Abstract
Deficits in the visual processing of faces in autism spectrum disorder (ASD) individuals may be due to atypical brain organization and function. Studies assessing asymmetric brain function in ASD individuals have suggested that facial processing, which is known to be lateralized in neurotypical (NT) individuals, may be less lateralized in ASD. Here we used functional near-infrared spectroscopy (fNIRS) to first test this theory by comparing patterns of lateralized brain activity in homologous temporal-occipital facial processing regions during observation of faces in an ASD group and an NT group. As expected, the ASD participants showed reduced right hemisphere asymmetry for human faces, compared to the NT participants. Based on recent behavioral reports suggesting that robots can facilitate increased verbal interaction over human counterparts in ASD, we also measured responses to faces of robots to determine if these patterns of activation were lateralized in each group. In this exploratory test, both groups showed similar asymmetry patterns for the robot faces. Our findings confirm existing literature suggesting reduced asymmetry for human faces in ASD and provide a preliminary foundation for future testing of how the use of categorically different social stimuli in the clinical setting may be beneficial in this population.
Introduction
Distinct cognitive and behavioral deficits observed in ASD may be attributed to altered patterns of functional hemispheric asymmetry[1]. Less asymmetry is represented by reduced hemisphere dominance and more bilateral recruitment of homologous regions of the cortex [2]. This could indicate inefficient cognitive functioning that may lead to observable deficits in domains like language and facial processing, which are typically lateralized in NTs [3]. Atypical patterns of asymmetry have been identified in ASD, but most of these studies have only investigated asymmetry for language processing, which is generally left lateralized in NT individuals [4]. Imaging studies have shown mixed results, including reduced asymmetry [4,5], reversed patterns of asymmetry [6], and typical asymmetry [7]. However, the theory of reduced asymmetry for hemisphere dominant tasks, such as language and facial processing, most consistently supports and explains the deficits characteristically observed in ASD.
Hemisphere dominance has been theorized to promote efficiency by avoiding duplication of functions in both hemispheres and allowing different categories of information to be processed in parallel [8]. Reduced asymmetry suggests that both hemispheres participate more equally in processing these tasks. As such, performance may suffer; both hemispheres are weakly activated, neither being dominant for the task[9]. In ASD, research has shown volume reductions in regions of the corpus callosum, the structure that connects the hemispheres and impacts the development of lateralization[10]. Alterations to this structure could impact functional asymmetry by interfering with the isolation required for each hemisphere to develop dominance in specific domains[11].
Imaging data has shown greater bilateral activation in ASD, compared to NT groups that demonstrate more left hemisphere activation, for receptive language tasks [5], as well as reduced leftward asymmetry in prefrontal regions for language production tasks [4]. Recent evidence suggests that the development of facial processing and language are jointly impacted [12–17],suggesting that asymmetry for both of these domains may complement each other.
Facial processing deficits in ASD appear in a range of behavioral studies from gender discrimination, recognition [18], emotion identification [19], and memory for faces [20]. Functional imaging has shown normal early visual processing in ASD[21], suggesting that facial processing deficits likely arise from problems with higher-level cognitive functioning, rather than general visual processing. The fusiform face area (FFA), a region in the fusiform gyrus, and the occipital face area (OFA), a region in the occipital cortex, are specific areas that are engaged during facial processing tasks and more active in the right hemisphere in NTs[22]. The results of functional magnetic resonance imaging (fMRI) studies investigating these regions in ASD are not consistent. However, much research suggests that individuals with ASD do not exhibit the same right hemisphere asymmetry as NTs during facial processing, such as having weaker activation in the FFA[23,24] and OFA [25]. Due to the widespread temporal-occipital activity around the FFA and OFA in response to facial stimuli in NTs [26], this broader facial network should be further examined in ASD to more generally identify reduced right hemisphere asymmetry for facial processing.
Although facial processing deficits are well established in ASD[27,28],research has just begun to explore whether these deficits are limited to human faces. Although individuals with high functioning ASD show reduced activation of the facial processing network in response to human faces, they elicit typical activation in this network in response to animal faces[25]. Additionally, research shows that children with ASD have increased verbal utterances towards robots than towards people in the clinical setting [29]. This is further supported by increased speech directed at robots compared to adults [30], suggesting that differential processing for non-human faces can potentially be used to improve socialization in ASD. These findings can be bolstered by assessing indices of how the brain responds to robot stimuli in ASD, and how these patterns may be similar to, or different from, those of NTs.
The novel studies examining interaction between individuals with ASD and interactive robots did not incorporate an NT group for comparison, but, because functional imaging research in NTs has shown comparable FFA activity for cat, cartoon, and human faces [31], we predict that they would also show similar patterns of functional activation for human and robot faces. In contrast, if individuals with ASD do not treat all faces similarly, we should be able to objectively demonstrate this through a comparison of asymmetry for human faces and robot faces in this population. Therefore, assessing lateralization patterns for human versus robot faces in both groups may contribute to an explanation for the increased interactions for robot over human counterparts in ASD.
