Abstract
There is some evidence in traditional medicine for the effectiveness of Thymus vulgaris (百里香 bǎi lǐ xiāng) in the treatment of anxiety in humans. The elevated plus-maze (EPM) has broadly been used to investigate anxiolytic and anxiogenic compounds. The present study investigated the effects of extract of T. vulgaris on rat behavior in the EPM. In the present study, the data were obtained from male Wistar rats. Animals were divided into four groups: saline group and T. vulgaris groups (50 mg/kg, 100 mg/kg, and 200 mg/kg infusion for 7 days by feeding). During the test period, the total distance covered by animals, the number of open- and closed-arm entries, and the time spent in open and closed arms of the EPM were recorded. T. vulgaris increased open-arm exploration and open-arm entry in the EPM, whereas extract of this plant has no effects on the total distance covered by animals and the number of closed-arm entries. The results of the present experiment indicate that T. vulgaris may have an anxiolytic profile in rat behavior in the EPM test, which is not influenced by the locomotor activity. Further research is required to determine the mechanisms by which T. vulgaris extract exerts an anxiolytic effect in rats.
Keywords: antioxidant, anxiety, elevated plus-maze, rat, Thymus vulgaris
Graphical abstract
1. Introduction
Anxiety disorders are among the most common psychiatric illnesses.1 Anxiety is characterized by a diffuse, unpleasant, vague sense of apprehension. It is often accompanied by autonomic symptoms, such as headache, perspiration, palpitations, tightness in the chest, and mild stomach discomfort.2 Benzodiazepines are the major class of compounds used to treat anxiety, and they have remained the most commonly prescribed drugs for anxiety.3 However, the realization that benzodiazepines present a narrow safety margin between the anxiolytic effect and those causing unwanted side effects has prompted many researchers to evaluate new compounds in the hope that other anxiolytic drugs will have less undesirable effects.3
Medicinal plants have been used from ancient times for their medicinal values as well as to impart flavor to food.4 Plants have been used in the management of illnesses since antiquity and has continuously grown over time as complementary medicine, because they are readily and cheaply available healthcare alternatives.5 Nowadays, there is a growing interest in the use of crude extracts and dry powder samples of medicinal and aromatic plants and for the development and preparation of alternative traditional medicine and food additives.6, 7 Drugs derived from traditional herbs may have possible therapeutic relevance in the treatment of anxiety. Research has been conducted to investigate natural anxiolytic agents in the search for an alternative.3
Approximately 150 species of Thymus are abundantly found, mainly in Asia, Africa, and North America. Recently, its range has been widely been extended to the Iberian Peninsula, with most of the species being endemic.4 Thymus vulgaris L. (百里香 bǎi lǐ xiāng; Lamiaceae) is a medicinal plant belonging to the Lamiaceae family.8, 9 In folk medicine, some Thymus spp. are used for their antihelminthic, expectorant, antiseptic, antispasmodic, antimicrobial, antifungal, antioxidative, antivirotic, carminative, sedative, and diaphoretic effects. They are usually administered by infusion or are used externally in baths to cure rheumatic and skin diseases.8, 10 Thyme contains high concentrations of phenols, including thymol (12–61%), carvacrol (0.4–20.6%), 1,8-cineole (0.2–14.2%), q-cymene (9.1–22.2%), linalool (2.2–4.8%), borneol (0.6–7.5%), a-pinene (0.9–6.6%), and camphor (0–7.3%). Carvacrol and thymol are the main phenolic components that are primarily responsible for its antioxidative activity.11 In addition, thyme oil is widely used in phytotherapy, most notably to treat and offer protection from acne, hypertension, infections, and cancers.12 The oil contains bioactive monoterpenes such as thymol, carvacrol, and linalool.13
There are a variety of animal tests for the investigation of anxiolytic effects of substances.14 The elevated plus-maze (EPM), a well-established animal test causing a fear status by comprehensible stimuli and the use of innate behavior of animals, is one of the most widely used models to assess anxiety in small rodents, and is a validated and reliable test for detecting both anxiolytic- and anxiogenic-like effects of agents.15, 16 In this animal model, an anxiolytic effect is evaluated by the relation of the entries into the open arms to the total entries and the time spent on the open arms of the EPM, in comparison with the same parameters of the control group. An increase of the time and proportion of the entries into the open arms without a changed locomotor activity is regarded as a powerful marker for an anxiolytic substance effect.17 Locomotor activity of the animals was assessed by measuring the total distance travelled by them.18, 19 There are no published reports in the literature about the effect of the extract of T. vulgaris on anxiety. On the basis of these considerations, this study was designed to characterize the anxiolytic-like activity of extract prepared from T. vulgaris leaves, using an EPM test.
