Abstract
There is limited evidence that ginger (生薑 shēng jiāng) powder consumption can relieve pain and inflammation because of its special phytochemical properties. This study is aimed at investigating the effect of ginger powder supplementation on some inflammatory markers in patients suffering from knee osteoarthritis. This is a double-blind randomized placebo-controlled clinical trial with a follow-up period of 3 months that was conducted on 120 outpatients with moderately painful knee osteoarthritis. Patients were randomly divided up into two groups: ginger group (GG) or placebo group (PG). Both groups received two identical capsules on a daily basis for 3 months. Each ginger capsule contained 500 mg of ginger powder; the placebo capsules had 500 mg of starch in them. Serum samples were collected prior to and after the intervention and were stored at −70 °C until the end of the study. Serum concentration of nitric oxide (NO) and hs-C reactive protein (hs-CRP) were measured using enzyme-linked immunosorbent assay kits. There was no significant difference between the two groups in terms of inflammatory markers (i.e., NO and hs-CRP) prior to the intervention. However, after 3 months of supplementation, serum concentration of NO and hs-CRP decreased in the GG. After 12 weeks, the concentration of these markers declined more in the GG than in the PG. Ginger powder supplementation at a dose of 1 g/d can reduce inflammatory markers in patients with knee osteoarthritis, and it thus can be recommended as a suitable supplement for these patients.
Keywords: C-reactive protein, Ginger, Nitric oxide, Osteoarthritis, Elderly
Graphical abstract
1. Introduction
Arthritis is a prevalent condition affecting millions of people around the world.1 Osteoarthritis (OA) or degenerative arthritis is the most common form of arthritis that is defined by degeneration of articular cartilage, joint pain and inflammation, and dysfunction particularly in older people.2, 3 Knee joints bear the largest part of the body weight, and they are most likely to be at risk of OA.4 As the prevalence of OA is high, and the nature of the disease is progressive, it is not surprising that it has had an important effect on the global economy.3, 5 Factors that can predispose people to OA of the knee are age, weight, body mass index (BMI), genetics, occupational activities, history of trauma, and physical work activities, especially kneeling, squatting, lifting, and climbing.6, 7, 8, 9
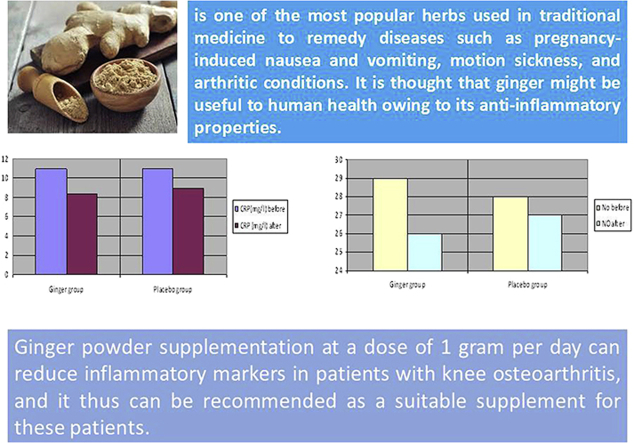
Treatment programs commonly involve nonpharmacological and pharmacological measures. If pain becomes debilitating, joint replacement surgery is to be used to ameliorate the quality of life.10 Generally, exercise and lifestyle modification are considered nonpharmacological measures,11 and analgesics are prescribed to relieve pain. However, there is increasing concern that some analgesics may cause serious complications. For instance, some cyclooxygenase-2 (COX-2) inhibitors such as nonsteroidal anti-inflammatory drugs have been shown to increase the risk of adverse cardiovascular and gastrointestinal events.12 Moreover, findings of two in vitro studies demonstrated that naproxen, indomethacin, and ibuprofen, which are the most frequently prescribed nonsteroidal anti-inflammatory drugs, block the synthesis of human cartilage matrix, and this is likely to increase the rate of degeneration of articular cartilage in OA.13, 14 This has led many researchers to attempt to find a remedy or modalities with negligible side effects and significant improvement in the symptoms.15 Ginger (生薑 shēng jiāng) is an underground rhizome of the plant Zingiber officinale, belonging to the family Zingiberaceae, which is one of the most popular herbs used in traditional medicine to remedy diseases such as pregnancy-induced nausea and vomiting, motion sickness, and arthritic conditions. It is thought that ginger might be useful to human health owing to its anti-inflammatory properties. The mechanism of its action is not clear, but it seems to inhibit the activation of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and inducible nitric oxide synthase (iNOS).16
C-reactive protein (CRP) is a plasma protein that increases in the systemic response to inflammatory events. Its rapid increase in synthesis within hours after tissue injury or infection suggests that it contributes to host defense and that it is part of the innate immune response. There is an association between elevation of this protein and future major cardiovascular events.17 Considering the role of ginger in reducing inflammation and given the fact that we could not find any study in the literature on the long-term effects of ginger on inflammation, this study was designed to determine the effect of daily supplementation of 1 g ginger powder for 3 months on inflammatory markers in older patients with knee OA.
