Abstract
Background
Rickettsia africae, the etiological agent of African tick bite fever, is widely distributed in sub-Saharan Africa. Contrary to reports of its homogeneity, a localized study in Asembo, Kenya recently reported high genetic diversity. The present study aims to elucidate the extent of this heterogeneity by examining archived Rickettsia africae DNA samples collected from different eco-regions of Kenya.
Methods
To evaluate their phylogenetic relationships, archived genomic DNA obtained from 57 ticks a priori identified to contain R. africae by comparison to ompA, ompB and gltA genes was used to amplify five rickettsial genes i.e. gltA, ompA, ompB, 17kDa and sca4. The resulting amplicons were sequenced. Translated amino acid alignments were used to guide the nucleotide alignments. Single gene and concatenated alignments were used to infer phylogenetic relationships.
Results
Out of the 57 DNA samples, three were determined to be R. aeschlimanii and not R. africae. One sample turned out to be a novel rickettsiae and an interim name of “Candidatus Rickettsia moyalensis” is proposed. The bonafide R. africae formed two distinct clades. Clade I contained 9% of the samples and branched with the validated R. africae str ESF-5, while clade II (two samples) formed a distinct sub-lineage.
Conclusions
This data supports the use of multiple genes for phylogenetic inferences. It is determined that, despite its recent emergence, the R. africae lineage is diverse. This data also provides evidence of a novel Rickettsia species, Candidatus Rickettsia moyalensis.
Author Summary
Rickettsia africae is a bacterium mainly vectored by Amblyomma and Rhipicephalus species of ticks. It is the etiological agent of African tick bite fever (ATBF), a spotted fever rickettsiosis that presents as an acute febrile illness characterized by petecheal skin hemorrhages, from which the name is derived. This bacterium is probably the most important in sub-Saharan Africa, including Kenya, in terms of incidence and prevalence. This notwithstanding, the disease is poorly understood and is often mistreated as malaria, and therefore qualifies as a highly neglected disease. This study examined the genetic relationships of R. africae collected from diverse eco-regions of Kenya. We present data that indicate high genetic diversity in Kenya’s R. africae and corroborate a recent study that reported similar genetic diversity in R. africae samples collected from a localized area in western Kenya. Importantly, we describe a divergent lineage and propose the name Candidatus Rickettsia moyalensis.
Introduction
Rickettsiae are obligate intracellular gram negative bacteria, belonging to the class alpha-proteobacteria. They are found in a diverse array of hosts ranging from vertebrates, arthropods, annelids, amoeba and plants. Based on a host perspective, the non-vertebrate-associated Rickettsia remain understudied and poorly characterized [1]. In contrast, the vertebrate-associated Rickettsia that are vectored by hematophagous arthropods such as ticks, fleas, lice and mites are better studied, are responsible for rickettsial diseases that are important cause of illness and death worldwide [2]. To date, this genus consists of 29 validated species and numerous partially characterized species [3,4], thus illustrating the difficulties of unravelling the composition of this seemingly homogeneous group of bacteria.
Phenotypic characters such as pathogenicity, growth temperature requirements, ability to polymerize host cell actin, and cross reactivity to somatic antigens of Proteus vulgaris strains (OX19 and OX2) and P. mirabilis OXK have been used to infer evolutionary relationships amongst rickettsiae [5]. From these criteria, the genus rickettsia was organized into three bio-types, namely, spotted fever group (SFG), typhus group (TG) and scrub typhus group (STG). The phenotypic characters have been found to be unreliable estimators of their phylogeny [6]. The advent of molecular tools brought major reorganizations in rickettsia taxonomy. For instance, by analysis of Rickettsia 16S rRNA (rrs), STG was removed from the genus Rickettsia and placed into its own genus, Orientia. This genus currently has only two species, O. tsutsugamushi [7] and a recently described O. chuto [8].
Several genes have been used for Rickettsia phylogenetic systematics: the rrs [5], gltA [9], 17kDa [10], ompA [11], ompB [12], sca4 [13], sca2 [14], and more recently, complete genomic sequences [15,16]. Currently, the genus delineates into four clades [17–19]: (i) The SFG which is the most derived, and consists of 23 validated species including R. africae, and numerous partially characterized species. Using whole genome approach, it has been realized that some of the members of rickettsiae such as R. helvetica do not fit in the SFG [16,20]). (ii) The transitional group (TRG) whose members are R. akari and R. felis. (iii) The TG which has only two members, namely, R. typhi and R. prowazekii; and (iv), the ancestral group (AG), whose members consists of R. bellii and R. canadensis. The use of the name TRG has been challenged [15,21]). In addition to the systemic of vertebrate-associated Rickettsia, clades associated with non-vertebrate Rickettsia have been described [16,19].
Of the rickettsiae, R. africae is probably the most important in Africa. It is the aetiological agent of African tick bite fever [22] and the most reported [23]. It has been reported in 22 sub-Saharan countries [3], the West Indies [24] and Oceania [25]. In Kenya, a recent surveillance study of rickettsiae in ticks identified 104 rickettsiae, of which 93% were R. africae, clearly, demonstrating its dominance [26]. R. africae is vectored by Amblyomma ticks, primarily A. variegatum and A. hebraeum. Infections have also been detected in many other ticks species [3,26–28] by PCR and not by competency studies.