2. Materials and methods
2.1. Preparation of plant extract
Leaves of T. vulgaris (百里香 bǎi lǐ xiāng) were collected in spring and identified at the Botanic Institute of this University. The plant material was dried at 40°C with air circulation, ground, and extracted with 70% ethanol by percolation at room temperature. The extract was then taken to the laboratory for the process of evaporation. The evaporation process involved complete removal of ethanol and water used for the extraction. The extracts were dried at 40°C under vacuum and finally freeze dried.20 Pharmacological assays were carried out with aqueous suspensions of the dried extract. The doses are expressed as milligrams of dried extract per kilogram of rat body weight. The extracts were redissolved in their solvents prior to each individual experiment.21
2.2. Animals
Male Wistar rats, weighing 230–250 g, were transported from the animal house to a room adjacent to the test laboratory 72 hours prior to the test. They were housed in groups of five per cage under a 12:12 dark/light cycle (lights on at 07:00 am) at 22 ± 2°C and given free access to food and water. Rats were randomly assigned to different treatment groups (n = 10). Animals were tested under the same experimental conditions. All experiments were carried out in a quiet room under controlled light conditions between 11:00 am and 3:00 pm. Behavioral observations were conducted in soundproof rooms at the same period of the day to reduce the confounding influence of diurnal variation on spontaneous behavior. Each animal was tested only once. All research and animal care procedures were approved by the Veterinary Ethics Committee of the Hamadan University of Medical Science, and were performed in accordance with international standards of animal welfare recommended by the Society for Neuroscience (Handbook for the Use of Animals in Neuroscience Research, 1997). The minimum number of animals and the minimum duration of observation required to obtain consistent data were used.
2.3. EPM test
The EPM design was similar to that originally described by Lister.22 In brief, the apparatus was composed of two open (50 cm × 10 cm × 1 cm) and two enclosed (50 cm × 10 cm × 50 cm) arms, which were radiated from a central platform (10 cm × 10 cm) to form a plus sign. A slightly raised edge on the open arms (1 cm) provided an additional grip for the animals. The plus-maze was elevated to a height of 50 cm above the floor level by a single central support. T. vulgaris extract were administered orally in three doses (50 mg/kg, 100 mg/kg, and 200 mg/kg infusion for 7 days by feeding). Then animal behavior in the EPM were videotaped for 10 minutes and saved on a computer.
The number of entries into and the time spent in each of the two types of arms were counted during a 10-minute test period. The open-arm entries and open-arm time were used as indices of anxiety, and the number of entries into the closed arms as an indicator of the reduction of spontaneous locomotion in rats. A rat was considered to have entered an arm when all its four paws were on that arm.
2.4. Statistical analysis
Results are expressed as mean ± standard error of the mean. The difference between the means was determined by one-way analysis of variance, followed by Tukey post hoc analysis. In all cases, differences were considered significant if p < 0.05.
3. Results
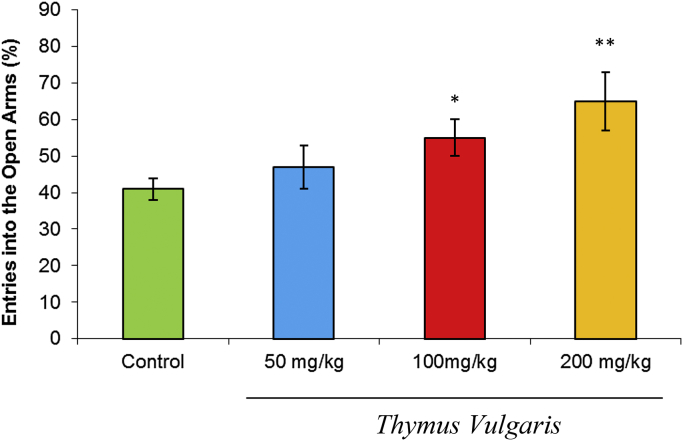
The effects of different doses of hydroalcoholic extract of T. vulgaris (百里香 bǎi lǐ xiāng) on the percentage of entries into the open arms are shown in Fig. 1. One-way analysis of variance indicated that, compared with the control group, extract of T. vulgaris caused an increase in the percentage of entries into the open arms. Tukey-post-test analysis showed that T. vulgaris exhibited a significant increase in the percentage of entries into the open arms at concentrations of 100 mg/kg (p < 0.05) and 200 mg/kg (p < 0.01), but not at 50 mg/kg, in comparison with the control group.
