Abstract
Maintenance of tissue-specific stem cells is vital for organ homeostasis and organismal longevity. Hematopoietic stem cells (HSCs) are the most primitive cell type in the hematopoietic system. They divide asymmetrically and give rise to daughter cells with HSC identity (self-renewal) and progenitor progenies (differentiation), which further proliferate and differentiate into full hematopoietic lineages. Mammalian ageing process is accompanied with abnormalities in the HSC self-renewal and differentiation. Transcriptional changes and epigenetic modulations have been implicated as the key regulators in HSC ageing process. The DNA damage response (DDR) in the cells involves an orchestrated signaling pathway, consisting of cell cycle regulation, cell death and senescence, transcriptional regulation, as well as chromatin remodeling. Recent studies employing DNA repair-deficient mouse models indicate that DDR could intrinsically and extrinsically regulate HSC maintenance and play important roles in tissue homeostasis of the hematopoietic system. In this review, we summarize the current understanding of how the DDR determines the HSC fates and finally contributes to organismal ageing.
Keywords: Hematopoietic stem cells, DNA damage response, Epigenetics, Ageing, P53
Introduction
In adult animals, tissue homeostasis is maintained by a hierarchy of different types of cells, ranging from tissue-specific stem cells, progenitors, to somatic cells with different functions [1]. Stem cells are the most primitive cell population in a specific tissue, which on the one hand self-renew to sustain the stem cell pool, and on the other hand differentiate to generate their somatic progenies [1], [2]. Dysregulation of self-renewal and differentiation of tissue-specific stem cells compromises the stem cell function, resulting in loss of tissue maintenance and organismal ageing [1], [3], [4].
Among all types of tissue-specific stem cells, hematopoietic stem cell (HSC) is considered as the prototype to study the functions of genes of interest in adult stem cell self-renewal and maintenance, as well as their roles in physiological ageing [5], [6]. Under unperturbed conditions, HSCs reside within their niches (bone marrow stromal cells) and are exposed to systematic environments consisting of cytokine, chemokine (Figure 1), and other factors [7]. The advantages of using the HSCs as the model to study stem cell ageing are mainly due to: (1) well-defined HSCs and their progenies with combinations of cell surface markers; (2) a panel of sophisticated in vitro assays to verify the HSC functions; and (3) the adoptive HSC transplantation assay as a gold standard to test stem cell functions [5]. Using naturally-aged wild type mice and genetically-modified premature ageing mouse models [8], [9], [10], [11], [12], [13], intrinsic and extrinsic factors contributing to the HSC ageing start to be unraveled [4], [14], [15], [16]. Among them, cell cycle regulators, transcriptional factors, epigenetic modulators, and metabolic pathways have been implicated as important regulators for HSC self-renewal and maintenance during ageing process [10], [12], [17], [18], [19], [20], [21], [22], [23].
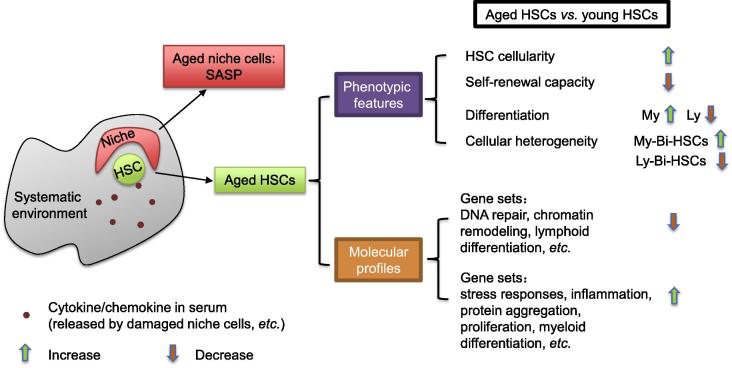
Figure 1.
Characteristics of aged HSCs
HSCs from aged mice show distinct phenotypic features and molecular profiles as compared to HSCs in young animals. The HSC ageing is a combinatorial effect from intrinsic determinants and extrinsic signals, such as niche cells and systematic environmental factors. HSC, hematopoietic stem cell; SASP, senescence-associated secretory phenotype; My, myeloid; Ly, lymphoid; My-Bi-HSC, myeloid-biased HSC; Ly-Bi-HSC, lymphoid-biased HSC.
DNA lesions in cells originate from endogenous cellular activities, such as DNA replication and mitochondrial respiration, as well as exogenous stimuli, such as therapeutic drugs against cancers and medical exposure to irradiation, posing direct threats to the integrity of the cellular genetic information [24], [25], [26]. If these DNA lesions could not be handled well, they will compromise cellular viability and drive the tumor formation [27], [28]. When it comes to the HSCs, improper repair of DNA lesions could negatively regulate the HSC maintenance and lead to HSC ageing [4], [8], [26]. Here, we concisely discuss the signatures defining “aged HSCs” and the role of genomic stability in HSC ageing.