In general, Rickettsia species are very closely related. Within the SFG where R. africae belongs, the mean nucleotide homogeneity with rrs, gltA, ompA, ompB and sca4 genes ranges from 82.2% to 99.8% [29]. Considering this high interspecies homogeneity, intraspecies differences are even smaller. For example, using the more variable multi spacer typing that combined dksA-xerC, mppA-purC, and rpmE-tRNAfMet spacer sequences, it was impossible to discriminate R. africae strains [30]. Using ompA and ompB genes, many groups have however reported heterogeneity [25,31,32]. A recent study reported an even higher heterogeneity of R. africae samples collected from a localized area in Western Kenya [33]. The study reported here sought to determine how widespread the heterogeneity of Kenya’s R. africae is by examining DNA samples collected from different eco-regions of Kenya.
Methods
Ethics statement
This study was carried out using ticks collected from domestic animals presented for slaughter. The tick samples were collected under protocol SSC#1248 that was reviewed and approved by the Animal Use Committee of the Kenya Medical Research Institute.
Sample acquisition and study site
Details of the areas the tick were collected from, method of collection, DNA extraction and preliminary genotyping have been published before [26] and are summarized in S1 Table.
Amplification of target genes
Sequence data for 57 tick-extracted DNA samples that had been identified as R. africae by comparison to 385 bp citrate synthase (gltA) gene, 530 bp outer membrane protein A gene (ompA) and 444 bp outer membrane protein B gene (ompB) were obtained from our laboratory's database from a previous study [26]. The sequences of both strands were re-checked for correctness and errors cleaned. Samples with short or missing sequences were re-amplified and re-sequenced. Two additional genes: the 450 bp 17kDa and 2700 bp sca4 genes were also amplified and sequenced.
Primers used to amplify target genes are listed in Table 1 and are previously described [34]. PCR reagents were obtained from Applied Biosystems (CA, USA) and reactions performed in a 25 μL reaction volume containing 10 μM of each primer, 200 μM of dNTP mix, 1.5U Taq polymerase and 2 mM MgCl2. Amplification was carried out in a DNA thermal cycler (HID Veriti) from Applied Biosystems (CA, USA). The following conditions were used for amplification: For ompA and gltA genes: 3 min of initial denaturation at 94°C, then 40 cycles at 94°C for 30 sec, 53°C for 30 sec, 68°C for 1 min. For the ompB gene: 3 min of initial denaturation at 95°C, then 40 cycles at 95°C for 30 sec, 50°C for 30 sec, 68°C for 1 min 30 sec. For the 17kDa gene: 5 min of initial denaturation at 94°C, then 35 cycles at 94°C for 30 sec, 50°C for 1 min, 68°C for 1 min. For sca4: 5 min of initial denaturation at 95°C, then 40 cycles at 95°C for 45 sec, 60°C for 30 sec and 68°C for 3 min. All the amplifications were then completed by holding for 7 min at 72°C. To ascertain correct product sizes, a portion of the amplicons (5μL) was run on a 1% (w/v) agarose gel containing ethidium bromide. Product sizes were estimated by comparing with a molecular mass standard (1kb plus ladder, Invitrogen, (CA, USA).
Table 1. Primers used for PCR and sequencing.
| Gene | Oligo Sequence (5'-3') | Amplicon size |
|---|---|---|
| gltA | 1,2CS49Fw: ACCTATACTTAAAGCAAGTATYGGT | 385bp |
| 1CS1234Rv: TCTAGGTCTGCTGATTTTTTGTTCA | ||
| 2CS1258Rv: ATTGCAAAAAGTACAGTGAACA | ||
| ompB | 1,2RAK1009Fw: ACATKGTTATACARAGTGYTAATGC | 444bp |
| 1OmpB1902Rv: CCGTCATTTCCAATAACTAACTC | ||
| 2RAK1452Rv: SGTTAACTTKACCGYTTATAACTGT | ||
| ompA | 1OmpAM50Fw: TTGCGTTATAACACTTTTTAAGTGA | 530bp |
| 1OmpA642Rv: ATTACCTATTGTTCCGTTAATGGCA | ||
| 2190-70Fw: ATGGCGAATATTTCTCCAAAA | ||
| 2190-701Rv: GTTCCGTTAATGGCAGCATCT | ||
| sca4 | 1D1Fw: ATGAGTAAAGACGGTAACCT | 2700bp |
| 1D3069Rv: TCAGCGTTGTGGAGGGGAAG | ||
| 2RrD749Fw: TGGTAGCATTAAAAGCTG | ||
| 2RrD2685Rv: TTCAGTAGAAGATTTAGT | ||
| 17kDa | 1Rp17kFw: AATGAGTTTTATACTTTACAAAATTCTAAAAACCA | 450bp |
| 1,2Rr2608Rv: CATTGTTCGTCAGGTTGGCG | ||
| 2Rr1175Fw: GCTCTTGCAACTTCTATGTT |
Fw = Foward primer; Rv = Reverse primer;
1 = primary PCR;
2 = secondary PCR and sequencing
Gene sequencing
The PCR products were purified using Isolate II PCR and Gel Kit (Bioline, UK) as recommended by the manufacturer. The purified PCR products were sequenced in both directions using the Big Dye Terminator Cycle Sequencing Kit v 3.1 (Applied Biosystems, CA, USA) and the sequences analyzed by capillary electrophoresis in a 3130 Genetic Analyzer (Applied Biosystems). The sequences were proofread, edited and assembled into consensus sequences using CLC Main Workbench v 7 (CLC Inc, Aarhus, Denmark), and used to query GenBank using the nucleotide Basic Local Alignment Search Tool (BLAST) [35].