2. Materials and methods
2.1. Study design and participants
This study was a double-blind randomized placebo-controlled clinical trial, with a 3-month follow-up, of 120 patients with knee OA visiting Khatam-ol-anbia Polyclinic in the city of Yazd in Iran. This study was performed in the period spanning November 2011 to May 2012.
Criteria for inclusion were age (between 50 years and 70 years) and diagnosis of OA verified by a rheumatologist according to the classification criteria of the American College of Rheumatology.18 Patients with any of the following were excluded: rheumatoid arthritis, inflammatory diseases, metabolic disorders (such as diabetes, cancer, or other serious diseases), signs or history of liver or kidney failure, treatment with oral corticosteroids within a period of 4 weeks prior to the trial, corticosteroids injection within 6 months prior to the experiment, fever > 38°C at screening, permanent consumption of ginger (生薑 shēng jiāng), allergy to ginger, unwillingness to continue the protocol, avoidance of consumption of > 20% of the ginger supplements, any serious complications, consumption of multivitamin, minerals, or other nutritional supplements, and consumption of analgesic medications.
A total of 120 patients meeting the above-mentioned criteria were randomly assigned to two groups (n = participants each): ginger group (GG) and placebo group (PG). Randomization was done via tables of random numbers that were sequential list prepared. The patients were asked to take two 500-mg capsules per day for 3 months. The GG and PG capsules contained powdered ginger and starch, respectively. Placebo and ginger capsules were similar in terms of color, odor, weight, and packing. All the capsules were manufactured in the Institute of Medicinal Plants in Tehran, Iran. Patients were examined at the beginning of the study and then again after 3 months in order to measure anthropometric indices. Moreover, the participants filled out a researcher-made questionnaire with questions on age, occupation, education, and obesity status. The patients were asked not to change their normal diet. The patients were also instructed to keep their daily use of the stairs to a minimum. Furthermore, a rheumatologist taught them some kinds of knee exercises.
2.2. Measurements
Anthropometric indices were obtained by a trained dietitian. The patients' weights were measured using digital scales with a readability of 0.1 kg and with the patients being minimally clothed, without shoes. Height was measured using a stadiometer with a readability of 0.5 cm and with the patients being in a standing position without shoes. To determine the obesity status, the BMI was calculated as weight (kg) divided by the square of height (m). All anthropometric measurements were obtained on the same day when blood specimens were taken.
2.3. Biochemical assessment
Both at the start of and 3 months after the experiment, 5 mL peripheral blood was taken from each patient in a nonfasting state through venipuncture of an antecubital vein (Suha, Iran). Serum samples were stored at −70°C until the end of the study. Serum CRP concentrations were measured using enzyme-linked immunosorbent assay kits (Biocore, Hamburg, Germany). Serum concentration of nitric oxide was measured using a colorimetric assay kit (Biocore).