FNIRS is a useful method for measuring and comparing cortical activation patterns in children and special populations because it allows participants to sit openly in a chair without being confined within a scanner or exposed to a magnetic field and loud noises. This noninvasive imaging technique has been used in individuals with ASD to assess interhemispheric connectivity during the resting state [32], and in prefrontal areas [33]and has suggested reduced connectivity in this population. In this preliminary experiment, we used fNIRS to compare the degree of asymmetry in the temporal-occipital regions in an ASD and an NT group for tasks that required processing of both human and robot faces. Because individuals with ASD show deficits in basic facial processing tasks, our first objective was to test the hypothesis that the ASD group in our sample would show reduced lateralization for human face stimuli relative to the NT group. Second, because individuals with ASD show improved interactions with robot faces, we wished to see whether these stimuli would show lateralization in the ASD group. Comparing patterns of activation for the robot faces in both groups could provide preliminary support for the successful use of robots in a clinical setting and encourage more effective methods of stimulating interaction.
Methods
Participants
Participants in this study included 8 males with a diagnosis of ASD and 12 NT males. All participants were between the ages of 7 and 36 (NT: M = 14.5, SD = 10.76; ASD: M = 15.6, SD = 9.55). To account for the heterogeneous nature of our sample, we ensured that each participant with ASD that was 22 years of age and younger was age matched with at least one NT participant within one year of age. For the one adult participant with ASD who was over 30 years old, we simply ensured we matched him with an NT participant within 10 years of age, since the significant developmental changes that occur early in life do not occur in adulthood. All procedures were conducted in accordance with the University of Nevada, Reno Institutional Review Board, following its approval of this study. Participants, or parents of participants under 18 years of age, signed written consent forms, and all participants provided verbal and non-verbal assent prior to and throughout the testing. Individuals in the ASD group had previously been diagnosed with ASD by a licensed clinical psychologist or doctor, not associated with this research. All participants in the ASD group had also been previously assessed with the Autism Diagnostic Observation Schedule (ADOS) by a clinical psychologist or speech pathologist qualified to administer this assessment. Individuals in the NT group had no previous diagnosis of ASD or a history of a medical disorder known to cause characteristics associated with ASD.
All participants were further assessed using the Wechsler Abbreviated Scale of Intelligence (WASI) to obtain a Full-2 IQ [34] (NT: M = 112.2, SD = 12.45; ASD: M = 95.9, SD = 14.95), the Edinburgh Handedness Inventory [35] to determine a laterality quotient (LQ) (NT: M = 82, SD = 15.21; ASD: M = 61.1, SD = 39.45), and the Gilliam Autism Rating Scale, Second Edition GARS-2[36]to obtain an Autism Index for the ASD group only (M = 89.9, SD = 22.75). Participants or parents of the participants under 18 years of age, filled out information for the Edinburgh Handedness Inventory. They indicated the individual’s hand dominance for 10 different everyday activities so that we could obtain an LQ for each participant. For the GARS-2, parents or caregivers provided current ratings of how often they observed the individual exhibit certain characteristics in the following categories: stereotyped behaviors, communication, and social interaction (Table 1).
Table 1. Participant characteristics.
| Participant Classification | Age | WASI Full-2 IQ | LQ (-100-100) | GARS-2 |
|---|---|---|---|---|
| NT 1 | 9 | 112 | 67 | N/A |
| NT 2 | 10 | 117 | 100 | N/A |
| NT 3 | 12 | 116 | 100 | N/A |
| NT 4 | 7 | 132 | 85 | N/A |
| NT 5 | 9 | 131 | 67 | N/A |
| NT 6 | 14 | 99 | 88 | N/A |
| NT 7 | 7 | 109 | 90 | N/A |
| NT 8 | 13 | 88 | 79 | N/A |
| NT 9 | 7 | 113 | 100 | N/A |
| NT 10 | 18 | 119 | 88 | N/A |
| NT 11 | 23 | 105 | 60 | N/A |
| NT 12 | 45 | 105 | 60 | N/A |
| ASD 1 | 10 | 81 | 100 | 74 |
| ASD 2 | 11 | 124 | 38 | 128 |
| ASD 3 | 9 | 104 | 71 | 59 |
| ASD 4 | 18 | 91 | -11 | 89 |
| ASD 5 | 11 | 83 | 100 | 89 |
| ASD 6 | 36 | 104 | 50 | 109 |
| ASD 7 | 8 | 81 | 100 | 81 |
| ASD 8 | 22 | 99 | 41 | N/A |
Stimuli and Procedure
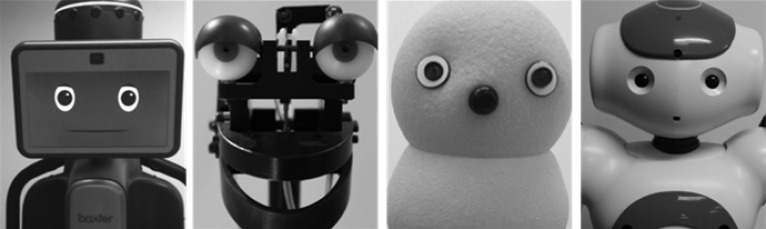
During the experiment, each participant was seated 57 cm away from a 17 inch Dell laptop, and all stimuli were presented using E-prime software [37]. To keep participants engaged in the stimuli, they were asked to do a 1-back task, in which they were instructed to press one key when the current stimulus repeated from the one prior and another key if it was different. The 1-back task was used to keep participants actively involved with the stimuli, rather than passively looking at the screen[38]. Face stimuli in the human task were acquired from a stimuli set used in previous research [39] and included pictures of 5 males and 5 females, without glasses or jewelry, showing neutral expressions. Facial stimuli with neutral expressions were chosen for two important reasons. First, they are more comparable to the emotionally neutral robot faces. More importantly, research suggests that individuals with ASD identify and process emotion differently than NTs [19,40,41]. Thus, emotionally neutral stimuli were used to eliminate the potential fora group difference in emotion identification to be a confounding variable. Face stimuli in the robot task were acquired from the UNR Robotics Research Lab, the Yale Social Robotics Lab, and the USC Interaction Lab and included pictures taken of 10 robots used in laboratory, research, or clinical settings that all had distinguishable heads and facial feature landmarks (Fig 1). All stimuli were grayscale, subtending 8° visual angle horizontally and 10° vertically, similar to the size of a real face viewed from about 100 cm away [42].