Phylogenetic data analysis
Six different alignments were generated: Five of them corresponded to sequences of each target gene (gltA, ompA, ompB, 17kDa and sca4), and one corresponding to the concatenated sequence of all the five genes as well as the validated rickettsia strains derived from GenBank (see S2 Table for names of reference strains and their accession numbers). To ensure the accuracy of these alignments, nucleotide sequences were translated to their respective amino acids using the translate tools in the CLC Main Workbench v7. Amino acid alignments were made using Muscle v 3.8 software [36]. The protein alignments were then used to guide the corresponding nucleotide alignments using TOPALi V2 software [37].
For phylogenetic inference, MEGA v7 software [38] was used to estimate the best substitution model as well as estimate for the Maximum Likelihood (ML) trees for the individual genes. The concatenated alignment tree was also estimated with the ML method using a General Time Reversal (GTR) nucleotide substitution model with a gamma distribution (GTR+G). For bayesian probability analysis, jModeltest v 2.1 [39] was used to determine the GTR+G as the best fit model for gltA, ompA, ompB, 17kDa and sca4 gene alignments. A partitioned analysis was then performed on the concatenated dataset using a bayesian Markov Chain Monte Carlo (MCMC) method implemented with MrBayes software v 3.2 [40]. The cluster confidence was given as posterior probabilities.
Results
Sample description
In total, 57 tick DNA samples were available for analysis. From these and as shown in supplementary information (S3 Table), 45 sequences were obtained for gltA (Genbank accession KX368721-KX368765), 57 for ompA (Accession: KX368868-KX368924), 44 for ompB (Accession: KX368823- KX368867), 57 for 17kDa (Accession: KX368766-KX368822) and 40 for sca4 (Accession: KX368925-KX368964). The gene sequences were subjected to BLAST analysis for a preliminary verification of their identity. BLAST results are shown in S1 Table.
Single gene topology trees and their ability to resolve study samples
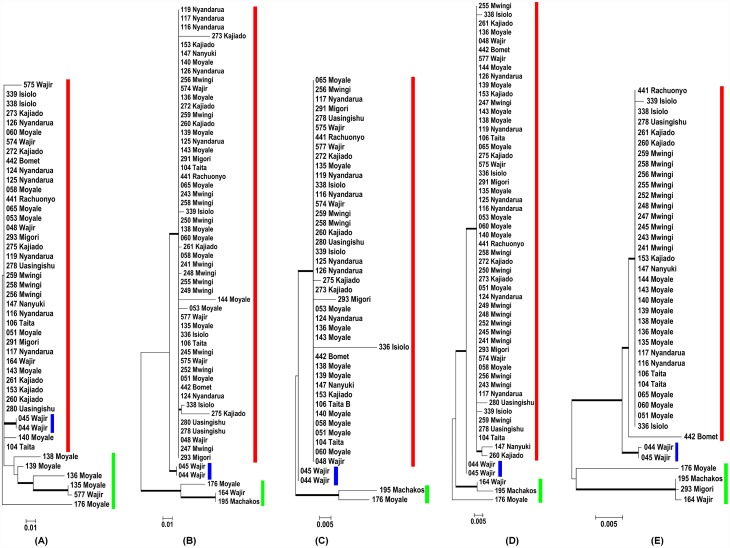
The topology of the gltA gene tree inferred with the ML method is shown in Fig 1, panel A. The tree shows unresolved evolutionary relationships (nodes with <50% bootstrap support values). Overlooking these clade credibility values, a major cluster (clade I) consisting of 89% of the sequences was observed in the more derived parts of the tree. The other two distinct clades were samples from the North Eastern part of Kenya. Clade II consisted of samples (044 and 045 from Wajir) that diverged as sister operational taxonomic units (OTUs). Clade III samples clustered at the basal part of the tree and came from Moyale (139, 135, 136 and 176) and Wajir (577).
Fig 1. Phylogeny of Rickettsia study samples isolated from diverse eco-regions of Kenya.
Maximum Likelihood trees were obtained from (A) gltA, (B) ompA, (C) ompB, (D) 17kDa and (E) sca4 partial nucleotide sequences. Members of clade I, II and III are shown beside the bolded red, blue and green lines respectively. Numbers at the nodes are bootstrap proportions with 1000 replicates. Only bootstrap values >50% are shown. The scale bar indicates the number of substitutions per nucleotide position.
In comparison to gltA, the ompA tree resolved majority of the nodes into three well supported groups (Fig 1, panel B). As in gltA, clade I had the majority of OTUs (52/57, 91%), and was the most derived. Majority of the nodes were unresolved, and the members clustered in a polytomy. As in gltA, clade II formed a dichotomy made of samples 044 and 045 from Wajir. Clade III constituted a cluster of 176_Moyale, 164_Wajir and 195_Machakos in the basal parts of the tree. In this clade, only 176_Moyale was shared with the gltA gene tree.
As shown in Fig 1, panel C, ompB also resolved the study OTUs into three groups. Clade I contained 40/44 (91%) members in a polytomy. Clade II contained the same sister OTUs (044 and 045) from Wajir. Clade III had the same samples as in the ompA gene tree. As with gltA, ompA and ompB genes, the topology of the 17kDa gene tree was consistent in delineating three clades (Fig 1, panel D). Clade I contained 52/57 (91%) of unresolved OTUs. Clade II consisted of sister OTUs (044 and 045) from Wajir, while clade III consisted of 164_Wajir, 195_Machakos and 176_Moyale
Compared to gltA, ompA, ompB and 17kDa, the sca4 gene tree was better resolved especially in delineation of clade I (Fig 1, panel E) which consisted of 35/40 (86%) OTUs. Clade II consisted of sister OTUs (044 and 045) from Wajir. Clade III consisted of 176_Moyale, 164_Wajir, and an additional member 293_Migori.