Characteristics of HSCs in ageing hematopoietic system
Compared to the young individuals, the frequency (percentage of HSCs within bone marrows) and absolute numbers of HSCs, which are phenotypically designated with defined surface markers, increase in naturally-aged individuals of mice and humans (Figure 1) [8], [29], [30]. However, HSCs in aged mice are defective in the self-renewal capacity [31]. The adoptive bone marrow transplantation assay is the “gold standard” to investigate the HSC functionality. Upon transplantation, HSCs are forced to enter the cell cycle and differentiate into different hematopoietic lineages [32]. The sequential transplantation with the HSCs from the primary transplantation could be further employed to test the robustness of HSCs in self-renewal. During the serial transplantation, HSCs get exhausted and step into an “aged” status [12], [33]. Using this serial adoptive transplantation assay, aged HSCs (HSCs from aged mice) showed limited repopulation ability to replenish the hematopoietic system in bone marrow-ablated congenic mice [12], [29]. The HSC transplantation assay indicates that the aged HSCs, in addition to a homing defect (a failure of transplanted donor HSCs trafficking to and engrafting in recipient bone marrows), only represent around 25% efficiency of HSCs from young animals [29].
Furthermore, aged HSCs have differentiation defects as well (Figure 1). Peripheral blood (PB) from aged mice contains a relative higher proportion of myeloid cells, such as Mac1 + and Gr1+ hematopoietic cells, as compared to the PB from young animals [29], [34], [35], which could be attributed to the higher proportion of myeloid progenitors generated in the bone marrow of aged mice [36]. The biased myeloid hematopoiesis in the aged mice is detrimental to hematopoietic system functions since the dysregulated output of lymphoid and myeloid cells would compromise the immunological response upon injury or infection in the aged animals and further promote ageing. This skewed differentiation is cell-autonomous, since transplanting aged HSCs to young mice could recapitulate the phenotypes of “ageing” hematopoietic compartments in these recipient mice [34], [36]. The increased ratio of myeloid vs. lymphoid hematopoietic cells in ageing is further attributed to altered heterogeneity in HSC compartments during the ageing process [37], [38]. Based on their differentiation capabilities, HSCs are further divided into lymphoid-biased HSCs (Ly-Bi HSCs), myeloid-biased HSCs (My-Bi HSCs), and balanced HSCs [17], [37]. The composition of HSC pools is shifted from Ly-Bi HSCs toward My-Bi HSCs during ageing.
In addition to the aforementioned phenotypically-defined characteristics, aged HSCs are distinct from young HSCs due to their unique transcriptomic and epigenomic features [39], [40], [41]. Aged HSCs are implicated with marked increase in the expression of genes involved in stress responses, inflammation, and protein aggregation, while the expression of factors responsible for DDR and chromatin remodeling is reduced (Figure 1) [40]. Accordingly, aged HSCs accumulate DNA lesions [8], are defective in protein homeostasis, and exhibit abnormal epigenetic landscapes on DNAs and histones [9], [39], [40]. DNA methylation is enriched specifically on the promoter regions of lymphoid and erythroid lineage genes [9]. On the contrary, promoters of genes responsible for the myeloid lineages exhibit reduced DNA methylation. This finding correlates with the skewed hematopoietic lineage output in aged mice [8], [36], [42]. Furthermore, hypo-methylated cysteines and active chromatin markers, such as H3K9me3 and H3K27me3, are enriched in the promoter regions of genes in the Gene Ontology categories of cell adhesion, proliferation, and ribosome, which are expressed higher in the aged HSCs than in the young ones [39]. Such transcriptional and epigenetic alterations could partially explain the phenotypic characteristics of aged HSCs, such as an increased mobilization, reduced homing ability, and loss of quiescence [29], [39].
DNA damage accumulation in HSC ageing
In somatic cells, loss of genomic integrity compromises the cellular viability and threatens the genetic information passage from parent cells to daughter cells [43]. Accumulation of genomic instability has been implicated in the hematopoietic malignancy, which could be derived from transformed HSCs [26], [28]. DNA lesions initiate DDR and induce chromatin remodeling, epigenetic modification, as well as transcriptional regulation, which consequently activate a series of cellular responses including DNA repair, cell cycle checkpoint, cellular senescence, and cell death [24], [25], [26], [43]. All of these pan-genome, epigenome, and transcriptome modifications definitely generate systematic outcome to shape the dynamics of the HSCs in the context of self-renewal and differentiation [44], [45].
The first direct link of DNA damage and HSC ageing comes from the analysis of double strand breakage (DSB) marker γ-H2AX in murine HSCs [8]. Rossi and colleagues investigated the DNA damages inside aged murine HSCs and noticed that aged HSCs accumulate high levels of DNA DSBs [8]. However, a recent study suggested that those γ-H2AX marked “DSB foci” may not be real DNA breaks. The “DSB foci” are nucleolar-associated and represent the residual replication stress during the HSC cycling [46]. The “γ-H2AX foci” severs as the chromatin repressive marker for the silencing of rDNA transcription, which compromises the ribosome biogenesis in aged HSCs [46]. Interestingly, Beerman et al. used the alkaline comet assay, which is extensively used in the field of DNA repair as the indicator for the DSBs and single strand breaks (SSBs) [47], [48], to compare the DNA damages in young and aged quiescent HSCs. As a result, they noticed that aged HSCs have a high degree of DNA breaks, as indicated with increased “Olive tail moment” [9], [48]. Similar to the murine HSCs, γ-H2AX antibody staining on human CD34+ HSCs and hematopoietic progenitors reveals an significant accumulation of DSBs during normal ageing process [49]. These data indicate that murine and human HSCs experience similar biological processes, namely genomic instability, during physiological ageing.
