Abstract
There has recently been much advancement in the diagnosis, treatment, and research of metabolic disorders, especially diabetes. Current research around the world is focused on finding an alternative source of treatment from natural resources for diabetic management, apart from the available synthetic medicines. The present study is a preliminary study of a polyherbal formulation using edible natural resources and an assessment of its antidiabetic activity. The formulation was screened for its phytochemical constituents, total phenols, flavonoids, and vitamin C content. It was also analyzed for its inhibitory effect against the digestive enzymes α-amylase and α-glucosidase, compared with the standard drug acarbose. The formulation showed the presence of major constituents such as steroids, cardiac glycosides, phenols, flavonoids, and saponins. It also had a high level of phenols (340 ± 2.5 mg/g), flavonoids (235.4 ± 8.3 mg/g), and vitamin C (470.8 ± 16.6 mg/g), and showed a half-maximal inhibitory concentration (IC50) value of 0.41 ± 0.03 mg/mL and 0.51 ± 0.01 mg/mL for amylase and glucosidase, respectively. The results showed that ADJ6 had a significant inhibitory activity on α-amylase and α-glucosidase; however, its inhibitory activity was less than that of acarbose. The plants that are formulated in ADJ6 possess potent antidiabetic activity. Thus, we found that ADJ6 is a potent lead for effective diabetic management; however, an evaluation of the formulation must be illustrated using an in vivo model.
Keywords: ADJ6, α-amylase, α-glucosidase, diabetes management, polyherbal formulation
Graphical abstract

1. Introduction
Type 2 diabetes or hyperglycemia, which is characterized by high blood glucose levels and glycosuria, is an endocrine disorder. It is responsible for the development of various other complications in the human body. It may occur for two main reasons: (1) deficient/insufficient secretion of insulin by the pancreatic beta cells1; or (2) decreased or lost sensitivity of the insulin receptors.2 These two factors are preceded by stress, obesity, and a sedentary lifestyle.3, 4 At present, diabetes management is a prime concern in the medical community. As a result of various factors, diabetes management is a challenging problem.5, 6, 7, 8
However, many medicinal plants in ancient Indian Medicine (Ayurveda) have been identified and used to reduce the hyperglycemic condition in the body. The herbs control the hyperglycemic condition and effectively maintain normal glucose levels from a long-term perspective. Many medicinal plants have recently been evaluated for their antihyperglycemic property and have been successfully proven for their effects.9, 10, 11 Numerous polyherbal formulations are also being evaluated for effective diabetes management. However, none of the formulations has made significant inroads in treatment alternatives. Thus, a new formulation has been developed that combines six different herbs that are used in daily life as foods and have significant antidiabetic activity. We combined the six plants based on previously available literature, indigenous knowledge, and various preliminary studies. Thus, the polyherbal formulation has been named “ADJ6”. Our aim was to investigate the antidiabetic potential of the polyherbal formulation (i.e., ADJ6) and prove its synergistic effect against various aspects involved in diabetes management.
2. Materials and methods
2.1. Materials
Chemicals such as soluble starch and porcine pancreatic α-amylase (PPA) were purchased from SRL Pvt. Ltd. (Mumbai, India). α-Glucosidase, 3,5-dinitrosalicylicacid (DNSA), and 2,4-dinitrophenyl hydrazine (DNPH) were obtained from HiMedia Laboratories (Mumbai, India). Para-nitrophenyl α-d-glucopyranoside and standards such as gallic acid, quercetin, and ascorbic acid were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Acarbose was purchased from Bayer Scientific (Leverkusen, Germany). All other reagents and chemicals were of analytical grade and procured locally in Chennai.
2.2. Identification of plants
The herbs were collected from the medicinal farm Frontier Mediville (Elavur, Gummidipoondi, India) and were submitted to the Plant Anatomy Research Centre (Tamil Nadu, India) for authentication. The authentication numbers have been provided in an additional file 1. The plant names, families, and parts used and their medicinal properties are listed in Table 1.
Table 1.