Fig. 1.
Effects of T. vulgaris extract (50 mg/kg, 100 mg/kg, and 200 mg/kg) on the percentage of entries into the open arms of the EPM during the 10-minute test session (n = 10). Data are expressed as mean ± SEM. Comparisons were made using ANOVA followed by post hoc Tukey's multiple comparison test. *p < 0.05. **p < 0.01. ANOVA = analysis of variance; EPM = elevated plus-maze; SEM = standard error of the mean.
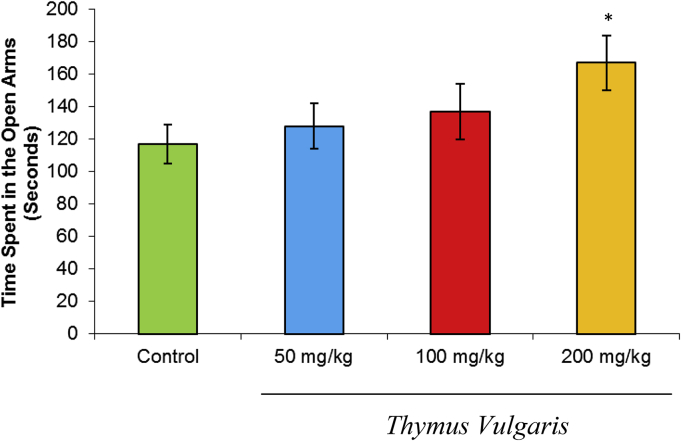
The effects of the different doses of T. vulgaris extract on the duration of time spent in the open arms are shown in Fig. 2. One-way analysis of variance indicated that, compared with the control group, the T. vulgaris extract-treated groups spent more time in the open arms. Tukey-post-test analysis showed that the extract-treated groups spent more time in the open arms at the dose of 200 mg/kg (p < 0.05).
Fig. 2.
Effects of T. vulgaris extract (50 mg/kg, 100 mg/kg, and 200 mg/kg) on the time spent in the open arms during the 10-minute test session (n = 10). *p < 0.05. **p < 0.01.
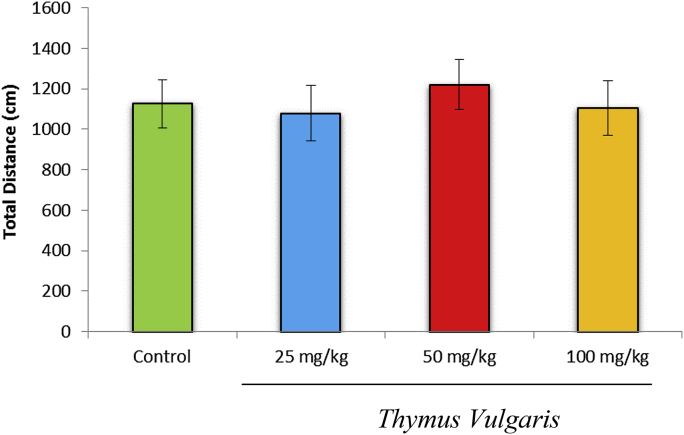
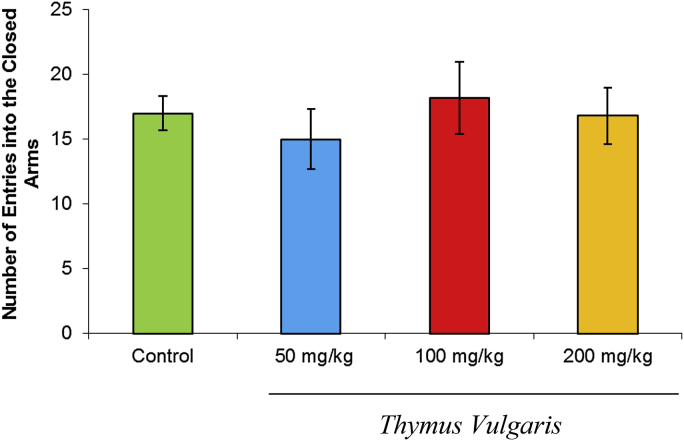
The total distance covered by the T. vulgaris extract-treated rats during the 10-minute test was not significantly (p > 0.05) different from controls (Fig. 3). The number of entries into the closed arms also was not significantly different between the T. vulgaris-treated group and the control group (Fig. 4).