How are these DNA breaks generated in HSCs under physiological conditions during the ageing process? Reactive oxygen species (ROS) generated from metabolic pathways in quiescent HSCs and replication errors during HSC proliferation could be the threats to genome integrity in HSCs [33], [50], [51]. Using different mouse models that harbor deficiencies in DNA repair pathways, it is found that loss of DNA repair factors results in accumulation of DNA damages in HSCs and severely- compromised capabilities of HSCs for self-renewal and differentiation under physiological conditions [8], [11], [52], [53], [54]. For examples, knockout mice with defects in DNA DSB repair and quenching the ROS (such as Atm-/- mice) or in resolving the replication fork stalls (knockout of Fancd2 pathway members) are ageing-prone and show defective hematopoiesis [51], [55], [56]. These findings strongly indicate that the proper repair of DNA damage is important for the maintenance of HSCs and protects against functional decline of HSCs during ageing [4], [8], [26], [45].
DNA repair pathway choices in HSCs
In order to fix DNA breaks, cells are equipped with different repair mechanisms or repair factors [24], [43]. The choice of pathways to repair a DNA lesion is highly dependent on the cell cycle phase and/or the physiological status of a cell [25], [57], [58]. Our knowledge of DNA repair in cell cycle stems from studies on the cycling somatic cells. Intriguingly, adults HSCs reside in quiescent status (G0) [14]. Two repair scenarios have been proposed to repair ionizing radiation (IR)-generated DSBs in HSCs in G0 phase [59], [60]. Passegué and her colleagues found that quiescent HSCs use the same repair program as in G1 phase of somatic cells, i.e., non-homologous end joining (NHEJ) pathway to repair the IR-generated DNA lesions [60]. In this scenario, upon DSB induction, protein complex comprising MRE11/RAD50/NBS1 is recruited to the DSB sites and activates ATM kinase, which phosphorylates MDC1/H2AX/53BP1/SMC1/KAP1 to alter the chromatin status around the DSBs and CHK2/p53 to initiate the cell cycle checkpoints and/or cell death signaling pathways [24], [61], [62], [63]. In addition, MRE11 nuclease resects the DNA strand at DSBs to generate micro-homology to facilitate the repair process [62], [64], [65]. This repair pathway is considered as a low fidelity repair choice, since abnormal chromosome fusions, loss of genetic material around the breakage sites, and accumulation of genetic mutations could happen following the repair [63]. Indeed, Passegué and her colleagues found that 53BP1 foci (a marker of NHEJ) rather than RAD51 (a marker of homologous recombination, HR) were prominently evident in IR-treated quiescent HSCs [60]. Consequently, chromosome analysis with spectral karyotyping (SKY) on the hematopoietic progenitors derived from IR-irradiated HSCs reveals a great increase in genome instabilities, including chromosome fusions [60]. However, this repair pathway could not effectively explain the accumulation of DNA break marker γ-H2AX in naturally-aged HSCs, because ligation of DNA breaks by NHEJ quenches the DDR signaling and thereby generates γ-H2AX-free HSCs [8], [42]. It has been proposed that quiescence is a cellular status when HSC loses its stringent control of repair machineries [42], [48]. Beerman et al. conducted the in vitro short-term culture of isolated quiescent HSCs [48]. After 24 h, a significant reduction in γ-H2AX-marked DSBs was noticed when the HSCs enter the cell cycle [48], suggesting that proliferating HSCs repair DSB better. G0 HSCs apparently express low levels of DDR genes as compared to proliferating HSCs (such as fetal liver HSCs) and progenitors [48]. In this way, DNA damage signaling may be attenuated in quiescent HSCs, which is consistent with the previous finding on accumulation of DNA breaks in aged HSCs [8], [42].
Once HSCs are mobilized and forced to enter cell cycle by in vivo administration of cytokine granulocyte-colony stimulating factor (G-CSF) or cultured in vitro in the presence of the stem cell factor (SCF), HSCs switch the repair mechanism from NHEJ toward HR [60]. In S/G2/M HSCs, MRN complex recruits ATM and resects the DSBs to generate the single strand overhangs, which can activate ATR/CHK1 kinase [63], [66]. RAD51 is then loaded onto the exposed single strands and forms DNA/protein filaments to initiate strand invasion into their homologous chromosomes [67]. As compared with NHEJ in G0/G1 cell cycle, HR is more stringent in keeping the genomic integrity. However, although HSCs can faithfully repair the DNA breaks when cycling [46], [48], [51], entry into cell cycle could be detrimental to the quality of HSCs because serial transplantation experiments in mice demonstrate that HSCs have limited replicative lifespan and multiple rounds of stress-induced HSC cycling can compromise self-renewal and differentiation capacity, leading to exhaustion of the HSC pool [12], [46].