Concatenating gltA, ompA, ompB, 17kDa and sca4 genes allows better phylogenetic resolution
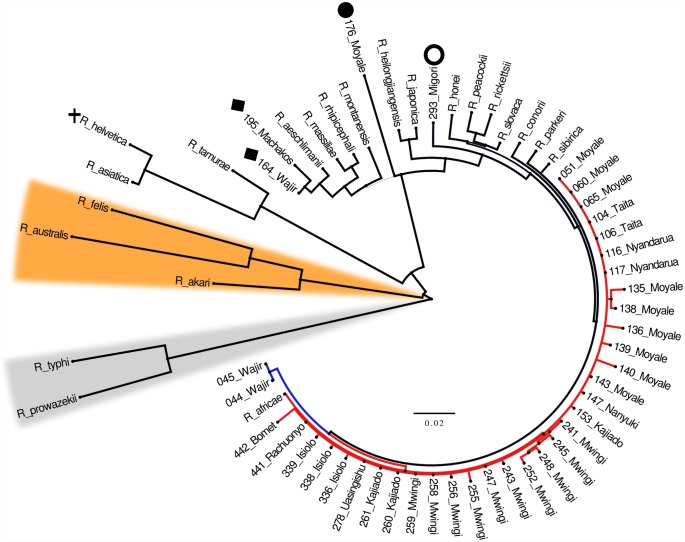
In order to improve the phylogenetic resolution of individual genes, the five genes were concatenated. Out of the 57 samples initially available for analysis, only 39 were included in the concatenation. The choice of the 39 was influenced by availability of the limiting gene (39 sca4 samples), and not missing more than one of the other four gene sequences. The concatenated sequences also included validated Rickettsia species available in GenBank. The concatenated tree constructed with Bayesian method is shown in Fig 2. The validated Rickettsia sequences delineated into the three known Rickettsia clades: TG, TRG and SFG. All the 39 study OTUs lay within the SFG clade, of which 33/39 (84%) clustered with the validated R. africae str ESF-5 shown as clade I. The two sister OTUs (044 and 045) from Wajir formed a distinct clade (clade II) that shared the most recent common ancestor with clade I. Two other samples (164_Wajir and 195_Machakos) previously identified as R. africae [26] delineated with the validated R. aeschlimanii. The positions of these OTUs held when tested by ML method (S1 Fig).
Fig 2. Bayesian probability tree of study samples with validated Rickettsia species.
The tree is based on partitioned concatenated datasets of gltA, ompA, ompB, 17kDa and sca4 partial nucleotide sequences. Amino acid alignments were used to guide the nucleotide alignments. The tree is estimated using a GTR+G substitution model as implemented in MrBayes v3.2. The tree is a consensus of 15,002 trees (post burn-in) pooled from two independent Markov Chain run in parallel. Thin lines indicate posterior probability values of < 1. Lineage diversity within the R. africae study samples is highlighted in red and blue to indicate clades i and ii respectively. Samples previously misclassified as R. africae are now classified as R. aeschlimanii (black diamond). Study sample 176_Moyale branches distinctly from other rickettsiae and is considered a novel rickettsia species and a provisional name "Candidatus rickettsia moyalensis" (black circle) is proposed. NB: Although 293_Migori (open circle) branched as a lone taxon, it clustered with R. aeschlimanii by Maximum Likelihood method. Non-spotted fever group lineages are highlighted orange for transition group and grey for typhus group. The status of R. helvetica (shown in black cross), originally in spotted fever group is now uncertain [20].
The position of 293_Migori was however tenuous, as it branched with R. aeschlimanii on ML method, but as a lone taxon between R. heilongjiangensis and R. slovaca on Bayesian analysis. Another interesting sample 176_Moyale branched as a lone taxon with total statistical support (a posterior probability value of 1), thus raising questions concerning its taxonomic status. We consider this sample as a novel rickettsiae and an interim name of “Candidatus Rickettsia Moyalensis” is proposed.