2.4. Dietary assessment
Dietary intake was assessed by trained dietitians. Data on 24-hour recalls were collected from all 120 participants prior to and after the intervention. The 24-hour recall is based on actual intake and may be used to estimate absolute rather than relative intake.19 However, this method is susceptible to recall bias, both for identification of foods eaten and for quantification of portion sizes. In order to reduce this type of error in this study, dietary data were collected by highly trained interviewers.20 Individuals were asked whether the day of recalls was a usual day or not. To enter the data into the computer, standard reference tables were used to convert household portions to grams.21 After coding, the dietary recall form was linked to a nutrient database (Nutritionist IV, Bruno, CA, USA). For mixed dishes, food groups were calculated taking account of their ingredients. The data related to Nutritionist IV were modified in accordance with the Iranian Food Composition Table.
2.5. Ethical considerations
The protocol was explained to all patients. The participants volunteered to participate in this study and had the possibility of withdrawing from the study at any moment. The Ethics-in-Research Commission of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved the study, and all patients gave written informed consent.
2.6. Statistical analysis
Data were submitted to SPSS 16.0.2 (2008; SPSS Inc., Chicago, IL, USA).22 Prior to the statistical comparison, a Kolmogorov–Smirnov test was used to demonstrate the distribution of quantitative data. Furthermore, the t test was used to draw a comparison between the data obtained from the two groups (i.e., between-groups comparison). In addition, Student t test was used for comparing the data obtained from each group before and after the intervention (i.e., within-groups comparison). The results of the protocol are reported as mean ± standard deviation because the distribution of the quantitative data was normal. All analyses were performed at the 0.05 level of significance (2-tailed).
3. Results
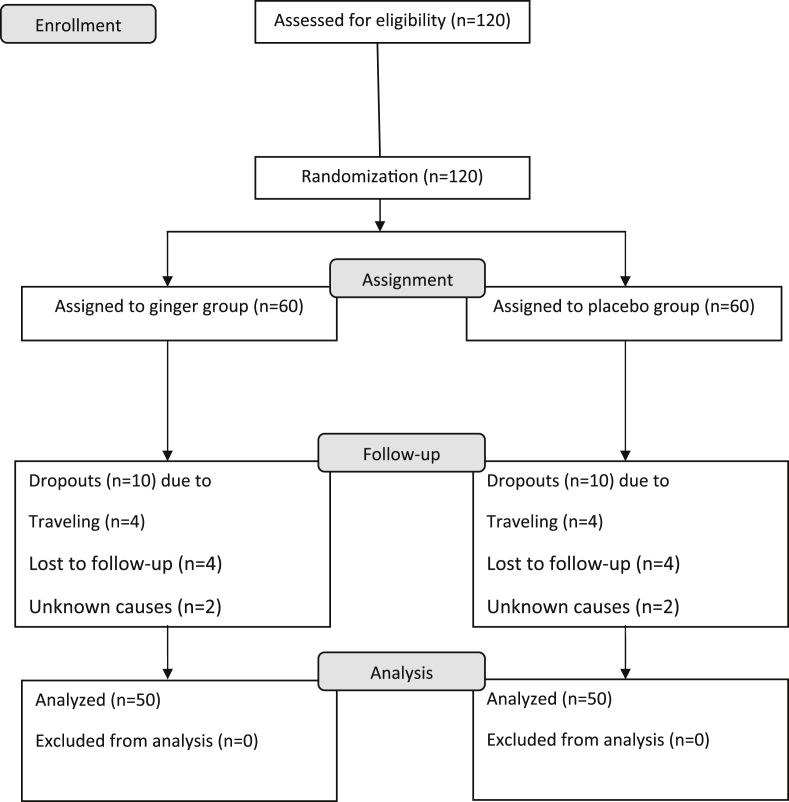
At the beginning of the study, 120 patients participated and were randomized. During the protocol, 20 patients—10 receiving ginger (生薑 shēng jiāng) and 10 receiving placebo—were excluded: eight of the participants withdrew because of a trip, eight due to loss of follow-up, and four for undisclosed reasons. Therefore, 100 patients completed the trial without protocol violations (Fig. 1).
Fig. 1.
Study flowchart.