p53-p21/PUMA pathway in HSC cell fate determination
DNA damages exhaust HSCs in terms of self-renewal and differentiation by reducing the HSC pool (quantity) and compromising HSC stemness (quality) [26], [35], [45]. Upon DNA damage, cells engage a serial of downstream cellular events including cell cycle arrest, apoptosis, and transcriptional reprogramming [43]. Faithful DNA repair preserves the HSC genome integrity and sustains HSC stem cell identity in proper self-renewal and differentiation. However, depending on the repair efficiency for certain DNA lesions, HSCs undertake different fates toward permanent cell cycle arrest (senescence), cell death (HSC elimination) [68], [69], and even differentiation [70], [71], [72], [73]. As the HSC fate determinant, the p53 pathway has been well studied in vivo [74], [75]. The p53 pathway is transiently activated after a single dose of IR or can be constantly activated in HSCs by persistent DNA damages, such as critically shortened telomeres [60], [69], [76]. Downstream of p53 signaling, p53 trans-activates p21 to promote the cell survival by initiating cell cycle arrest for DNA repair or cellular senescence, while induced expression of the p53 upregulated modulator of apoptosis (PUMA) by p53 is responsible for cellular clearance (Figure 2) [13], [68]. Inhibition of either branch of p53-p21 or p53-PUMA benefits HSC self-renewal and maintenance in several cases of HSC ageing mice models [74], [77], [78], [79]. Complete p53 loss renders cytoprotective effects on IR-damaged HSCs [80] and promotes symmetric division of HSCs to expand the HSC pool [74], [81], [82]. However, these p53-null HSCs show defective differentiation and are tumor-prone, indicating that p53 null compromises the HSC quality [74], [78]. In this regard, the balance of p21 and PUMA downstream of p53 signaling is essential for the maintenance of HSCs and hematopoietic system [75], [83].
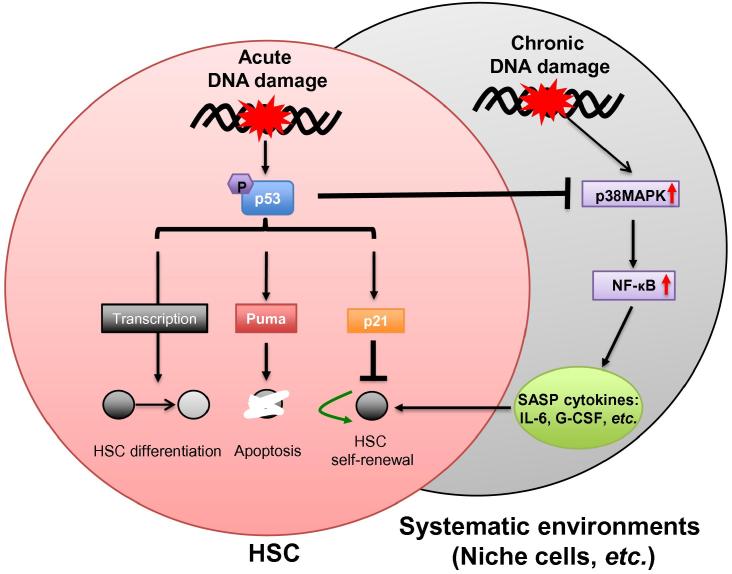
Figure 2.
p53 signaling in HSC fate determination toward ageing
Hematopoietic stem cell fates are determined intrinsically with molecular events insides a HSC and extrinsically by its residing environments, including HSC niche cells and circulating serum factors. p53 signaling plays important roles in HSC fate determination upon DNA damage. After acute DNA damage induction, cell cycle arrest/senescence and cell death pathways medicated by p53-p21 and p53-PUMA determine the HSC pool dynamics. Transcriptional regulation by stabilized p53 exerts additional impact on the HSC transcriptome and leads to HSC differentiation, etc. Furthermore, senescent or heavily-damaged cells secrete pro-inflammatory cytokines (SASP cytokines) to drive HSCs into cycling, which consequently triggers HSC exhaustion. SASP is proposed to be dependent on p38MAPK-NF-κB signaling. In this scenario, p53 signaling plays beneficial roles in inhibiting the SASP by suppressing the p38MAPK activation and subsequent NF-κB transcriptional activation. HSC, hematopoietic stem cell; SASP, senescence-associated secretory phenotype; G-CSF, granulocyte-colony stimulating factor.
Compared to hematopoietic progenitors, murine HSCs are resistant to acute DNA damage induction and prone to survival [60]. This is likely due to a high expression level of the pro-survival genes in murine HSCs [60]. In response to acute DNA damage, HSCs tend to be arrested and reside in the senescent status, suggesting that p53-p21 branch is activated in HSCs preferably to limit HSC self-renewal [77], [84]. Loss of p21 in the mouse model with persistent DNA damage (the 3rd generation of Terc−/− mice; G3 Terc−/−) with critically-shortened telomeres could partially rescue ageing phenotypes by improving the repopulation capacity and self-renewal of HSCs [77]. Furthermore, activated p53-PUMA branch is responsible for the HSC death upon lethal dose of γ-irradiation (10 Gy), since loss of PUMA protects the HSCs and extends the lifespan of irradiated mice [85]. The constitutive activation of p53 signaling in mice lines expressing p53 phosphorylation mutations (T21D and S23D), a C-terminal truncated p53 allele, or other gene mutations confers premature ageing of hematopoietic systems [13], [86], [87]. Genetic ablation of Puma restores the viability of HSCs, indicating p53-PUMA limiting the HSC pool size [85], [86]. These data point to a promising therapeutic strategy to protect HSCs and prolong healthy lifespan with p21 or PUMA inhibitors. However, it is of note that p21-null HSCs exhibit self-renewal defects in serial transplantation assay, while Puma-null HSCs are superior to their wild type controls [86], [88]. These findings indicate that p53-p21 and p53-PUMA have differential roles in mediating HSC fates and ageing due to different extents of DNA lesions (Figure 2).