Fig. 3.
Effects of T. vulgaris extract (50 mg/kg, 100 mg/kg, and 200 mg/kg) on the total distance covered by the rats during the 10-minute test session (n = 10 in each group).
Fig. 4.
Effects of T. vulgaris extract (50 mg/kg, 100 mg/kg, and 200 mg/kg) on the number of closed-arm entries during the 10-minute test session (n = 10).
4. Discussion
Plants were used for medicinal purposes long before recorded history, and their utilization in medication is still well disseminated around the world.23 Many plants exert recognized medicinal effects on the central nervous system, and are able to act on chronic conditions such as anxiety and depression that do not respond well to conventional therapeutic treatments.24 Various types of herbal medicines have been used as anxiolytics in different parts of the world.3 On the basis of these considerations, the purpose of this study was to characterize the anxiolytic-like activity of the hydroalcoholic extract prepared from T. vulgaris (百里香 bǎi lǐ xiāng). The results of the present study demonstrated that the extract of T. vulgaris increased the percentage of both the entries and the time spent in the open arms of the maze. Therefore, the extract was able to produce anxiolytic effect in rats after the 1-week oral administration. The effect of T. vulgaris was not induced by changes in motor activity at these doses, because the total distance covered by the rats was not altered. An increase in the time and proportion of the entries into the open arms lacking a changed locomotor activity is confirmed as a potent sign of an anxiolytic substance effect.15
Many phytomedicines exert their beneficial effects through the additive or synergistic action of several chemical compounds acting at a single target site or at multiple target sites.25 T. vulgaris is a well-known herb that is widely cultivated in many regions of the world.13, 26 T. vulgaris is used mainly as a food seasoning, but also as a source of essential oils that are used in perfumery and as a worming and bactericidal agent in medicine.27 Additionally, thyme is known to contain a high concentration of phenolic compounds, such as thymol and carvacrol, which are found in its essential oils. Fecka and Turek28 found phenolic compounds in wild thyme, with caffeic acid and rosmarinic acid derivatives being the most important of these. Several authors already reported that flavonoid groups exhibited a wide range of biological activities, such as antioxidant, anti-inflammatory, antimicrobial, antiangiogenic, anticancer, and anti-allergic effects.29, 30, 31 Reports indicate that the volatile oils of thyme are among the main essential oils used in the food industry and in cosmetics as preservatives and antioxidants.32 T. vulgaris essential oil is a mixture of monoterpenes. The main compounds of this oil are the natural terpenoid thymol and its phenol isomer carvacrol,33 which have antioxidative, antimicrobial, antitussive, expectorant, antispasmodic, and antibacterial effects.34, 35 Accordingly, it has been reported that ethanol thyme extract may be used as a natural antioxidant to prolong the stability of oils.36 In addition, it could be concluded that the essential oil of T. vulgaris has a potential antioxidant activity and a protective effect against toxicity of aflatoxins, and this protection is dose dependent.37
Extensive research has been conducted to reveal multiple neural substrates and mechanisms that contribute to the etiology of depression and anxiety, among which the imbalance between oxidation and the antioxidant defense system has gained attention.38 Some studies have demonstrated the role of oxidative stress in anxiety of rodents.39, 40 Furthermore, it has recently been reported that two agents that induce oxidative stress, l-buthionine-(S,R)-sulfoximine and xanthine plus xanthine oxidase (X + XO), cause increased anxiety-like behavior in rats.41 Moreover, increasing evidence suggests that the impairment of antioxidant defense and neuronal cell death are important in the process of emotional disorders, such as depression and anxiety.38 Accordingly, Masood et al42 reported that the induction of oxidative stress in mouse hypothalamus occurs in parallel with anxiety. Consumption of diets with high levels of sucrose was reported to increase the oxidation of proteins in the frontal cortex and to cause anxiety in rats. Increased anxiety has been correlated positively with the increase of reactive oxygen species levels. In