Fig 1. Examples of stimuli.
The robot face task consisted of robots with distinct head shapes and key facial feature landmarks. Reprinted from Elaine Short under a CC BY license, with permission from Elaine Short, original copyright 2014.
There were 10 blocks of human faces and 10 blocks of robot faces. Each block consisted of 15 stimuli presented successively. The sequences of presentations per trial that participants viewed for the 1-back task were created as follows: From the set of 10 possible images for the stimuli type, 15 image presentations, and whether or not the image would repeat from the previous, were determined randomly. More specifically, each image was labeled 1–10 and, for the first presentation, an image from 1–10 was randomly generated. For the following image, whether it would repeat was determined randomly. If it was a repeat, the same image would be used for the second position, but if it was not a repeat, an image would be randomly generated again. The process repeated for the 15 presentations per trial. Finally, this entire process was repeated for 10 total blocks. This procedure was performed identically for the human and the robot trials. Each participant received the same sequence of stimuli and trials. Each stimulus remained on the monitor for 1000 ms, with fixed 500 ms interstimulus intervals that were not dependent on key responses. After the last stimulus of each block, there was an 8 second rest period. This resting time gave the hemodynamic response adequate time to return back to baseline [43–45], while still keeping the total experiment time comfortable for the patient population. The order in which the blocks were presented was randomized. The entire experimental session lasted about 12 minutes. Prior to beginning fNIRS recording, each participant ran through 4 randomized blocks (60 stimuli) of the behavioral task once for practice (for results of behavioral task, see S1 Fig).
FNIRS set up
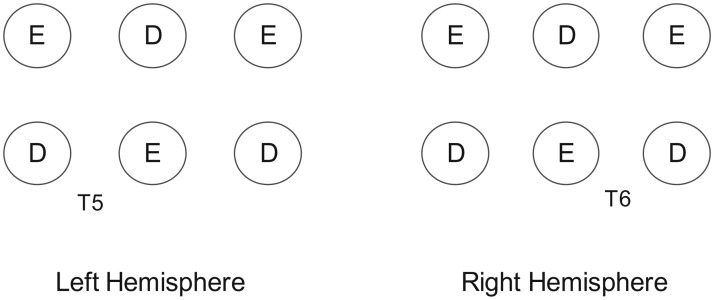
Oxygenated (HbO) and deoxygenated (HbR) hemoglobin levels were measured with a continuous wave fNIRS system (TechEn CW6 fNIRS System, Milford, MA), measuring two wavelengths (690 nm and 830 nm) that were sampled at 50 Hz (20 ms). Each participant’s head was measured using the international 10–20 system as a reference for probe placement [46]. Specifically, channels in the left hemisphere were placed such that one emitter-detector pair was directly over T5, with six other channels placed around it, and channels in the right hemisphere were placed symmetrically, with one directly over T6, for a total of 14 channels (Fig 2). Bilateral temporal areas include T5 and T6, and asymmetry for faces has previously been detected in these regions with fNIRS[47]. The path of light from the emitter to the detector travels in a shallow banana shaped path that only penetrates approximately half the distance between the optodes[48], and it is therefore recommended that the distance between each emitter and detector be at least 2.5 cm. Thus, we set optical channels at 2.6 cm in distance in a 2x3 lattice attached to a custom-made headband to ensure that the configuration was constant within each hemisphere for all participants. Prior to beginning the experiment, the channels were screened for clear respiratory patterns at both wavelengths. If the signal was not clear enough to see the respiratory pattern and cardiac pulsation, we adjusted the setup on the participant’s head. Any channels that remained noisy were noted for later exclusion from data analysis.
Fig 2. Array depicting placement of emitter (E) and detector (D) probes over back of head.
Analysis
Oxygenation concentration changes from baseline from raw signals were obtained using the modified Beer-Lambert approach [49] and analyzed with the HomER2 software package [50]. Raw fNIRS data were pre-processed with a low pass filter of 0.5 Hz to eliminate respiratory noise, heart pulsation, and high frequency noise in the signal. We visually inspected the waveforms for motion artifacts, but none were found, and we did not apply any further motion artifact removal techniques. As HbO has previously been shown to correlate best with blood flow compared to HbR and total hemoglobin values [51], we focused on HbO signals for analysis (for sample raw data, see S2 Fig).
Data was obtained for each 20 ms of recording, which we averaged to get values for each second. To compare relative HbO values across channels and participants, we normalized the raw scores into Z-scores, as this allows data to be averaged regardless of unit[52,53]. We based these scores on the baseline rest period starting from 5 seconds prior to the task period. Thus, each z-score represents the change of the hemodynamic response during the presentation of the faces from baseline. Z-scores were calculated for every channel at each second as follows: (xtask−mbaseline)/s [47]. xtask represents the raw data at each second during the task period, mbaseline represents the mean of the raw data during the last 5 seconds of the baseline period, and s represents the standard deviation of the raw data during baseline.