Persistent DNA damage creates a pro-ageing environment for HSCs
Intrinsic defects in repairing DNA damages and their contributions to compromised HSC self-renewal and ageing process have been extensively studied in recent years. However, ageing environments, such as mis-regulated cytokine factor secretion and altered stem cells niches, can all affect HSC maintenance (Figure 1) [4], [76], [89], [90]. An interesting example of the environmental impact on the HSC self-renewal and differentiation comes from the parabiosis assay by surgical connection between young and aged mice with the circulatory blood system [91], [92]. In this assay, young and aged HSCs are unanimously exposed to a common systematic environment, such as serum factors and osteoblast niches. Multi-organ analysis shows that the interconnection of young and aged mice significantly improves tissue homeostasis including HSCs and hematopoietic system of the aged mice. These data strongly indicate that a young systematic environment can rejuvenate the aged HSCs [91], [92]. Although the search for the key factors in the systematic environment that contribute to the ageing and rejuvenation of stem cells is still ongoing, these findings conceptually prove that the HSCs, in addition to the intrinsic regulation, could be functionally modulated by the environmental cues. On the other hand, parabiosis assay highlights a novel concept that HSC ageing could be delayed or partially reversed by rejuvenating serum factors [69], [91], [92].
Does DNA damage generate a systematic change and promote the HSC ageing? The analysis of HSCs from G3 Terc−/− mice unveils some hints on this question. The critically-shortened telomeres in G3 Terc−/− mice can be recognized as persistent DNA breaks, which constantly activate the p53-p21/PUMA pathway in tissue-specific stem cells and their somatic progenies [77], [89], [93]. Ju et al. analyzed the interplay between HSCs and their niches, i.e., mesenchymal stem cell (MSC)-derived bone marrow stromal cells. In G3 Terc−/− mice, the functionality of both MSCs and bone marrow stromal cells is compromised. In addition, the high level of G-CSF cytokine in the G3 Terc−/− mice serum significantly reduces the engraftment of HSCs in bone marrow niches [76]. G-CSF inhibition leads to the improved engraftment and functionality of HSCs. These findings suggest that a cellular response from those “damaged” cells with persistent DNA lesions caused by telomere shortening could alter the local environment (such as HSC niches) or systematic environment (i.e., cytokines or chemokines in serum) and confer a deleterious effect on self-renewal and maintenance of HSCs [69], [76], [89].
How persistent DNA damage signaling can change the systematic environment? One possibility could be the senescence-associated secretory phenotype (SASP) [94], [95]. Campisi and colleagues found that senescent cells, although permanently arrested in cell cycle, is metabolically active in producing inflammatory factors (SASP cytokines), such as IL-6, IL-10, INF γ, and G-CSF [94]. Furthermore, not only senescent cells, but also cells with persistent DNA damages, exhibit SASP and secrete pro-inflammatory cytokines to change systematic environment in the animal tissues [96]. Activated NF-κB signaling has been implicated in SASP, since inhibition of NF-κB signaling by knocking down of p65 greatly alleviates the expression of SASP cytokines [97], [98], [99]. Furthermore, activation of p38MAPK kinase activity by various stimuli promotes SASP induction. p38MAPK sits upstream of NF-κB signaling and regulates the NF-κB activity. Interestingly, although p53 is not required for initiating SASP, p53 restrains SASP once the cellular senescence is established, since p53-null cells show enhanced expression of SASP cytokines [99]. The inhibitory effects of SASP by p53 could be attributed to the fact that p53 restrains p38MAPK activity via its DDR-independent activity. In this sense, p53 DDR-independent signaling may provide protective roles in maintaining HSC homeostasis by inhibiting SASP in the systematic environment of hematopoietic system (Figure 2).