Plants and their parts used for the polyherbal formulation.
| Serial Number | Binomial name | Common name | Family name | Part used | Activity | Reference no. |
|---|---|---|---|---|---|---|
| 1 | Momordica charantia | Bitter gourd | Cucurbitaceae | Whole fruit | Antihyperglycemic effect, analgesic, antipyretic, hypotriglyceridemic, hypocholesterolemic, antiulcerative, antiproliferative, wound healing | 12, 13, 14, 15, 16, 17, 18, 19 |
| 2 | Psidium guajava | Guava | Myrtaceae | Whole fruit | Hypolipidaemic, hepatoprotective, antihyperglycemic, renal protective, antioxidant | 20, 21, 22, 23, 24 |
| 3 | Phyllanthus emblica | Amla | Phyllanthaceae | Pulp | Antihyperglycemic, hypolipidemic, antiapoptotic, anti-inflammatory, chondroprotective, endothelial dysfunction | 25, 26, 27, 28, 29 |
| 4 | Trigonella foenum-graecum | Fenugreek | Fabaceae | Seeds | Antihyperglycemic, neuroprotective, immunomodulatory, | 30, 31, 32, 33 |
| 5 | Syzygium cumini | Jamun | Myrtaceae | Seeds | Antihyperglycemic, antiproliferative, chemoprotective | 34, 35, 36, 37, 38 |
| 6 | Gymnema sylvestre | Gymnema | Asclepiadaceae | Leaves | Antihyperglycemic effect | 15, 39, 40, 41, 42 |
2.3. Preparation of ADJ6
The plant parts were minced individually using a mixer. The individual plants were then freeze-dried using a lyophilizer to minimize the loss of bioactive components. The freeze-dried powder was stored in an air-tight container at room temperature until further use. The traditional Ayurvedic methods of preparing herbal formulations are primarily aqueous (it is likely that the peptides, proteins and glycans possessing antidiabetic activity would be denatured in organic solvents.). The powders of each plant were mixed together in a specific proportion, soaked in water for 24 hours, and filtered. The filtrate was used for further analysis. Fresh extracts were prepared when needed using the specific proportion.
2.4. Pancreatic α-amylase inhibition assay
The inhibition assay was performed using the DNSA method.43 The assay mixture consisted of 500 μL of 0.02M sodium phosphate buffer [containing 6mM sodium chloride (NaCl), pH 6.9], 0.05 units of PPA solution, and ADJ6 at a concentration of 0.1–1.5 mg/mL (w/v). The assay mixture was preincubated at 37°C for 20 minutes. After incubation, 250 μL of 0.5% (v/v) starch solution in the aforementioned buffer was added to the tubes and incubated for 15 minutes at 37°C. The reaction was terminated by adding 1 mL of dinitrosalicylic acid reagent and then incubated in a boiling water bath for 10 minutes. The tubes were cooled and the absorbance was measured at 540 nm (Shimadzu UV-VIS 1800 spectrophotometer; Shimadzu Corporation, Kyoto, Japan). A tube with PPA but without ADJ6 served as the control with 100% enzyme activity. Acarbose, an amylase inhibitor, was the positive control.
2.5. α-Glucosidase inhibition activity
The assay was performed with slight modifications using α-glucosidase from Saccharomyces cerevisiae.44 The assay mixture consisted of 150 μL of 0.1M sodium phosphate buffer (containing 6mM NaCl, pH 6.9), 0.1 unit of α-glucosidase, and ADJ6 at a concentration of 0.1–1.5 mg/mL (w/v). The assay mixture was preincubated at 37°C for 10 minutes. After incubation, 50 μL of 2mM para-nitrophenyl α-d-glucopyranoside in 0.1M sodium phosphate buffer was added to the mixture and incubated at 37°C for 25 minutes. The reaction was terminated by adding 50 μL of 0.1M sodium carbonate (Na2CO3). The yellow color that developed was measured at 405 nm (Bio-Rad microplate reader; Bio-Rad Laboratories, California, USA). The tube with α-glucosidase but without ADJ6 served as the control with 100% enzyme activity, and acarbose served as the positive control.