Table 1 illustrates the baseline characteristics of the participants prior to the intervention in both groups. As shown, the patients were predominantly women, but the frequency of men and women between groups was statistically insignificant (p = 0.83). There was no significant difference between the two groups in terms of age, weight, height, and BMI. Similarly, the two groups were not significantly different in terms of sex, obesity, education, and occupational status.
Table 1.
Comparison of baseline characteristics for the variables under investigation in ginger and placebo groups.
| Variables | Ginger group (n = 50) | Placebo group (n = 50) | p |
|---|---|---|---|
| Age (y) | 57.98 ± 6.2 | 59.1 ± 6.1 | 0.3 |
| Weight (kg) | 67.94 ± 7.0 | 66.3 ± 5.9 | 0.21 |
| Height (cm) | 161.4 ± 6.4 | 161.2 ± 7.0 | 0.9 |
| BMI (kg/m2) | 26.1 ± 2.9 | 25.5 ± 2.0 | 0.2 |
| Sex | |||
| Men | 3 (6) | 7 (14) | 0.31 |
| Women | 47 (94) | 43 (86) | |
| Obesity status | |||
| Obese | 31 (62) | 33 (66) | 0.83 |
| Nonobese | 19 (38) | 17 (34) | |
| Education | |||
| Illiterate | 20 (40) | 18 (36) | 0.60 |
| Elementary school graduate | 10 (20) | 12 (24) | |
| Middle and high school graduate | 15 (30) | 18 (36) | |
| University graduate | 5 (10) | 2 (4) | |
| Occupational status | |||
| Employed | 18 (36) | 20 (40) | 0.40 |
| Unemployed | 32 (64) | 30 (60) | |
Data are presented as n (%) or mean ± SD.
BMI = body mass index.
Table 2 presents the effect of ginger and starch on CRP and NO of the patients at the beginning of and 3 months after the study. As can be seen, there was no significant difference between the two groups in CRP and NO concentration prior to the intervention. However, after supplementation of ginger, a significant decrease was observed in serum concentration of CRP and NO in the GG, but not in the other group. At the end of the study, CRP and NO concentration decreased more in the experimental group than in the control group.
Table 2.
Comparison of mean of NO and CRP concentration in both ginger and placebo groups prior to and after intervention.
| Variables | Ginger group | Placebo group | pa |
|---|---|---|---|
| Energy (kcal/d) | |||
| Prior to | 1904.2 ± 325.4 | 1859.7 ± 272.2 | 0.6 |
| After | 2010.4 ± 401.0 | 1997.4 ± 213.1 | 0.3 |
| pb | 0.9 | 0.2 | |
| Fiber (g/d) | |||
| Prior to | 9.2 ± 4.2 | 10.9 ± 9.9 | 0.5 |
| After | 12.3 ± 5.9 | 7.8 ± 8.2 | 0.9 |
| p | 0.8 | 0.6 | |
| Cholesterol (mg/d) | |||
| Prior to | 302.02 ± 102.82 | 310.21 ± 123.02 | 0.08 |
| After | 295.02 ± 87.82 | 301.02 ± 95.12 | 0.6 |
| p | 0.7 | 0.5 | |
| NO (μmol/L) | |||
| Prior to | 29.02 ± 0.82 | 29.21 ± 1.02 | 0.53 |
| After | 26.02 ± 1.82 | 27.02 ± 0.32 | <0.001 |
| Change | −3.0 ± 0.72 | −2.01 ± 0.19 | <0.001 |
| p | <0.001 | <0.001 | |
| CRP (mg/L) | |||
| Prior to | 11.06 ± 1.43 | 11.21 ± 1.20 | 0.56 |
| After | 8.47 ± 1.62 | 9.66 ± 1.31 | <0.001 |
| Change | −2.58 ± 1.47 | −1.54 ± 1.12 | <0.001 |
| p | <0.001 | <0.001 | |
CRP = C-reactive protein; NO = nitric oxide.
Student t test.
Paired t test.
Table 2 also gives the means for some daily dietary intake in the two groups prior to and after the intervention. No statistically significant difference was found between pre- and postintervention figures for daily energy and fiber and cholesterol intake. In other words, between-group and within-group comparisons did not show any significant difference in daily energy and fiber and cholesterol intake.