Phylogeny of R. africae study OTUs and those collected previously in Kenya
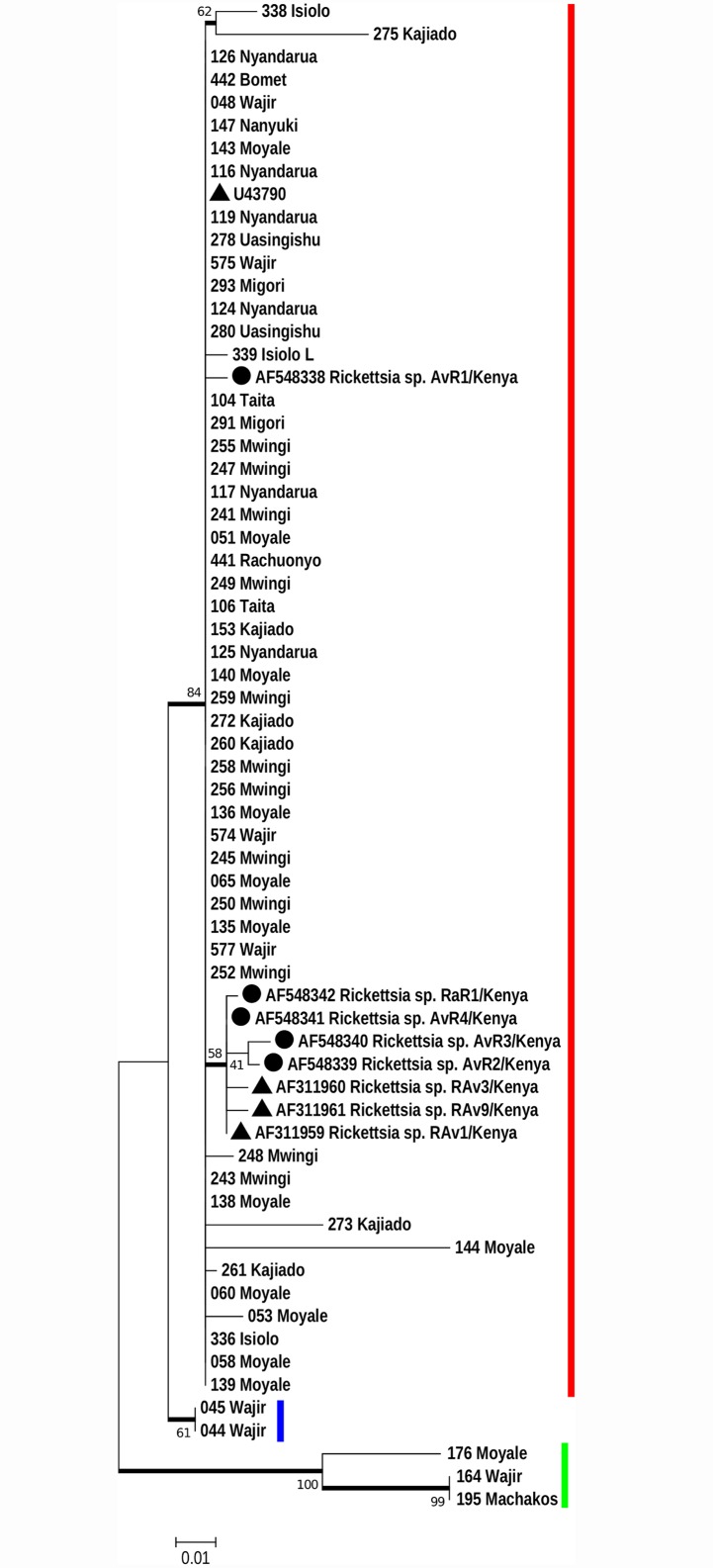
The ompA gene sequences obtained from our study samples and those published previously collected in Kenya [23,31] were used to generate a phylogenetic tree (Fig 3). With this tree, three clades were discernible. Clade I contained majority of the OTUs 61/66 (92.4%) and a cluster of other 7 that formed a subclade within clade I, that consisted of OTUs published in previous studies. Clade II consisted of sister OTUs (044 and 045) from Wajir, and a basal clade III that consisted of members 176_Moyale, 164_Wajir, and 195_Machakos. Clearly, five of our sequences (044, 045 and 164 from Wajir, 176 Moyale and 195 Machakos) are distinct from those described previously.
Fig 3. Phylogeny of Rickettsia sequences from this study and those collected previously in Kenya [23, 31].
ompA nucleotide sequences of study isolates and other R. africae reported from previous studies [23,31] were analysed by Maximum Likelihood method using MEGA v7 based on the Hasegawa-Kishino-Yano (HKY) model of substitution. The tree has a log likelihood ratio of -1049 and involved all codon positions. Members of clade I, II and III are shown beside the bolded red, blue and green lines respectively. Sequences from Parola et al 2001 [23] are shown as black triangles and those from Macaluso et al 2003 [31] by black circles. Numbers at the nodes are bootstrap proportions with 1000 replicates. Only bootstrap values >50% are shown. The scale bar indicates the number of substitutions per nucleotide position. Clearly, five of our sequences (044, 045 and 164 from Wajir, 176 Moyale and 195 Machakos) are distinct from those described previously.
Discussion
In this study, a combination of relatively conserved (gltA, 17kD) and variable (ompA, ompB, and sca4) genes were used to infer the evolutionary topology of R. africae using DNA samples obtained from ticks [26]. This work expands on a recent study that reported a significant heterogeneity of R. africae samples collected from a localized area in Western Kenya [33]. We also report on possible existence of a sub-lineage within the R. africae samples, as well as identify a putative novel Rickettsia species that was associated with R. appendiculatus tick collected from a cow in Moyale County, in Northern Kenya.
Due to its intracellular lifestyle, rickettsiae are highly dependent on their primary tick vectors and tend to be selective for the tick species they infect. For example, R. africae are primarily vectored by the Amblyomma species [41] but infections have also been found in Rhipicephalus and Hyalomma ticks [3]. As shown in S1 Table, 84% of the R. africae sequences came from Amblyomma and Rhipicephalus ticks. The remaining 16% came from H. truncatum and other unspeciated Hyalomma ticks. Nevertheless, without competency studies, it is difficult to say which of these findings were true infections.