2.6. Qualitative phytochemical analysis
The formulation ADJ6 was analyzed for the presence of amino acids, steroids, cardiac glycosides, phenols, tannins, terpenoids, alkaloids, flavonoids, saponins, carbohydrates, reducing sugar, and anthrones.45
2.7. Evaluation of bioactive constituents
2.7.1. Estimation of total phenolic content
Total phenolic content was determined using gallic acid as the reference standard.46 One milliliter of sample (0.2–1 mg/mL) was added with 0.5 mL of Folin–Ciocalteau reagent and incubated at room temperature for 10 minutes. Furthermore, 2.5 mL of saturated Na2CO3 solution was added and incubated at room temperature for 30 minutes. The resultant color was measured at 750 nm versus a blank containing distilled water and Folin–Ciocalteau reagent. Total phenolic content was expressed in gallic acid equivalents using the equation T = CXV/M (T = Total Phenolic Content (mg/g) of extract as gallic acid equivalents, C = Concentration of Gallic acid established from the calibration curve, V = Volume of the extract solution in ml, M = weight of extract in grams).
2.7.2. Determination of total flavonoid content
Total flavonoid content was determined with slight modifications using quercetin as the reference standard.47 One milliliter of sample (0.2–1 mg/mL) was added with 0.5 mL of 1.2% aluminum chloride in 10% methanol, 0.5 mL of 1M potassium acetate, and made up to 3 mL with distilled water. The mixture was incubated for 30 minutes in the dark, and the absorbance was read at 415 nm. Aluminum chloride without the sample alone served as the blank. Total flavonoid content was expressed in gram quercetin equivalents.
2.7.3. Determination of vitamin C
Vitamin C was determined using the method described by Roe,48 but with modification, using ascorbic acid as the reference compound. Fifty microliters of sample (0.2–1 mg/mL) was added with 150 μL of 5% TCA (Trichloroacetic acid). The tubes were centrifuged at 3500 g for 5 minutes. The mixture was added with 500 μL of 10mM DNPH and 500 μL of 4% thiourea and incubated at room temperature for 3 hours. Postincubation 2.5 mL of 85% sulfuric acid was added and the absorbance was measured at 530 nm. The result was expressed in milligrams per gram of ascorbic acid.
2.8. Statistical analysis
All experiments were performed in three different sets with each set duplicated. The data are expressed as the mean ± the standard deviation (SD).
3. Results and discussion
3.1. Inhibition of α-amylase and α-glucosidase
α-Amylase (EC 3.2.1.1) randomly cleaves the α-(1–4) glycosidic linkages of amylase to yield dextrin, maltose, or maltotriose,49 whereas α-glucosidase (EC 3.2.1.20) hydrolyzes the terminal nonreducing 1-4 linked α-glucose to release glucose molecules.50 Incubation of the α-amylase with the substrate leads to the generation of maltose; however, the addition of the ADJ6 [inhibitory effect (IC50), 0.41 ± 0.03 mg/mL) significantly inhibited the liberation of maltose in a dose-dependent manner, compared with acarbose (IC50, 0.2 ± 0.01 mg/mL) (Fig. 1). The IC50 of α-glucosidase was 0.51 ± 0.01 mg/mL, whereas that of acarbose was 0.39 ± 0.02 mg/mL (Fig. 2).
Fig. 1.
α-Amylase inhibition assay.
Fig. 2.
α-Glucosidase inhibition assay.
3.2. Phytochemical analysis
The aqueous extract of the formulation also showed the presence of major phytochemical constituents such as phenols, flavonoids, steroids, cardiac glycosides, and saponins (Table 2).
Table 2.
Phytochemical analysis.