4. Discussion
This study found that, in the patients who suffered from moderate knee OA, the serum concentration of CRP and NO 3 months after ginger (生薑 shēng jiāng) supplementation was significantly different from the data obtained at the beginning of the study. This finding is consistent with that of recent studies on ginger supplementation on anti-inflammatory mediators. Lee et al23 found that 6-gingerol had an anti-inflammatory effect on mouse macrophages. This active constituent of ginger decreased iNOS and TNF-α expression by inhibiting I-kappaB alpha phosphorylation, nuclear factor-kappa B (NF-κB) nuclear activation, and protein kinase C-alpha translocation.
In another study, Jung et al24 showed that ginger hexane extract (GHE) significantly inhibited the excessive production of NO, protein, and mRNA expression of iNOS in lipopolysaccharide (LPS)-stimulated BV-2 cells, a mouse microglial cell line, and attenuated their gene expressions. Furthermore, GHE inhibited mRNA expression and protein induction of COX-2 in BV-2 cells stimulated through LPS. The only thing that was not affected by GHE was the production of PGE2 or COX-2 expression in microglial cells. Also, GHE significantly suppressed the production of TNF-α and IL-1β and reduced their gene expressions in these cells.24
In another in vitro study carried out by Shimoda et al25 to evaluate the anti-inflammatory activity of red ginger extract (RGE), Z. officinale var. Rubra in mouse leukemic monocytes (RAW 264.7 cells) stimulated by LPS, it was found that RGE (100 μg/mL) significantly inhibited NO production. Moreover, dosage of 3 μg/mL and 10 μg/mL significantly inhibited PGE2 production. Separation of RGE by means of bioassay showed that [6]-shogaol and gingerdiols were capable of inhibiting NO production.25
After 10 weeks of ginger supplementation and resistance training, Atashak et al26 reported significant decreases in the mean values of CRP in three of the groups in their experiment: resistance training plus ginger (RTGI), resistance training plus placebo (RTPL), and ginger (GI). CRP concentration declined 35.1%, 28.3%, and 21.2% in RTGI, RTPL, and GI groups, respectively.26
A recent study by Han et al27 found that treatment of Raw 264.7 cells, a murine macrophage-like cell line, with 150 ng/mL and 200 ng/mL of 12-dehydrogingerdione significantly suppressed LPS-stimulated production of NO. Moreover, this treatment suppressed the LPS-stimulated increase in iNOS levels. However, treatment of cells with LPS without 12-dehydrogingerdione significantly increased NO production.
In another study, daily supplementation of 2 g ginger for 2 months was found to significantly decrease serum concentration of hs-C reactive protein (hs-CRP), TNF- α, and IL-6 in the GG compared with the baseline in type 2 diabetes patients.28 These factors did not change in the PG during the protocol. In other words, covariance analysis revealed that the level of hs-CRP and TNF- α lowered more in the blood of the group receiving ginger than in the blood of the placebo-receiving patients. However, the level of IL-6 remained unchanged in both groups.
The results of the present research do not concur with the findings of a few similar studies. Ueda et al22 studied the in vitro effect of ginger extract on cytokines produced from a macrophage-like cell line. After an oral administration of 100–1000 μg/mL squeezed ginger extract (SGE) to mice, the production of TNF-α and IL-6 and MCP-1 increased in mice cells. When the mice were orally administered with the SGE or its ethanol-insoluble for a single time or two, TNF-α production increased in peritoneal cells; however, administration for more than four times had opposite results. In addition, oral administration of the SGE for several times increased immune resistance but caused an anti-inflammatory activity.
A possible mechanism whereby ginger reduces inflammation in patients with OA is as follows: activation of synovial cells in the joints leads to the release of two key cytokines involved in the inflammation and degradation of joints, TNF-α and IL-1β. Both mediators induce NF-κB, which is a ubiquitous eukaryotic transcription factor with a pivotal role in inflammatory pathways.29 This nuclear factor brings about inflammation by activating iNOS, COX-2 pathway, and lipoxygenase pathway and by inducing the secretion of inflammatory cytokines.