another study, oxidative stress in the hippocampus of adult rats was reported to be anxiogenic.43 Interestingly, the induction of oxidative stress by a nonpharmacological method also leads to anxiety-like behavior in rats.40 Moreover, the increase in anxiety-like behavior is reversed by treatment with the antioxidant tempol, suggesting direct involvement of oxidative stress in mediating anxiety-like behavior in rats.41 Tempol is a water-soluble and cell-membrane-permeable molecule,44 with demonstrated antioxidant activity in various biological systems.45 Moreover, it has been shown that oxidative stress (l-buthionine-(S,R)-sulfoximine)-induced anxiety-like behavior was prevented with antioxidant tempol treatment in rats.40 The antioxidant effect of plant extracts in vitro is probably caused by their ability to act as reducing agents and free radical scavengers or as quenchers of singlet oxygen formation. Some authors ascertained the fact that phenolic compounds were able to chelate metal ions. Melidou et al46 found that intracellular binding of iron is responsible for the protection offered by flavonoids against H2O2-induced DNA damage. On the other hand, complexation of plant extracts with metal ions results in a significant reversal from antioxidant to pro-oxidant properties for the resulting complexes.47
It has been reported that thyme oils find wide use in dietary supplementations due to their antioxidant property.48 Several researchers have evaluated the antioxidant properties of extracts from different herbs and spices in lipid systems.49 Phenolic monoterpenes in thyme, thymol, and carvacrol are the primary compounds that have been reported to possess a high antioxidant activity.32, 50 Youdim and Deans51 measured changes in antioxidant enzyme activity of different organs during the lifetime of rats. They found out that dietary supplementation of T. vulgaris reduced the unfavorable age-related decline in activities of superoxide dismutase in the liver and heart of old rats. These results highlighted the benefit of T. vulgaris as a dietary antioxidant. It is very interesting that even the drying methods of plants used for preparation of extracts could be responsible for the content of phenolics and flavonoids, as well as for the antioxidant activity of extracts.52
In the present study, T. vulgaris extract was found to decrease the level of anxiety in animals. T. vulgaris oil also contains bioactive monoterpenes such as linalool.13 According to this finding, linalool inhalation has been shown to reduce anxiety.53 The presence of linalool and linalyl acetate in the plant extract supports the claim that the extract has sedative effect.54 In another study, it has been shown that kaempferol induces anxiolytic activities in the EPM test in mice.55 In addition, it has been shown that carvacrol presents anxiolytic effects in the plus maze test, which are not influenced by the locomotor activity in the open-field test.56 It is possible that these components play essential roles in the anxiolytic properties of T. vulgaris in the EPM test.
As mentioned previously, thyme contains thymol and carvacrol compounds.32, 50 It has been reported that thymol exhibits significant anticonvulsant and antiepileptogenic properties.57 The similarity of action of thymol and GABA suggests that this terpenoid acts centrally by mimicking or facilitating GABA action.58 Furthermore, it is known that thymol acts as a positive modulator of the GABA (A) receptor.59 Moreover, it has been shown that the anxiolytic-like effect of carvacrol in mice is involvement with GABAergic transmission.56 GABA is widely known to be involved in the etiology of anxiety, hence the short-term effectiveness of diazepam, a GABA agonist, in relieving anxiety.60
5. Conclusion
In conclusion, our results demonstrate that oral administration of T. vulgaris (百里香 bǎi lǐ xiāng) extract may have an anxiolytic profile in rats. The presence of polyphenols, flavonoids, and essential oil in the extract of T. vulgaris reinforces the anxiolytic effects of this plant observed in this study. Possibly, the anxiolytic activity observed in this work was not only dependent on the flavonoid or essential oil content, but also related to other substances with antioxidant activity. Further studies would be necessary to evaluate the contribution of other substances to the activity shown, as it still remains to be determined which components were responsible for these effects.