| Name of the constituent | Inference |
|---|---|
| Amino acids | − |
| Steroids | + |
| Cardiac Glycosides | + |
| Phenols | + |
| Tannins | − |
| Terpenoids | − |
| Alkaloids | − |
| Flavonoids | + |
| Saponins | + |
| Carbohydrates | − |
| Reducing sugar | + |
| Anthrones | − |
3.3. Total phenol, flavonoid, and vitamin C content
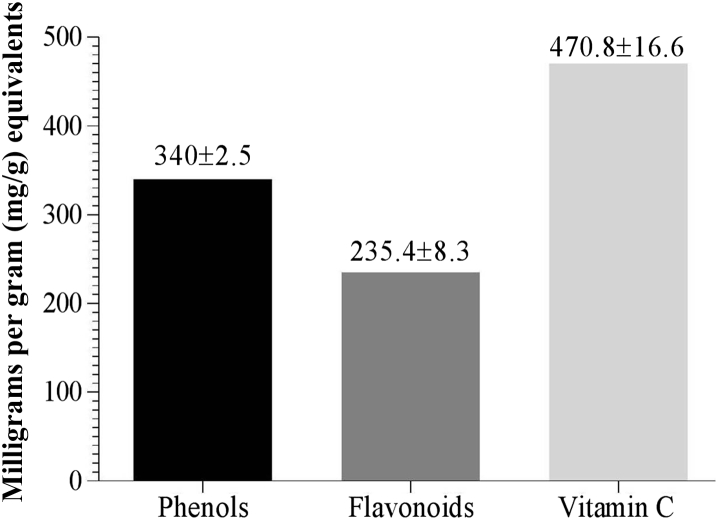
The aqueous extract of the formulation was estimated for total phenol, flavonoids, and vitamin C content. The amount of phenols was 340 ± 2.5 mg/g equivalents of gallic acid. The amount of flavonoids was 235.4 ± 8.3 mg/g of quercetin equivalents, whereas the level of vitamin C was the highest at 470.8 ± 16.6 mg/g of ascorbic acid equivalents (Fig. 3).
Fig. 3.
Estimation of phenols, flavonoids, and vitamin C.
The analyses suggested that the IC50 values of ADJ6 against α-glucosidase and α-amylase at various concentrations were less than those for the standard drug acarbose. Acarbose is a potent inhibitor of α-amylase and α-glucosidase, but adverse effects have occurred in the gastrointestinal tract because of excessive inhibition of the amylase enzyme that results in flatulence and diarrhea.51 Use of acarbose for the treatment of diabetes by dietary control has reportedly resulted in hepatitis.52, 53, 54 Acarbose treatment also elevates liver enzyme levels, whereas its withdrawal normalizes levels.55
There has recently been a push to find an alternative source of treatment for type 2 diabetes, especially from medicinal plants that have high potency and fewer adverse effects than preexisting drugs.56, 57, 58, 59, 60, 61 Excessive inhibition of pancreatic amylase can lead to abnormal bacterial fermentation of carbohydrate foods in the colon, which may lead to adverse digestive disorders.51, 62 Plant-based compounds and products derived from natural sources have been reported for their potent inhibitory activities of the enzymes, but have fewer or no side effects.56, 57, 58, 59, 60, 61 The polyherbal formulation ADJ6, although it has slightly less inhibitory activity than acarbose, will likewise have fewer or no adverse effects because it is completely derived from plant sources. Because ADJ6 is rich in phytochemical constituents (i.e., phenols, flavonoids, and vitamin C), it will be helpful in the management of diabetes and will decrease the risk of other chronic disorders that are associated with diabetes.
The present study elucidates that the polyherbal formulation ADJ6 can be used for effectively managing type 2 diabetes mellitus. The activities of the individual components of the plants are well known for their insulin mimetic role and for their role in reducing insulin resistance.