Ginger contains gingerols and shogaols, which are pungent phenolic compounds with anti-inflammatory properties.30, 31 These substances decrease the two proinflammatory factors of TNF-α and IL-1β in osteoarthritic cartilage. In synoviocytes, these compounds decrease the IL-1β- or TNF-α-induced expression of TNF-α mRNA and protein, the TNF-α-induced production of COX2, and the TNF-α-induced activation of the NF-κB. In addition, these crucial compounds suppress the synthesis of prostaglandin and leukotriene by inhibiting the COX-2 and lipoxygenase pathways and also inflammation-involved pathways.32, 33 This way, they can diminish the inflammation.
5. Conclusion
The present work is significant in three ways. (1) We examined the long-term effect of supplementation of ginger powder on inflammatory markers in patients with knee OA. (2) The study was a double-blind randomized clinical trial, meaning that neither the researchers nor the patients were aware of the content of capsules. (3) The protocol involved a control group that was similar to the GG in all measurements.
It is also important to note the limitation in that the present study did not measure neuronal sensitization, Immunoglobulin G (IgG), Immunoglobulin M (IgM), and did not determine ginger capsule's formulation. Sample attrition resulting from patient withdrawal may be regarded as another limitation of the protocol. Further research into this topic could evaluate the effect of supplementation of 1 g/d ginger capsule on lipid profile, coagulation factors, other inflammatory mediators, and neuronal sensitization. Future research can also consider the effect of 1 g/d ginger capsule on oxidative stress-related parameters.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We thank the patients who took part in this project. Special thanks to the chancellor of Yazd Central laboratory, Dr Akhavan and staff in this laboratory, particularly, Mr Khajeh Afzali and Mr Salimi for their cooperation in blood sampling and performing biochemical tests.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Natarajan S. To assess the efficacy & safety of NILIN™ SR tablets in the management of osteoarthritis of knee. Int J Pharm Life Sci. 2012;3:1413–1423. [Google Scholar]
- 2.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp J Intern Med. 2011;2:205–212. [PMC free article] [PubMed] [Google Scholar]
- 3.Leach M.J., Kumar S. The clinical effectiveness of ginger (Zingiber officinale) in adults with osteoarthritis. Int J Evid-Based Healthc. 2008;6:311–320. doi: 10.1111/j.1744-1609.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 4.Toda Y., Tsukimura N. A six-month follow up of a randomized trial comparing the efficacy of a lateral-wedge insole with subtalar strapping and an in-shoe lateral-wedge insole in patients with varus deformity osteoarthritis of the knee. Arthritis Rheum. 2004;50:3129–3136. doi: 10.1002/art.20569. [DOI] [PubMed] [Google Scholar]
- 5.Leask A. Will o' the wisp: CCN4 as a novel molecular target in osteoarthritis. J Cell Commun Signal. 2011;5:51–52. doi: 10.1007/s12079-010-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer K.T. Occupational activities and osteoarthritis of the knee. Br Med Bull. 2012;102:147–170. doi: 10.1093/bmb/lds012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McWilliams D., Leeb B., Muthuri S., Doherty M., Zhang W. Occupational risk factors for osteoarthritis of the knee: a meta-analysis. Osteoarthr Cartil. 2011;19:829–839. doi: 10.1016/j.joca.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Franklin J., Ingvarsson T., Englund M., Lohmander S. Association between occupation and knee and hip replacement due to osteoarthritis: a case-control study. Arthritis Res Ther. 2010;12:2–9. doi: 10.1186/ar3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goker B., Doughan A.M., Schnitzer T.J., Block J.A. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43:988–994. doi: 10.1002/1529-0131(200005)43:5<988::AID-ANR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Haghighi A., Tavalaei N., Owlia M.B. Effects of ginger on primary knee osteoarthritis. Indian J Rheumatol. 2006;1:3–7. [Google Scholar]