The gltA gene codes for citrate synthase, an enzyme ubiquitously found in nearly all living cells, and is central in energy metabolism [42]. Evolutionary history inferred from this gene demonstrated low sequence divergence and yielded poorly supported clades with unresolved nodes (Fig 1, panel A). This is expected considering that, even within the Rickettsia genus, gltA gives poor interspecies resolution especially for the more derived branches of the SFG [9]. Nevertheless, three unsupported groupings (Fig 1, panel A, shown by red, blue and green lines) were discernible. It could be argued that the lack of resolution emanated from using a small fragment (385 bp) compared to 1234 bp that was used by Roux et al [9]. The Roux study aimed to develop gltA gene as a phylogenetic marker for Rickettsia species. From their generated phylogenies, the resolution decreased within the more recently emerged species, of which R. africae is a member. Our study that focused on intra-species variation within the R. africae lineage had similar problems with gltA and it is doubtful that a longer gltA that failed to resolve the recently evolved rickettsiae would have resolved variation within the study OTUs. We think longer fragments may have increased the number of variable characters within the gene but not the resolution.
As shown in Fig 1 (panels B, C and D), gene trees derived from ompA, ompB and 17kDa had very similar topologies and the three groupings seen in gltA tree were better supported. This topological concordance gives credence to the derived gene trees. By BLAST analysis, majority of the OTUs clustering in clade I were identical to R. africae reference strain ESF (S1 Table).
Tree topologies derived from individual genes identified clades II and III as outliers (Fig 1, blue and green lines). Interestingly, for all the genes, clade II had only two members (044_Wajir and 045_Wajir) that appeared as a sub-lineage within R. africae. With four of the five genes analysed, BLAST analysis of the two samples showed highest identity to R. africae (accession no. HQ335126) with 99% for gltA, >99% for ompA (accession no. HQ335132), 99% for 17kDa (accession no. KF646137) and 100% for sca4 (accession no. CP001612). BLAST analysis of the ompB gene showed highest homology (98%) to R. mongolotimonae (accession no. DQ097083). Within our study OTUs, members of clade III were the most genetically distant. With the Sca4, one member (293_Migori) that had consistently been in clade I was placed in clade III.
From the foregoing, the limitations of gene trees constructed from single genes are evident and give credence to recommendations to use a variety of genes sampled from different regions of the genome as the best practice for phylogeny assignment [43]. Fig 2 shows a Bayesian tree of study OTUs and the validated Rickettsia species derived from a concatenated sequence of gltA, ompA, ompB, 17kDa and sca4. The concatenated tree confirms the branching orders of clade I and authenticates members of clade II (044_ and 045_Wajir) as being a sub-lineage of R. africae. This derivation was confirmed by ML method (S1 Fig). Only after concatenation is the picture clearer that, the majority of members populating clade III in single gene analysis are not R. africae. Samples 164_Wajir and 195_Machakos (both associated with H. truncatum ticks from a cow) delineate with R. aeschlimanii, while 293_Migori appeared as a lone taxon (Fig 2).
Since sample 176_Moyale did not branch with any of the validated species in the concatenated tree (Fig 2), its sequence was compared to isolates available in GenBank. With the 276 bp gltA gene, it was most identical to R. heilongjiangensis (97.0%). With this gene, a homology of 99.9% is required in order for the sample to qualify as R. heilongjiangensis [29]. With the 489 bp fragment of ompA gene, the DNA was most identical to Candidatus R. amblyommii (97.0%) against the required homology of 98.8%. With the ompB gene, the 267 bp fragment was most identical to Rickettsia rhipicephali (99.0%) against a required homology of 99.2% to qualify as R. rhipicephali. With the sca4 gene, the 1846 bp was most similar to R. africae str ESF5 (97.0%) against the required homology of 99.3%. For the 17kDa, the closest homology was with Rickettsia raoultii str Khabarovsk (97.0%) identity. There are no published homology requirements for 17kDa gene. Given this level of nucleotide sequence divergence from validated Rickettsia species, our results support the consideration of 176_Moyale as a sample from a new Rickettsia species. Until grown in culture, and its biology elucidated, we propose that this sample be identified as “Candidatus R. moyalensis”.
Two other studies in Kenya have reported genetic heterogeneity within the R. africae lineage, one in Maasai Mara game reserve [31] and another in rural farming community in Asembo, Nyanza province [33]. The current study extends these findings and identifies Northern Kenya (Moyale and Wajir) as harbouring more heterogeneous R. africae or completely new species (Fig 2 and S1 Fig) compared to other regions. To determine homology of our study OTUs and those described as R. africae variants in previous studies in Kenya [23, 31], ompA gene sequences were compared. As shown in Fig 3, five of our sequences (044, 045 and 164 from Wajir, 176 Moyale and 195 Machakos) are distinct from those described previously. Unfortunately, we did not sequence a second region of ompA and ompB genes that were associated with significant sequence variation [33]. The Rickettsia species dynamics in Kenya are probably being moulded by: 1) the highly mobile nomadic populations that move across wide geographical borders that create opportunities for mixing of rickettsiae from different livestock species (goats, sheep, cows, donkeys and camels). 2) The encroachment of wildlife habitats by nomadic pastoralists that introduce previously isolated Rickettsia species in wildlife to livestock. We speculate that these factors could be responsible for the observed genetic variations within the Kenyan R. africae, as well providing opportunity for genetic mixing that may result in creation of new lineages.