4. Conclusion
The plants that are used in the formulation have been long used for food and medicinal purposes. Our results suggest that ADJ6 inhibits the enzymes α-amylase and α-glucosidase and contains a high amount of phytochemical constituents (i.e., total phenols, flavonoids, and vitamin C). Thus, ADJ6 may be used in the management of type 2 diabetes mellitus with few or no side effects. However, further studies of ADJ6 using in vitro and in vivo models need to be performed to elucidate its insulin mimetic activity and reduction of insulin resistance.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank ISM-NP Lab, AUKBC Research Centre, MIT Campus of Anna University (Chennai, India) for their help in performing the α-glucosidase inhibitory assays. We would also like to thank the research scholars and nontechnical staff of our department.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jtcme.2014.12.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rorsman P. Review: insulin secretion: function and therapy of pancreatic beta-cells in diabetes. Br J Diabetes Vasc Dis. 2005;5:187–191. [Google Scholar]
- 2.Pfeifer M.A., Halter J.B., Porte D., Jr. Insulin secretion in diabetes mellitus. Am J Med. 1981;70:579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- 3.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz M.S., Chadha A. Type 2 diabetes mellitus in childhood: obesity and insulin resistance. J Am Osteopath Assoc. 2008;108:518–524. [PubMed] [Google Scholar]
- 5.Blonde L. Current challenges in diabetes management. Clin Cornerstone. 2005;7(suppl 3):S6–S17. doi: 10.1016/s1098-3597(05)80084-5. [DOI] [PubMed] [Google Scholar]
- 6.Venkataraman K., Kannan A.T., Mohan V. Challenges in diabetes management with particular reference to India. Int J Diabetes Dev Ctries. 2009;29:103–109. doi: 10.4103/0973-3930.54286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown M.T., LeRoth D. Overcoming challenges in type 2 diabetes management to improve patient outcomes. Expert Rev Endocrinol Metab. 2010;5:741–751. doi: 10.1586/eem.10.45. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy M., Roberts A. Complex type 2 diabetes mellitus—management challenges and pitfalls. Aust Fam Physician. 2013;42:207–210. [PubMed] [Google Scholar]
- 9.Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 10.Modak M., Dixit P., Londhe J., Ghaskadbi S., Devasagayam T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan V., Najmi A.K., Akhtar M., Aqil M., Mujeeb M., Pillai K.K. A pharmacological appraisal of medicinal plants with antidiabetic potential. J Pharm Bioallied Sci. 2012;4:27–42. doi: 10.4103/0975-7406.92727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed I., Adeghate E., Sharma A.K., Pallot D.J., Singh J. Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes Res Clin Pract. 1998;40:145–151. doi: 10.1016/s0168-8227(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 13.Gürbüz I., Akyüz C., Yeşilada E., Sener B. Anti-ulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J Ethnopharmacol. 2000;71:77–82. doi: 10.1016/s0378-8741(99)00178-6. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed I., Lakhani M.S., Gillett M., John A., Raza H. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2001;51:155–161. doi: 10.1016/s0168-8227(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 15.Mishra L.C. CRC Press LLC; 2000 N.W. Corporate Blvd., Boca Raton, Florida 33431, USA: 2003. Scientific Basis for Ayurvedic Therapies. [Google Scholar]
- 16.Virdi J., Sivakami S., Shahani S., Suthar A.C., Banavalikar M.M., Biyani M.K. Antihyperglycemic effects of three extracts from Momordica charantia. J Ethnopharmacol. 2003;88:107–111. doi: 10.1016/s0378-8741(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 17.Prasad V., Jain V., Girish D., Dorle A.K. Wound-healing property of Momordica charantia L. fruit powder. J Herb Pharmacother. 2006;6:105–115. doi: 10.1080/j157v06n03_05. [DOI] [PubMed] [Google Scholar]
- 18.Patel R., Mahobia N., Upwar N., Waseem N., Talaviya H., Patel Z. Analgesic and antipyretic activities of Momordica charantia Linn. fruits. J Adv Pharm Technol Res. 2010;1:415–418. doi: 10.4103/0110-5558.76441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao P.C., Liaw C.C., Hwang S.Y. Antiproliferative and hypoglycemic cucurbitane-type glycosides from the fruits of Momordica charantia. J Agric Food Chem. 2013;61:2979–2986. doi: 10.1021/jf3041116. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez-Escrig A., Rincón M., Pulido R., Saura-Calixto F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J Agric Food Chem. 2001;49:5489–5493. doi: 10.1021/jf010147p. [DOI] [PubMed] [Google Scholar]