The secreted inflammatory factors from these cells with persistent DNA damages, may be detrimental to the HSC self-renewal and maintenance. For example, G-CSF mobilizes the HSCs out of bone marrow niches and impairs their engraftment [76], [100]. IL6, INFα, and INF γ have been implicated in promoting the cell cycle entry of HSCs, resulting in HSC exhaustion toward ageing [90], [101]. Tissues in aged animals are enriched in senescent somatic cells that contain persistent DNA damages. Furthermore, ageing process positively correlates with increased inflammatory responses [102], which may further ameliorate the HSC maintenance and promote ageing [103]. Recent studies indicate that interfering with SASP by blocking the cytokine production pathway or eliminating cytokine-producing cells in tissues greatly improves the tissue function and animal lifespan. Clearance of p16Ink4a-positive senescent cells in progeroid BubR1 mutant mice could substantially delay the onset of ageing phenotypes and even rejuvenate the ageing tissues when such cellular clearance was applied in late-life of BubR1 mutants [104]. Furthermore, Chang et al. employed ABT263, a specific chemical inhibitor for Bcl-2 and Bcl-xL, to induce the apoptosis of senescent HSCs. They found that ABT263 treatment restores the functionality of HSCs in the sub-lethally irradiated wild type mice and naturally-aged mice, thus greatly improving the healthy lifespan of mice [105]. The data further confirm the assumption that senescent cells with persistent DNA damage can establish a systematic environment driving HSC ageing.
Conclusions and perspectives
Integrities of HSC pool and HSC quality are considered as the key factors contributing to the organismal ageing in mammals [4], [29]. DDR plays essential roles in the maintenance of these two HSC features [8], [44], [45], [52], [68]. Deficiency in DNA repair results in the accumulation of unrepaired DNA breaks in aged HSCs, while persistent DNA breaks in hematopoietic cells and HSC niches create a pro-inflammatory environment to promote HSC entry into cell cycle and proliferation, which consequently exhausts HSCs [69]. It would be very tricky to experimentally modify those ageing HSCs with genetic approaches in order to achieve the better DNA repair and functional improvement. Instead, using bio-active cytokines to interfere with the systematic ageing environment or using small chemical molecules to specially remove the “aged” HSCs would be the reliable and practical strategies to rejuvenate the ageing HSCs and prolong the healthy lifespan of mammals [105], [106].
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
TL is currently supported by the National Natural Science Foundation of China (Grant No. 81571380), and the Natural Science Foundation of Zhejiang Province – China (Grant No. LY16H080009). ZJ is supported by the National Natural Science Foundation of China (Grant Nos. 81130074, 81420108017, and 81525010). TL and ZJ are both funded by the National Key R&D Plan from the Ministry of Science and Technology of China (Grant No. SQ2016ZY05002341). ZQW is partially supported by the Deutsche Forschungsgemeinschaft (DFG), Germany.
Handled by Xingzhi Xu
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.Weissman I.L. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.He S., Nakada D., Morrison S.J. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 3.Signer R.A., Morrison S.J. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orkin S.H., Zon L.I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikkola H.K., Orkin S.H. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 7.Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi D.J., Bryder D., Seita J., Nussenzweig A., Hoeijmakers J., Weissman I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 9.Beerman I., Bock C., Garrison B.S., Smith Z.D., Gu H., Meissner A. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12:413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Challen G.A., Sun D., Mayle A., Jeong M., Luo M., Rodriguez B. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell. 2014;15:350–364. doi: 10.1016/j.stem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S., Yajima H., Huynh H., Zheng J., Callen E., Chen H.T. Congenital bone marrow failure in DNA-PKcs mutant mice associated with deficiencies in DNA repair. J Cell Biol. 2011;193:295–305. doi: 10.1083/jcb.201009074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janzen V., Forkert R., Fleming H.E., Saito Y., Waring M.T., Dombkowski D.M. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 13.Dumble M., Moore L., Chambers S.M., Geiger H., Van Zant G., Goodell M.A. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J., Astle C.M., Harrison D.E. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 15.Oshima M., Iwama A. Epigenetics of hematopoietic stem cell aging and disease. Int J Hematol. 2014;100:326–334. doi: 10.1007/s12185-014-1647-2. [DOI] [PubMed] [Google Scholar]
- 16.Rossi D.J., Jamieson C.H., Weissman I.L. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Challen G.A., Boles N.C., Chambers S.M., Goodell M.A. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo M., Jeong M., Sun D., Park H.J., Rodriguez B.A., Xia Z. Long non-coding RNAs control hematopoietic stem cell function. Cell Stem Cell. 2015;16:426–438. doi: 10.1016/j.stem.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Zhou J., Liu D., Dong F., Cheng H., Wang W. ATF4 plays a pivotal role in the development of functional hematopoietic stem cells in mouse fetal liver. Blood. 2015;126:2383–2391. doi: 10.1182/blood-2015-03-633354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Diao D., Shi Z., Zhu X., Gao Y., Gao S. SIRT6 controls hematopoietic stem cell homeostasis through epigenetic regulation of Wnt signaling. Cell Stem Cell. 2016;18:495–507. doi: 10.1016/j.stem.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Chu Y., Wang W., Yuan W. MTORC signaling in hematopoiesis. Int J Hematol. 2016;103:510–518. doi: 10.1007/s12185-016-1944-z. [DOI] [PubMed] [Google Scholar]