Conflict of interest
There is no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the staff of the Neurophysiology Research Center for helping us to carry out this project. This research was supported by a grant (Grant number: 8812154596) of the Hamadan University of Medical Sciences, Hamadan, Iran.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Stein D.J., Hollander E., Rothbaum B.O. 2nd ed. American Psychiatric Publishing, Inc.; Washington, DC: 2009. Textbook of Anxiety Disorders. [Google Scholar]
- 2.Kaplan H.I., Sadoch B.J. 8th ed. Lippincott, Williams and Wilkins; Baltimore: 2005. Comprehensive Textbook of Psychiatry. [Google Scholar]
- 3.Grundman O., Nakajima J., Seo S., Butterweck V. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J Ethnopharmacol. 2007;110:406–411. doi: 10.1016/j.jep.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Hossain M.A., AL-Raqmi K.A., AL-Mijizy Z.H., Weli A.M., Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;9:705–710. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussmann R.W., Paul S., Aserat W., Paul E. Plant use in Odo-Bulu and Demaro, Bale Region, Ethiopia. J Ethnobiol Ethnomed. 2011;7:28–81. doi: 10.1186/1746-4269-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oke B., Aslim C., Ozturk S., Altundag G. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009;112:874–879. [Google Scholar]
- 7.Rota M.C., Herrera A., Martinez R.M., Sotomayor J.A., Jordan M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19:681–687. [Google Scholar]
- 8.Al-Bayati F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J Ethnopharmacol. 2008;116:403–406. doi: 10.1016/j.jep.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Dogu-Baykut E., Gunes G., Decker E.A. Impact of shortwave ultraviolet (UV-C) radiation on the antioxidant activity of thyme (Thymus vulgaris L.) Food Chem. 2014;157:167–173. doi: 10.1016/j.foodchem.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Fachini-Queiroz F.C., Kummer R., Estevão-Silva C.F. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012:657026. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhl S.R. Technomic Publishing Co Inc.; Lancaster: 2000. Handbook of Spices, Seasonings, and Flavorings. Chapter 4. [Google Scholar]
- 12.Zarzuelo A., Crespo E. The medicinal and non-medicinal uses of thyme. In: Stahl-Biskup E., Saez F., editors. Thyme: The Genus Thymus. Taylor & Francis; London, UK: 2003. pp. 263–292. [Google Scholar]
- 13.Ahmad A., van Vuuren S., Viljoen A. Unravelling the complex antimicrobial interactions of essential oils—the case of Thymus vulgaris (thyme) Molecules. 2014;19:2896–2910. doi: 10.3390/molecules19032896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciolino N.R., Smith J.M., Stranahan A.M. Galanin mediates features of neural and behavioral stress resilience afforded by exercise. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.09.029. S0028-3908:00348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skelly M.J., Weiner J.L. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav. 2014;4:468–483. doi: 10.1002/brb3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenborger L., Levone B.R., da Silva E.S. The microinjection of a cannabinoid agonist into the accumbens shell induces anxiogenesis in the elevated plus-maze. Pharmacol Biochem Behav. 2014;124:160–166. doi: 10.1016/j.pbb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Pellow S., Chopin P., File S.E., Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 18.Burghardt P.R., Wilson M.A. Microinjection of naltrexone into the central, but not the basolateral, amygdala blocks the anxiolytic effects of diazepam in the plus maze. Neuropsychopharmacology. 2006;31:1227–1240. doi: 10.1038/sj.npp.1300864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drapier D., Bentué-Ferrer D., Laviolle B. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176:202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Silva L.F., Lima E.S., Vasconcellos M.C. In vitro and in vivo antimalarial activity and cytotoxicity of extracts, fractions and a substance isolated from the Amazonian plant Tachia grandiflora (Gentianaceae) Mem Inst Oswaldo Cruz. 2013;108:501–507. doi: 10.1590/0074-0276108042013017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaffer S. Understanding local Mediterranean diets: a multidisciplinary pharmacological and ethnobotanical approach. Pharmacol Res. 2005;52:353–366. doi: 10.1016/j.phrs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 23.Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of Ayurveda. Pharmacogn Rev. 2014;8:73–80. doi: 10.4103/0973-7847.134229. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco M.M., Costa C.A., Freire A.O., Santos J.G., Costa J.M. Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine. 2009;16:265–270. doi: 10.1016/j.phymed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Adwan G., Abu-Shanab B., Adwan K., Abu-Shanab F. Antibacterial effects of nutraceutical plants growing in Palestine on Pseudomonas aeruginosa. Turk J Biol. 2006;30:239–242. [Google Scholar]
- 26.Cerda A., Martínez M.E., Soto C. The enhancement of antioxidant compounds extracted from Thymus vulgaris using enzymes and the effect of extracting solvent. Food Chem. 2013;139:138–143. doi: 10.1016/j.foodchem.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Dawidowic A.I., Rado E., Wianowska D., Mardarowicz M., Gawdzik J. Application of PLE for the determination of essential oil components from Thymus vulgaris L. Talanta. 2008;76:878–884. doi: 10.1016/j.talanta.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 28.Fecka I., Turek S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008;108:1039–1053. doi: 10.1016/j.foodchem.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Anyasor G.N., Ogunwenmo K.O., Oyelana O.A., Akpofunure B.E. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl (Costaceae) Afr J Biotechnol. 2010;9:4880–4884. [Google Scholar]
- 30.Popovic-Milenkovic M.T., Tomovic M.T., Brankovic S.R., Ljujic B.T., Jankovic S.M. Antioxidant and anxiolytic activities of Crataegus nigra Wald. et Kit. berries. Acta Pol Pharm. 2014;71:279–285. [PubMed] [Google Scholar]
- 31.Barile E., Bonanomi G., Antignani V. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry. 2007;68:596–603. doi: 10.1016/j.phytochem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Achour S., Khelifi E., Attia Y., Ferjani E., Noureddine Hellal A. Concentration of antioxidant polyphenols from Thymus capitatus extracts by membrane process technology. J Food Sci. 2012;77:C703–C709. doi: 10.1111/j.1750-3841.2012.02696.x. [DOI] [PubMed] [Google Scholar]
- 33.Amiri H. Essential oils composition and antioxidant properties of three Thymus species. Evid Based Complement Alternat Med. 2012;2012:728065. doi: 10.1155/2012/728065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoferl M., Buchbauer G., Jirovetz L. Correlation of antimicrobial activities of various essential oils and their main aromatic volatile constituents. J Essential Oil Res. 2009;21:459–463. [Google Scholar]
- 35.The European Scientific Cooperative on Phytotherapy, in collaboration with Georg Thieme . 2nd ed. 2007. ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products. [Google Scholar]
- 36.Zaborowska Z., Przygoński K., Bilska A. Antioxidative effect of thyme (Thymus vulgaris) in sunflower oil. Acta Sci Pol Technol Aliment. 2012;11:283–291. [PubMed] [Google Scholar]
- 37.El-Nekeety A.A., Mohamed S.R., Hathout A.S., Hassan N.S., Aly S.E., Abdel-Wahhab M.A. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induced oxidative stress in male rats. Toxicon. 2011;57:984–991. doi: 10.1016/j.toxicon.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Ding L., Zhang C., Masood A. Protective effects of phosphodiesterase 2 inhibitor on depression- and anxiety-like behaviors: involvement of antioxidant and anti-apoptotic mechanisms. Behav Brain Res. 2014;268:150–158. doi: 10.1016/j.bbr.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Almeida A.A., de Carvalho R.B., Silva O.A., de Sousa D.P., de Freitas R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol Biochem Behav. 2014;118:69–78. doi: 10.1016/j.pbb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Vollert C., Zagaar M., Hovatta I. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Salim S., Sarraj N., Taneja M., Saha K., Tejada-Simon M.V., Chugh C. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Masood A., Nadeem A., Mustafa S.J., O'Donnell J.M. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Souza C.G., Moreira J.D., Siqueira I.R. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31(1 suppl):S76–S84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- 45.Francischetti I.M., Gordon E., Bizzarro B. Tempol, an intracellular antioxidant, inhibits tissue factor expression, attenuates dendritic cell function, and is partially protective in a murine model of cerebral malaria. PLoS One. 2014;9:e87140. doi: 10.1371/journal.pone.0087140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melidou M., Riganakos K., Galaris D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. Free Radic Biol Med. 2005;39:1591–1600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Yang U.J., Park T.S., Shim S.M. Protective effect of chlorophyllin and lycopene from water spinach extract on cytotoxicity and oxidative stress induced by heavy metals in human hepatoma cells. J Toxicol Environ Health A. 2013;76:1307–1315. doi: 10.1080/15287394.2013.851632. [DOI] [PubMed] [Google Scholar]
- 48.Dal Bosco A., Gerencsér Z., Szendrő Z. Effect of dietary supplementation of spirulina (Arthrospira platensis) and thyme (Thymus vulgaris) on rabbit meat appearance, oxidative stability and fatty acid profile during retail display. Meat Sci. 2014;96:114–119. doi: 10.1016/j.meatsci.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 49.Samotyja U., Małecka M. Antioxidant activity of blackcurrant seeds extract and rosemary extracts in soybean oil. Eur J Lipid Sci Technol. 2010;112:1331–1336. [Google Scholar]
- 50.Undeğer U., Başaran A., Degen G.H., Başaran N. Antioxidant activities of major thyme ingredients and lack of (oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells at low levels of carvacrol and thymol. Food Chem Toxicol. 2009;47:2037–2043. doi: 10.1016/j.fct.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Youdim K.A., Deans S.G. Dietary supplementation of thyme (Thymus vulgaris L.) essential oil during the lifetime of the rat: its effects on the antioxidant status in liver, kidney and heart tissues. Mech Ageing Dev. 1999;109:163–175. doi: 10.1016/s0047-6374(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 52.Hamrouni-Sellami I., Rahali F.Z., Rebey I.B., Bourgou S., Limam F., Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2013;6:806–817. [Google Scholar]
- 53.Souto-Maior F.N., Carvalho F.L., Morais L.C., Netto S.M., de Sousa D.P., Almeida R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol Biochem Behav. 2011;100:259–263. doi: 10.1016/j.pbb.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 54.Barocelli E., Calcina F., Chiavarini M. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “Grosso” essential oil. Life Sci. 2004;76:213–223. doi: 10.1016/j.lfs.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Grundmann O., Nakajima J., Kamata K., Seo S., Butterweck V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine. 2009;16:295–302. doi: 10.1016/j.phymed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 56.Melo F.H., Venâncio E.T., de Sousa D.P. Anxiolytic-like effect of carvacrol (5-isopropyl-2-methylphenol) in mice: involvement with GABAergic transmission. Fundam Clin Pharmacol. 2010;24:437–443. doi: 10.1111/j.1472-8206.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- 57.Sancheti J., Shaikh M.F., Chaudhari R. Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Aunyn Schmiedebergs Arch Pharmacol. 2014;387:59–66. doi: 10.1007/s00210-013-0917-5. [DOI] [PubMed] [Google Scholar]
- 58.Waliwitiya R., Belton P., Nicholson R.A., Lowenberger C.A. Effects of the essential oil constituent thymol and other neuroactive chemicals on flight motor activity and wing beat frequency in the blowfly Phaenicia sericata. Pest Manag Sci. 2010;66:277–289. doi: 10.1002/ps.1871. [DOI] [PubMed] [Google Scholar]
- 59.García D.A., Vendrell I., Galofré M., Suñol C. GABA released from cultured cortical neurons influences the modulation of t-[(35)S]butylbicyclophosphorothionate binding at the GABAA receptor effects of thymol. Eur J Pharmacol. 2008;600:26–31. doi: 10.1016/j.ejphar.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Komaki A., Khaledi Nasab Z., Shahidi S., Sarihi A., Salehi I., Ghaderi A. Anxiolytic effects of acute injection of hydro-alcoholic extract of lettuce in the elevated plus-maze task in rats. Avicenna J Neurol Psych Physiol. 2014;1:e18695. [Google Scholar]