- 21.Gutiérrez R.M., Mitchell S., Solis R.V. Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2008;117:1–27. doi: 10.1016/j.jep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Rai P.K., Jaiswal D., Mehta S., Watal G. Anti-hyperglycaemic potential of Psidium guajava raw fruit peel. Indian J Med Res. 2009;129:561–565. [PubMed] [Google Scholar]
- 23.Rai P.K., Mehta S., Watal G. Hypolipidaemic and hepatoprotective effects of Psidium guajava raw fruit peel in experimental diabetes. Indian J Med Res. 2010;131:820–824. [PubMed] [Google Scholar]
- 24.Lin C.Y., Yin M.C. Renal protective effects of extracts from guava fruit (Psidium guajava L.) in diabetic mice. Plant Foods Hum Nutr. 2012;67:303–308. doi: 10.1007/s11130-012-0294-0. [DOI] [PubMed] [Google Scholar]
- 25.Ihantola-Vormisto A., Summanen J., Kankaanranta H., Vuorela H., Asmawi Z.M., Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63:518–524. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 26.Sumantran V.N., Kulkarni A., Chandwaskar R. Chondroprotective potential of fruit extracts of Phyllanthus emblica in osteoarthritis. Evid Based Complement Alternat Med. 2008;5:329–335. doi: 10.1093/ecam/nem030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnaveni M., Mirunalini S. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol. 2010;21:93–105. doi: 10.1515/jbcpp.2010.21.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Akhtar M.S., Ramzan A., Ali A., Ahmad M. Effect of amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int J Food Sci Nutr. 2011;62:609–616. doi: 10.3109/09637486.2011.560565. [DOI] [PubMed] [Google Scholar]
- 29.Usharani P., Fatima N., Muralidhar N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes Metab Syndr Obes. 2013;6:275–284. doi: 10.2147/DMSO.S46341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bin-Hafeez B., Haque R., Parvez S., Pandey S., Sayeed I., Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 31.Tripathi U.N., Chandra D. The plant extracts of Momordica charantia and Trigonella foenum-graecum have anti-oxidant and anti-hyperglycemic properties for cardiac tissue during diabetes mellitus. Oxid Med Cell Longev. 2009;2:290–296. doi: 10.4161/oxim.2.5.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moorthy R., Prabhu K.M., Murthy P.S. Anti-hyperglycemic compound (GII) from fenugreek (Trigonella foenum-graecum Linn.) seeds, its purification and effect in diabetes mellitus. Indian J Exp Biol. 2010;48:1111–1118. [PubMed] [Google Scholar]
- 33.Kumar P., Kale R.K., McLean P., Baquer N.Z. Antidiabetic and neuroprotective effects of Trigonella foenum-graecum seed powder in diabetic rat brain. Prague Med Rep. 2012;113:33–43. doi: 10.14712/23362936.2015.35. [DOI] [PubMed] [Google Scholar]
- 34.Prince P.S., Menon V.P., Pari L. Hypoglycaemic activity of Syzigium cumini seeds: effect on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol. 1998;61:1–7. doi: 10.1016/s0378-8741(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 35.Prince P.S.M., Kamalakkannan N., Menon V.P. Syzigium cumini seed extracts reduce tissue damage in diabetic rat brain. J Ethnopharmacol. 2003;84(2–3):205–209. doi: 10.1016/s0378-8741(02)00316-1. [DOI] [PubMed] [Google Scholar]
- 36.Kochhar A., Nagi M. Effect of supplementation of traditional medicinal plants on blood glucose in non-insulin-dependent diabetics: a pilot study. J Med Food. 2005;8:545–549. doi: 10.1089/jmf.2005.8.545. [DOI] [PubMed] [Google Scholar]
- 37.Arun R., Prakash M.V., Abraham S.K., Premkumar K. Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J Ethnopharmacol. 2011;134:329–333. doi: 10.1016/j.jep.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Aqil F., Gupta A., Munagala R. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. Nutr Cancer. 2012;64:428–438. doi: 10.1080/01635581.2012.657766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baskaran K., Kizar Ahamath B., Radha Shanmugasundaram K., Shanmugasundaram E.R. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol. 1990;30:295–300. doi: 10.1016/0378-8741(90)90108-6. [DOI] [PubMed] [Google Scholar]