For each hemisphere, we calculated the average HbO levels across channels at each second with these normalized scores [54]excluding channels that did not meet our predetermined criteria for an acceptable signal, as well as corresponding channels in the opposite hemisphere. This provided average values at each time point for the left and right hemisphere. To account for the rise of the hemodynamic response from resting levels [55,56], we then took the peak HbO level from those averages in the 3 to 10 second time frame after the face stimuli onset. Finally, there were data values representing peak activation for the left and right hemispheres for each participant in both the task for human faces and the task for robot faces. To test our first hypothesis that the ASD group would show less asymmetry than the NT group for human faces, we conducted a 2x2 repeated-measures ANOVA for the human faces condition, with a between-subjects factor of diagnosis (ASD and NT) and a within-subjects factor of hemisphere (left and right). To test our second question of whether the ASD group would show more comparable asymmetry to the NT group, we conducted a 2x2 repeated-measures ANOVA for the robot faces condition, also with a between-subjects factor of diagnosis and a within-subjects factor of hemisphere. We conducted these as two separate analyses because the first analysis served to use our sample to support the preexisting literature, while the second analysis served to test a novel question. The participants’ variables of age, IQ, and LQ were later included as covariates to assess their impact, if any, on the overall findings.
Results
Analyses of the assessments showed that the groups did not significantly differ on age or LQ. There was a significant difference in IQ between groups, but further analysis showed that, of the vocabulary and matrix reasoning subcomponents that made up the IQ score, only vocabulary significantly differed between groups. Although groups are generally matched on IQ to control for general cognitive abilities, the diagnostic profile for ASD suggests that matching groups for performance on tests that utilize language skills may not be appropriate [57]. Language is characteristically aberrant in this population, and lower scores in vocabulary subcomponents of IQ tests are not unusual [57–61]. Therefore, we felt that it was only critical that the groups did not significantly differ on the matrix reasoning task, a measure of abilities that are generally not impaired in ASD [62].
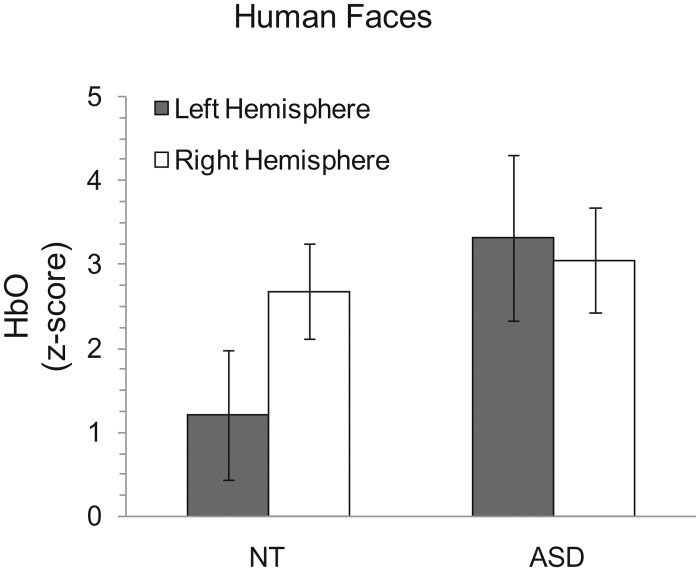
After excluding all the channels due to poor signals, we found that the number of channels excluded in each group did not significantly differ (NT channel exclusions: M = 1.25, SE = .392; ASD channel exclusions: M = 1.5, SE = .378; t(18) = -.437, p = .389). The analysis of the fNIRS data for our investigation of the human faces showed that there was no main effect of hemisphere (F(1, 18) = 3.085, p = .096) or diagnosis (F(1, 18) = 1.476, p = .24). However, the interaction between hemisphere activity and diagnosis was significant(F(1, 18) = 6.12, p = .012) (Fig 3). Post-hoc pairwise comparisons, using Bonferroni correction for multiple comparisons, indicated that the NT group exhibited greater activation in the right hemisphere than the left hemisphere (p = .002), and there was no significant difference between the hemispheres for the ASD group (p = .649). Neither the left nor right hemisphere showed a significant difference between diagnoses (p = .105 and p = .712, respectively).
Fig 3. HbO z-scores for the human faces condition for both groups.
The NT group shows greater right hemisphere lateralization than the ASD group. All error bars represent standard error of the mean.
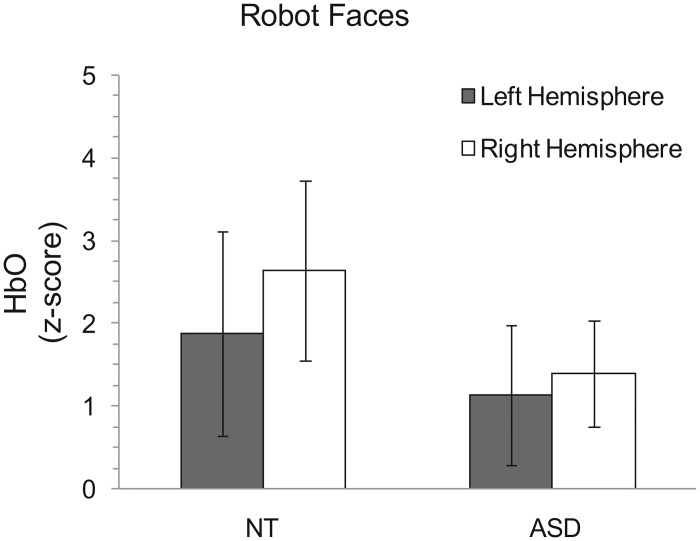
The analysis for our investigation of the robot faces showed that there was no main effect of hemisphere (F(1, 18) = 1.241, p = .28) or diagnosis (F(1, 18) = .444, p = .514). Additionally, there was no interaction between diagnosis and lateralization for robot faces (F(1, 18) = .298, p = .592) (Fig 4).