- 22.Qian P., He X.C., Paulson A., Li Z., Tao F., Perry J.M. The Dlk1-Gtl2 locus preserves LT-HSC function by inhibiting the PI3K-mTOR pathway to restrict mitochondrial metabolism. Cell Stem Cell. 2016;18:214–228. doi: 10.1016/j.stem.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J.Y., Nakada D., Yilmaz O.H., Tothova Z., Joseph N.M., Lim M.S. MTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7:593–605. doi: 10.1016/j.stem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Amours D., Jackson S.P. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- 25.Branzei D., Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 26.Kenyon J., Gerson S.L. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res. 2007;35:7557–7565. doi: 10.1093/nar/gkm1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeijmakers J.H. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 28.Hoeijmakers J.H. Genome maintenance mechanisms are critical for preventing cancer as well as other aging-associated diseases. Mech Ageing Dev. 2007;128:460–462. doi: 10.1016/j.mad.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Geiger H., Denkinger M., Schirmbeck R. Hematopoietic stem cell aging. Curr Opin Immunol. 2014;29:86–92. doi: 10.1016/j.coi.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Pang W.W., Price E.A., Sahoo D., Beerman I., Maloney W.J., Rossi D.J. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roobrouck V.D., Ulloa-Montoya F., Verfaillie C.M. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Kondo M., Wagers A.J., Manz M.G., Prohaska S.S., Scherer D.C., Beilhack G.F. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 33.Kamminga L.M., van Os R., Ausema A., Noach E.J., Weersing E., Dontje B. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23:82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- 34.Sudo K., Ema H., Morita Y., Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi D.J., Bryder D., Weissman I.L. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi D.J., Bryder D., Zahn J.M., Ahlenius H., Sonu R., Wagers A.J. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller-Sieburg C.E., Sieburg H.B., Bernitz J.M., Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012;119:3900–3907. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun D., Luo M., Jeong M., Rodriguez B., Xia Z., Hannah R. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers S.M., Shaw C.A., Gatza C., Fisk C.J., Donehower L.A., Goodell M.A. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinclair D.A., Oberdoerffer P. The ageing epigenome: damaged beyond repair? Ageing Res Rev. 2009;8:189–198. doi: 10.1016/j.arr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi D.J., Seita J., Czechowicz A., Bhattacharya D., Bryder D., Weissman I.L. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6:2371–2376. doi: 10.4161/cc.6.19.4759. [DOI] [PubMed] [Google Scholar]
- 43.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandal P.K., Blanpain C., Rossi D.J. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol. 2011;12:198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- 45.Blanpain C., Mohrin M., Sotiropoulou P.A., Passegue E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Flach J., Bakker S.T., Mohrin M., Conroy P.C., Pietras E.M., Reynaud D. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klaude M., Eriksson S., Nygren J., Ahnstrom G. The comet assay: mechanisms and technical considerations. Mutat Res. 1996;363:89–96. doi: 10.1016/0921-8777(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 48.Beerman I., Seita J., Inlay M.A., Weissman I.L., Rossi D.J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15:37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rube C.E., Fricke A., Widmann T.A., Furst T., Madry H., Pfreundschuh M. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naka K., Muraguchi T., Hoshii T., Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 51.Walter D., Lier A., Geiselhart A., Thalheimer F.B., Huntscha S., Sobotta M.C. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 52.Nijnik A., Woodbine L., Marchetti C., Dawson S., Lambe T., Liu C. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 53.Bender C.F., Sikes M.L., Sullivan R., Huye L.E., Le Beau M.M., Roth D.B. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parmar K., Kim J., Sykes S.M., Shimamura A., Stuckert P., Zhu K. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28:1186–1195. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 56.Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 57.Zhao B., Zhang W.D., Duan Y.L., Lu Y.Q., Cun Y.X., Li C.H. Filia is an ESC-specific regulator of DNA damage response and safeguards genomic stability. Cell Stem Cell. 2015;16:684–698. doi: 10.1016/j.stem.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahuja A.K., Jodkowska K., Teloni F., Bizard A.H., Zellweger R., Herrador R. A short G1 phase imposes constitutive replication stress and fork remodelling in mouse embryonic stem cells. Nat Commun. 2016;7:10660. doi: 10.1038/ncomms10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambrosio S., Di Palo G., Napolitano G., Amente S., Dellino G.I., Faretta M. Cell cycle-dependent resolution of DNA double-strand breaks. Oncotarget. 2016;7:4949–4960. doi: 10.18632/oncotarget.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohrin M., Bourke E., Alexander D., Warr M.R., Barry-Holson K., Le Beau M.M. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruhn C., Zhou Z.W., Ai H., Wang Z.Q. The essential function of the MRN complex in the resolution of endogenous replication intermediates. Cell Rep. 2014;6:182–195. doi: 10.1016/j.celrep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Saidi A., Li T., Weih F., Concannon P., Wang Z.Q. Dual functions of Nbs1 in the repair of DNA breaks and proliferation ensure proper V(D)J recombination and T-cell development. Mol Cell Biol. 2010;30:5572–5581. doi: 10.1128/MCB.