- 40.Kanetkar P., Singhal R., Kamat M. Gymnema sylvestre: a memoir. J Clin Biochem Nutr. 2007;41:77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arun L.B., Arunachalam A.M., Arunachalam K.D., Annamalai S.K., Kumar K.A. In vivo anti-ulcer, anti-stress, anti-allergic, and functional properties of gymnemic acid isolated from Gymnema sylvestre R Br. BMC Complement Altern Med. 2014;14:70. doi: 10.1186/1472-6882-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav M., Lavania A., Tomar R., Prasad G.B., Jain S., Yadav H. Complementary and comparative study on hypoglycemic and antihyperglycemic activity of various extracts of Eugenia jambolana seed, Momordica charantia fruits, Gymnema sylvestre, and Trigonella foenum graecum seeds in rats. Appl Biochem Biotechnol. 2010;160:2388–2400. doi: 10.1007/s12010-009-8799-1. [DOI] [PubMed] [Google Scholar]
- 43.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 44.Kim Y.M., Jeong Y.K., Wang M.H., Lee W.Y., Rhee H.I. Inhibitory effect of pine extract on alpha-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005;21:756–761. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Trease G.E., Evans W.C. London; Academic Press: 1989. A Textbook of Pharmacognosy; pp. 22–40. [Google Scholar]
- 46.McDonald S., Prenzler P.D., Antolovich M., Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. [Google Scholar]
- 47.Cameron G.R., Milton R.F., Allan J.W. Measurement of flavonoids in plant sample. Lancet. 1943;179 [Google Scholar]
- 48.Roe J.H. Comparative analyses for ascorbic acid by the 2,4-dinitrophenylhydrazine method with the coupling reaction at different temperatures: a procedure for determining specificity. J Biol Chem. 1961;236:1611–1613. [PubMed] [Google Scholar]
- 49.Eichler H.G., Korn A., Gasic S., Pirson W., Businger J. The effect of a new specific α-amylase inhibitor on post-prandial glucose and insulin excursions in normal subjects and type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1984;26:278–281. doi: 10.1007/BF00283650. [DOI] [PubMed] [Google Scholar]
- 50.Chiba S. Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci Biotechnol Biochem. 1997;61:1233–1239. doi: 10.1271/bbb.61.1233. [DOI] [PubMed] [Google Scholar]
- 51.Kast R.E. Acarbose related diarrhea: increased butyrate upregulates prostaglandin E. Inflamm Res. 2002;51:117–118. doi: 10.1007/pl00012389. [DOI] [PubMed] [Google Scholar]
- 52.Andrade R.J., Lucena M., Vega J.L. Acarbose-associated hepatotoxicity. Diabetes Care. 1998;21:2029–2030. doi: 10.2337/diacare.21.11.2029. [DOI] [PubMed] [Google Scholar]
- 53.Diaz-Gutierrez F.L., Ladero J.M., Diaz-Rubio M. Acarbose-induced acute hepatitis. Am J Gastroenterol. 1998;93:481. doi: 10.1111/j.1572-0241.1998.481_1.x. [DOI] [PubMed] [Google Scholar]
- 54.Fujimoto Y., Ohhira M., Miyokawa N., Kitamori S., Kohgo Y. Acarbose-induced hepatic injury. Lancet. 1998;351:340. doi: 10.1016/S0140-6736(05)78337-9. [DOI] [PubMed] [Google Scholar]
- 55.Gentile S., Turco S., Guarino G., Sasso F.C., Torella R. Aminotransferase activity and acarbose treatment in patients with type 2 diabetes. Diabetes Care. 1998;22:1217–1218. doi: 10.2337/diacare.22.7.1217. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S.C., Ahmad S.A. Traditional medicinal plants: ancient and modern approach. Anc Sci Life. 1992;12:197–200. [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuda H., Morikawa T., Yoshika M. Antidiabetogenic constituents from several natural medicines. Pure Appl Chem. 2002;74:1301–1308. [Google Scholar]
- 58.Vaidya A.D., Devasagayam T.P. Current status of herbal drugs in India: an overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harbilas D., Martineau L.C., Harris C.S. Evaluation of the antidiabetic potential of selected medicinal plant extracts from the Canadian boreal forest used to treat symptoms of diabetes: part II. Can J Physiol Pharmacol. 2009;87:479–492. doi: 10.1139/y09-029. [DOI] [PubMed] [Google Scholar]
- 60.Petrovska B.B. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahomoodally M.F. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid Based Complement Alternat Med. 2013;2013:14. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Apostolidis E., Kwon Y.I., Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.