Fig 4. HbO z-scores for the robotic face condition for both groups.
The groups do not differ in lateralized activation. All error bars represent standard error of the mean.
In each case, these results were upheld after adding age, IQ, and LQ as covariates. Finally, the GARS-2 scores for all ASD participants excluding one, who did not have a caretaker to provide scoring, did not predict left or right hemisphere activation for the ASD group in either the human faces condition (left: r(5) = .685, p = .089; right: r(5) = .346, p = .447) or the robot faces condition (left: r(5) = .116, p = .805; right: r(5) = .552, p = .229). Shapiro-Wilk tests confirmed that the residuals passed normality checks for both the human faces (SW (df = 20) = .971, p = .771) and the robot faces (SW (df = 20) = .924, p = .117).
Discussion
This exploratory research used fNIRS to assess patterns of temporal-occipital brain activation in response to face stimuli in a group of NT individuals compared to a group with ASD. We tested two hypotheses regarding facial processing and asymmetry. First, we wanted to assess whether individuals with ASD elicit less lateralized activation in this facial processing region than NTs during facial processing tasks for typical human faces. Second, we wanted to determine whether lateralized activation patterns were similar between groups when viewing robot faces. As predicted, the NT group showed right hemisphere lateralization for the human faces, but the ASD group did not (Fig 3). Remarkably, however, brain activation patterns for the ASD group did not differ from the NT group for the robot faces (Fig 4). Taken together, these novel findings encourage future investigations for attributing characteristic behaviors in ASD to atypical functional asymmetry.
Previous reports suggesting functional alterations to asymmetry in ASD subjects in response to facial stimuli are currently limited. Here, we sought to bolster these studies and enhance the current understanding of abnormal asymmetry in this population. Reports of posterior corpus callosum structural abnormalities in ASD [63,64] are supported by these findings of reduced functional asymmetry for human faces, as deficits in this subsection could have inhibited development of asymmetry in face processing regions. Additionally, this finding complements the existing reports of reduced asymmetry for word processing in homologous regions because these processes generally arise in symmetrical regions of the cortex [65]. The second main finding that the ASD group's pattern of activation for the robot faces did not differ from the NTs, which suggests that the ASD group may be processing this category of faces similarly to NTs. Therefore, abnormal asymmetry is not globally observed in ASD. If the behavioral studies that showed individuals with ASD interact more with robots than humans [29]also tested an NT group, they may find that the interactions towards robots were comparable between groups, providing behavioral support for the data presented here.
Based on research that suggests NTs generalize across different categories of faces[31], this may not hold true in ASD. These individuals seem to process the robot faces similar to the way NTs do, with regards to lateralization. One possible explanation is that individuals with ASD exhibit more lateralized activity for objects and are processing robots more as objects, rather than as people. It has been suggested that they have greater social interest in object-like stimuli than human-like stimuli and that this interest in objects increases as they get older, so that they have even greater attraction to objects than NT children [66]. Additionally, research has supported typical, or even superior, object processing for individuals with ASD, compared to NTs [67,68]. For example, the N290, an event related potential (ERP) component that has shown face sensitivity, has faster responses to faces than objects in NTs, but faster responses to objects than faces in individuals with ASD [69].
Because we only assessed asymmetry for human and robot faces, it is difficult to reach the conclusion that the ASD participants were processing the robot faces like objects. A future study should incorporate an additional assessment of asymmetry in both groups for object stimuli that are complex enough to also be processed holistically or by their features, such as houses. This would help provide more insight as to the reason why individuals with ASD process robot faces differently than human faces. Additionally, longer rest periods can be incorporated. Although literature suggests that 8 seconds is adequate for the hemodynamic signal to return to baseline [43–45], other research suggests that a longer period of 12–15 seconds is ideal for allowing the signal to fully return to baseline [70]. Another limitation is the relatively small sample size. This exploratory study was intended to shed light on recent observations in the clinical setting with a more objective and theoretical framework. However, these findings warrant and encourage additional studies with larger sample sizes to further pursue these questions and validate the main findings and covariate analyses. The heterogeneous age sample we used could have influenced the results, as age has been shown to impact the degree of lateralization in NTs [2,71]. However, it is not clear exactly when lateralization progresses, or whether these changes are strictly due to maturational change [72]. Thus, we decided to incorporate a wider age range to help reduce any biases. Additionally, we believed that including both children and adults better represented the full breadth of developmental and learned changes that occur in both populations.
The results from this experiment lend support to the hypothesis that individuals with ASD show reduced functional lateralization while processing human faces. Similar patterns of asymmetrical activation in both groups when viewing robot faces suggests that facial processing may not be abnormal in ASD for all categories of face stimuli. This research can potentially be used to explain and determine cognitive performance in individuals with ASD, based on the patterns of abnormal functional asymmetry. Future studies can build on these results by assessing asymmetry for other lateralized domains that are associated with functions impacted in ASD.