Conclusion
This study provides new information regarding the phylogenetic relationships of the R. africae lineage. This lineage, though only recently emerged [30] is clearly undergoing diversification, as observed in the branching order of the samples studied. A definite sub-lineage of R. africae (samples 044 and 045 from Wajir) was identified. It was impossible to confirm the placement of sample 293_Migori. Additional sequence data will be required to resolve the ambiguity. Lastly, a putative novel rickettsiae (sample 176_Moyale) with a proposed name of “Candidatus Rickettsia moyalensis” was identified. Further work will be needed to determine its prevalence in Kenya and its implications to human and/or animal disease.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
A General Time Reversal with Gamma distribution (GTR+G) model was used to infer phylogeny of concatenated partial sequences of gltA, ompA, ompB, 17kDa and sca4 nucleotide sequences. Amino acid alignments were used to guide the nucleotide alignments. The tree with the highest log likelihood (-8252.6475) is shown. Study samples that are bonafide R. africae aggregate in clades I and II. Samples previously misclassified as R. africae are now classified as R. aeschlimanii (black diamond). Study sample 176_Moyale branches distinctly from other rickettsiae and is considered a novel rickettsia species provisionally named "Candidatus rickettsia moyalensis" (black circle). With this method, 293_Migori (open circle) clusters with R. aeschlimanii. Numbers at the nodes are bootstrap proportions with 1000 replicates. Only bootstrap values >50% are shown. SFG = spotted fever group, TRG = transition group, TG = typhus group. The status of R. helvetica (shown in black cross), originally in spotted fever group is now uncertain [20].
(TIF)
Acknowledgments
We thank the staff of the Basic Science Laboratory, Walter Reed Project for their considerable help with this study. We are grateful to Ms Elizabeth Vitalis for reading the manuscript and making useful remarks. This work is published with the permission of the Director, Kenya Medical Research Institute.
Disclaimer
The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The U.S. Government has the right to retain a nonexclusive, royalty free license and to any copyright covering this paper.
Data Availability
The sequences for the study OTUs are publicly available in the Genbank and can be accessed using accession numbers provided in the manuscript (S3 Table).
Funding Statement
Financial support for this study was from a grant from the US Armed Forces Health Surveillance Center, Division of Global Emerging Infection Surveillance Operations. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proc Biol Sci. 2006;273: 2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10: 694–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin Microbiol Rev. 2013;26: 657–702. 10.1128/CMR.00032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LPSN. List of prokaryotic names with standing in nomenclature [Internet]. [cited 18 March 2016]. Available: http://www.bacterio.net/rickettsia.html
- 5.Roux V, Raoult D. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res Microbiol. 1995;146: 385–396. [DOI] [PubMed] [Google Scholar]
- 6.Fournier PE, Raoult D. Current knowledge on phylogeny and taxonomy of rickettsia spp. Ann N Y Acad Sci. 2009;1166: 1–11. 10.1111/j.1749-6632.2009.04528.x [DOI] [PubMed] [Google Scholar]
- 7.Tamura a, Ohashi N, Urakami H, Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45: 589–591. [DOI] [PubMed] [Google Scholar]
- 8.Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48: 4404–4409. 10.1128/JCM.01526-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47: 252–261. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BE, Tzianabos T. Comparative sequence analysis of a genus-common Rickettsial antigen gene. J Bacteriol. 1989;171: 5199–5201. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=210341&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier PE, Roux V, Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int J Syst Bacteriol. 1998;48 Pt 3: 839–849. [DOI] [PubMed] [Google Scholar]
- 12.Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol. 2000;50: 1449–1455. [DOI] [PubMed] [Google Scholar]
- 13.Sekeyova Z, Roux V, Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of “gene D”, which encodes an intracytoplasmic protein. Int J Syst Evol Microbiol. 2001;51: 1353–1360. [DOI] [PubMed] [Google Scholar]
- 14.Ngwamidiba M, Blanc G, Ogata H, Raoult D, Fournier PE. Phylogenetic study of Rickettsia species using sequences of the autotransporter protein-encoding gene sca2. Ann N Y Acad Sci. 2005;1063: 94–99. [DOI] [PubMed] [Google Scholar]
- 15.Merhej V, Raoult D. Rickettsial evolution in the light of comparative genomics. Biol Rev. 2011;86: 379–405. 10.1111/j.1469-185X.2010.00151.x [DOI] [PubMed] [Google Scholar]
- 16.Murray GGR, Weinert LA, Rhule EL, Welch JJ. The Phylogeny of Rickettsia Using Different Evolutionary Signatures: How Tree-Like is Bacterial Evolution? Syst Biol. 2016;65: 265–279. 10.1093/sysbio/syv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillespie JJ, Beier MS, Rahman MS, Ammerman NC, Shallom JM, Purkayastha A, et al. Plasmids and Rickettsial evolution: Insight from Rickettsia felis. PLoS One. 2007;2: e266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillespie JJ, Driscoll TP, Verhoeve VI, Utsuki T, Husseneder C, Chouljenko VN, et al. Genomic Diversification in Strains of Rickettsia felis Isolated from Different Arthropods. Genome Biol Evol. 2014;7: 35–56. 10.1093/gbe/evu262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinert L a, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009;7: 6 10.1186/1741-7007-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driscoll T, Gillespie JJ, Nordberg EK, Azad AF, Sobral BW. Bacterial DNA sifted from the Trichoplax adhaerens (Animalia: Placozoa) genome project reveals a putative rickettsial endosymbiont. Genome Biol Evol. 2013;5: 621–645. 10.1093/gbe/evt036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournier P, Belghazi L, Robert C, Elkarkouri K, Richards AL, Ogawa M, et al. Variations of Plasmid Content in Rickettsia felis. PLoS One. 2008;3: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly PJ, Beati L, Mason PR, Matthewman L a, Roux V, Raoult D. Rickettsia africae sp. nov., the etiological agent of African tick bite fever. Int J Syst Bacteriol. 1996;46: 611–614. [DOI] [PubMed] [Google Scholar]
