end points. This means that false-positive results may have arisen from multiple testing. Fourth, the strong and independent associations between raised biomarker levels and clinical end points observed in this large cohort of HIV-positive persons do not necessarily mean that these biomarkers are valuable for risk stratification of individual patients [41].
No study has thus far provided irrefutable evidence for a causal relationship between activated inflammation/coagulation and clinical events during HIV infection. Among HIV-positive persons with early HIV infection enrolled in the Strategic Timing of Antiretroviral Treatment (START) trial, immediate ART reduced the incidence of AIDS-defining events and cancer as compared to treatment deferral until CD4+ T-cell counts dropped below 350 cells/mm3 [42]. Because ART decreases inflammation and coagulation by suppressing HIV replication [16, 43, 44], START trial findings are consistent with the hypothesis of activated inflammation and coagulation as a contributing cause of morbidity, although the benefits of immediate ART may also have been mediated by other mechanisms. The Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE) trial will randomize 6500 HIV-positive individuals who have a low-to-moderate CVD risk and are receiving ART to start receiving statin therapy or placebo. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER), which recruited HIV-uninfected individuals with elevated hsCRP levels at baseline, statins were found to reduce the risk of CVD by 50% [45]. However, because REPRIEVE and JUPITER have CVD as the main study end point, it is impossible to determine whether the clinical benefit of statin therapy is mediated by a reduction in cholesterol level, a reduction of inflammation, or a combination of both [17, 46]. Three additional placebo-controlled trials of antiinflammatory therapies to reduce risk of cardiovascular events started enrolling participants: the Canakinumab Antiinflammatory Thrombosis Outcomes Study (CANTOS), which is assessing a monoclonal antibody against interleukin 1β [47]; the Cardiovascular Inflammation Reduction Trial (CIRT), which is assessing low-dose methotrexate among HIV-uninfected persons [48]; and the NCT01949116 trial, which is assessing methotrexate among HIV-positive persons. By deploying therapies that directly target inflammation, these trials will be important to test the inflammatory hypothesis of CVD. However, their results will not address whether enhanced inflammation and coagulation is causally linked to cancer and other non–AIDS-defining morbidities.
To conclude, IL-6 is a stronger predictor of fatal events than of fatal and nonfatal CVD and non–AIDS-defining cancer. There is a need for clinical end point–driven trials to determine whether antithrombotic and antiinflammatory therapies to lower levels of IL-6, hsCRP, and D-dimer can reduce morbidity and mortality in treated HIV infection. Such trials would not only determine the potential role of adjuvant antiinflammatory and antithrombotic therapies in the management of HIV infection, but would also elucidate whether enhanced inflammation and coagulation are causally linked to the development of AIDS-defining and non–AIDS-defining morbidity.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the Strategies for Management of Anti-Retroviral Therapy (SMART) and Evaluation of Subcutaneous Proleukin in a Randomized International Trial (ESPRIT) participants and investigators (see the article by El-Sadr et al [12] for the complete list of SMART investigators and the article by Abrams et al [18] for the complete list of ESPRIT investigators).
A. H. B., J. L. O., A. N. P., J. D. N., B. G., and J. D. L. conceived the study. J. L. O. and A. N. P. performed all statistical analyses. A. H. B. drafted the manuscript. All authors contributed to data interpretation, critically revised the manuscript, and approved the final version.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Financial support. This work was supported by the National Institutes of Health (grants U01AI46957 and U01AI068641 to the ESPRIT and SMART and grants U01AI042170 and U01AI46362 to the SMART), the Research Council at Rigshospitalet, and the Danish National Research Foundation (grant DNRF126).