Supporting Information
No significant main effect of condition (F(1, 18) = 1.088, p = .311) or diagnosis (F(1, 18) = 2.335, p = .144). No significant interaction (F(1, 18) = .743, p = .4). All error bars represent standard error of the mean.
(TIF)
FNIRS data from sample NT participant that includes HbO and HbR time course from each channel.
(TIF)
(DOCX)
(XLSX)
Acknowledgments
We would like to thank Dr. Debra Vigil, Dr. Jaclyn Stephens, Dr. Filiz Gözenman, Rafal Skiba, Dr. Kevin Jones, Dr. Dexter Jung, Kenneth Burleigh, Brenda Juarez, Emily Chau, Jennifer Geddes, and Drew Foglesong for their contributions to this project. During the early, troubleshooting stages of this project, we sought help from several NIRS researchers who graciously shared their experiences and advice using this technology, and we would specifically like to thank Dr. Luca Pollonini for his words of wisdom. With the help of the UNR Robotics Research Lab (Jessie Smith), the Yale Social Robotics Lab (Brian Scassellati and Henny Admoni), and the USC Interaction Lab (Maja Matarić and Elaine Short), we were able to obtain the robot images. We greatly appreciate the support and commitment from the families and participants of this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by The Integrative Neuroscience Center of Biomedical Research Excellence award, funded by National Institute of General Medical Sciences of the National Institutes of Health (P20 GM103650). CJ was awarded the support for participant compensation.
References
- 1.Lo YC, Soong WT, Gau SS, Wu YY, Lai MC, Yeh FC, et al. (2011) The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: a study using diffusion spectrum imaging tractography. Psychiatry Res 192: 60–66. 10.1016/j.pscychresns.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 2.Bergerbest D, Gabrieli JD, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, et al. (2009) Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. Neuroimage 45: 237–246. 10.1016/j.neuroimage.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossion B, Joyce CA, Cottrell GW, Tarr MJ (2003) Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage 20: 1609–1624. [DOI] [PubMed] [Google Scholar]
- 4.Kleinhans NM, Muller RA, Cohen DN, Courchesne E (2008) Atypical functional lateralization of language in autism spectrum disorders. Brain Res 1221: 115–125. 10.1016/j.brainres.2008.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, et al. (2010) Decreased left posterior insular activity during auditory language in autism. AJNR Am J Neuroradiol 31: 131–139. 10.3174/ajnr.A1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flagg EJ, Cardy JE, Roberts W, Roberts TP (2005) Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci Lett 386: 82–87. [DOI] [PubMed] [Google Scholar]
- 7.Knaus TA, Tager-Flusberg H, Mock J, Dauterive R, Foundas AL (2012) Prefrontal and occipital asymmetry and volume in boys with autism spectrum disorder. Cogn Behav Neurol 25: 186–194. 10.1097/WNN.0b013e318280e154 [DOI] [PubMed] [Google Scholar]
- 8.Deacon TW (1998) The symbolic species: The co-evolution of language and the brain.: WW Norton & Company. [Google Scholar]
- 9.Renteria ME (2012) Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet 15: 401–413. 10.1017/thg.2012.13 [DOI] [PubMed] [Google Scholar]
- 10.Frazier TW, Hardan AY (2009) A meta-analysis of the corpus callosum in autism. Biol Psychiatry 66: 935–941. 10.1016/j.biopsych.2009.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jancke L, Wunderlich G, Schlaug G, Steinmetz H (1997) A case of callosal agenesis with strong anatomical and functional asymmetries. Neuropsychologia 35: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 12.Behrmann M, Plaut DC (2014) Bilateral hemispheric processing of words and faces: evidence from word impairments in prosopagnosia and face impairments in pure alexia. Cereb Cortex 24: 1102–1118. 10.1093/cercor/bhs390 [DOI] [PubMed] [Google Scholar]
- 13.Behrmann M, Plaut DC (2013) Distributed circuits, not circumscribed centers, mediate visual recognition. Trends Cogn Sci 17: 210–219. 10.1016/j.tics.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Plaut DC, Behrmann M (2011) Complementary neural representations for faces and words: a computational exploration. Cogn Neuropsychol 28: 251–275. 10.1080/02643294.2011.609812 [DOI] [PubMed] [Google Scholar]
- 15.Cantlon JF, Pinel P, Dehaene S, Pelphrey KA (2011) Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex 21: 191–199. 10.1093/cercor/bhq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, et al. (2010) How learning to read changes the cortical networks for vision and language. Science 330: 1359–1364. 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- 17.De Winter FL, Zhu Q, Van den Stock J, Nelissen K, Peeters R, de Gelder B, et al. (2015) Lateralization for dynamic facial expressions in human superior temporal sulcus. Neuroimage 106: 340–352. 10.1016/j.neuroimage.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 18.Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, et al. (2006) Configural processing in autism and its relationship to face processing. Neuropsychologia 44: 110–129. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys K, Minshew N, Leonard GL, Behrmann M (2007) A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia 45: 685–695. [DOI] [PubMed] [Google Scholar]