- 23.Parola P. Rickettsioses in sub-Saharan Africa. Ann N Y Acad Sci. 2006;1078: 42–47. [DOI] [PubMed] [Google Scholar]
- 24.Kelly PJ. Rickettsia africae in the West Indies. Emerg Infect Dis. 2006;12: 224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldin C, Mediannikov O, Davoust B, Cabre O, Barré N, Raoult D, et al. Emergence of Rickettsia Africae, Oceania. Emerg Infect Dis. 2011;17: 100–102. 10.3201/eid1701.101081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutai BK, Wainaina JM, Magiri CG, Nganga JK, Ithondeka PM, Njagi ON, et al. Zoonotic Surveillance for Rickettsiae in Domestic Animals in Kenya. Vector-Borne Zoonotic Dis. 2013;13: 360–366. 10.1089/vbz.2012.0977 [DOI] [PubMed] [Google Scholar]
- 27.Maina AN. Sero-epidemiology and molecular characterization of Rickettsiae infecting humans, selected animals and arthropod vectors in Asembo, western Kenya, 2007–2010 Alice Ngonyo Maina A thesis submitted in partial fulfilmentfor the degree of Doctor of Philosop [Internet]. Jomo Kenyatta University of Agriculture and Technology. 2012. Available: http://ir.jkuat.ac.ke/handle/123456789/1016
- 28.Socolovschi C, Matsumoto K, Marie J Lou, Davoust B, Raoult D, Parola P. Identification of Rickettsiae, Uganda and Djibouti [2]. Emerg Infect Dis. 2007;13: 1508–1509. 10.3201/eid1310.070078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41: 5456–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fournier P-E, Raoult D. Identification of rickettsial isolates at the species level using multi-spacer typing. BMC Microbiol. 2007;7: 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macaluso KR, Davis J, Alam U, Korman A, Rutherford JS, Rosenberg R, et al. Spotted fever group Rickettsiae in ticks from the masai mara region of Kenya. Am J Trop Med Hyg. 2003;68: 551–553. [DOI] [PubMed] [Google Scholar]
- 32.Parola P, Inokuma H, Camicas JL, Brouqui P, Raoult D. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg Infect Dis. 2001;7: 1014–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maina AN, Jiang J, Omulo S a., Cutler SJ, Ade F, Ogola E, et al. High Prevalence of Rickettsia africae Variants in Amblyomma variegatum Ticks from Domestic Mammals in Rural Western Kenya: Implications for Human Health. Vector-Borne Zoonotic Dis. 2014;14: 693–702. 10.1089/vbz.2014.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang J, Blair PJ, Felices V, Moron C, Cespedes M, Anaya E, et al. Phylogenetic analysis of a novel molecular isolate of spotted fever group Rickettsiae from northern Peru: Candidatus Rickettsia andeanae. Ann N Y Acad Sci. 2005;1063: 337–342. [DOI] [PubMed] [Google Scholar]
- 35.Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ. Basic Local Alignment Search Tool. J Mol Biol. 1990;215: 403–410. [DOI] [PubMed] [Google Scholar]
- 36.Edgar RC, Drive RM, Valley M. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne I, Lindner D, Bayer M, Husmeier D, Mcguire G, Marshall DF, et al. TOPALi v2: A rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics. 2009;25: 126–127. 10.1093/bioinformatics/btn575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posada D. jModelTest: Phylogenetic model averaging. Mol Biol Evol. 2008;25: 1253–1256. 10.1093/molbev/msn083 [DOI] [PubMed] [Google Scholar]
- 40.Ronquist F, Teslenko M, Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3. 2 : Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst Biol. 2012;61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fournier P-E, El Karkouri K, Leroy Q, Robert C, Giumelli B, Renesto P, et al. Analysis of the Rickettsia africae genome reveals that virulence acquisition in Rickettsia species may be explained by genome reduction. BMC Genomics. 2009;10: 166 10.1186/1471-2164-10-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiegand G, Remingtonl SJ. Citrate Synthase: Structure, Control, and Mechanism. Annu Rev Biophys Biophys Chem. 1986;15: 97–117. [DOI] [PubMed] [Google Scholar]
- 43.Cummings MP, Otto SP, Wakeley J. Sampling properties of DNA sequence data in phylogenetic analysis. Mol Biol Evol. 1995;12: 814–822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
A General Time Reversal with Gamma distribution (GTR+G) model was used to infer phylogeny of concatenated partial sequences of gltA, ompA, ompB, 17kDa and sca4 nucleotide sequences. Amino acid alignments were used to guide the nucleotide alignments. The tree with the highest log likelihood (-8252.6475) is shown. Study samples that are bonafide R. africae aggregate in clades I and II. Samples previously misclassified as R. africae are now classified as R. aeschlimanii (black diamond). Study sample 176_Moyale branches distinctly from other rickettsiae and is considered a novel rickettsia species provisionally named "Candidatus rickettsia moyalensis" (black circle). With this method, 293_Migori (open circle) clusters with R. aeschlimanii. Numbers at the nodes are bootstrap proportions with 1000 replicates. Only bootstrap values >50% are shown. SFG = spotted fever group, TRG = transition group, TG = typhus group. The status of R. helvetica (shown in black cross), originally in spotted fever group is now uncertain [20].
(TIF)
Data Availability Statement
The sequences for the study OTUs are publicly available in the Genbank and can be accessed using accession numbers provided in the manuscript (S3 Table).