Potential conflicts of interest. A. N. P. received payment for speaking at national human immunodeficiency virus (HIV) meetings funded by Gilead (in Spain and Austria) in the past year, attended an advisory board meeting for Abbvie about 2–3 years ago, was funded by GSK Biologicals to perform modeling analyses of the potential impact of a partially effective HIV vaccine in southern Africa (the project ended about 1.5 years ago), and received funding from Ashfield Communications in his role of chair of the HIV Therapy Conference in Glasgow. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rodger AJ, Fox Z, Lundgren JD et al. . Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis 2009; 200:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulware DR, Hullsiek KH, Puronen CE et al. . Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges AH, Weitz JI, Collins G et al. . Markers of inflammation and activation of coagulation are associated with anaemia in antiretroviral-treated HIV disease. AIDS 2014; 28:1791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges ÁH, Silverberg MJ, Wentworth D et al. . Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duprez DA, Neuhaus J, Kuller LH et al. . Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordell AD, McKenna M, Borges ÁH et al. . Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Béténé A, Dooko C, De Wit S et al. . Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 67:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W et al. . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledwaba L, Tavel JA, Khabo P et al. . Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012; 7:e24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grund B, Baker JV, Deeks SG et al. . Relevance of Interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016; 11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neaton JD, Neuhaus J, Emery S. Soluble biomarkers and morbidity and mortality among people infected with HIV: summary of published reports from 1997 to 2010. Curr Opin HIV AIDS 2010; 5:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sadr WM, Lundgren JD, Neaton JD et al. . CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res 2014; 2:288–94. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990; 265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009; 113:2878–87. [DOI] [PubMed] [Google Scholar]
- 16.Rajasuriar R, Wright E, Lewin SR. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr Opin HIV AIDS 2015; 10:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duprez DA, Otvos J, Tracy RP et al. . High-density lipoprotein subclasses and noncardiovascular, noncancer chronic inflammatory-related events versus cardiovascular events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2015; 4:e002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrams D, Lévy Y, Losso MH et al. . Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361:1548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borges AH, O'Connor JL, Phillips AN et al. . Factors associated with D-dimer levels in HIV-infected individuals. PLoS One 2014; 9:e90978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lifson AR, Rhame FS, Belloso WH et al. . Reporting and evaluation of HIV-related clinical endpoints in two multicenter international clinical trials. HIV Clin Trials 2006; 7:125–41. [DOI] [PubMed] [Google Scholar]
- 21.Kowalska JD, Friis-Møller N, Kirk O et al. . The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–23. [DOI] [PubMed] [Google Scholar]
- 22.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modelling marginal distributions. J Am Stat Assoc 1989; 84:1065–73. [Google Scholar]
- 23.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 2012; 379:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian G, Mi J, Wei X et al. . Circulating interleukin-6 and cancer: A meta-analysis using Mendelian randomization. Sci Rep 2015; 5:11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008; 359:1897–08. [DOI] [PubMed] [Google Scholar]
- 26.Wensley F, Gao P, Burgess S et al. . Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 2011; 342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst 2010; 102:202–6. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342:836–43. [DOI] [PubMed] [Google Scholar]
- 29.Pai JK, Pischon T, Ma J et al. . Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004; 351:2599–610. [DOI] [PubMed] [Google Scholar]
- 30.Reingold J, Wanke C, Kotler D et al. . Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr 2008; 48:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuhaus J, Jacobs DR Jr, Baker JV et al. . Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenorio AR, Zheng Y, Bosch RJ et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cushman M, Lemaitre RN, Kuller LH et al. . Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1999; 19:493–8. [DOI] [PubMed] [Google Scholar]
- 34.Ford ES, Greenwald JH, Richterman AG et al. . Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010; 24:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folsom AR, Delaney JA, Lutsey PL et al. . Associations of factor VIIIc, D-dimer, and plasmin-antiplasmin with incident cardiovascular disease and all-cause mortality. Am J Hematol 2009; 84:349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson EM, Singh A, Hullsiek KH et al. . Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis 2014; 210:1396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French MA, Cozzi-Lepri A, Arduino RC, Johnson M, Achhra AC, Landay A; INSIGHT SMART Study Group. Plasma levels of cytokines and chemokines and the risk of mortality in HIV-infected individuals: a case-control analysis nested in a large clinical trial. AIDS 2015; 29:847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borges ÁH, O'Connor JL, Phillips AN et al. . Factors associated with plasma IL-6 levels during HIV infection. J Infect Dis 2015; 212:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Il'yasova D, Colbert LH, Harris TB et al. . Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev 2005; 14:2413–8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 1999; 19:1427–32. [PubMed] [Google Scholar]
- 41.Wang TJ, Gona P, Larson MG et al. . Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006; 355:2631–9. [DOI] [PubMed] [Google Scholar]
- 42.INSIGHT START Study Group Lundgren JD, Babiker AG et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker JV, Neuhaus J, Duprez D et al. . Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr 2011; 56:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain V, Hartogensis W, Bacchetti P et al. . Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013; 208:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker PM, Danielson E, Fonseca FA et al. . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–207. [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep 2013; 15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011; 162:597–605. [DOI] [PubMed] [Google Scholar]
- 48.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost 2009; 7:332–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.