- 20.Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C (1998) Memory for faces in children with autism. Child Neuropsychology 4: 187–198. [Google Scholar]
- 21.Hadjikhani N, Chabris CF, Joseph RM, Clark J, McGrath L, Aharon I, et al. (2004) Early visual cortex organization in autism: an fMRI study. Neuroreport 15: 267–270. [DOI] [PubMed] [Google Scholar]
- 22.Rossion B, Schiltz C, Crommelinck M (2003) The functionally defined right occipital and fusiform "face areas" discriminate novel from visually familiar faces. Neuroimage 19: 877–883. [DOI] [PubMed] [Google Scholar]
- 23.Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY (2004) Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 43: 481–490. [DOI] [PubMed] [Google Scholar]
- 24.Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. (2005) fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia 43: 373–385. [DOI] [PubMed] [Google Scholar]
- 25.Whyte EM, Behrmann M, Minshew NJ, Garcia NV, Scherf KS (2015) Animal, but not human, faces engage the distributed face network in adolescents with autism. Dev Sci. [DOI] [PubMed] [Google Scholar]
- 26.Pierce K, Haist F, Sedaghat F, Courchesne E (2004) The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain 127: 2703–2716. [DOI] [PubMed] [Google Scholar]
- 27.Dawson G, Webb SJ, McPartland J (2005) Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol 27: 403–424. [DOI] [PubMed] [Google Scholar]
- 28.Weigelt S, Koldewyn K, Kanwisher N (2012) Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev 36: 1060–1084. 10.1016/j.neubiorev.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 29.Scassellati B, Admoni H, Mataric M (2012) Robots for use in autism research. Annu Rev Biomed Eng 14: 275–294. 10.1146/annurev-bioeng-071811-150036 [DOI] [PubMed] [Google Scholar]
- 30.Kim ES, Berkovits LD, Bernier EP, Leyzberg D, Shic F, Paul R, et al. (2013) Social robots as embedded reinforcers of social behavior in children with autism. J Autism Dev Disord 43: 1038–1049. 10.1007/s10803-012-1645-2 [DOI] [PubMed] [Google Scholar]
- 31.Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N (2000) Response properties of the human fusiform face area. Cogn Neuropsychol 17: 257–280. 10.1080/026432900380607 [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Fan Y, Guo H, Huang D, He S (2014) Reduced interhemispheric functional connectivity of children with autism spectrum disorder: evidence from functional near infrared spectroscopy studies. Biomed Opt Express 5: 1262–1274. 10.1364/BOE.5.001262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narita N, Saotome A, Higuchi H, Narita M, Tazoe M, Sakatani K (2012) Impaired prefrontal cortical response by switching stimuli in autism spectrum disorders. J Pediatr Neurol 10: 087–094. [Google Scholar]
- 34.Wechsler D (1999) Wechsler abbreviated scale of intelligence. New York, NY. [Google Scholar]
- 35.Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 36.Gilliam JE (2006) Gilliam autism rating scale.: Pro-ed.
- 37.(1999) E-prime. In: Psychology Software Tools I, editor.
- 38.Wang X, Yang J, Shu H, Zevin JD (2011) Left fusiform BOLD responses are inversely related to word-likeness in a one-back task. Neuroimage 55: 1346–1356. 10.1016/j.neuroimage.2010.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith FW, Schyns PG (2009) Smile through your fear and sadness: transmitting and identifying facial expression signals over a range of viewing distances. Psychol Sci 20: 1202–1208. 10.1111/j.1467-9280.2009.02427.x [DOI] [PubMed] [Google Scholar]
- 40.Baron-Cohen S, Spitz A, Cross P (1993) Do children with autism recognise surprise? A research note. Cognition & Emotion 7: 507–516. [Google Scholar]
- 41.Hadjikhani N, Joseph RM, Manoach DS, Naik P, Snyder J, Dominick K, et al. (2009) Body expressions of emotion do not trigger fear contagion in autism spectrum disorder. Soc Cogn Affect Neurosci 4: 70–78. 10.1093/scan/nsn038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiao JH, Cottrell G (2008) Two fixations suffice in face recognition. Psychol Sci 19: 998–1006. 10.1111/j.1467-9280.2008.02191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser V, Bauernfeind G, Kreilinger A, Kaufmann T, Kubler A, Neuper C, et al. (2014) Cortical effects of user training in a motor imagery based brain-computer interface measured by fNIRS and EEG. Neuroimage 85 Pt 1: 432–444. 10.1016/j.neuroimage.2013.04.097 [DOI] [PubMed] [Google Scholar]
- 44.Leff D, Koh PH, Aggarwal R, Leong J, Deligianni F, Elwell C, et al. (2006) Optical mapping of the frontal cortex during a surgical knot-tying task, a feasibility study Medical imaging and augmented reality: Springer; pp. 140–147. [Google Scholar]
- 45.Malonek D, Grinvald A (1996) Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272: 551–554. [DOI] [PubMed] [Google Scholar]
- 46.Klem GH, Luders HO, Jasper HH, Elger C (1999) The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52: 3–6. [PubMed] [Google Scholar]
- 47.Ichikawa H, Kitazono J, Nagata K, Manda A, Shimamura K, Sakuta R, et al. (2014) Novel method to classify hemodynamic response obtained using multi-channel fNIRS measurements into two groups: exploring the combinations of channels. Front Hum Neurosci 8: 480 10.3389/fnhum.2014.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellicer A, del Carmen Bravo M. Near-infrared spectroscopy: a methodology-focused review; 2011. Elsevier; pp. 42–49. [DOI] [PubMed] [Google Scholar]