- 11.Focht B.C. Move to improve: how knee osteoarthritis patients can use exercise to enhance quality of life. ACSM Health Fit J. 2012;16:24–28. doi: 10.1249/FIT0b013e318264cae8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaksch W., Dejaco C., Schirmer M. 4 years after withdrawal of rofecoxib: where do we stand today? Rheumatol Int. 2008;28:1187–1195. doi: 10.1007/s00296-008-0650-4. [DOI] [PubMed] [Google Scholar]
- 13.Mamdani M. The changing landscape for COX-2 inhibitors–a summary of recent events. Healthc Q. 2005;8:24–26. doi: 10.12927/hcq..17052. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein F.E., Faich G., Goldstein J.L. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. J Am Med Assoc. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 15.Zakeri Z., Izadi S., Bari Z., Soltani F., Narouie B., Ghasemi-rad M. Evaluating the effects of ginger extract on knee pain, stiffness and difficulty in patients with knee osteoarthritis. J Med Plants Res. 2011;5:3375–3379. [Google Scholar]
- 16.Lee H.Y., Park S.H., Lee M. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKβ activity for NF-κB activation and suppresses NF-κB-regulated expression of inflammatory genes. Br J Pharmacol. 2012;167:128–140. doi: 10.1111/j.1476-5381.2012.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black S., Kushner I., Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 18.Grzanna R., Lindmark L., Frondoza C.G. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 19.Willett W. Oxford University Press; New York: 1998. Nutritional Epidemiology. [Google Scholar]
- 20.Larkin F., Metzner H., Guire K. Comparison of three consecutive-day and three random-day records of dietary intake. J Am Diet Assoc. 1991;91:1538. [PubMed] [Google Scholar]
- 21.Ghaffarpour M., Houshiar-Rad A., Kianfar H. Nashre Olume Keshavarzy; Tehran: 1999. The Manual for Household Measures, Cooking Yields Factors and Edible Portion of Foods. [Google Scholar]
- 22.Ueda H., Ippoushi K., Takeuchi A. Repeated oral administration of a squeezed ginger (Zingiber officinale) extract augmented the serum corticosterone level and had anti-inflammatory properties. Biosci Biotechnol Biochem. 2010;74:2248–2252. doi: 10.1271/bbb.100456. [DOI] [PubMed] [Google Scholar]
- 23.Lee T.-Y., Lee K.-C., Chen S.-Y., Chang H.-H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382:134–139. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- 24.Jung H.W., Yoon C.H., Park K.M., Han H.S., Park Y.K. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem Toxicol. 2009;47:1190–1197. doi: 10.1016/j.fct.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Shimoda H., Shan S.-J., Tanaka J. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J Med Food. 2010;13:156–162. doi: 10.1089/jmf.2009.1084. [DOI] [PubMed] [Google Scholar]
- 26.Atashak S., Peeri M., Jafari A. Effects of 10 week resistance training and ginger consumption on C-reactive protein and some cardiovascular risk factors in obese men. Physiol Pharmacol. 2010;14:318–328. [Google Scholar]
- 27.Han Y., Song C., Koh W. Anti-inflammatory effects of the Zingiber officinale Roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated raw 264.7 cells. Phytother Res. 2012;27:1200–1205. doi: 10.1002/ptr.4847. [DOI] [PubMed] [Google Scholar]
- 28.Mahluji S., Ostadrahimi A., Mobasseri M., Attari V.E., Payahoo L. Anti-inflammatory effects of Zingiber officinale in type 2 diabetic patients. Age (yr) 2013;3:273–276. doi: 10.5681/apb.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julie S., Jurenka M. Anti-inflammatory properties of curcumin, a major constituent. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 30.Dugasani S., Pichika M.R., Nadarajah V.D., Balijepalli M.K., Tandra S., Korlakunta J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol,[8]-gingerol,[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Venkatesha S.H., Berman B.M., Moudgil K.D. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg Med Chem. 2011;19:21–29. doi: 10.1016/j.bmc.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ameye L.G., Chee W.S.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res Ther. 2006;8:127. doi: 10.1186/ar2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegazy H.G. Ameliorative effects of ginger and α-lipoic acid on oxidative stress and inflammation in senile female rats. Afr J Pharm Pharmacol. 2011;5:1096–1105. [Google Scholar]