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y.G., Saidi A., Frappart P.O., Min W., Barrucand C., Dumon-Jones V. Conditional deletion of Nbs1 in murine cells reveals its role in branching repair pathways of DNA double-strand breaks. EMBO J. 2006;25:5527–5538. doi: 10.1038/sj.emboj.7601411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Symington L.S. vol. 6. Cold Spring Harb Perspect Biol; 2014. End resection at double-strand breaks: mechanism and regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie A., Kwok A., Scully R. Role of mammalian Mre11 in classical and alternative nonhomologous end joining. Nat Struct Mol Biol. 2009;16:814–818. doi: 10.1038/nsmb.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jazayeri A., Falck J., Lukas C., Bartek J., Smith G.C., Lukas J. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 67.Baumann P., West S.C. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 68.Sperka T., Wang J., Rudolph K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 69.Behrens A., van Deursen J.M., Rudolph K.L., Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol. 2014;16:201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss C.N., Ito K. DNA damage: a sensible mediator of the differentiation decision in hematopoietic stem cells and in leukemia. Int J Mol Sci. 2015;16:6183–6201. doi: 10.3390/ijms16036183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inomata K., Aoto T., Binh N.T., Okamoto N., Tanimura S., Wakayama T. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 72.Sherman M.H., Bassing C.H., Teitell M.A. Regulation of cell differentiation by the DNA damage response. Trends Cell Biol. 2011;21:312–319. doi: 10.1016/j.tcb.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santos M.A., Faryabi R.B., Ergen A.V., Day A.M., Malhowski A., Canela A. DNA-damage-induced differentiation of leukaemic cells as an anti-cancer barrier. Nature. 2014;514:107–111. doi: 10.1038/nature13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Elf S.E., Miyata Y., Sashida G., Liu Y., Huang G. P53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y., Elf S.E., Asai T., Miyata Y., Liu Y., Sashida G. The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior. Cell Cycle. 2009;8:3120–3124. doi: 10.4161/cc.8.19.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ju Z., Jiang H., Jaworski M., Rathinam C., Gompf A., Klein C. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 77.Sperka T., Song Z., Morita Y., Nalapareddy K., Guachalla L.M., Lechel A. Puma and p21 represent cooperating checkpoints limiting self-renewal and chromosomal instability of somatic stem cells in response to telomere dysfunction. Nat Cell Biol. 2012;14:73–79. doi: 10.1038/ncb2388. [DOI] [PubMed] [Google Scholar]
- 78.Begus-Nahrmann Y., Lechel A., Obenauf A.C., Nalapareddy K., Peit E., Hoffmann E. P53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet. 2009;41:1138–1143. doi: 10.1038/ng.426. [DOI] [PubMed] [Google Scholar]
- 79.Choudhury A.R., Ju Z., Djojosubroto M.W., Schienke A., Lechel A., Schaetzlein S. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 80.Marusyk A., Porter C.C., Zaberezhnyy V., DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cicalese A., Bonizzi G., Pasi C.E., Faretta M., Ronzoni S., Giulini B. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 82.Insinga A., Cicalese A., Faretta M., Gallo B., Albano L., Ronzoni S. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc Natl Acad Sci U S A. 2013;110:3931–3936. doi: 10.1073/pnas.1213394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonizzi G., Cicalese A., Insinga A., Pelicci P.G. The emerging role of p53 in stem cells. Trends Mol Med. 2012;18:6–12. doi: 10.1016/j.molmed.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y., Schulte B.A., LaRue A.C., Ogawa M., Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu H., Shen H., Yuan Y., XuFeng R., Hu X., Garrison S.P. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu D., Ou L., Clemenson G.D., Jr, Chao C., Lutske M.E., Zambetti G.P. Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol. 2010;12:993–998. doi: 10.1038/ncb2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belle J.I., Langlais D., Petrov J.C., Pardo M., Jones R.G., Gros P. P53 mediates loss of hematopoietic stem cell function and lymphopenia in Mysm1 deficiency. Blood. 2015;125:2344–2348. doi: 10.1182/blood-2014-05-574111. [DOI] [PubMed] [Google Scholar]
- 88.Cheng T., Rodrigues N., Shen H., Yang Y., Dombkowski D., Sykes M. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 89.Song Z., Ju Z., Rudolph K.L. Cell intrinsic and extrinsic mechanisms of stem cell aging depend on telomere status. Exp Gerontol. 2009;44:75–82. doi: 10.1016/j.exger.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Baldridge M.T., King K.Y., Goodell M.A. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 92.Conboy M.J., Conboy I.M., Rando T.A. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schaetzlein S., Kodandaramireddy N.R., Ju Z., Lechel A., Stepczynska A., Lilli D.R. Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell. 2007;130:863–877. doi: 10.1016/j.cell.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodier F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salminen A., Kauppinen A., Kaarniranta K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Chien Y., Scuoppo C., Wang X., Fang X., Balgley B., Bolden J.E. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freund A., Patil C.K., Campisi J. P38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tesio M., Oser G.M., Baccelli I., Blanco-Bose W., Wu H., Gothert J.R. Pten loss in the bone marrow leads to G-CSF-mediated HSC mobilization. J Exp Med. 2013;210:2337–2349. doi: 10.1084/jem.20122768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baldridge M.T., King K.Y., Boles N.C., Weksberg D.C., Goodell M.A. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 103.King K.Y., Goodell M.A. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang J., Wang Y., Shao L., Laberge R.M., Demaria M., Campisi J. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sinha M., Jang Y.C., Oh J., Khong D., Wu E.Y., Manohar R. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]