- 49.Chance B, Anday E, Nioka S, Zhou S, Hong L, Worden K, et al. (1998) A novel method for fast imaging of brain function, non-invasively, with light. Opt Express 2: 411–423. [DOI] [PubMed] [Google Scholar]
- 50.Huppert TJ, Diamond SG, Franceschini MA, Boas DA (2009) HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt 48: D280–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshi Y, Kobayashi N, Tamura M (2001) Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol (1985) 90: 1657–1662. [DOI] [PubMed] [Google Scholar]
- 52.Schroeter ML, Zysset S, Kruggel F, von Cramon DY (2003) Age dependency of the hemodynamic response as measured by functional near-infrared spectroscopy. Neuroimage 19: 555–564. [DOI] [PubMed] [Google Scholar]
- 53.Shimada S, Hiraki K (2006) Infant's brain responses to live and televised action. Neuroimage 32: 930–939. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Sun J, Sun B, Luo Q, Gong H (2014) Studying hemispheric lateralization during a Stroop task through near-infrared spectroscopy-based connectivity. J Biomed Opt 19: 57012 10.1117/1.JBO.19.5.057012 [DOI] [PubMed] [Google Scholar]
- 55.Ichikawa H, Kanazawa S, Yamaguchi MK, Kakigi R (2010) Infant brain activity while viewing facial movement of point-light displays as measured by near-infrared spectroscopy (NIRS). Neurosci Lett 482: 90–94. 10.1016/j.neulet.2010.06.086 [DOI] [PubMed] [Google Scholar]
- 56.Nakato E, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R (2011) Distinct differences in the pattern of hemodynamic response to happy and angry facial expressions in infants—a near-infrared spectroscopic study. Neuroimage 54: 1600–1606. 10.1016/j.neuroimage.2010.09.021 [DOI] [PubMed] [Google Scholar]
- 57.Lincoln AJ, Courchesne E, Kilman BA, Elmasian R, Allen M (1988) A study of intellectual abilities in high-functioning people with autism. J Autism Dev Disord 18: 505–524. [DOI] [PubMed] [Google Scholar]
- 58.Allen MH, Lincoln AJ, Kaufman AS (1991) Sequential and simultaneous processing abilities of high-functioning autistic and language-impaired children. J Autism Dev Disord 21: 483–502. [DOI] [PubMed] [Google Scholar]
- 59.Narita T, Koga Y (1987) Neuropsychological assessment of childhood autism.
- 60.Rumsey JM, Hamburger SD (1988) Neuropsychological findings in high-functioning men with infantile autism, residual state. J Clin Exp Neuropsychol 10: 201–221. [DOI] [PubMed] [Google Scholar]
- 61.Venter A, Lord C, Schopler E (1992) A follow-up study of high-functioning autistic children. J Child Psychol Psychiatry 33: 489–507. [DOI] [PubMed] [Google Scholar]
- 62.Allen G, Courchesne E (2003) Differential Effects of Developmental Cerebellar Abnormality on Cognitive and Motor Functions in the Cerebellum: An fMRI Study of Autism. American Journal of Psychiatry 160: 262–273. [DOI] [PubMed] [Google Scholar]
- 63.Chung MK, Dalton KM, Alexander AL, Davidson RJ (2004) Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage 23: 242–251. [DOI] [PubMed] [Google Scholar]
- 64.Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, et al. (2006) Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry 60: 218–225. [DOI] [PubMed] [Google Scholar]
- 65.Dundas EM, Plaut DC, Behrmann M (2013) The joint development of hemispheric lateralization for words and faces. J Exp Psychol Gen 142: 348–358. 10.1037/a0029503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maestro S, Muratori F, Cavallaro MC, Pecini C, Cesari A, Paziente A, et al. (2005) How young children treat objects and people: an empirical study of the first year of life in autism. Child Psychiatry Hum Dev 35: 383–396. [DOI] [PubMed] [Google Scholar]
- 67.Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ (2008) Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism 12: 457–472. 10.1177/1362361308096402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, et al. (1998) The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. J Child Psychol Psychiatry 39: 747–753. [PubMed] [Google Scholar]
- 69.Webb SJ, Dawson G, Bernier R, Panagiotides H (2006) ERP evidence of atypical face processing in young children with autism. J Autism Dev Disord 36: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plichta M, Heinzel S, Ehlis A-C, Pauli P, Fallgatter A (2007) Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: a parametric validation study. Neuroimage 35: 625–634. [DOI] [PubMed] [Google Scholar]
- 71.Lohmann H, Drager B, Muller-Ehrenberg S, Deppe M, Knecht S (2005) Language lateralization in young children assessed by functional transcranial Doppler sonography. Neuroimage 24: 780–790. [DOI] [PubMed] [Google Scholar]
- 72.Groen MA, Whitehouse AJ, Badcock NA, Bishop DV (2012) Does cerebral lateralization develop? A study using functional transcranial Doppler ultrasound assessing lateralization for language production and visuospatial memory. Brain Behav 2: 256–269. 10.1002/brb3.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No significant main effect of condition (F(1, 18) = 1.088, p = .311) or diagnosis (F(1, 18) = 2.335, p = .144). No significant interaction (F(1, 18) = .743, p = .4). All error bars represent standard error of the mean.
(TIF)
FNIRS data from sample NT participant that includes HbO and HbR time course from each channel.
(TIF)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.